Spatial Heterogeneity of the Microbial Community in the Surface Sediments in the Okinawa Trough
Abstract
1. Introduction
2. Materials and Methods
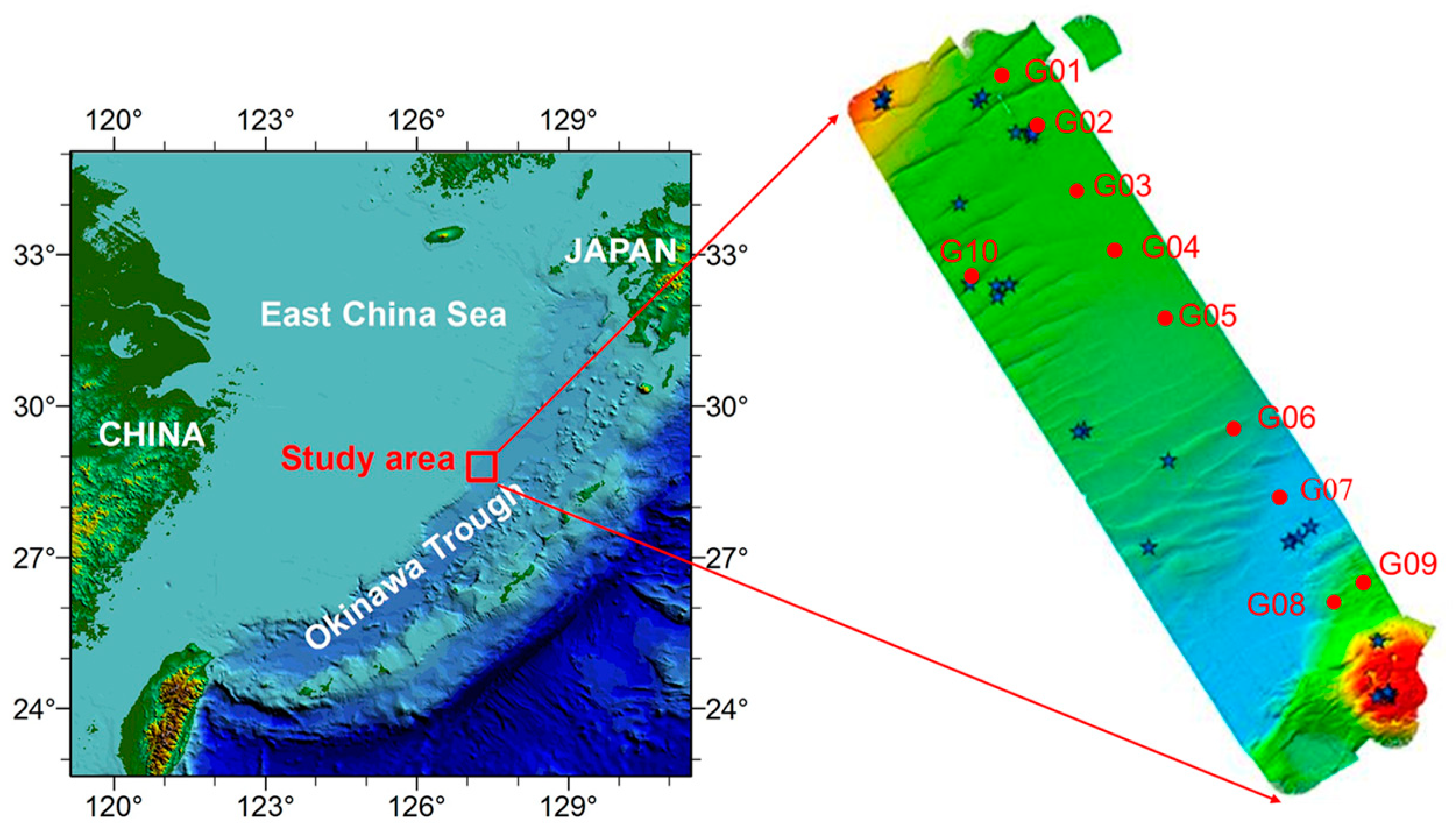
2.1. Sample Collection
2.2. Geochemical Analyses
2.3. DNA Extraction and 16S rRNA Sequencing
2.4. Analysis of 16S rRNA Gene Sequences
2.5. Statistical Analyses
3. Results
3.1. Environmental Parameters at the Sampling Sites
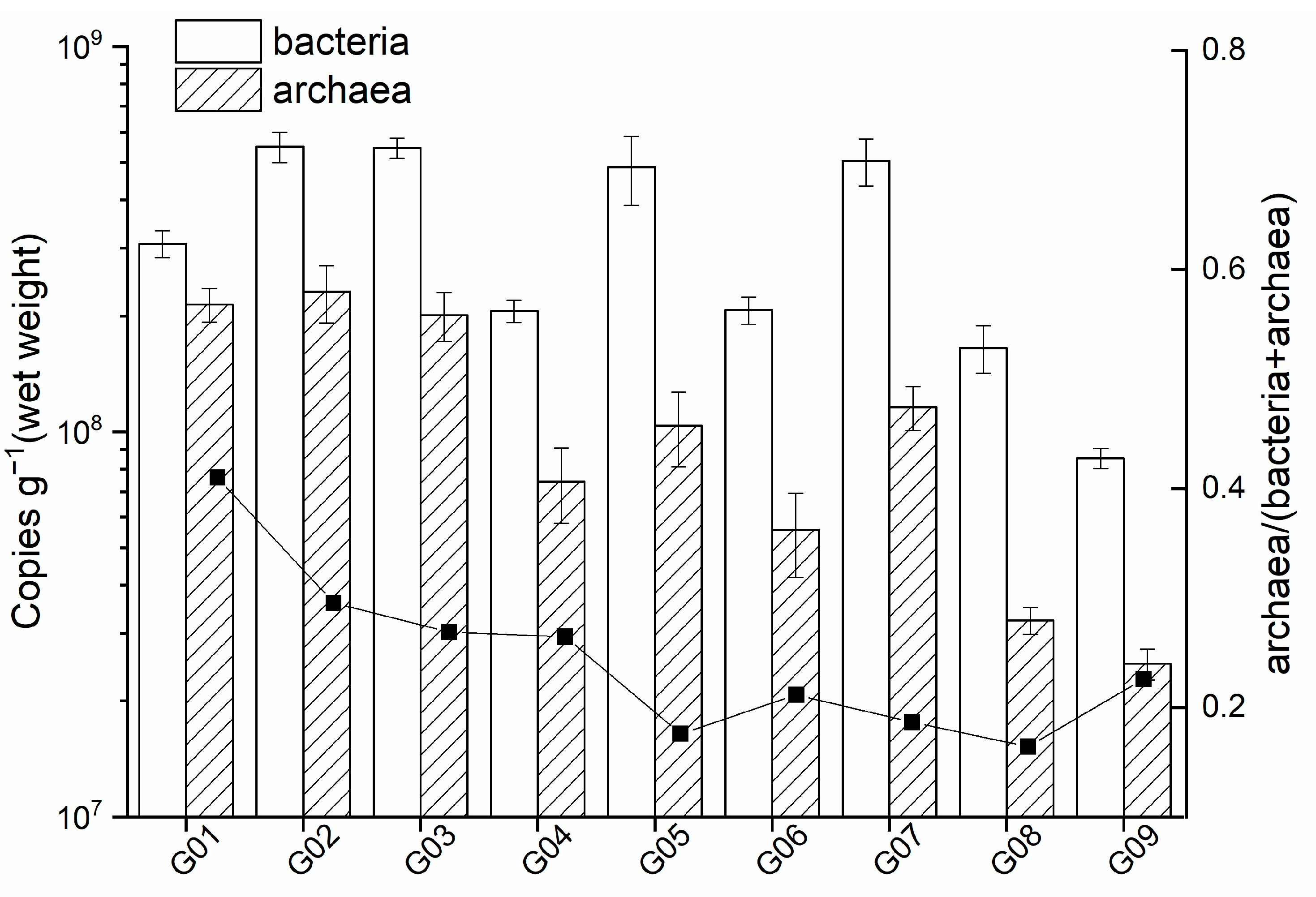
3.2. Abundance of Microbial Community in OT Sediments
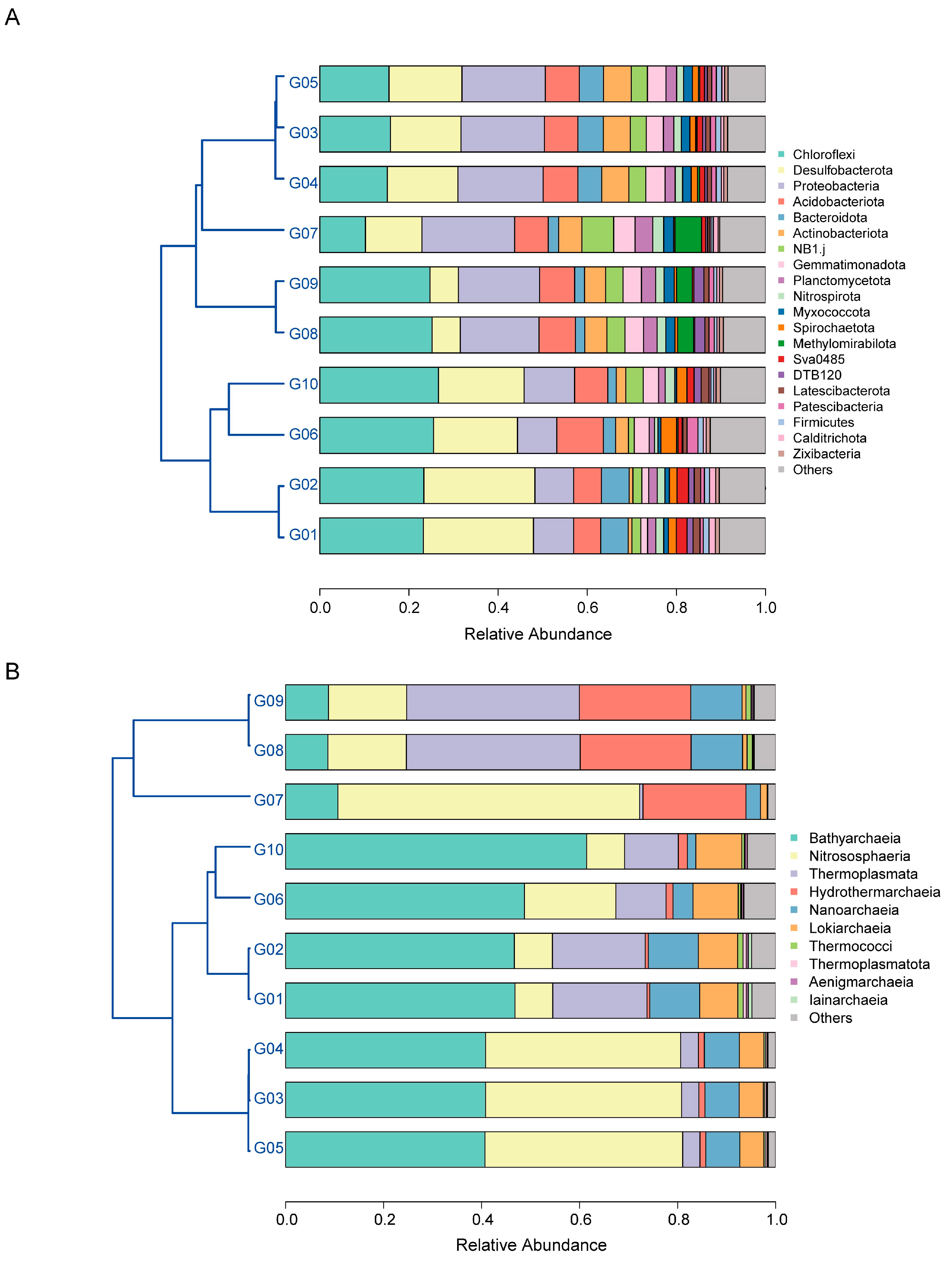
3.3. Diversity of Archaeal and Bacterial Community
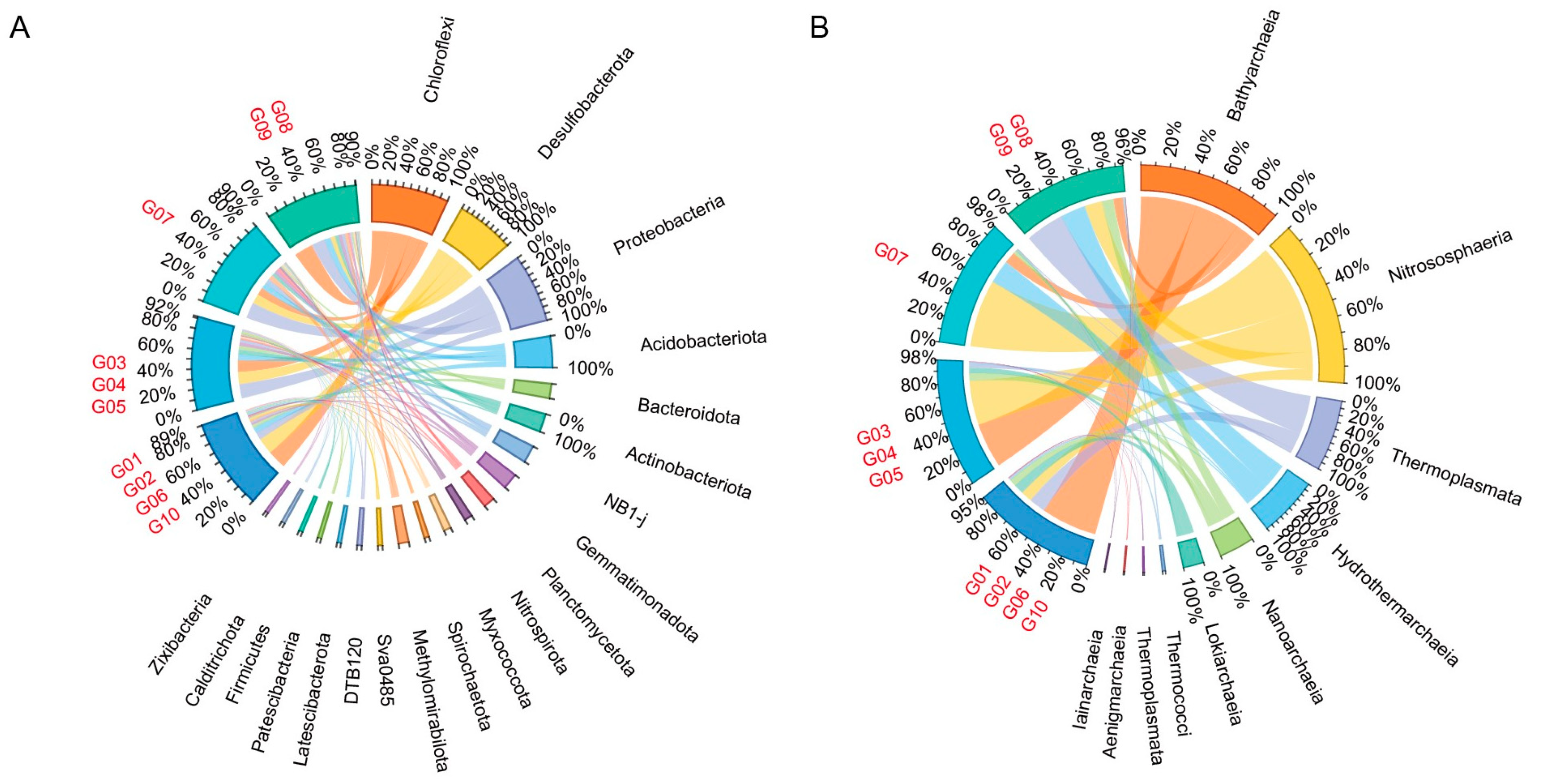
3.4. Microbes Involved in Biogeochemical Cycles
3.5. Spatial Distributions of Microbial Communities and Their Relationships with Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Learman, D.R.; Henson, M.W.; Thrash, J.C.; Temperton, B.; Brannock, P.M.; Santos, S.R.; Mahon, A.R.; Halanych, K.M. Biogeochemical and Microbial Variation across 5500 km of Antarctic Surface Sediment Implicates Organic Matter as a Driver of Benthic Community Structure. Front. Microbiol. 2016, 7, 284. [Google Scholar] [CrossRef]
- Stuart, C.T.; Rex, M.A.; Etter, R.J. (Eds.) Large-scale spatial and temporal patterns of deep-sea benthic species diversity. In Ecosystems of the Deep Oceans; Elsevier: Amsterdam, The Netherlands, 2003; pp. 295–312. [Google Scholar]
- Ramirez-Llodra, E.; Brandt, A.; Danovaro, R.; De Mol, B.; Escobar, E.; German, C.R.; Levin, L.A.; Martinez Arbizu, P.; Menot, L.; Buhl-Mortensen, P.; et al. Deep, diverse and definitely different: Unique attributes of the world’s largest ecosystem. Biogeosciences 2010, 7, 2851–2899. [Google Scholar] [CrossRef]
