Bio-Engineers of Marine Animal Forests: Serpulidae (Annelida) of the Biostalactite Fields in the Submarine Cave “lu Lampiùne” (Mediterranean Sea, Italy)
Abstract
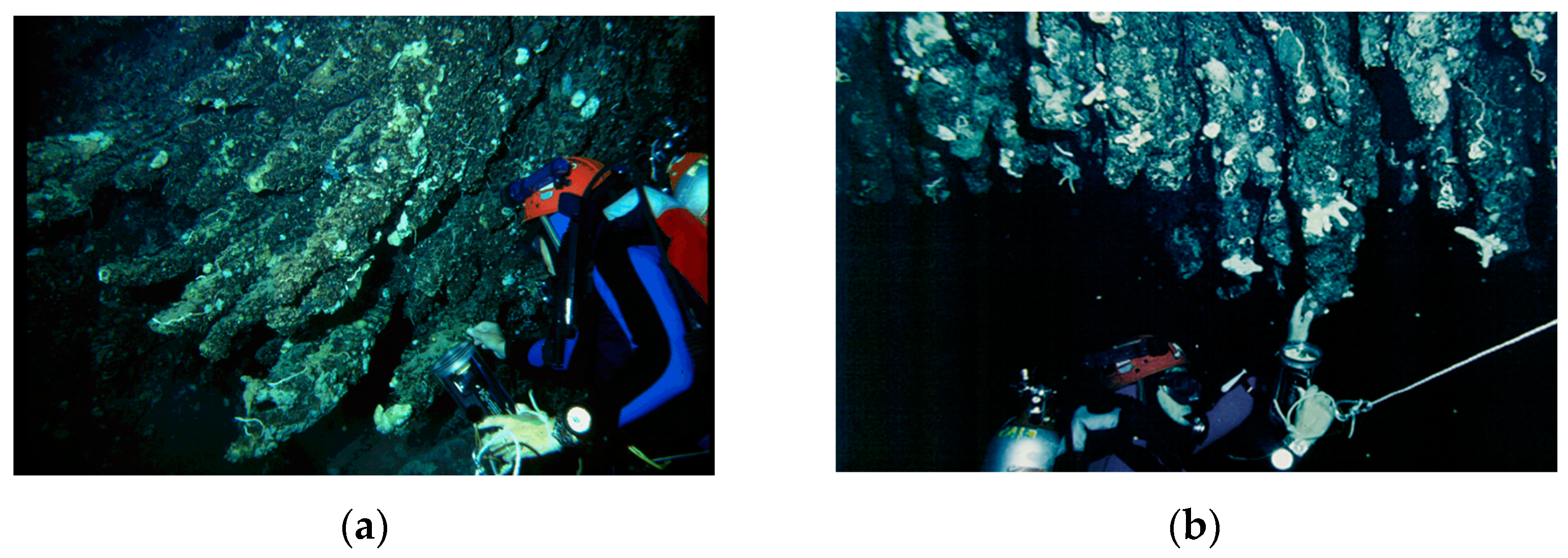
1. Introduction
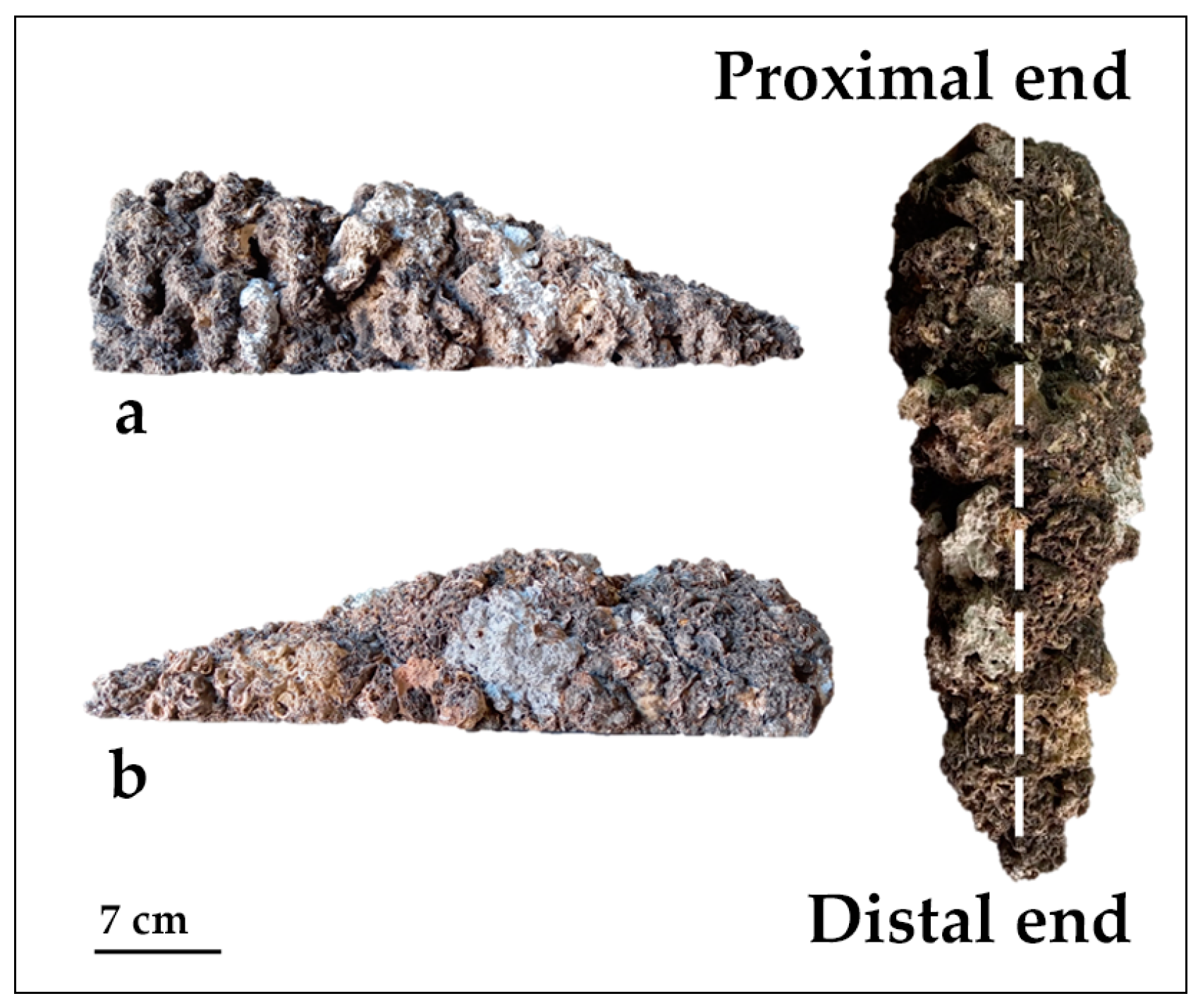
2. Materials and Methods
- -
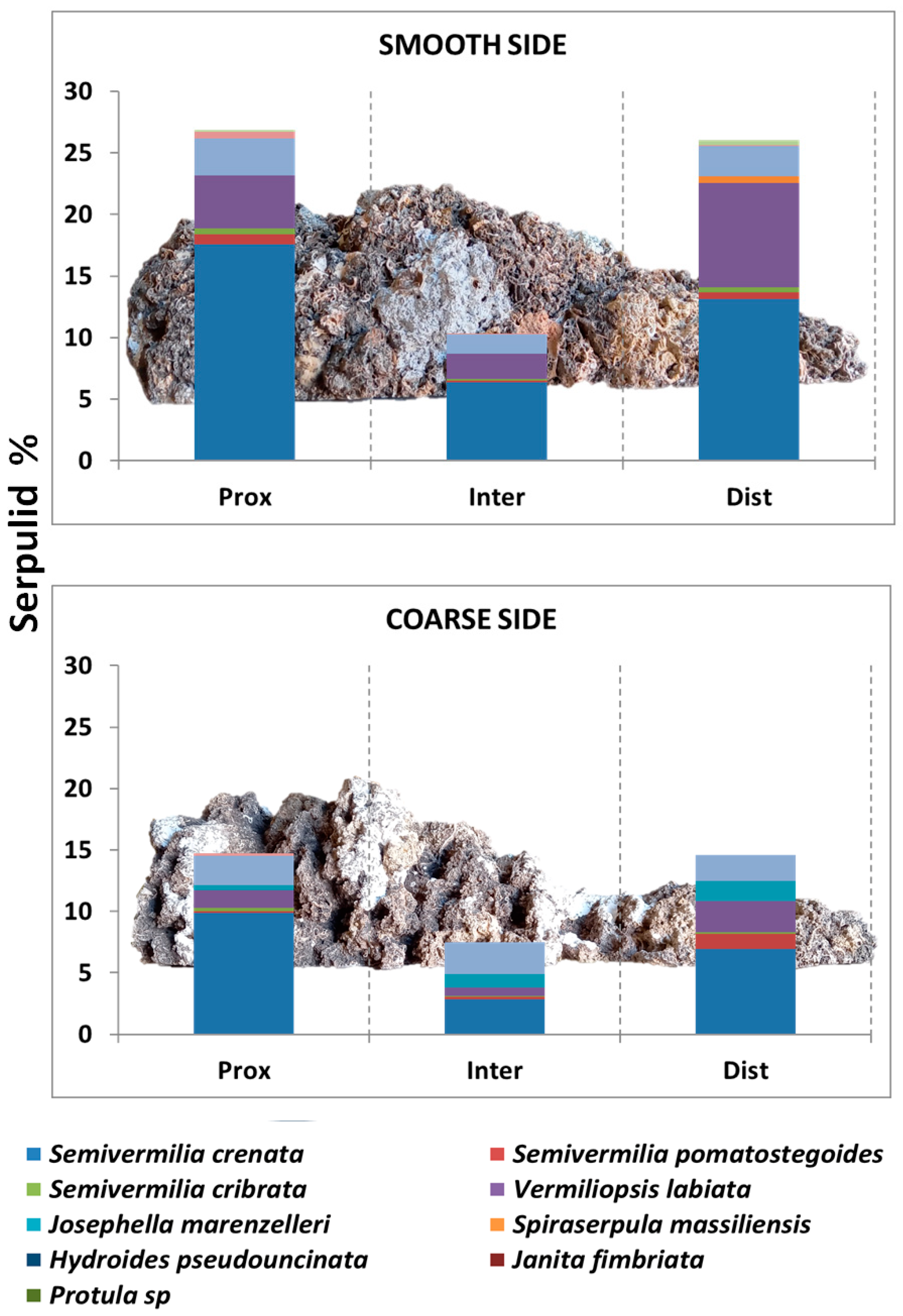
- on the two opposite sides of the BST, recognized on the basis of different textures (coarse vs. smooth) of the outer surface.
- -
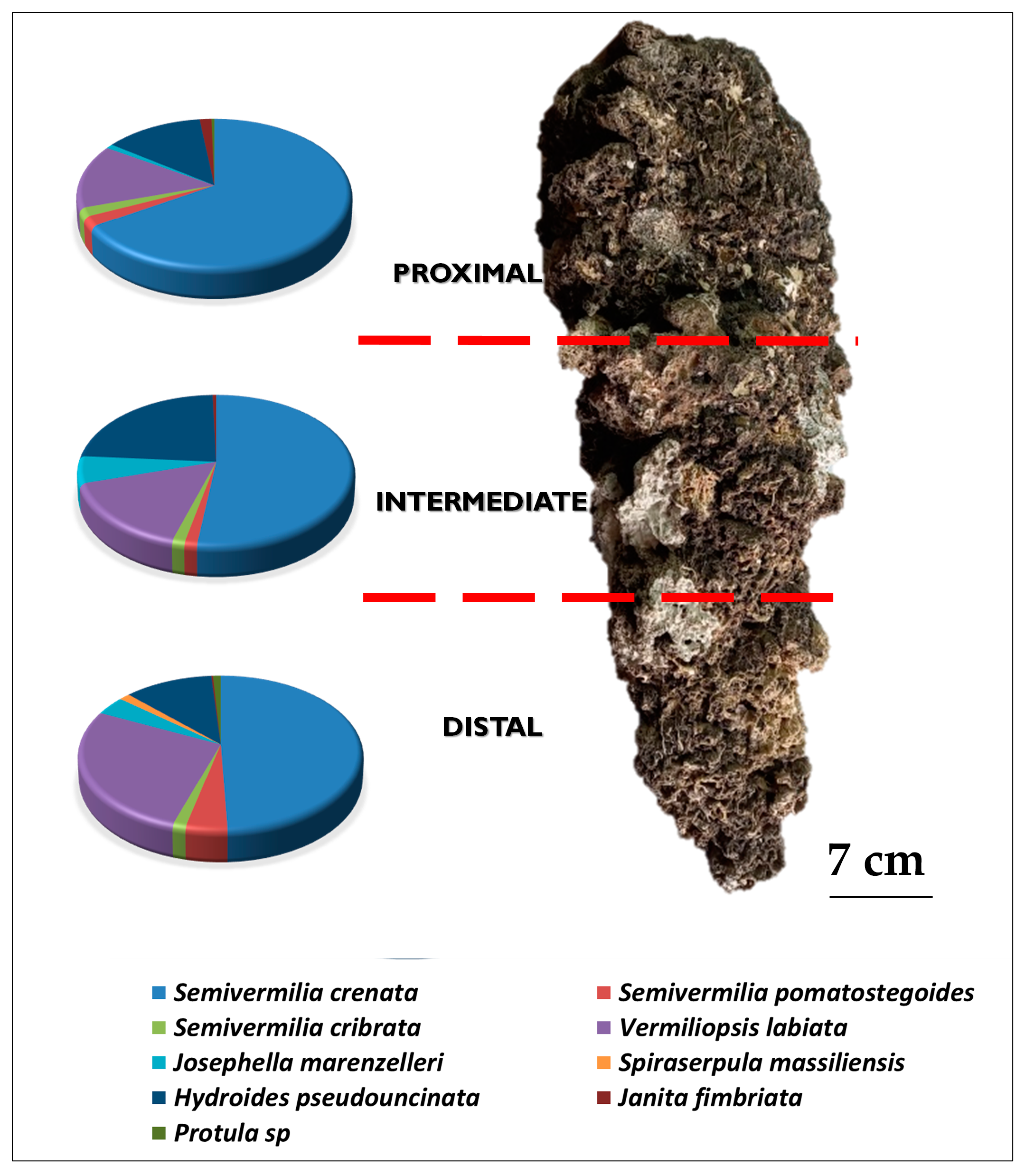
- for each side, at proximal, intermediate, and distal positions along the longitudinal axis of the BST, in relation to the point of detachment from the ceiling.
3. Results
4. Discussion
5. Conclusions
- Sciaphilic Serpulidae dominated in terms of number of taxa and abundances on the studied BST from “lu Lampiùne” submarine cave;
- Semivermilia crenata and Vermiliopsis labiata, characteristic of the dark cave, although not exclusive, largely contribute to the increase in the roughness of the BST surface providing crevices and microcavities;
- In an early phase of the cave colonization, the relatively high food supply likely led larger species (Protula) with a gregarious behavior to promote the formation and growth of the BST;
- Remarkably, the large Protula tubes that built the core of BTS and allowed it to grow 6000 years ago have now been replaced by smaller-sized Serpulidae
- As indicated by 14C dating, the accretion of Protula tubes started to slow down about 3000 years ago, with a shift in Serpulidae bioconstructors, likely due to environmental changes;
- Among the superimposed species, S. crenata and V. labiata currently act as secondary builders, while others, including smaller specimens such as Josephella marenzelleri, act as binders coating the surface of the underlying invertebrate skeletons and filling crevices of the BST surface;
- Negligible water dynamics, the absence of light-dependent competitors, as well as a low metabolic requirement linked to the small size may have facilitated the success of small-sized constructors in the dark sector of the cave.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riedl, R. Biologie der Meereshöhlen; Paul Parey: Hamburg, Germany, 1966. [Google Scholar]
