1. Introduction
Biofouling on submerged maritime structures can lead to increased fuel consumption, higher frictional resistance, reduced maneuverability, and greater maintenance and cleaning costs [
1]. Marine pollution caused by antifouling substances is now regarded as one of the world’s most serious problems. As a result, scientists and environmentalists are increasingly concerned about the environmental degradation caused by these chemicals [
2]. Marine constructions frequently use antifouling paint to prevent biofouling. By 2021, the market of antifouling coatings and paints is expected to be valued at USD 9.22 billion [
3]. In the coming years, the global market for antifouling coatings is anticipated to increase significantly. As indicated by a report published in November 2024, the market is projected to develop at a compound annual growth rate (CAGR) of 8.2% from 2024 to 2030, reaching a size of USD 16.40 billion [
4]. As mentioned by a report published in October 2024, the market is expected to grow at a compound annual growth rate (CAGR) of 8.22% from 2024 to 2034, surpassing USD 22.68 billion. According to these forecasts, the antifouling coatings market is expected to grow steadily due to rising demand for these products in a variety of applications [
5].
The practice of antifouling for boats and ships dates back to ancient Greek and Roman times. Early antifouling coatings, including grease, tar, brimstone, and sulfur pitch, were later developed in the United Kingdom. Irgarol 1051 on the French Riviera was the first known instance of booster biocides contaminating waters near the coast [
6]. To combat iron ship corrosion, copper sheathing—used over a century ago—prevented fouling by releasing toxic metal ions. This innovation eventually led to the development of antifouling coatings in the mid-1800s [
7,
8]. Despite recent legislative changes, copper oxide has been the most widely used antifouling agent in the UK for the past ten years, following in sequence of application by TBT, Irgarol 1051, diuron, dichlofluanid, and zinc [
9].
Significant levels (till 1700 ng per liter) were identified in the nearby marinas. Several antifouling substances (biocides), that are found in high boating activity regions, exist in combination form [
10]. Irgarol 1051 was discovered in 13 of 26 saltwater samples collected from various marine areas, making it the most frequently occurring antifouling component. The highest ever measured concentration was 4.0 µg L
−1. It was discovered to indicate ecological risk from strains in the Arctic [
11].
Following a restriction on tributyle-tin (TBT) use due to its negative environmental effects, Irgarol 1051 became the most well-known biocide. This resulted in a revenue loss of USD 147 million in the Arcachon Bay area [
12]. At low concentrations (up to 10 ng/L), it was also discovered that other mollusk types’ wild populations were impacted [
13]. The European Community, in 1989, implemented a regulation that prohibited the application of tributyle-tin on ships shorter than 25 m [
14].
As the principal producers in aquatic ecosystems, algae are essential. It is believed that half of the world’s primary production, which provides the minerals and energy needed to maintain planetary ecosystems, is produced by algae and cyanobacteria [
15]. The origins of microalgae vary according to species, size, and the structure of the cell wall, as well as the many enzymes that break them down. These species also range in terms of how sensitive and reactive they are to organotin chemicals. As a result, several species have been observed to develop tolerance to organotin chemicals, either breaking them down or accumulating them [
16].
Irgarol 1051 is a diamino-1,3,5-triazine that is 1,3,5-triazine-2,4-diamine carrying a N-tert-butyl, N’-cyclopropyl, and a methyl-sulfanyl group at position 6. It has a role as an antifouling biocide, a xenobiotic, and an environmental contaminant. It is an aryl sulfide, a member of cyclopropanes, and a diamino-1,3,5-triazine. It is functionally related to a 1,3,5-triazine-2,4-diamine. It derives from a hydride of 1,3,5-triazine (source: National Library of Medicine). There are structural similarities between Irgarol 1051 and other s-triazine derivatives like simazine and atrazine. Among the main functional groups of Irgarol are the methylthio group, alkylamino substituents, and the s-triazine core, which all work together to effectively block photosystem II (PSII) in aquatic plants and algae. Atrazine and simazine both function as PSII inhibitors and share this mechanism. However, unlike atrazine, Irgarol is more hydrophobic, preventing the attraction of freshwater and marine algae. Irgarol 1051, an s-triazine-based herbicide, is frequently used in European coastal waters. Additionally, it has been demonstrated that Irgarol 1051 is extremely harmful to freshwater and marine microalgal growth and also to the initial phases of macroalgae zoospore formation [
17]. For instance, at sub-nanomolar dosages, Irgarol has an impact on the algal population [
18]. Irgarol 1051 is very detrimental to aquatic life, especially primary producers, because it suppresses PS II [
19]. Antifouling Irgarol showed a highly destructive impact on different marine algae—especially in ports with high shipping activities—due to its high concentrations in marine ports. It showed highly adverse effects on the growth, amino acids, and total chlorophyll content of different marine algae [
7,
11].
The monitoring study by Muñoz et al. [
20] on Irgarol toxicity among 12 mico-organic pollutants on Spain’s beach showed that antifouling Irgarol exceeds the adverse effect threshold for marine species. The average concentration of Irgarol in Spain’s beach was around 0.36 ng·L
−1. However, studies have shown that this compound has a modest potential for bioconcentration, posing minimal risk to humans and predators consuming fish. On the other hand, Cima and Varello [
21] demonstrated that Irgarol exhibits toxicity to animals. Given its widespread use in the marine environment for many years, there is an undeniable risk to coastal biocoenoses due to chemical interactions between Irgarol and various ligands, complex formation, and bioaccumulation within trophic chains. Paints often contain Irgarol, an antifouling chemical, to stop fouling organisms from attaching to and growing on ship hulls or other underwater structures. Worldwide limitations on incorporating them in antifouling paints have been brought by worries about their toxicity to aquatic creatures that are not intended targets [
22].
It is common for organisms in high-shipment marine environments to be simultaneously exposed to a range of biocides that have the ability to interact and affect vulnerable species in an antagonistic, additive, or synergistic manner [
23]. The European Union created the Biocidal Agents Directive to permit the use of biocidal chemicals inside its boundaries. The unified data standards have been used by the EU for both new and ongoing biocide programs. Each antifouling agent submitted for registration was required to provide a baseline set of data [
24]. The prohibition on the use of antifouling systems containing Irgarol-1051 officially came into effect on 1 January 2023 [
25].
Due to the synergy between various nutrients and antioxidant components, the two marine algae species in this study possess an excellent nutritional profile. Unlike many plant-based food sources, they provide essential nutrients, proteins, and vitamins, including vitamin D and B12. In contrast,
chlorella products offer these elements and more. Compared to other plant-based diets,
Chlorella sp. has higher levels of iron and folate Aquaculture can safely and sustainably use
Chlorella sp. as it does not contain any hazardous compounds [
26,
27]. Due to the high concentration of vital amino acids, protein, fats, carbs, vitamins, and pigments found in
Dunaliella sp., it is recommended that fish use this natural feed. It is said to be abundant in beneficial antioxidants such as vitamin B
12, β-carotene, zeaxanthin, and omega-3 polyunsaturated fatty acids [
28].
The purpose of the work was to assess the harmful effects of Irgarol 1051, a biocide frequently found in antifouling systems, against two marine algae species: Dunaliella bardawil and Chlorella salina. The objective of the study was to investigate how Irgarol 1051 affected algal growth and metabolite composition in a lethal and sublethal impact. In order to accomplish this, comprehensive analyses, including IR spectroscopy, protein profiling, and evaluations of antioxidant activity, and the employed laboratory tests have been carried out to determine the concentration at which 50% of algae were affected (EC50). Additionally, the study used spatial analysis to monitor the proliferation of algae in Egyptian coastal waters in order to pinpoint areas where these algae are most prevalent, especially in front of the governorates of the Nile Delta.
Spatial analysis plays a vital role in mapping the distribution of Dunaliella bardawil and Chlorella salina along the Egyptian Mediterranean coast. Using remote sensing, GIS, and other spatial tools, researchers can monitor the proliferation of these algae, identify high-density zones, and analyze environmental factors influencing their growth. This approach helps pinpoint optimal locations for potential commercial cultivation, assess ecological impacts, and track seasonal variations. In regions like the Nile Delta, where coastal conditions fluctuate, spatial analysis provides valuable data for sustainable management and conservation efforts.
3. Results and Discussion
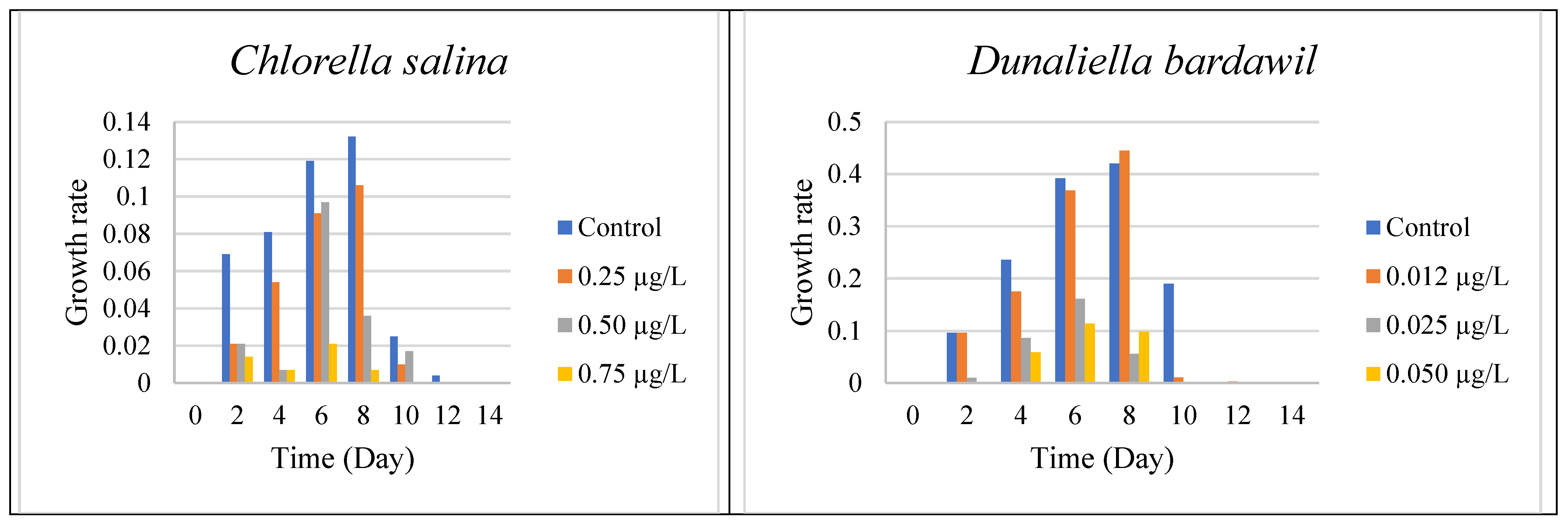
On the Mediterranean coast of Egypt, the mass concentration of algae, which is represented by chlorophyll, varied from east to west. Using geostatistical studies and remote sensing, we examined the possible locations for algal development throughout the Mediterranean coast of Egypt. The two tested algal species” Chlorella salina and Dunaliella bardawil “were significantly inhibited at all the tested Irgarol doses, which correlated with the biocide’s increasing toxicity.