- Kallmeyer, J.; Pockalny, R.; Adhikari, R.; Smith, D.; D’Hondt, S. Global distribution of microbial abundance and biomas in subseafloor sediment. Proc. Natl. Acad. Sci. USA 2012, 109, 16213–16216. [Google Scholar] [CrossRef] [PubMed]
- D’Hondt, S.; Pockalny, R.; Fulfer, V.M.; Spivack, A.J. Subseafloor life and its biogeochemical impacts. Nat. Commun. 2019, 10, 3519. [Google Scholar] [CrossRef] [PubMed]
- Probandt, D.; Knittel, K.; Tegetmeyer, H.E.; Ahmerkamp, S.; Holtappels, M.; Amann, R. Permeability shapes bacterial communities in sublittoral surface sediments. Environ. Microbiol. 2017, 19, 1584–1599. [Google Scholar] [CrossRef]
- Orsi, W.D. Ecology and evolution of seafloor and subseafloor microbial communities. Nat. Rev. Microbiol. 2018, 16, 671–683. [Google Scholar] [CrossRef]
- Hoshino, T.; Doi, H.; Uramoto, G.I.; Wörmer, L.; Adhikari, R.R.; Xiao, N.; Morono, Y.; D’Hondt, S.; Hinrichs, K.U.; Inagaki, F. Global diversity of microbial communities in marine sediment. Proc. Natl. Acad. Sci. USA 2020, 117, 27587–27597. [Google Scholar] [CrossRef]
- Shinjo, R.; Kato, Y. Geochemical constraints on the origin of bimodal magmatism at the Okinawa Trough, an incipient back-arc basin. Lithos 2000, 54, 117–137. [Google Scholar]
- Glasby, G.; Notsu, K. Submarine hydrothermal mineralization in the Okinawa Trough, SW of Japan: An overview. Ore Geol. Rev. 2003, 23, 299–339. [Google Scholar]
- Sun, Z.; Wei, H.; Zhang, X.; Shang, L.; Yin, X.; Sun, Y.; Xu, L.; Huang, W.; Zhang, X. A unique Fe-rich carbonate chimney associated with cold seeps in the Northern Okinawa Trough, East China Sea. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2015, 95, 37–53. [Google Scholar]
- Sun, Z.; Wu, N.; Cao, H.; Xu, C.; Liu, L.; Yin, X.; Zhang, X.; Geng, W.; Zhang, X. Hydrothermal metal supplies enhance the benthic methane filter in oceans: An example from the Okinawa Trough. Chem. Geol. 2019, 525, 190–209. [Google Scholar]