- Harmelin, J.G.; Vacelet, J.; Vasseur, P. Les grottes sous-marines obscures: Un milieu extrême et un remarquable biotope refuge. Téthys 1985, 11, 214–229. [Google Scholar]
- Bianchi, C.N.; Sanfilippo, R. Policheti serpuloidei. In Grotte Marine. Cinquant’anni di Ricerche in Italia; Cicogna, F., Bianchi, C.N., Ferrari, G., Forti, P., Eds.; Ministero dell’Ambiente e della Tutela del Territorio: Roma, Italy, 2003; pp. 175–185. [Google Scholar]
- Gerovasileiou, V.; Bianchi, C.N. Mediterranean Marine Caves: A Synthesis of Current Knowledge. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–87. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean bioconstructions along the Italian coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar]
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. (Eds.) Marine Animal Forests. The Ecology of Benthic Biodiversity Hotspots; Springer Nature: Berlin/Heidelberg, Germany, 2017; p. 1350. [Google Scholar]
- Guido, A.; Rosso, A.; Sanfilippo, R.; Miriello, D.; Belmonte, G. Skeletal vs. microbialite geobiological role in bioconstructions of confined marine environments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 593, 110920. [Google Scholar]
- Gerovasileiou, V.; Dimitriadis, C.; Arvanitidis, C.; Voultsiadou, E. Taxonomic and functional surrogates of sessile benthic diversity in Mediterranean marine caves. PLoS ONE 2017, 12, e0183707. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar]
- Laborel, J. Le concrétionnement algal ‘‘Coralligène’’ et son importance géomorphologique en Méditerranée. Rec. Trav. St. Mar. Endoume 1961, 23, 37–60. [Google Scholar]
- Cocito, S.; Federghini, F.; Sgorbini, S. Bioconstructions promote biodiversity: Lessons from bryozoans and other invertebrates. Biol. Mar. Mediterr. 2001, 8, 175–180. [Google Scholar]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006, 44, 123–195. [Google Scholar]
- Casellato, S.; Stefanon, A. Coralligenous habitat of the Northern Adriatic Sea: An overview. Mar. Ecol.-Evol. Persp. 2008, 29, 321–341. [Google Scholar] [CrossRef]