To determine the effective concentration (EC50) of Irgarol 1051 for Chlorella salina and Dunaliella bardawil, the organisms were cultured under varying concentrations of the compound. The results indicated that both algae perished within two days of exposure. Dunaliella bardawil exhibited more severe adverse effects compared to Chlorella salina. Experiments conducted under different concentrations revealed that both organisms experienced significant stress and perished by the sixth day. The effective concentration (EC50) for C. salina was approximately 0.5 µg·L−1 on the eighth day, whereas for D. bardawil, it was much lower at 0.025 µg·L−1. These results indicate that D. bardawil is more sensitive to Irgarol 1051 than C. salina. The tested Irgarol 1051 doses for C. salina were 0.25, 0.5, and 0.75 µg L−1, while for D. bardawil they were 0.012, 0.025, and 0.05 µg·L−1 (one higher and one lower dose for each algal species).
The results presented in
Figure 1 and
Supplementary Materials Table S1 indicate that algal growth steadily increased until the eighth day for both organisms, reaching its peak compared to the controls. However, the growth rates varied depending on the Irgarol concentration, with
Dunaliella bardawil experiencing greater growth inhibition than
Chlorella salina.
A two-way ANOVA test was used to determine the effect of Irgarol 1051 on the growth of Chlorella salina and Dunaliella bardawil during the 14 days. The study found a dose-dependent decrease in algal growth, with higher levels of Irgarol 1051 causing stronger growth inhibition (p < 0.01). Least Significant Difference (LSD) testing revealed significant differences between the control and treatment groups. The impact was more evident in D. bardawil, indicating a greater sensitivity to the biocide than Chlorella salina.
However, Gatidou et al. [
34] observed that Irgarol 1051 inhibits the growth of
Dunaliella tertiolecta at concentrations greater than 0.8 µg·L
−1 and kills almost all of the cells at 3.0 µg·L
−1. Similarly, Kaamoush and El-Agawany [
35] reported comparable findings, indicating that Dunaliella salina exhibited growth stimulation at the lowest concentration of the antifouling agent Irgarol (0.012 µg·L
−1). However, as the Irgarol concentration increased, a progressive inhibition in growth was observed. The findings obtained are in line with those explained by Anita et al. [
36] who demonstrated that at doses below 100 ng L
−1, Irgarol kills 50% of the phytoplankton. It has been demonstrated that Irgarol 1051 is an acutely dangerous chemical for cyanobacterial growth since it inhibits cell growth at doses ranging from 5 to 10 µg·L
−1 at 4 days [
33,
34,
35,
36,
37].
3.1. Infrared Spectroscopy
IR spectroscopy enables rapid examination with tiny quantities of material while providing good analytical quality. It provides a signature of the macromolecular makeup of the cells. Chemometrics has advanced to the point where it is possible to quantify not just large cellular pools like protein, lipid, and carbohydrate but also small components to consider.
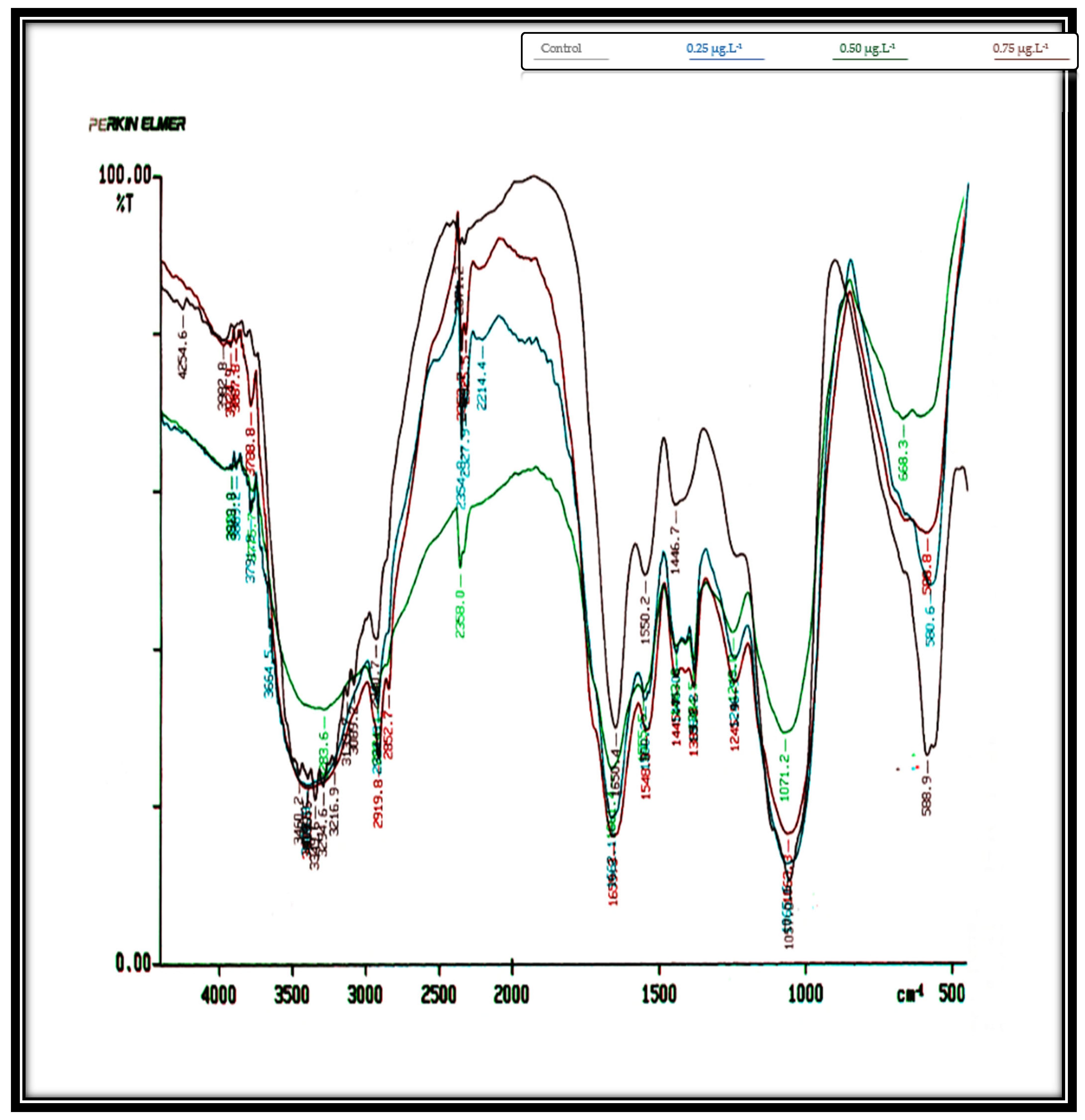
Table 1 records the obtained infrared spectra of the investigated two algae,
C. salina and
D. bardawil, which were cultivated for 8 days while being treated with Irgarol 1051, an antifouling agent.
Figure 2 shows the graphs of these spectra. The entire biochemical makeup of the algae cells was represented in these data. These displayed the band assignments that were derived from analyses of macromolecules and whole cell organelles in the 4250–500 cm
−1 range. The overall count of spectrum ranges that are able to explain the chemical standards of the two examined algae at the regions between 4250 and 500 cm
−1 was indicated by the obtained spectra.
The IR spectra of untreated (control)
Chlorella salina cells displayed a total of 16 peaks. At Irgarol concentrations of 0.25, 0.50, and 0.75 µg·L
−1, the analysis revealed the presence of 16, 15, and 12 peaks, respectively. The majority of the peaks are found between 3500 and 2750 cm
−1 in frequency. While the majority of the peaks in the treated cultures were observed at frequencies between 1750 and 1060 cm
−1 (five peaks), the control culture had eight peaks. In the frequency range of 3500–2750 cm
−1, the control culture exhibited eight peaks. However, at the same frequencies, only six peaks were observed in cultures treated with Irgarol at concentrations of 0.25, 0.50, and 0.75 µg·L
−1, respectively. Furthermore,
Table 1 data clearly shows that six new peaks occurred at concentrations of 0.25 µg·L
−1 and 0.50 µg·L
−1 of Irgarol, four new peaks appeared at Irgarol concentrations ranging from 0.50 µg·L
−1 to 0.75 µg·L
−1, with only two new peaks detected.