- Nunoura, T.; Oida, H.; Nakaseama, M.; Kosaka, A.; Ohkubo, S.B.; Kikuchi, T.; Kazama, H.; Hosoi-Tanabe, S.; Nakamura, K.-i.; Kinoshita, M. Archaeal diversity and distribution along thermal and geochemical gradients in hydrothermal sediments at the Yonaguni Knoll IV hydrothermal field in the Southern Okinawa Trough. Appl. Environ. Microbiol. 2010, 76, 1198–1211. [Google Scholar] [PubMed]
- Yanagawa, K.; Breuker, A.; Schippers, A.; Nishizawa, M.; Ijiri, A.; Hirai, M.; Takaki, Y.; Sunamura, M.; Urabe, T.; Nunoura, T. Microbial community stratification controlled by the subseafloor fluid flow and geothermal gradient at the Iheya North hydrothermal field in the Mid-Okinawa Trough (Integrated Ocean Drilling Program Expedition 331). Appl. Environ. Microbiol. 2014, 80, 6126–6135. [Google Scholar] [PubMed]
- Wang, L.; Yu, M.; Liu, Y.; Liu, J.; Wu, Y.; Li, L.; Liu, J.; Wang, M.; Zhang, X.-H. Comparative analyses of the bacterial community of hydrothermal deposits and seafloor sediments across Okinawa Trough. J. Mar. Syst. 2018, 180, 162–172. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Wu, N.; Sun, Z.; Liu, C.; Zhen, Y.; Xin, Y.; Zhang, X.; Geng, W.; Cao, H.; et al. Diversity of Anaerobic Methane Oxidizers in the Cold Seep Sediments of the Okinawa Trough. Front. Microbiol. 2022, 13, 819187. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Sun, Z.; Xu, C.; Zhang, X.; Qin, S.; Geng, W.; Cao, H.; Zhai, B.; Li, X.; et al. Potential coupling of microbial methane, nitrogen, and sulphur cycling in the Okinawa Trough cold seep sediments. Microbiol. Spectr. 2024, 12, e03490-23. [Google Scholar] [CrossRef]
- Wu, N.; Xu, C.; Li, A.; Cao, H.; Chen, Y.; Zhang, X.; Geng, W.; Zhai, B.; Li, Q.; Sun, Z. Oceanic carbon cycle in a symbiotic zone between hydrothermal vents and cold seeps in the Okinawa Trough. Geosyst. Geoenviron. 2022, 1, 100059. [Google Scholar] [CrossRef]