- Rossi, S.; Bramanti, L. (Eds.) Perspectives on the Marine Animal Forests of the World; Springer Nature: Berlin/Heidelberg, Germany, 2020; p. 530. [Google Scholar]
- Onorato, R.; Forti, P.; Belmonte, G.; Costantini, A.; Poto, M. La grotta sottomarina lu Lampiùne: Novità esplorative e prime indagini ecologiche. Thalass. Salentina 2003, 26, 55–64. [Google Scholar]
- Belmonte, G.; Ingrosso, G.; Poto, M.; Quarta, G.; D’elia, M.; Onorato, O.; Calcagnile, L. Biogenic stalactites in submarine caves at the Cape of Otranto (SE Italy): Dating and hypothesis on their formation. Mar. Ecol. 2009, 30, 376–382. [Google Scholar] [CrossRef]
- MacIntyre, I.G.; Videtich, P.E. Pseudostalactites from Submarine Cave Near Columbus Cay, Belize Barrier-Reef Complex-Evidence of Extensive Submarine Lithification. AAPG Bull. 1979, 63, 489. [Google Scholar]
- Guido, A.; Mastandrea, A.; Rosso, A.; Sanfilippo, R.; Russo, F. Micrite precipitation induced by reducing sulfate bacteria in serpulid bioconstructions from submarine caves (Syracuse, Sicily). Rend. Online Soc. Geol. Ital. 2012, 21, 933–934. [Google Scholar]
- Guido, A.; Heindel, K.; Birgel, D.; Rosso, A.; Mastandrea, A.; Sanfilippo, R.; Russo, F.; Peckmann, J. Pendant bioconstructions cemented by microbial carbonate in submerged marine caves (Holocene, SE Sicily). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 388, 166–180. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Guido, A.; Mastandrea, A.; Russo, F.; Riding, R.; Taddei Ruggiero, E. Metazoan/microbial biostalactites from present day submarine caves in the Mediterranean Sea. Mar. Ecol. 2015, 36, 1277–1293. [Google Scholar] [CrossRef]
- Guido, A.; Jimenez, C.; Achilleos, K.; Rosso, A.; Sanfilippo, R.; Hadjioannou, L.; Petrou, A.; Russo, F.; Mastandrea, A. Cryptic serpulid-microbialite bioconstructions in the Kakoskali submarine cave (Cyprus, Eastern Mediterranean). Facies 2017, 63, 21. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Guido, A.; Gerovasileiou, V. Serpulid communities from two marine caves in the Aegean Sea, eastern Mediterranean. J. Mar. Biol. Assoc. 2017, 97, 1059–1068. [Google Scholar] [CrossRef]