Methyl group, methylene group, and methyl group Vas C-H are represented by the eight number of peaks that emerged in the 3500–2750 cm
−1 control zone, mostly from lipids and fatty acids, in combination with V C=O of ester functional groups [
38]. The spectral bands ranging from 1153~1020 cm
−1 may be definitively attributed to carbohydrates, mostly because of C-O-C stretching vibrations. However, there is a band at 1080 cm
−1 that also includes contributions from phosphorylated compounds including nucleic acids and carbohydrates [
39]. In the treated cultures, most of these significant chemical compounds disappeared. However, in
Chlorella salina, no noticeable effect was observed for molecules within the frequency range of 1060–500 cm
−1, which corresponds to the N, C, O, and H bonds of polysaccharides. The asymmetric stretching mode of phosphorylated molecules, including nucleic acids, and the contributions from carbohydrates are shown by the band at 1080 cm
−1 [
40].
At frequencies between 1750 and 1060 cm
−1, an equal number of peaks (5) appeared across all the treated concentrations of Irgarol 1051. This suggests that two new compounds emerged at these frequencies in all the treated samples compared to the control. These peaks correspond to amides associated with proteins and the phosphodiester backbone of DNA and RNA. Additionally, bands arising from P=O asymmetric stretching or C-H ring bending provided further structural information [
41]. Under these frequencies, two bands appeared new under all the tested concentrations of Irgarol.
At frequencies 2750 to 1060 cm
−1, the total peaks at control were four: eight at 0.25 µg·L
−1, seven at 0.50 µg·L
−1, and six at 0.75 g/L Irgarol 1051. These findings demonstrate that the majority of the novel compounds discovered at these frequencies are amides connected with protein, as well as ether functional groups from lipids and fatty acids [
42]. This phenomenon is also related to neutral lipids’ protective actions towards stresses [
43].
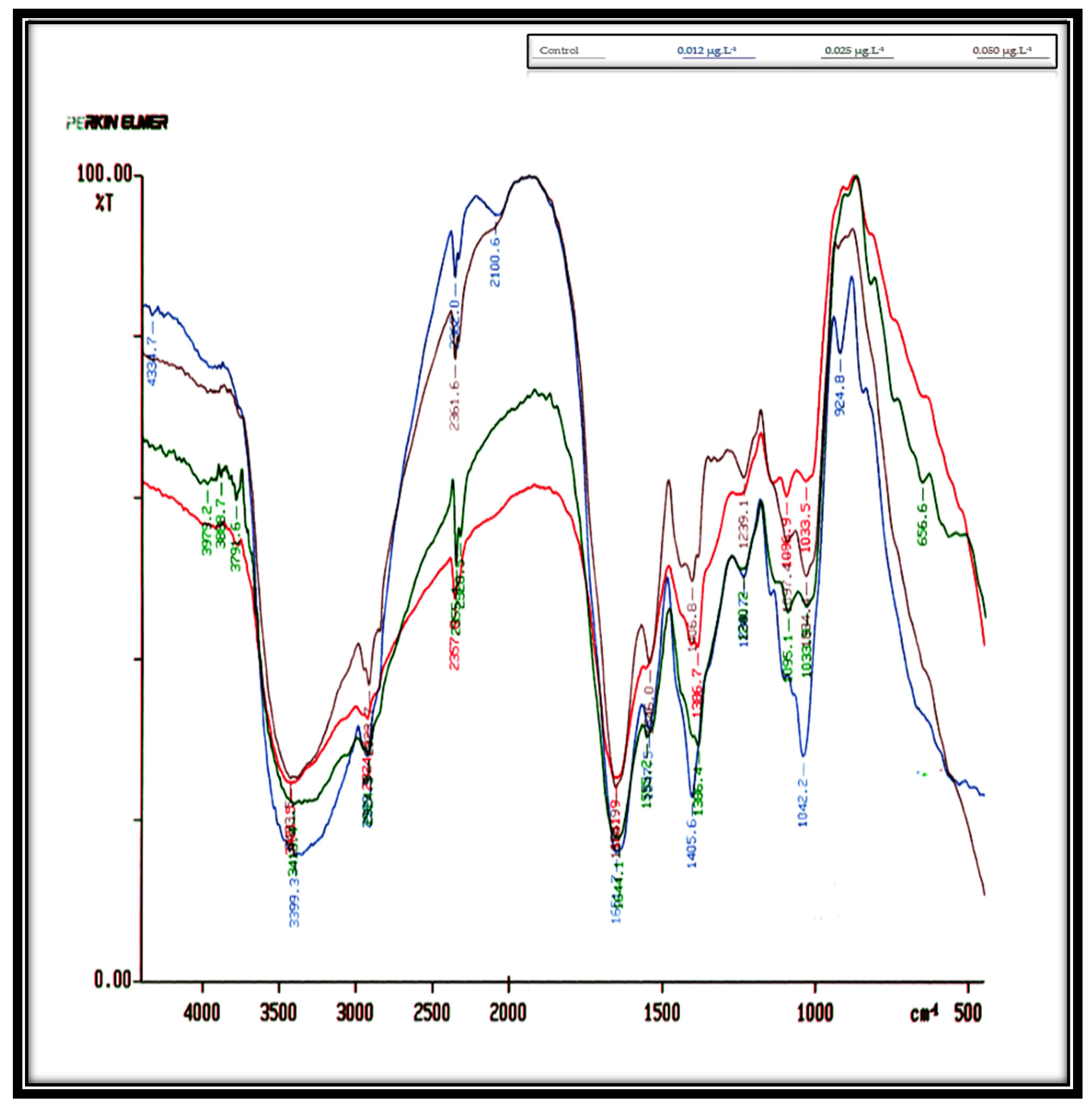
Table 2,
Figure 3 show the influence of concentrations of 0.012, 0.025, and 0.050 µg·L
−1 Irgarol on
D. bardawil’s IR spectra cell cultures for eight days. These findings indicate that
D. bardawil was more severely affected by Irgarol toxicity than
C. salina. In the untreated control culture, a total of 14 peaks were observed. However, as the concentration of Irgarol increased, the number of peaks significantly decreased, indicating potential structural or biochemical alterations in the treated samples. As compared to the controlled culture, the total number of peaks at concentration 0.012 µg·L
−1 Irgarol was eleven peaks, meaning that three peaks vanished. At concentration 0.025 µg·L
−1 Irgarol, nine peaks appeared simultaneously, six peaks vanished, and only one peak appeared in the region of 1060–500 cm
−1 (i.e., polysaccharides). At an Irgarol concentration of 0.050 µg·L
−1, only seven peaks were visible, with seven peaks simultaneously disappearing. The majority of the vanished peaks were located between 4250 and 3500 cm
−1 in frequency. This indicates that the phosphodiester backbone of the nucleic acids, the methyl and methylene groups, and the amides linked to proteins vanished. This could be the reason why
C. salina is less sensitive to Irgarol 1051 than
D. bardawil.
A comparison with the control revealed that some peaks disappeared, new ones emerged, and others remained unchanged. The positions of some of the cell compounds’ side chains may have changed, or some compounds with large molecular weights may have vanished in favor of compounds with lower molecular weights, explaining the development of new peaks under Irgarol stress. These findings align with those of Dao et al. (2017) [
41], who observed that in the infrared (IR) spectra of
Chlorella sp. and Scenedesmus acutus cultures treated with varying concentrations of Pb, there was a noticeable increase in lipids and carbohydrates, likely linked to detoxification mechanisms. Meanwhile, other components, such as proteins and phosphorylated molecules, showed a decrease. These latter effects were related to either the metal’s direct effects on the synthesis/degradation of the algae or the indirect effects of produced ROS during Pb treatment. The results also match with the findings of El-Agawany and Kaamoush [
44] who demonstrated that an infrared (IR) analysis of
Dunaliella tertiolecta cultures treated with varying zinc concentrations revealed alterations in peak number and creation of different compounds. The frequencies 3500–2750 cm
−1, which correspond to the Vas C-H of methyl groups, Vas C-H of methylene groups, and Vas C-H of methylene groups, were the only ones that did not alter at any of the amounts examined [
45], together with V C=O of ester functional groups, which are mostly derived from fatty acids and lipids [
38]. These findings may demonstrate that the antifouling agent concentration and the type of organism are the primary determinants of the harmful effect of Irgarol. The emergence and disappearance of certain compounds were primarily influenced by factors such as the level of the stress agent, the slow synthesis rate of specific compounds, the positioning of certain side chains, the breakdown of complex molecules into simpler ones, and the type of organism under study [
46,
47].
In regard to statistical analysis, we have completed a thorough evaluation of the IR spectrum alterations using statistical comparisons. We assessed how Irgarol 1051 concentration and treatment time affected peak variations using a two-way ANOVA test. Both C. salina and D. bardawil showed statistically significant (p < 0.05) peak modifications (disappearance, appearance, or shift), according to the data. Our findings are supported by the statistical analysis, which shows that these spectrum alterations are a direct result of Irgarol exposure rather than being random.
3.2. Total Soluble Protein Profile Bands
Microalgae are regarded as significant supplies for the commercial production of protein because of their high protein content, so they are used as a particular fish meal in the aquaculture sector [
48]. Proteins contain sequential copies of genetic information, translating nucleotide bases into long chains of amino acids. Comparing protein profiles allows for a clearer understanding of the differences and similarities between treated and untreated algae, helping to identify biochemical changes induced by treatment [
49].