- Lee, C.K.; Barbier, B.A.; Bottos, E.M.; McDonald, I.R.; Cary, S.C. The Inter-Valley Soil Comparative Survey: The ecology of Dry Valley edaphic microbial communities. ISME J. 2012, 6, 1046–1057. [Google Scholar] [CrossRef]
- Coolen, M.J.L.; Hopmans, E.C.; Rijpstra, W.I.C.; Muyzer, G.; Schouten, S.; Volkman, J.K.; Sinninghe Damsté, J.S. Evolution of the methane cycle in Ace Lake (Antarctica) during the Holocene: Response of methanogens and methanotrophs to environmental change. Org. Geochem. 2004, 35, 1151–1167. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Canonical ordination. In Numerical Ecology With R; Springer International Publishing: Cham, Switzerland, 2011; pp. 203–297. [Google Scholar]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Wang, M.; Qiao, Y.; Zheng, Y.; Zhang, X.-H. Bacterial and archaeal communities in sediments of the north Chinese marginal seas. Microb. Ecol. 2015, 70, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Oni, O.E.; Schmidt, F.; Miyatake, T.; Kasten, S.; Witt, M.; Hinrichs, K.-U.; Friedrich, M.W. Microbial communities and organic matter composition in surface and subsurface sediments of the Helgoland mud area, North Sea. Front. Microbiol. 2015, 6, 1290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; St John, E.; Anantharaman, K.; Reysenbach, A.-L. Global patterns of diversity and metabolism of microbial communities in deep-sea hydrothermal vent deposits. Microbiome 2022, 10, 241. [Google Scholar] [CrossRef]
- Jungbluth, S.P.; Amend, J.P.; Rappé, M.S. Metagenome sequencing and 98 microbial genomes from Juan de Fuca Ridge flank subsurface fluids. Sci. Data 2017, 4, 170037. [Google Scholar] [CrossRef]