- Jimenez, C.; Achilleos, K.; Petrou, A.; Hadjioannou, L.; Guido, A.; Rosso, A.; Gerovasileiou, V.; Albano, P.; Di Franco, D.; Andreou, V.; et al. A dream within a dream: Kakoskali Cave, a unique marine ecosystem in Cyprus (Levantine Sea). In Marine Caves of the Eastern Mediterranean Sea. Biodiversity, Threats and Conservation; Öztürk, B., Ed.; Turkish Marine Research Foundation (TUDAV): Istanbul, Türkiye, 2019; Publication no. 53; pp. 91–110. [Google Scholar]
- Fagerstrom, J.A. The Evolution of Reef Communities; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Palma, R.M.; Angeleri, M.P. Early Cretaceous serpulid limestones: Chachao Formation, Neuquen Basin, Argentina. Facies 1992, 27, 175–178. [Google Scholar] [CrossRef]
- ten Hove, H.A.; Van Den Hurk, P. A review of recent and fossil serpulid ‘reefs’; actuopalaeontology and the ‘Upper Malm’ serpulid limestones in NW Germany. Geol. Mijnb. 1993, 72, 23–67. [Google Scholar]
- Friebe, J.G. Serpulid-bryozoan-foraminiferal biostromes controlled by temperate climate and salinity: Middle Miocene of the Styrian Basin, Austria. Facies 1994, 30, 51–62. [Google Scholar] [CrossRef]
- Cirilli, S.; Iannace, A.; Jadoul, F.; Zamparelli, V. Microbial serpulids buildups in Norian–Rhaetian of the Western Mediterranean area: Ecological response of marginal communities to stressed environments. Terra Nova 1999, 11, 195–202. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; Rzhavsky, A.V.; ten Hove, H.A. Serpulidae Rafinesque, 1815. In Handbuch der Zoologie, Annelida, Band 2 Pleistoannelida, Sedentaria II; Westheide, W., Ed.; De Gruyter: Berlin, Germany, 2020; pp. 213–275. [Google Scholar]
- Georgieva, M.N.; Paull, C.K.; Little, C.T.S.; McGann, M.; Sahy, D.; Condon, D.; Lundsten, L.; Pewsey, J.; Caress, D.W.; Vrijenhoek, R.C. Discovery of an extensive deep-sea fossil serpulid reef associated with a cold seep, Santa Monica Basin California. Front. Mar. Sci. 2019, 6, 115. [Google Scholar] [CrossRef]
- Rouse, G.W.; Pleijel, F. Polychaetes; Oxford University Press: New York, NY, USA, 2001; p. 354. [Google Scholar]
- Vinn, O.; ten Hove, H.A.; Mutvei, H.; Kirsimaee, K. Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida). Zool. J. Linn. Soc. 2008, 154, 633–650. [Google Scholar] [CrossRef]