The total protein profile bands of untreated (control) and treated organisms under different concentrations of Irgarol 1051 were recorded in
Supplementary Materials Tables S2–S4 for
Chlorella salina and
Supplementary Materials Tables S5–S7 for
Dunaliella bardawil, as well as
Supplementary Materials Figures S1–S3 for both algal species. These data show that some protein bands remained unaltered, while some looked new and others disappeared when compared to the control. Algae are found in a variety of environments, ranging from fresh water to a hypersaline water system. These organisms can normally survive in conditions with drastic changes in their surroundings. However, in response to the stress of these environments, changes in enzyme activity and thallus shape are common [
50,
51]. Consequently, modifications to these species’ protein structures may also be connected to methods of adaptation. Stress-related symptoms of protein synthesis have been demonstrated in algae during this phase [
52]. The findings showed that protein synthesis in response to stress in
C. salina and
D. bardawil indicated the presence of stress-specific proteins, influenced by certain environmental stress factors.
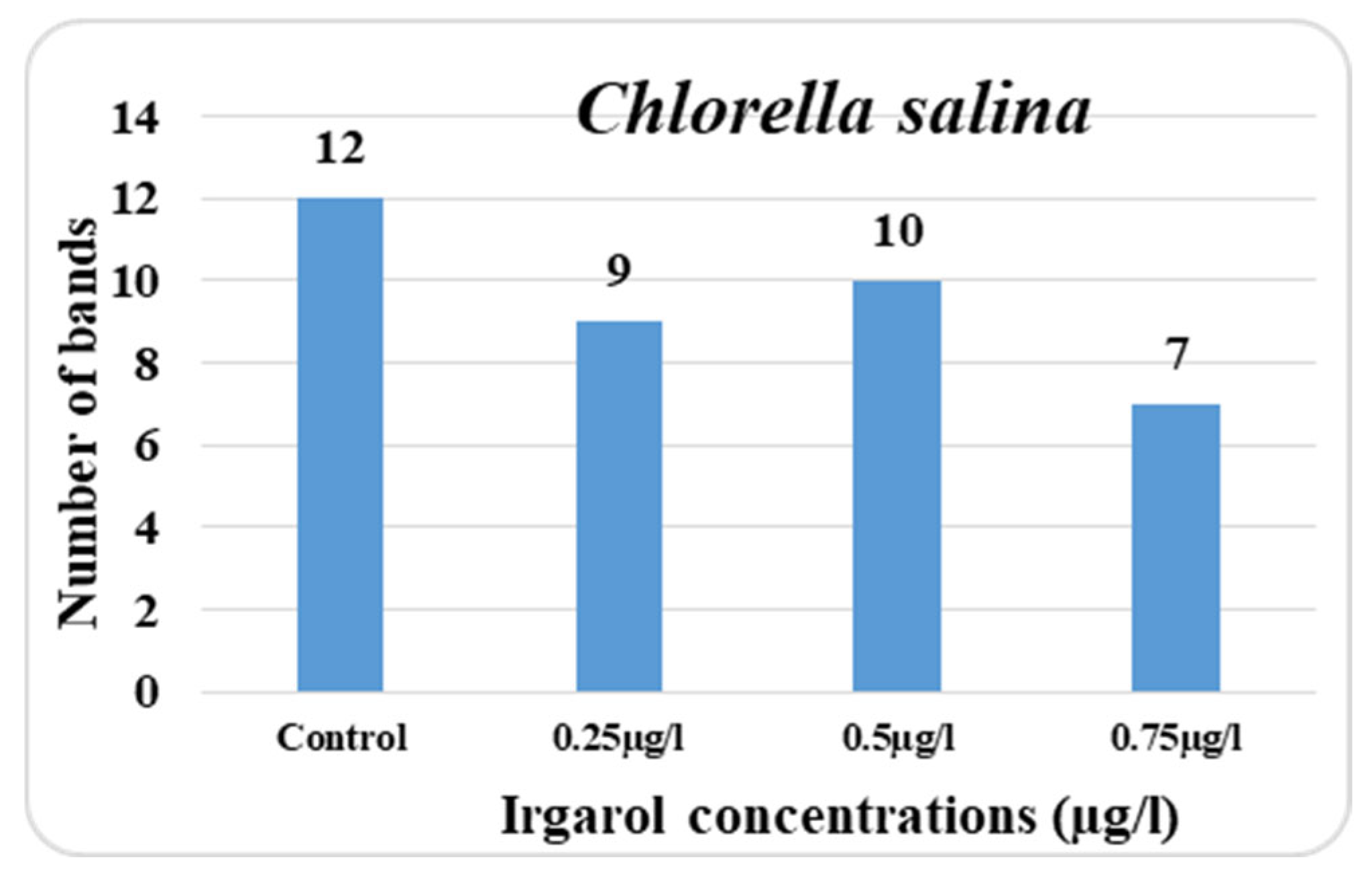
In the control culture of
Chlorella salina, the total number of protein bands detectable on the gel plate was 12; however, in the treatment cultures, this number was reduced depending on the dose of Irgarol. The lower the number of bands in comparison to the control, the higher the concentration of Irgarol. It is also obvious from the data collected that the majority of the bands appeared in the range between 212 and 41 KDa. At 0.25 µg·L
−1 concentration, there were nine bands overall, while at 0.75 µg·L
−1 concentration, there were seven bands total. At concentrations of 0.25, 0.50, and 0.75 µg·L
−1, the number of new bands in
C. salina was three, four, and three. The disappearing bands, on the other hand, were six, six, and eight for concentrations of 0.25, 0.50, and 0.75 µg·L
−1, respectively (
Figure 4 and
Figure 5).
Mohy El-Din and Abdel-Karee [
49] observed that the greatest number of missing bands identified in the sea algae
C. salina after copper and cadmium treatments was eight and five, respectively, which could be associated with DNA alterations. The total number of polymorphic bands in
C. salina culture was 9, 10, and 11 with concentrations of 0.25, 0.50, and 0.75 µg·L
−1, respectively, while the number of bands that stayed unaltered was 6, 6, and 4 at the same concentrations. Mohy El-Din et al. [
53] reported that there were successive modifications to polymorphic bands and a decrease in protein fingerprinting in
Pterocladia capillacea and
Ulva lactuca, which could be the result of the presence of heavy metals having a deleterious effect on enzymes involved in protein production.
Ultimately, the percentage reduction in the total number of protein bands, compared to the control, was 25.0%, 16.67%, and 41.67%, respectively. On the other hand, there are now 9, 10, and 11 bands, respectively, more polymorphic bands. The number of unaltered bands at concentrations of 0.75 µg·L−1, 0.25, and 0.50 µg·L−1 was four, respectively. This could suggest that concentration 0.75 µg·L−1 is more hazardous than concentrations 0.25 and 0.50 µg·L−1.
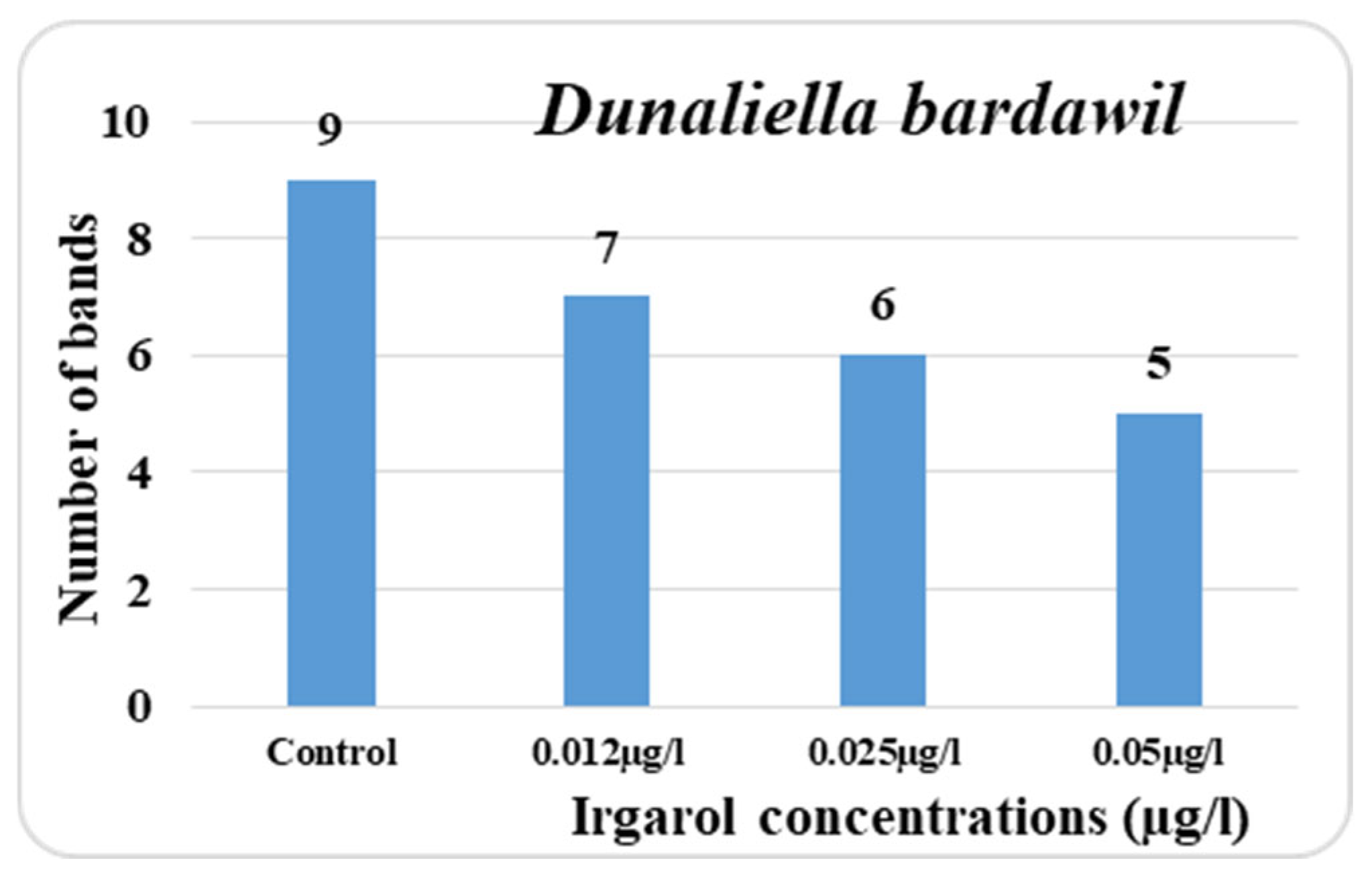
The obtained data indicated that known antifouling Irgarol is more harmful to
D. bardawil than
C. salina as shown in
Supplementary Materials Figures S1 and S2. While the doses of 0.012, 0.025, and 0.050 µg·L
−1 produced seven, six, and five bands, respectively, the total number of bands in the untreated cultures (control) was nine. Among all the concentrations tested, only one band appeared as a new addition. In addition, the three distinct concentrations produced the appearance of two, three, and six more bands in comparison to the control. Additionally, the treatment concentrations had a significant impact on the polymorphic bands, which reached three, four, and seven in that order.