- Carr, S.A.; Jungbluth, S.P.; Eloe-Fadrosh, E.A.; Stepanauskas, R.; Woyke, T.; Rappé, M.S.; Orcutt, B.N. Carboxydotrophy potential of uncultivated Hydrothermarchaeota from the subseafloor crustal biosphere. ISME J. 2019, 13, 1457–1468. [Google Scholar] [CrossRef]
- Kato, S.; Nakano, S.; Kouduka, M.; Hirai, M.; Suzuki, K.; Itoh, T.; Ohkuma, M.; Suzuki, Y. Metabolic Potential of As-yet-uncultured Archaeal Lineages of Candidatus Hydrothermarchaeota Thriving in Deep-sea Metal Sulfide Deposits. Microbes Environ. 2019, 34, 293–303. [Google Scholar] [CrossRef]
- Vilhelmsson, O.; Sigurbjornsdottir, M.A.; Thorsteinsdottir, G.V.; Cascone, M.; Corso, D.; Tonietti, L.; Migliaccio, F.; Nappi, N.; Ricciardelli, A.; Selci, M. Diversity of Thermophilic Prokaryotes. In Thermophilic Anaerobes: Phylogeny, Physiology and Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 21–90. [Google Scholar]
- Qi, Y.-L.; Chen, Y.-T.; Xie, Y.-G.; Li, Y.-X.; Rao, Y.-Z.; Li, M.-M.; Xie, Q.-J.; Cao, X.-R.; Chen, L.; Qu, Y.-N.; et al. Analysis of nearly 3000 archaeal genomes from terrestrial geothermal springs sheds light on interconnected biogeochemical processes. Nat. Commun. 2024, 15, 4066. [Google Scholar] [CrossRef]
- Maszenan, A.; Seviour, R.; Patel, B.; Janssen, P.; Wanner, J. Defluvicoccus vanus gen. nov., sp. nov., a novel Gram-negative coccus/coccobacillus in the ‘Alphaproteobacteria’ from activated sludge. Int. J. Syst. Evol. Microbiol. 2005, 55, 2105–2111. [Google Scholar] [CrossRef]
- Freches, A.; Fradinho, J.C. The biotechnological potential of the Chloroflexota phylum. Appl. Environ. Microbiol. 2024, 90, e01756-23. [Google Scholar] [CrossRef]