- Vinn, O.; Kupriyanova, E.K. Evolution of a dense outer protective tube layer in serpulids (Polychaeta, Annelida). Carnets Géol./Noteb. Geol. 2011, 5, 137–147. [Google Scholar] [CrossRef]
- Vinn, O. Biomineralization in Polychaete Annelids: A Review. Minerals 2021, 11, 1151. [Google Scholar] [CrossRef]
- Zabala, M.; Riera, T.; Gili, J.M.; Barange, M.; Lobo, A.; Peñuelas, J. Water flow, trophic depletion, and benthic macrofauna impoverishment in a submarine cave from the Western Mediterranean. Mar. Ecol. 1989, 10, 271–287. [Google Scholar] [CrossRef]
- Bussotti, S.; Terlizzi, A.; Fraschetti, S.; Belmonte, G.; Boero, F. Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Mar. Ecol. Prog. Ser. 2006, 325, 109–119. [Google Scholar] [CrossRef]
- Antonioli, F.; Silenzi, S.; Frisia, S. Tyrrhenian Holocene palaeoclimate trends from spelean serpulids. Quat. Sci. Rev. 2001, 20, 1661–1670. [Google Scholar] [CrossRef]
- Rosso, A.; Sanfilippo, R.; Guido, A.; Gerovasileiou, V.; Ruggiero, E.T.; Belmonte, G. Colonisers of the dark: Biostalactite-associated metazoans from “lu Lampiùne” submarine cave (Apulia, Mediterranean Sea). Mar. Ecol. 2021, 42, e12634. [Google Scholar] [CrossRef]
- Onorato, R.; Palmisano, G. Otranto: La grotta sottomarina de “lu Lampiune”. Itiner. Speleol. II 1990, 2, 84–90. [Google Scholar]
- Montefalcone, M.; Oprandi, A.; Azzola, A.; Morri, C.; Bianchi, C.N. Serpulid reefs and their role in aquatic ecosystems: A global review. Adv. Mar. Biol. 2022, 92, 1–54. [Google Scholar]
- ten Hove, H.A. Different causes of mass occurrence in serpulids. In Biology and Systematic of Colonial Organisms, Systematics Association Special; Larwood, G., Rosen, B.R., Eds.; Academic Press: London, UK, 1979; Volume 11, pp. 281–298. [Google Scholar]
- Bianchi, C.N.; Morri, C. Studio bionomico comparativo di alcune grotte marine sommerse; definizione di una scala di confinamento. Mem. Istit. Ital. Speleolog. 1994, 6 (Suppl. S2), 107–123. [Google Scholar]
- Fornós, J.J.; Forteza, V.; Martínez-Taberner, A. Modern polychaete reefs in western Mediterranean lagoons: Ficopomatus enigmaticus (Fauvel) in the Albufera of Menorca, Balearic Islands. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 128, 175–186. [Google Scholar]