Eventually, the three tested concentrations of Irgarol each had an effect on four, two, and four bands, which remained unaffected. In comparison to the control, the percentage decrease in the number of bands was 22.2%, 33.3, and 44.4%, respectively. The data collected also clearly shows that in
D. bardawil, the majority of the disappearing bands appeared in the low molecular weight region, and hence at the high Rf value region. Conversely, the majority of the unaltered bands were observed in the vicinity of low Rf values. The fact that
D. bardawil is more susceptible to the antifouling Irgarol than
C. salina may be explained by this map. El Taher [
54] recognized that an organism’s ability to survive under stressful conditions could be enhanced by the creation or accumulation of newly evolved proteins. Furthermore, Exss-Sonne et al. [
55] came to the conclusion that the synthesis and build-up of novel proteins may enable algae to tolerate stress conditions. These findings are consistent with the observations for both kinds of algae. The results align with the findings of El-Agawany and Kaamoush [
44], who stated that the stress effects of heavy metals on
Dunaliella tertiolecta lead to biochemical modifications in certain molecules, particularly proteins.
Bands were dispersed throughout the gel plates in the total soluble protein profile for the two treated organisms and the control at the three varied Irgarol doses. The two studied organisms had different sums of all the bands that emerged on the gel plates, indicating that the number of bands depends primarily on the species and concentration of the stressing substance. However, it came to light through Sinha and Hader [
56] that
Anabaena sp. cultured under stressful conditions did not exhibit any alterations in their protein pattern. In contrast, numerous authors have noted that there was a noticeable alteration in the protein bands’ composition under stress [
57]. Additionally, El-Agawany et al. [
58] observed that two additional appeared bands in
Spirulina platensis cultivated under stress that were absent from the unaltered culture. Salah El-Din’s [
59] findings demonstrated that the majority of algae species share physiological processes related to the production or biodegradation of certain macromolecules. The different variations in total soluble protein bands in stressed algae appear to be explained by this finding. According to Exss-Sonne et al. and Ahmed, 2010 [
55,
60], the organism can develop new protein production or accumulation to increase its stress tolerance. These findings are almost in agreement with those of
C. salina and
D. bardawil. The antifouling chemical Irgarol 1051 had a more deleterious impact on the protein profile in the wall-less alga
D. bardawil than in the walled alga
C. salina.
3.3. Activity of Total Antioxidants
Aerobic metabolism continuously generates reactive oxygen species (ROS), which are unstable free radicals that can damage lipids, proteins, and DNA. This oxidative stress contributes to cellular damage and plays a key role in the development of various diseases. Several types of photosynthetic cyanobacteria and green microalgae can produce antioxidants in response to oxidative stress induced by metals, acidity, pollution, ultraviolet radiation, and nutritional constraints [
61].
Living organisms have developed an intricate antioxidant system in order to fend against ROS and minimize their damage. Enzymatic and non-enzymatic chemicals are included in these antioxidant systems. The total antioxidant activity of the system is represented by the sum of these antioxidants [
47]. The collaboration of several antioxidants provides more protection versus bio-reactive oxygen damage than any single component alone. Thus, the total antioxidant capacity may provide more meaningful biological information than individual component measurements since it takes into account the overall impact of all antioxidants inside the organism’s body cells [
62].
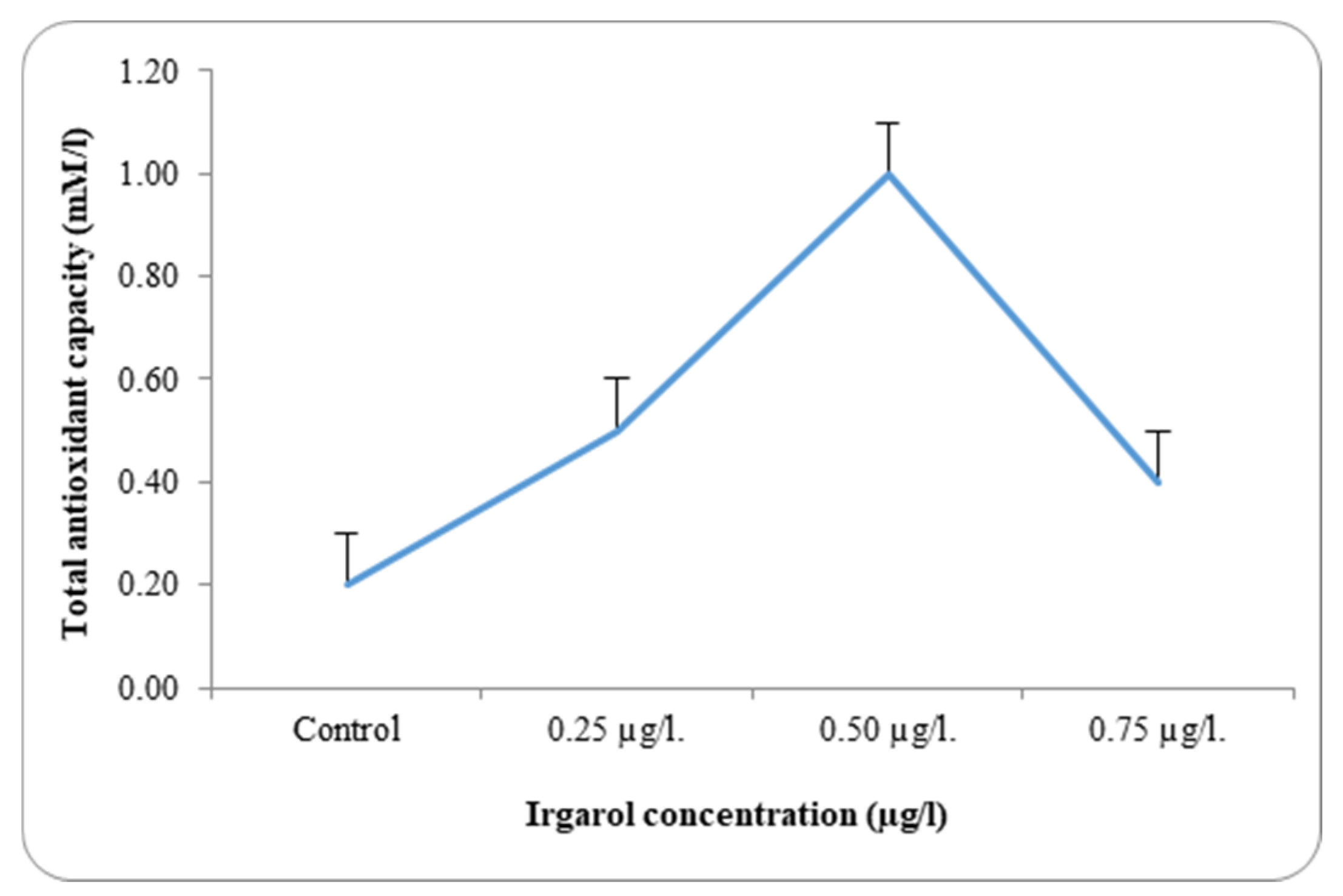
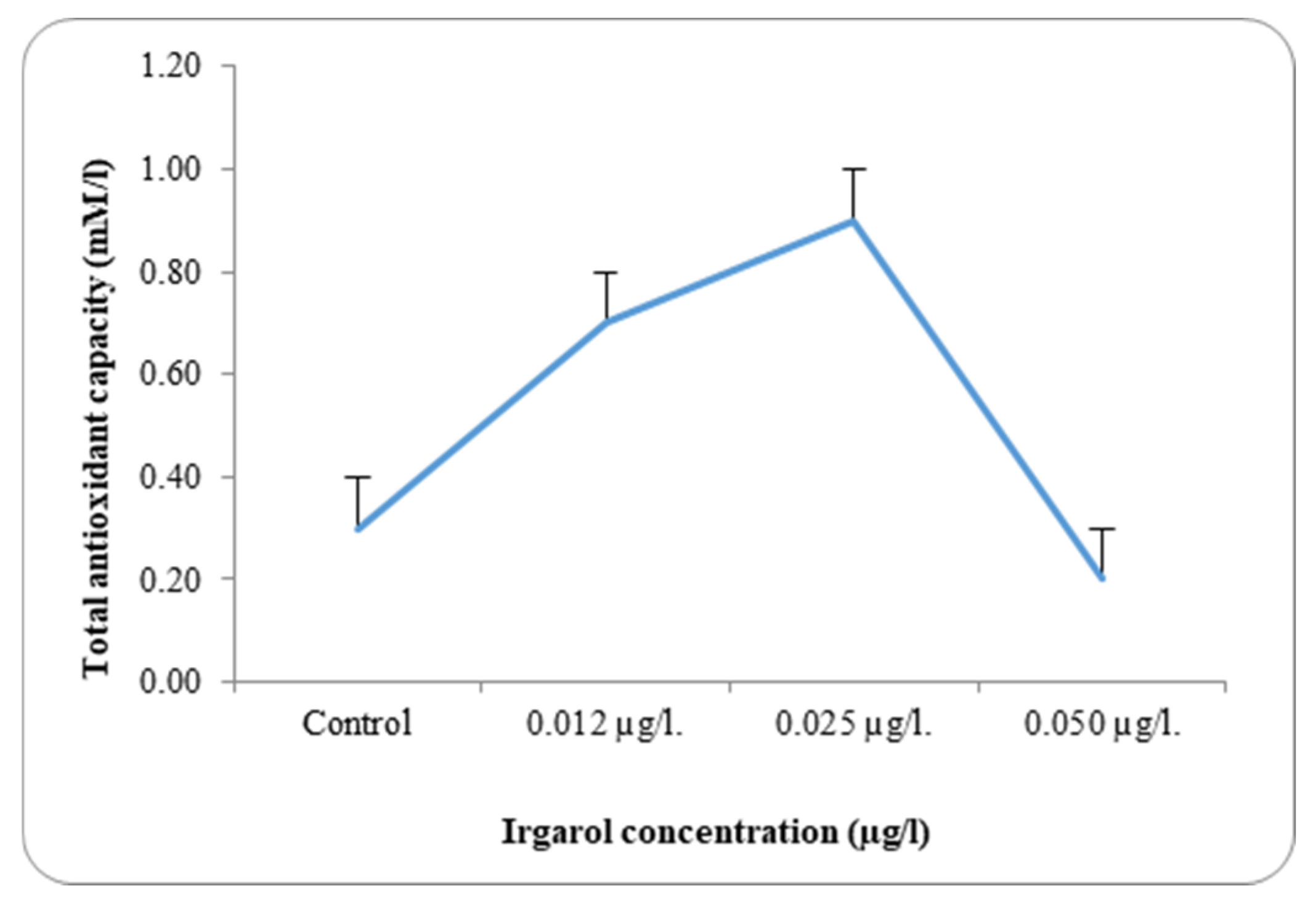
Table 3 and
Figure 6 and
Figure 7 present the results of the antioxidant capacity of
Chlorella salina and
Dunaliella bardawil at control and under different Irgarol 1051 concentrations. Reflecting on the study’s findings regarding resistance, it is clear that species with greater stress tolerance often exhibit significantly higher antioxidant enzyme activity. The results showed that
C. salina was more effective than
D. bardawil at dissociating exogenous H
2O
2. The state of balance between the oxidative and antioxidative capacity determines the fate of the organism under oxidative stress. While Irgarol concentrations were 0.50 µg·L
−1 for
C. salina and 0.025 µg·L
−1 for
D. bardawil, there was a substantial increase in total antioxidant activity when compared to controls. The total antioxidants’ activities are mostly determined by the stress impact, the period of cultivation, and the species evaluated [
47].