- Petriglieri, F.; Kondrotaite, Z.; Singleton, C.; Nierychlo, M.; Dueholm Morten, K.D.; Nielsen Per, H. A comprehensive overview of the Chloroflexota community in wastewater treatment plants worldwide. mSystems 2023, 8, e0066723. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.; Rinna, J.; Roussel, E.G.; Fry, J.C.; Weightman, A.J.; Parkes, R.J. Prokaryotic functional diversity in different biogeochemical depth zones in tidal sediments of the Severn Estuary, UK, revealed by stable-isotope probing. FEMS Microbiol. Ecol. 2010, 72, 179–197. [Google Scholar] [CrossRef]
- Lloyd, K.G.; Schreiber, L.; Petersen, D.G.; Kjeldsen, K.U.; Lever, M.A.; Steen, A.D.; Stepanauskas, R.; Richter, M.; Kleindienst, S.; Lenk, S. Predominant archaea in marine sediments degrade detrital proteins. Nature 2013, 496, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Lazar, C.S.; Baker, B.J.; Seitz, K.; Hyde, A.S.; Dick, G.J.; Hinrichs, K.U.; Teske, A.P. Genomic evidence for distinct carbon substrate preferences and ecological niches of B athyarchaeota in estuarine sediments. Environ. Microbiol. 2016, 18, 1200–1211. [Google Scholar]
- Meng, J.; Xu, J.; Qin, D.; He, Y.; Xiao, X.; Wang, F. Genetic and functional properties of uncultivated MCG archaea assessed by metagenome and gene expression analyses. ISME J. 2014, 8, 650–659. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Pan, J.; Liu, Y.; Duan, C.-H.; Li, M. Genomic and transcriptomic insights into methanogenesis potential of novel methanogens from mangrove sediments. Microbiome 2020, 8, 94. [Google Scholar] [CrossRef]
- Vigneron, A.; L’Haridon, S.; Godfroy, A.; Roussel Erwan, G.; Cragg Barry, A.; Parkes, R.J.; Toffin, L. Evidence of Active Methanogen Communities in Shallow Sediments of the Sonora Margin Cold Seeps. Appl. Environ. Microbiol. 2015, 81, 3451–3459. [Google Scholar] [CrossRef]
- Maltby, J.; Sommer, S.; Dale, A.W.; Treude, T. Microbial methanogenesis in the sulfate-reducing zone of surface sediments traversing the Peruvian margin. Biogeosciences 2016, 13, 283–299. [Google Scholar] [CrossRef]