- Scicchitano, G.; Monaco, C. Grotte carsiche e linee di costa sommerse tra Capo Santa Panagia e Ognina (Siracusa, Sicilia sudorientale). Alp. Mediterr. Quat. 2006, 19, 187–194. [Google Scholar]
- Bianchi, C.N.; Aliani, S.; Morri, C. Present day serpulid reefs, with reference to an on going research project on Ficopomatus enigmaticus. Publ. Serv. Géol. Luxemb. 1995, 29, 61–65. [Google Scholar]
- Bosence, D.W.J. Recent serpulid reefs, Connemara, Eire. Nature 1973, 242, 40–41. [Google Scholar]
- Denitto, F.; Licciano, M. Recruitment of Serpuloidea (Annelida: Polychaeta) in a marine cave of the Ionian Sea (Italy, central Mediterranean). J. Mar. Biol. Assoc. 2006, 86, 1373–1380. [Google Scholar]
- Harmelin, J.G.; Vacelet, J. Clues to deep-sea biodiversity in a nearshore cave. Vie Milieu 1997, 47, 351–354. [Google Scholar]
- Bianchi, C.N.; Cattaneo-Vietti, R.; Cinelli, F.; Morri, C.; Pansini, M. Lo studio biologico delle grotte sottomarine: Conoscenze attuali e prospettive. Boll. Mus. Ist. Biol. Univ. Genova 1996, 60, 41–69. [Google Scholar]
- Zibrowius, H. Remarques sur la faune sessile des grottes sous-marines et de l’étage bathyal en Méditerranée. Rapp. Comm. Int. Mer Médit. 1971, 20, 243–245. [Google Scholar]
- Vacelet, J.; Boury-Esnault, N.; Harmelin, J.G. Hexactinellid cave, a unique deep-sea habitat in the scuba zone. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1994, 41, 965–973. [Google Scholar] [CrossRef]
- Passelaigue, F. Les Migrations Journalières du Mysidacé Marin Cavernicole Hemimysis Speluncola. Comparaison Avec les Migrations Verticales du Plancton. Ph.D. Thesis, Université d’Aix-Marseille II, Marseille, France, 1989. [Google Scholar]
- Rosso, A.; Sanfilippo, R.; Taddei Ruggiero, E.; Di Martino, E. Faunas and ecological groups of Serpuloidea, Bryozoa and Brachiopoda from submarine caves in Sicily (Mediterranean Sea). Boll. Della Soc. Paleontol. Ital. 2013, 52, 167–176. [Google Scholar]
- Belloni, S.; Bianchi, C.N. Policheti di alcune grotte marine della Penisola Sorrentina (Golfo di Napoli). Boll. Mus. Ist. Biol. Univ. Genova 1982, 50, 118–127. [Google Scholar]