In
C. salina, total antioxidant activity increased by 2.5-, 5.0-, and 2.0-fold at Irgarol concentrations of 0.25, 0.50, and 0.75 µg·L
−1, respectively, compared to the control. The outcomes achieved are very similar to those obtained by Ajitha et al. [
63] who proved that exposure to zinc and mercury in
C. vulgaris led to a notable increase in total antioxidant concentrations at baseline, which gradually decreased with acute and long-term exposure. Knauert and Knauer [
64] supported the acquired outcomes, stating that intracellular ROS accumulated in the microalgae
C. vulgaris as a reaction to Cu toxicity. Consistent with the findings,
Anabaena doliolum microalga cells have been shown to exhibit a similar trend in ROS formation in response to Zn
2+, achieving their maximal antioxidant doses greater than 0.7 mg·L
−1 [
65].
In
C. salina, total antioxidant activity decreased at an Irgarol concentration of 0.75 µg·L
−1 but remained higher than under normal conditions (control). In contrast, the activity of these antioxidants increased by 2.3- and 3-fold, respectively, in the case of
D. bardawil, concentrations 0.012 and 0.025 µg·L
−1 Irgarol 1051 as compared to the control, while it declined by 1.0-fold at 0.050 µg·L
−1 Irgarol. These findings may validate the hypothesis of the study, which states that
C. salina is more efficient than
D. bardawil at dissociating exogenous H
2O
2. However, in the case of
C. salina and
D. bardawil, the maximal activity for these antioxidants were attained at concentrations of 0.50 µg·L
−1 and 0.025 µg·L
−1 of Irgarol 1051, respectively. Lipids, nucleic acids, and protein oxidation are strongly linked to the detrimental effects of ROS accumulation on cellular levels, which eventually result in changes to cell structure. Data demonstrating a concentration-dependent decrease in protein content in
C. vulgaris cells in the presence of different Zn concentrations support the obtained results. The highest concentration of protein was found at high zinc levels, whereas lower concentrations showed a rise in protein content. This is likely due to the upregulation of stress proteins, such as antioxidant enzymes, to enhance defense against toxicity or possibly indicate the maximal defense threshold [
63].
There was a discernible rise in the overall antioxidant activity in both examined algae as compared to the control. A detailed analysis of the data revealed that antioxidant activity increased with rising Irgarol concentrations in both algae species, consistently exceeding the levels observed in their respective controls. The concentration of Irgarol at 0.50 µg·L−1 for C. salina and 0.025 µg·L−1 for D. bardawil showed the greatest increase in magnitude. At higher Irgarol concentrations, total antioxidant activity showed a slight decline in D. bardawil but remained elevated compared to the control in C. salina.
Belghith et al. [
66] reported similar results, indicating that the microalgae halophilic
Dunaliella salina treated with varying doses of Cd (II) demonstrated an increase in overall antioxidant capacity. The improvement of biological components engaged in defense mechanisms against Cd toxicity may be the cause of this increase. Additionally, Jobby et al. [
67] showed that the activity of particular antioxidant enzymes was significantly reduced in
Chlorella vulgaris cells exposed to different concentrations of Cr (VI).
The acquired results provide strong support for the findings of El-Agawany et al. [
58], who indicated that an increase in the overall activity of antioxidants in
Spirulina platensis is typically observed under any stress. Additionally, they noted that under mild stress, the antioxidants’ high levels of activity prevented the peroxidation breakdown of proteins and lipids by scavenging reactive oxygen species (ROS). Additionally, Jungklang [
68] noted that plants under stress frequently experience antioxidant stress due to an imbalance between the production of reactive oxygen species (ROS) and the antioxidants’ capacity to quench them. According to reports, plants with high antioxidant levels are more resistant to oxidative damage because they are able to control the redox balance within their cells [
69]. This is demonstrated by the fact that overall antioxidant levels, which include both enzymatic and non-enzymatic antioxidants, generally increased as stress levels increased, suggesting a cellular ability to withstand the stress. If the level of stress is not so severe as to exceed the concentration limit that could harm or kill the organism, then this conclusion can be supported. A similar outcome was also noted for
D. bardawil, where a decrease in total antioxidant activity was reported at greater Irgarol 1051 concentrations. Actually, oxidative damage is promoted by environmental contaminants, particularly heavy metals, in two ways: either by raising ROS concentrations within cells or by decreasing the antioxidant capacity of cells [
70].
3.4. Mapping Mass Concentration of Algae Along the Egyptian Mediterranean Sea
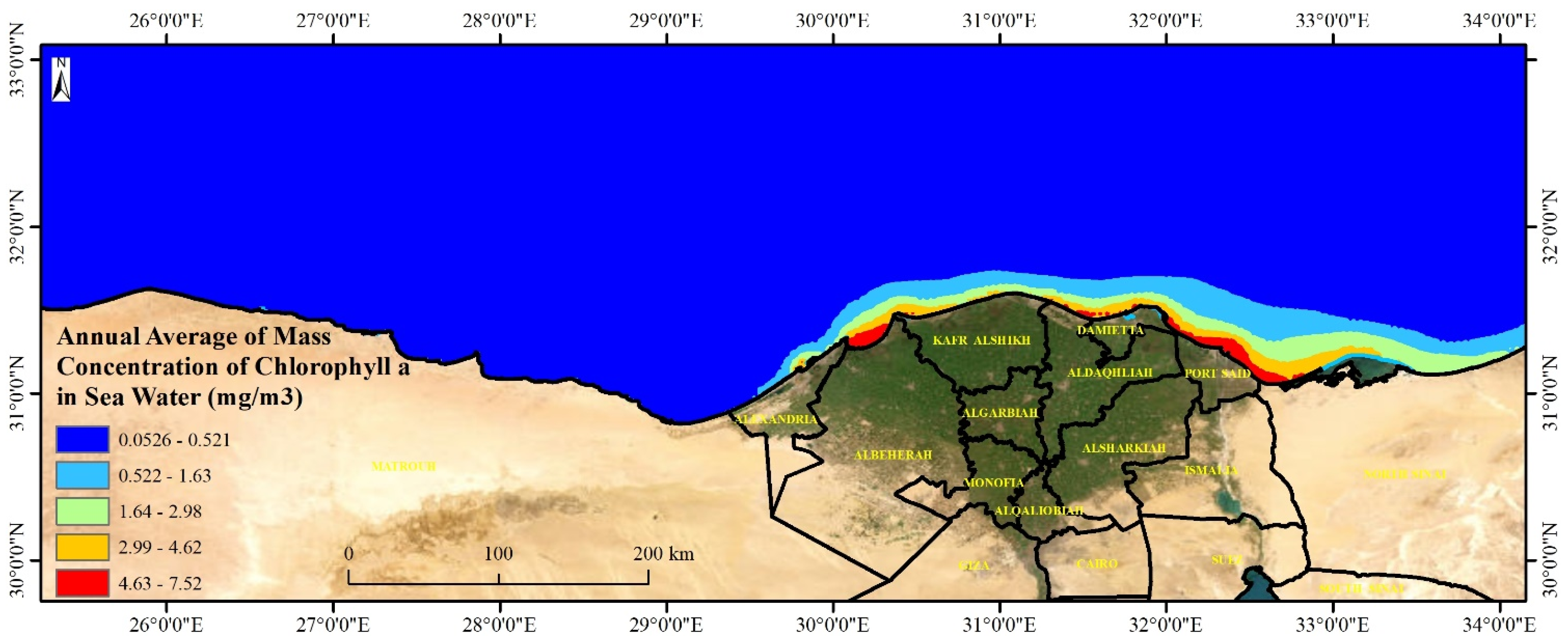
This section represents the potentiality of algal growth including
Chlorella salina and
Dunaliella bardawil along the Mediterranean seawater. Mapping the potential growth of
Chlorella salina and
Dunaliella bardawil along the Mediterranean Sea is crucial for environmental monitoring, aquaculture, pollution control, and climate change research. These algae play a key role in marine food webs and serve as indicators of water quality, nutrient levels, and ecosystem health. High algal growth areas, particularly near the Nile Delta, are vulnerable to eutrophication and pollution, making mapping essential for assessing the impact of nutrient runoff and toxic biocides like Irgarol 1051. Additionally, these algae support aquaculture, biofuel production, and pharmaceuticals, making their distribution important for economic sustainability. Understanding algal growth patterns also aids in carbon sequestration studies and helps policymakers develop strategies for marine conservation and coastal management. By integrating spatial analysis with environmental assessments, researchers can balance economic development and ecosystem preservation in Mediterranean coastal regions. The levels of Algae represented by chlorophyll-a mass concentration along the Egyptian Mediterranean coast ranged from 0.05 to 7.52 with a mean value of 0.17 mg·m
−3. The highest values were recorded east and middle of the Egyptian coast (
Figure 8). The mass concentration of chlorophyll-a along the shoreline is generally decreasing from east to west, fluctuating from 1 mg·m
−3 at the eastern coast to 6.5 mg·m
−3 at the middle Nile Delta coast with an obvious decrease moving westward reaching the lowest values at the west coast (<0.1 mg·m
−3). Low levels (<0.08 mg·m
−3) were reported in the coastal and deep water away from the shoreline.