- Xiao, K.-Q.; Beulig, F.; Kjeldsen, K.U.; Jørgensen, B.B.; Risgaard-Petersen, N. Concurrent Methane Production and Oxidation in Surface Sediment from Aarhus Bay, Denmark. Front. Microbiol. 2017, 8, 1198. [Google Scholar] [CrossRef]
- Xiao, K.-Q.; Beulig, F.; Røy, H.; Jørgensen, B.B.; Risgaard-Petersen, N. Methylotrophic methanogenesis fuels cryptic methane cycling in marine surface sediment. Limnol. Oceanogr. 2018, 63, 1519–1527. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Cao, S.; Hao, Q.; Liu, C.; Li, Y. Anaerobic oxidation of methane driven by different electron acceptors: A review. Sci. Total Environ. 2024, 946, 174287. [Google Scholar] [PubMed]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 2015, 526, 587–590. [Google Scholar] [PubMed]
- Ruff, S.E.; Biddle, J.F.; Teske, A.P.; Knittel, K.; Boetius, A.; Ramette, A. Global dispersion and local diversification of the methane seep microbiome. Proc. Natl. Acad. Sci. USA 2015, 112, 4015–4020. [Google Scholar]
- Zhu, B.; Karwautz, C.; Andrei, S.; Klingl, A.; Pernthaler, J.; Lueders, T. A novel Methylomirabilota methanotroph potentially couples methane oxidation to iodate reduction. mLife 2022, 1, 323–328. [Google Scholar] [PubMed]
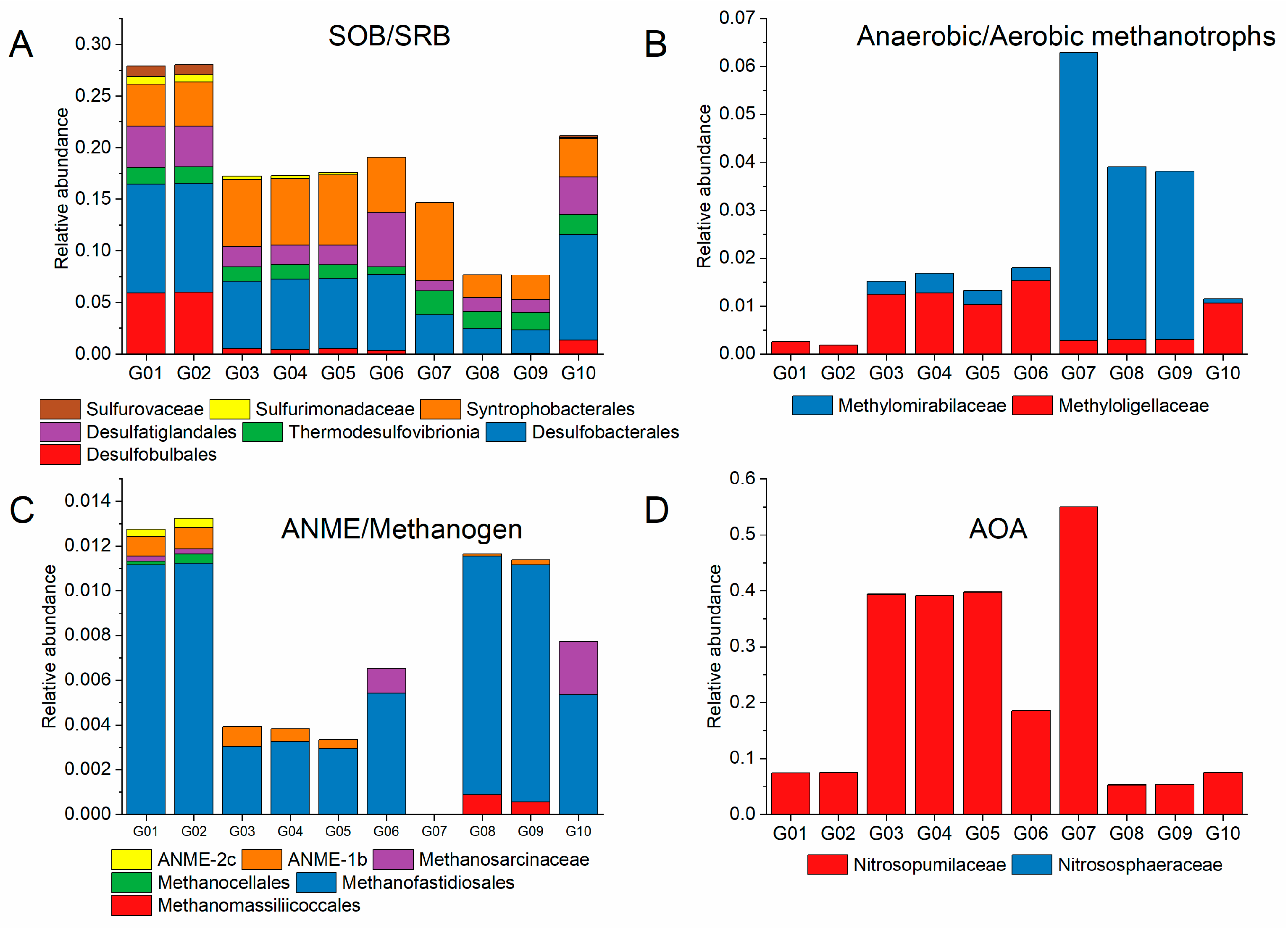
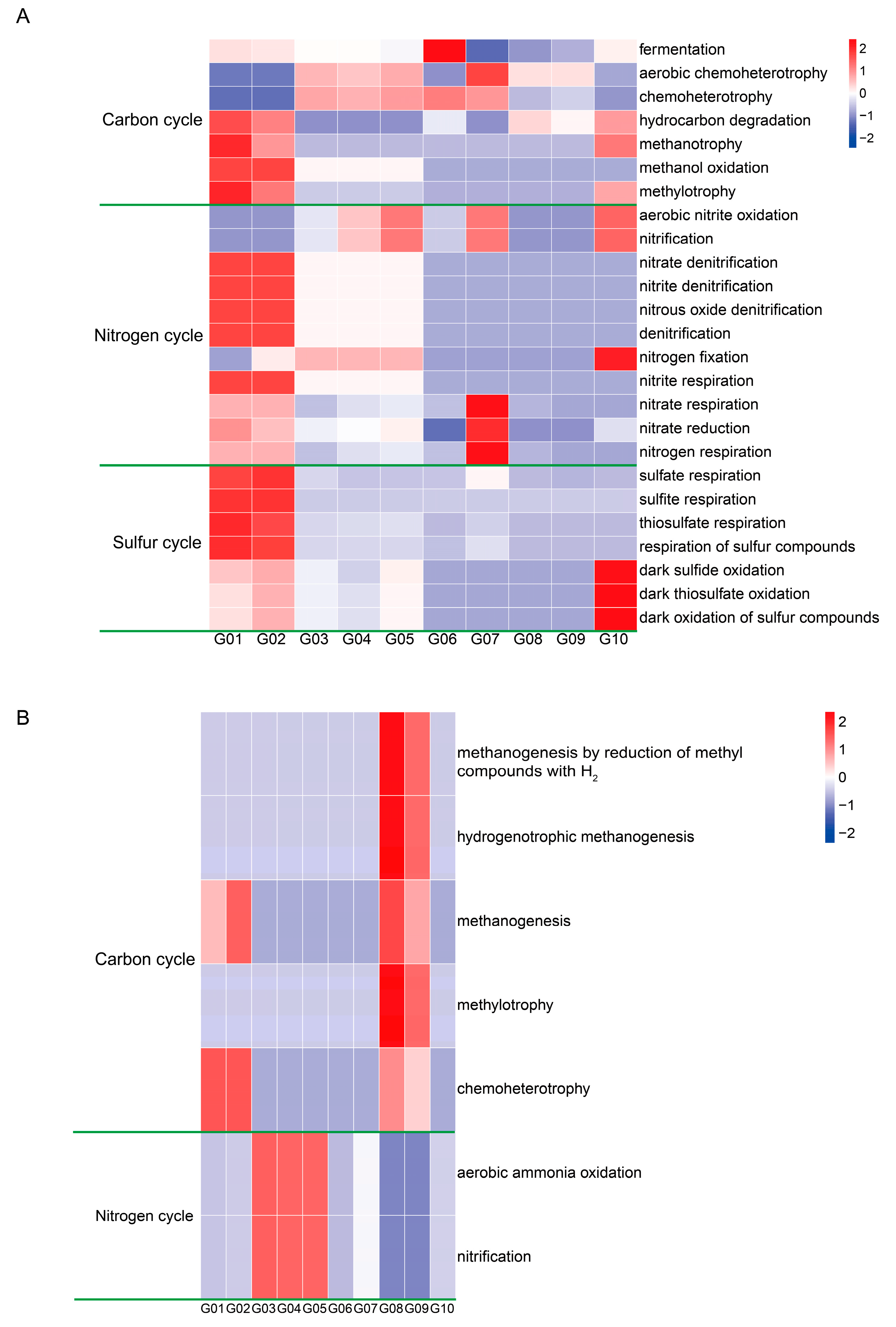
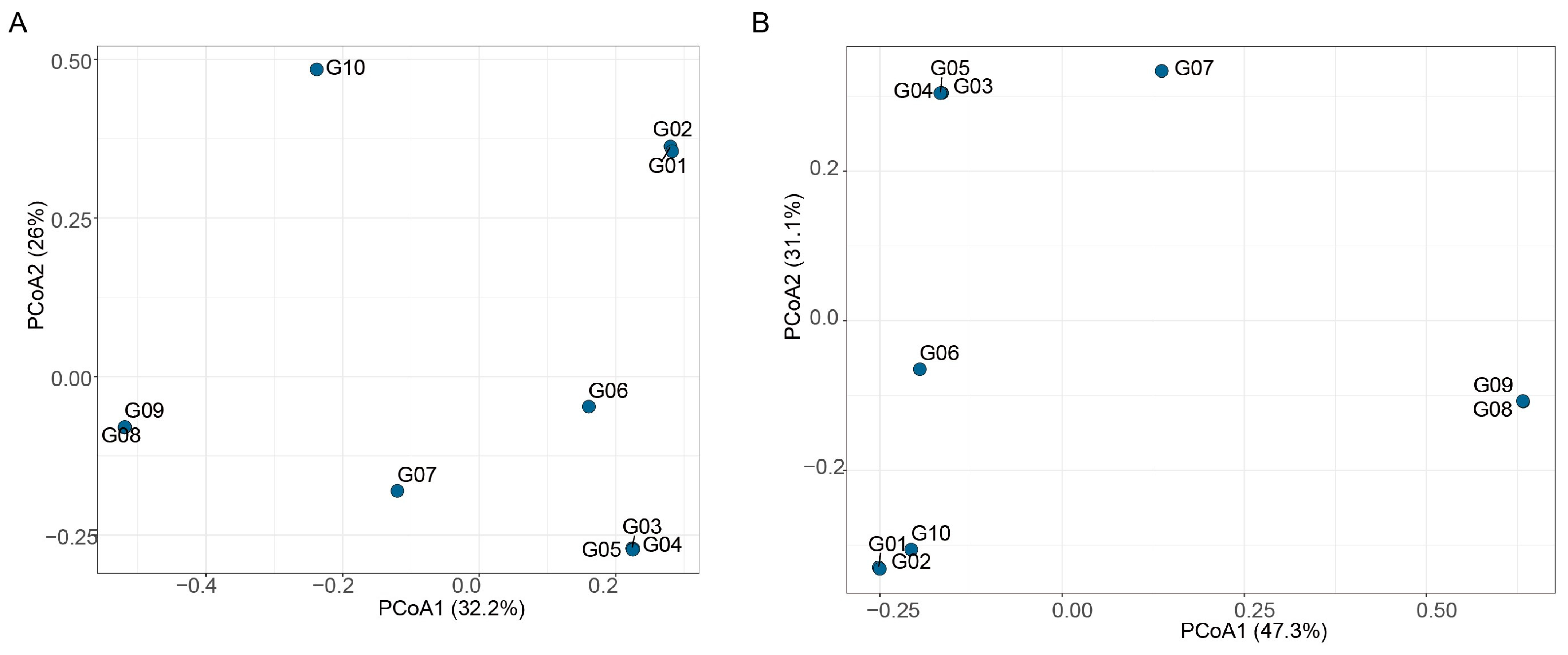

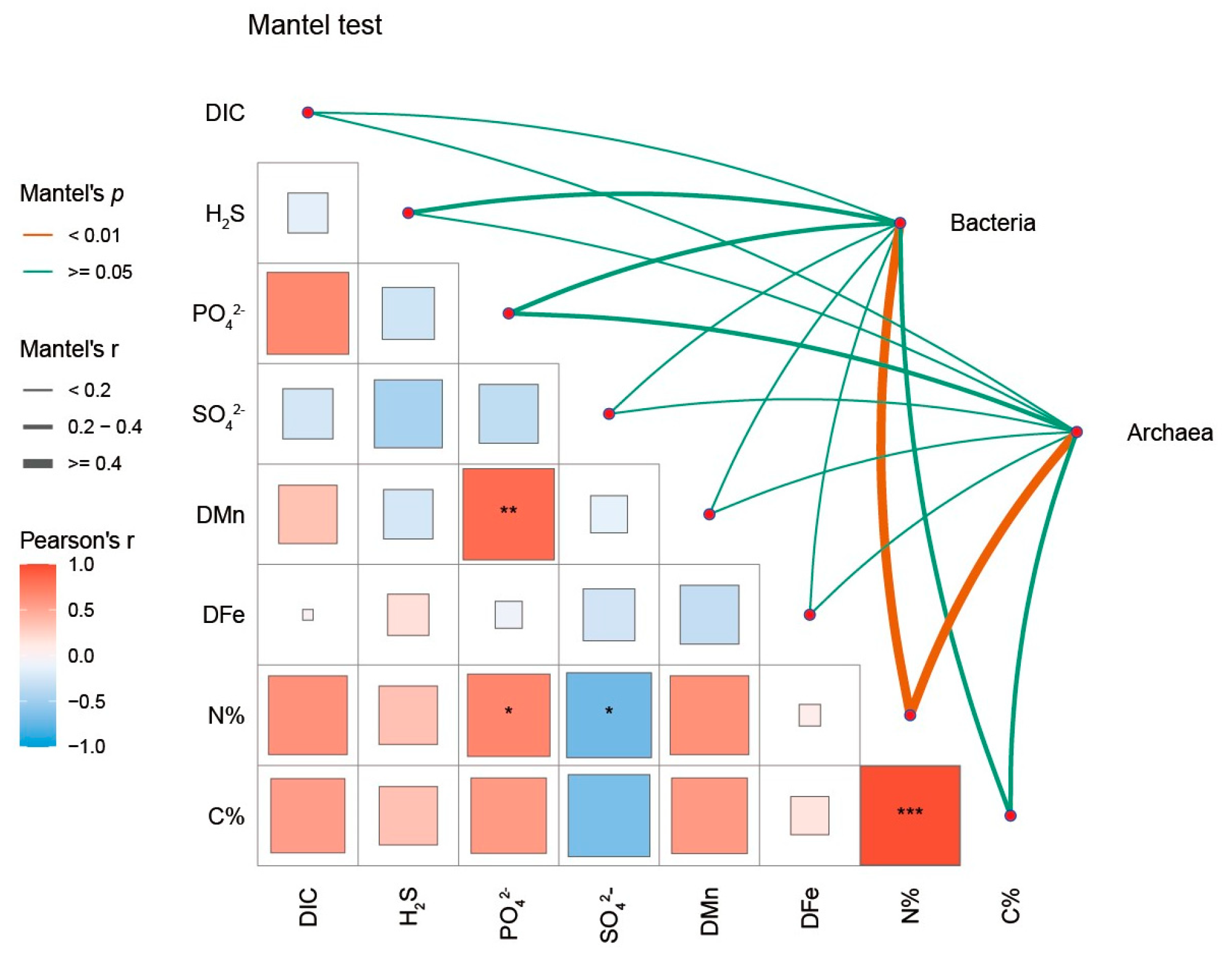
| Site | Water Depth (m) | CH4 (µM) | DIC (mM) | DOC (mM) | H2S (µM) | NO3− (nM) | PO42− (µM) | NH4+ (µM) | SO42− (Mm) | dMn (µM) | dFe (µM) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G01 | 922 | 3.56 | 64.57 | 27.22 | |||||||
| G02 | 956 | 26.26 | 1.84 | 1.46 | 1.27 | 1.40 | 28.27 | 27.43 | 4.62 | 19.85 | |