- Bianchi, C.N. Policheti Serpuloidei. In Guide per il Riconoscimento Delle Specie Animali Delle Acque Lagunari e Costiere Italiane, Collana del Progetto Finalizzato “Promozione Della Qualità Dell’ambiente”; serie AQ/1/96, 5; C.N.R.: Roma, Italy, 1981; p. 187. [Google Scholar]
- Guido, A.; Mastandrea, A.; Rosso, A.; Sanfilippo, R.; Tosti, F.; Riding, R.; Russo, F. Commensal symbiosis between agglutinated polychaetes and sulfate reducing bacteria. Geobiology 2014, 12, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Quarta, G.; D’Elia, M.; Belmonte, G.; Braione, E.; Scrimieri, L.; Calcagnile, L. 14C dating on marine bio-constructions from a submarine cave in the Adriatic Sea. Nucl. Instrum. Methods Phys. Res. B 2019, 455, 234–237. [Google Scholar] [CrossRef]
- Quarta, G.; D’Elia, M.; Calcagnile, L.; Belmonte, G.; Ingrosso, G. Reconstructing the formation mechanism of submarine biogenic stalactites: The contribution of AMS. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 1244–1247. [Google Scholar] [CrossRef]
- Sket, B. The ecology of anchihaline caves. Trends Ecol. Evol. 1996, 11, 221–225. [Google Scholar] [CrossRef]
- Harmelin, J.G. Bryozoaires des grottes sous-marines obscures de la région marseillaise, faunistique et écologie. Téthys 1969, 1, 793–806. [Google Scholar]
- Pérès, J.M.; Picard, J. Notes sommaires sur le peuplement des grottes sous-marines de la région de Marseille. Comptes Rendu Soc. Biogeogr. 1949, 227, 42–45. [Google Scholar]
- Pérès, J.M.; Picard, J. Nouveau manuel de bionomie benthique de la Mer Méditerranée. Rec. Trav. Stn. Marittime Endoume 1964, 47, 3–137. [Google Scholar]
- Balduzzi, A.; Bianchi, C.N.; Boero, F.; Cattaneo-Vietti, R.; Pansini, M.; Sarà, M. The suspension-feeder communities of a Mediterranean Sea cave. Sci. Mar. 1989, 53, 387–395. [Google Scholar]
- Harmelin, J.G. Patterns in the distribution of bryozoans in the Mediterranean marine caves. Stygologia 1986, 2, 10–25. [Google Scholar]
- Zibrowius, H. Étude morphologique, systématique et écologique des Serpulidae (Annelida Polychaeta) de la région de Marseille. Rec. Trav. Stn. Marittime Endoume 1968, 43, 81–252. [Google Scholar]