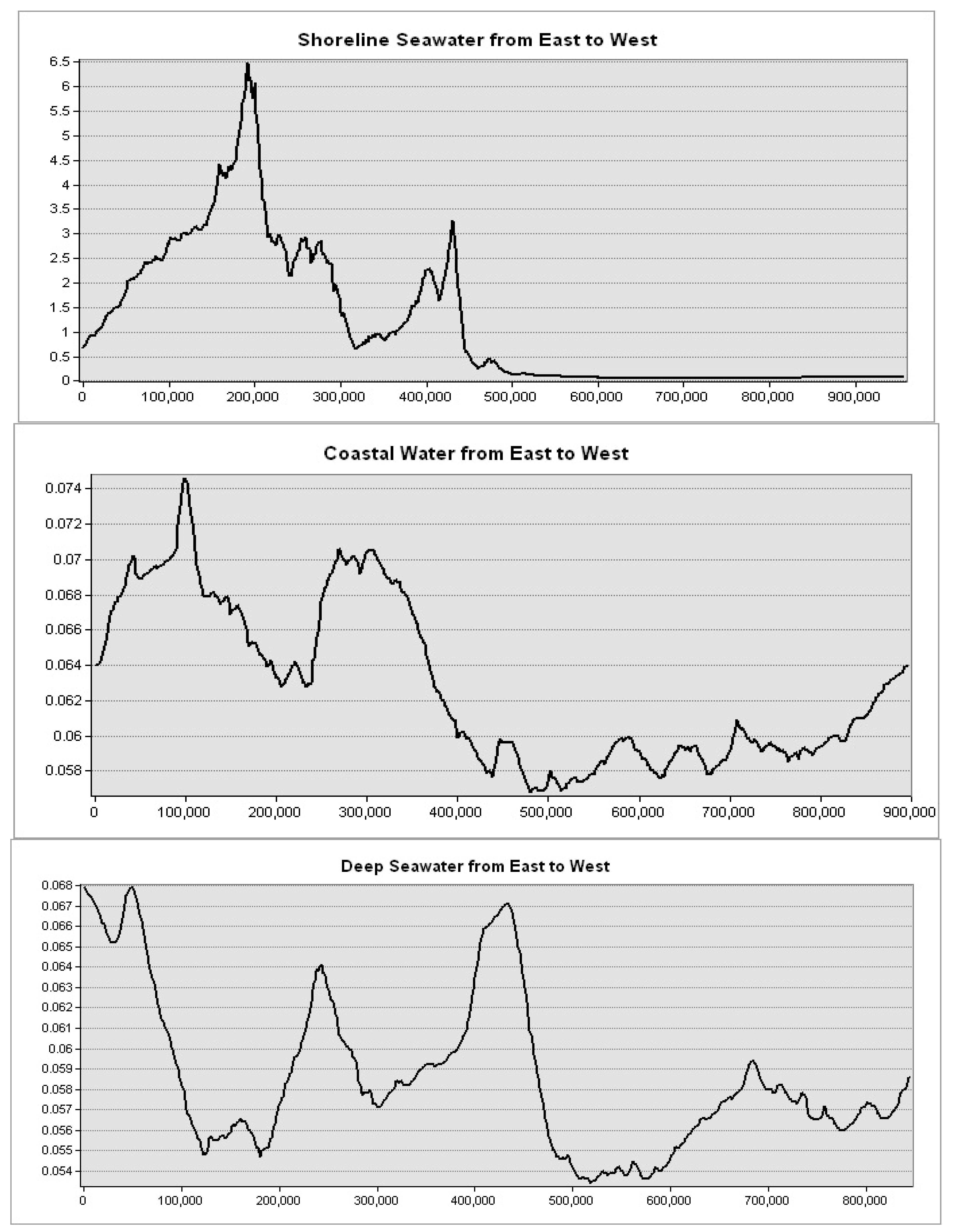
Levels of mass chlorophyll-a at the Mediterranean Sea governorates are decreasing with depth, showing the highest levels at the shoreline seawater (5 km from the shore) and the lowest levels at the deep water (200 km away from the shore) (
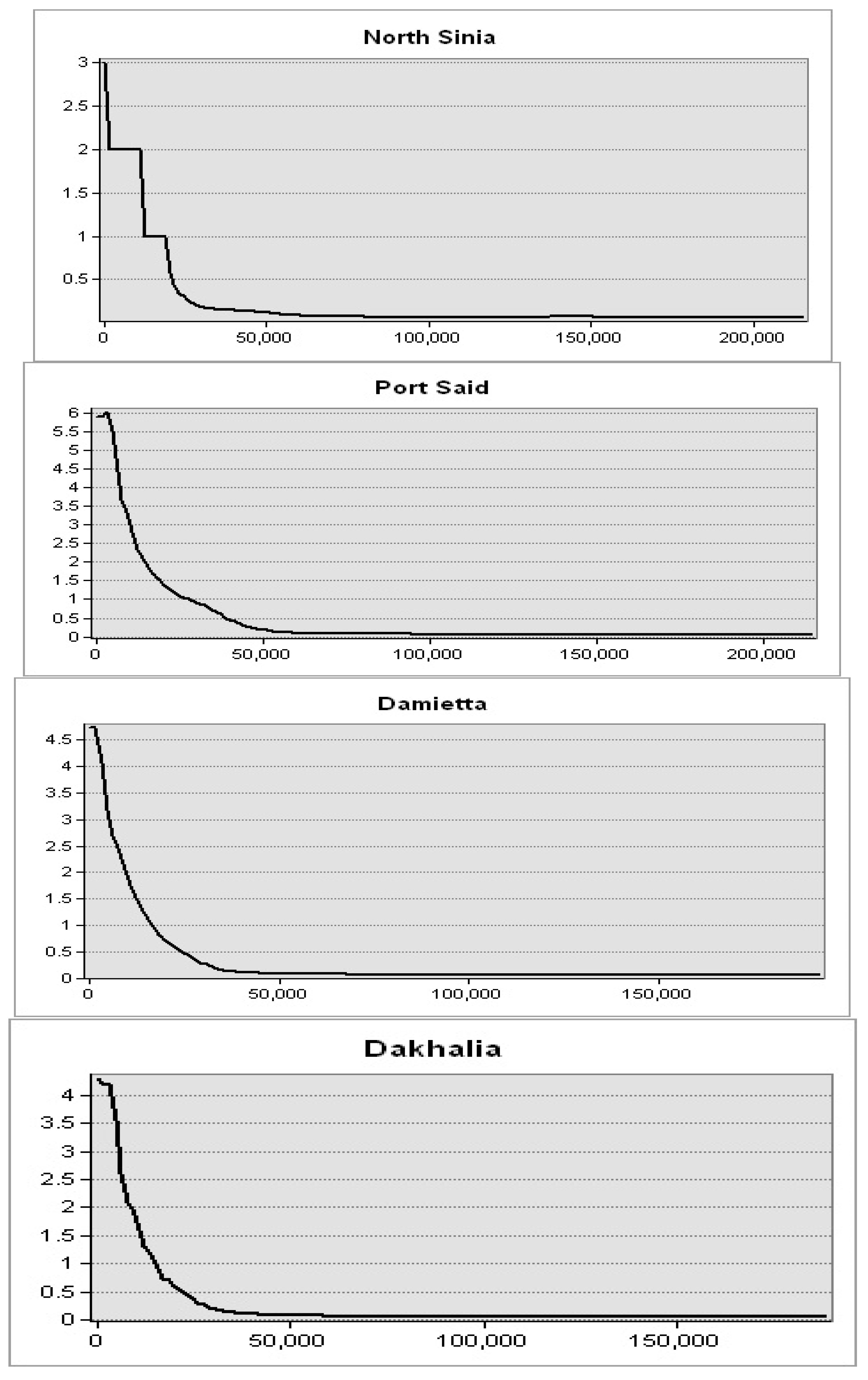
Figure 9). This spatial trend was obvious in seawater in front of the Mediterranean Sea governorates. Further, the highest levels were observed in seawater of the Nile delta governorates: Port Said, Damietta, and Dakhalia shores reporting 6, 4.5, and 4 mg·m
−3, respectively (
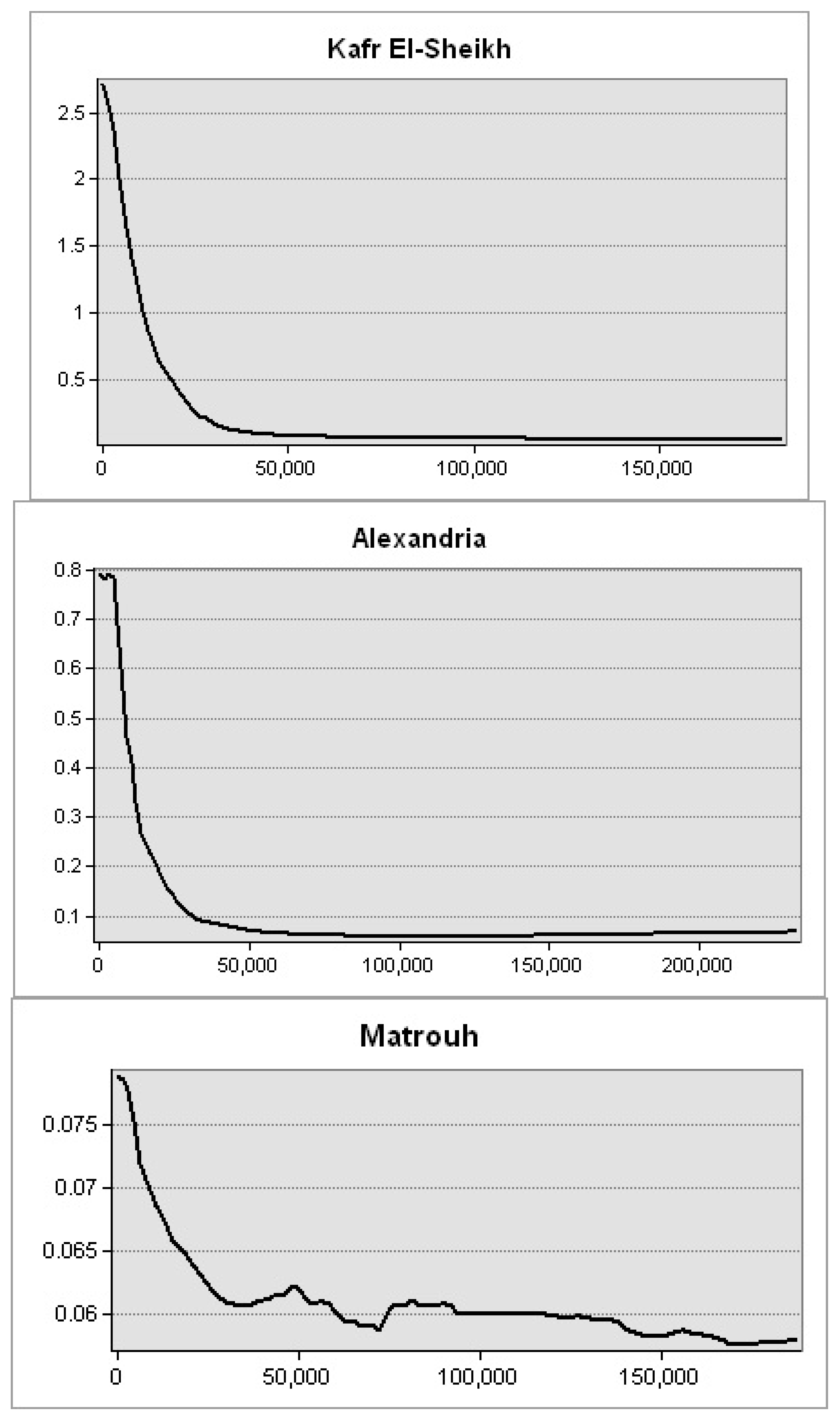
Figure 10). The lowest levels were observed along Matrouh and Alexandria shores reporting 0.075 and 0.8 mg·m
−3, respectively (
Figure 11). The plenty of mass chlorophyll-a in front of the Egyptian northern governorates can be ordered as follows: Port Said > Damietta > Dakahlia > North Sinia > Kafr El-Sheikh > Alexandria > Matrouh.
The high chlorophyll-a concentration in seawater along the Delta governorates’ shores of Egypt can be explained by several key factors such as nutrient enrichment, eutrophication, seasonal dynamics, and hydrological conditions. The Nile River delivers high levels of nitrogen and phosphorus from agricultural runoff and urban wastewater into the Mediterranean, boosting phytoplankton growth. These nutrients are essential for primary production, especially near river mouths [
71]. Extensive human activities, including the use of fertilizers and discharges from urban areas, contribute to nutrient overloading, leading to eutrophication and algal blooms. Nutrient inputs vary seasonally, with river runoff peaking in autumn and winter, further supporting chlorophyll-a variability across different periods. Coastal plumes and limited water mixing near the southeastern Mediterranean coastline trap nutrients in localized areas, intensifying chlorophyll-a concentrations [
72]. These interconnected processes, influenced by both natural and anthropogenic factors, make the coastal waters of Egypt’s Delta region hotspots for high chlorophyll-a levels.
4. Conclusions
This study evaluated the harmful impacts of Irgarol 1051, a biocide antifouling compound, on the levels of some important metabolites such as protein profiles, I.R. spectroscopy of total cell constituents, and total antioxidant activities in two marine algal species; Chlorella salina and Dunaliella bardawil. It also tested the potential sites for algal growth along the Egyptian Mediterranean coast using remote sensing and geostatistical analyses. The findings showed that Irgarol 1051 concentrations for C. salina were 0.25, 0.5, and 0.75 µg·L−1, whereas for D. bardawil, they were 0.012, 0.025, and 0.05 µg·L−1. There was a notable inhibition of algal growth rate for the two tested species at all the tested Irgarol concentrations, which corresponded to the increasing toxicity of this biocide.
The obtained I.R. spectra of the primary cell constituents revealed the disappearance of some peaks and the appearance of new ones, while others remained virtually unchanged. Protein synthesis in the stress adaptation mechanism of C. salina and D. bardawil after a time of stress demonstrated the presence of species-stressed proteins for a specific environmental stress factor (i.e., Irgarol compounds). The number of protein profile bands depends primarily on the species type and concentration of the stressed substance. The antifouling chemical Irgarol 1051 had a more negative impact on the protein profile in the wall-less alga D. bardawil than in the walled alga C. salina.