| G03 | 1010 | 1.56 | 2.09 | 0.87 | 0.61 | 384.78 | 4.29 | 56.48 | 27.63 | 49.83 | 26.38 |
| G04 | 1042 | 0.70 | 1.93 | 1.20 | 0.46 | 17.39 | 1.90 | 103.11 | 27.73 | 56.58 | 10.96 |
| G05 | 1076 | 1.26 | 1.80 | 0.70 | 0.46 | 302.17 | 3.73 | 46.69 | 28.46 | 171.73 | 13.60 |
| G06 | 1107 | 3.59 | 2.17 | 0.75 | 0.12 | 10.87 | 2.47 | 38.63 | 28.71 | 46.42 | 10.14 |
| G07 | 1167 | 2.10 | 0.38 | 6.21 | 42.92 | 27.41 | 170.59 | 1.05 | |||
| G08 | 1078 | 0.59 | 1.72 | 0.46 | 0.39 | 50.00 | 0.59 | 6.67 | 29.50 | 8.31 | 6.22 |
| G09 | 995 | 0.46 | 1.66 | 0.63 | 0.22 | 228.26 | 0.84 | 4.48 | 27.89 | 3.19 | 15.10 |
| G10 | 1030 | 0.73 | 1.86 | 0.73 | 1.48 | 1.02 | 3.39 | 27.66 | 36.87 | 7.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wu, N.; Xu, C.; Xin, Y.; Li, J.; Zhang, X.; Zhou, Y.; Sun, Z. Spatial Heterogeneity of the Microbial Community in the Surface Sediments in the Okinawa Trough. J. Mar. Sci. Eng. 2025, 13, 653. https://doi.org/10.3390/jmse13040653
Chen Y, Wu N, Xu C, Xin Y, Li J, Zhang X, Zhou Y, Sun Z. Spatial Heterogeneity of the Microbial Community in the Surface Sediments in the Okinawa Trough. Journal of Marine Science and Engineering. 2025; 13(4):653. https://doi.org/10.3390/jmse13040653
Chicago/Turabian StyleChen, Ye, Nengyou Wu, Cuiling Xu, Youzhi Xin, Jing Li, Xilin Zhang, Yucheng Zhou, and Zhilei Sun. 2025. "Spatial Heterogeneity of the Microbial Community in the Surface Sediments in the Okinawa Trough" Journal of Marine Science and Engineering 13, no. 4: 653. https://doi.org/10.3390/jmse13040653
APA StyleChen, Y., Wu, N., Xu, C., Xin, Y., Li, J., Zhang, X., Zhou, Y., & Sun, Z. (2025). Spatial Heterogeneity of the Microbial Community in the Surface Sediments in the Okinawa Trough. Journal of Marine Science and Engineering, 13(4), 653. https://doi.org/10.3390/jmse13040653