- Ten Hove, H.A.; Kupriyanova, E. Taxonomy of Serpulidae (Annelida, Polychaeta): The state of affairs. Zootaxa 2009, 2036, 1–126. [Google Scholar]
- D’Elia, M.; Quarta, G.; Calcagnile, L.; Belmonte, G. Study of the formation of biogenic speleothems found in submarine caves at the cape of Otranto, Italy, by 14C AMS. Nucl. Instrum. Methods Phys. Res. B 2007, 259, 395–397. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Chintiroglou, C.C.; Vafidis, D.; Koutsoubas, D.; Sini, M.; Dailianis, T.; Issaris, Y.; Akritopoulou, E.; Dimarchopoulou, D.; Voultsiadou, E. Census of biodiversity in marine caves of the Eastern Mediterranean Sea. Mediterr. Mar. Sci. 2015, 16, 245–265. [Google Scholar] [CrossRef]


| Positions | -BST- Whole Surface (n. Specimens) | -BST- Coarse Surface (n. Specimens) | -BST- Smooth Surface (n. Specimens) |
|---|---|---|---|
| ThProximal | 520 | 184 | 336 |
| Intermediate | 223 | 94 | 129 |
| Distal | 509 | 183 | 326 |
| Total specimens | 1252 | 461 | 791 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licciano, M.; Belmonte, G. Bio-Engineers of Marine Animal Forests: Serpulidae (Annelida) of the Biostalactite Fields in the Submarine Cave “lu Lampiùne” (Mediterranean Sea, Italy). J. Mar. Sci. Eng. 2025, 13, 639. https://doi.org/10.3390/jmse13040639
Licciano M, Belmonte G. Bio-Engineers of Marine Animal Forests: Serpulidae (Annelida) of the Biostalactite Fields in the Submarine Cave “lu Lampiùne” (Mediterranean Sea, Italy). Journal of Marine Science and Engineering. 2025; 13(4):639. https://doi.org/10.3390/jmse13040639
Chicago/Turabian StyleLicciano, Margherita, and Genuario Belmonte. 2025. "Bio-Engineers of Marine Animal Forests: Serpulidae (Annelida) of the Biostalactite Fields in the Submarine Cave “lu Lampiùne” (Mediterranean Sea, Italy)" Journal of Marine Science and Engineering 13, no. 4: 639. https://doi.org/10.3390/jmse13040639
APA StyleLicciano, M., & Belmonte, G. (2025). Bio-Engineers of Marine Animal Forests: Serpulidae (Annelida) of the Biostalactite Fields in the Submarine Cave “lu Lampiùne” (Mediterranean Sea, Italy). Journal of Marine Science and Engineering, 13(4), 639. https://doi.org/10.3390/jmse13040639