The activity of total antioxidants altered with various doses of Irgarol increased gradually, if at all, throughout the tests. Total antioxidant activity in both examined algae increased significantly when compared to the control group. A careful inspection of the outputs revealed that as Irgarol concentration increased, the antioxidant activity increased, which was higher in both algae than in the corresponding controls except at the highest concentration.
Spatial investigation indicated that the levels of mass chlorophyll-a at the Mediterranean Sea governorates are decreasing with depth showing the highest levels at the shoreline seawater and the lowest levels at the deep water. The highest levels were observed in seawater of the Nile delta governorates: Port Said, Damietta, and Dakhalia shores reporting 6, 4.5, and 4 mg·m−3, respectively. The lowest levels were observed along Matrouh and Alexandria shores reporting 0.075 and 0.8 mg·m−3, respectively. The high chlorophyll-a concentration in seawater along the Delta governorates’ shores of Egypt can be explained by several key factors: nutrient enrichment and eutrophication, from the River Nile at Damietta and Rasheed branches.
The overall outcomes of this investigation show that Irgarol 1051 has the potential to have a negative influence on all the studied metabolites of the two algae species at environmentally relevant concentrations. Furthermore, this reinforces the importance of spatial analyses of algae in maximizing both economic and environmental benefits.
The study reveals the toxic effects of Irgarol 1051 on marine algae species Chlorella salina and Dunaliella bardawil, which are crucial for the marine food web. The decline of these algae could lead to cascading effects on other marine species.
The study also raises concerns about Irgarol 1051’s potential for bioaccumulation and biomagnification, potentially causing higher concentrations in top predators and further harm.
The spatial analysis reveals high algal growth areas near the Nile Delta along the Egyptian coast, potentially vulnerable to the effects of Irgarol 1051 due to nutrient runoff and eutrophication.
The presence of a toxicant and increased antioxidant activity in the algae indicate stress, which is often used as a biomarker of environmental contamination, suggesting that Irgarol 1051 is inducing a physiological stress response in these organisms.
The study on Irgarol 1051, a biocide, focuses on its short-term effects on two algal species. It suggests that future research should explore the long-term, and chronic effects on these algae, including reproduction, genetic changes, and potential recovery.
It also highlights the importance of investigating the effects on other marine organisms, such as other algae, zooplankton, fish, and benthic invertebrates, to assess the broader ecosystem risks.
The study also highlights the potential synergistic effects of Irgarol 1051 in combination with other pollutants, such as heavy metals, pesticides, or other antifouling agents.
The study emphasizes the need for the development of alternative antifoulants and the need for spatial modeling and risk assessment to minimize the environmental impact of Irgarol 1051. It also suggests the influence of eutrophication on the toxicity of Irgarol 1051 and the sensitivity of algae.
The spatial analysis provided valuable insights into the algal distribution and potential Irgarol 1051 impact zones. Future studies should explore the use of advanced geospatial techniques, such as higher-resolution satellite data and machine learning for improved classification. Incorporating additional environmental factors like sea surface temperature and nutrient levels could enhance the assessment of eutrophication hotspots. Furthermore, hydrodynamic models may offer a better understanding of pollutant dispersion and its impact on coastal ecosystems.