Tidal Exclusion Barriers Fragment an Invertebrate Community into Taxonomically and Functionally Distinct Estuarine and Wetland Assemblages
Abstract
1. Introduction
2. Materials and Methods
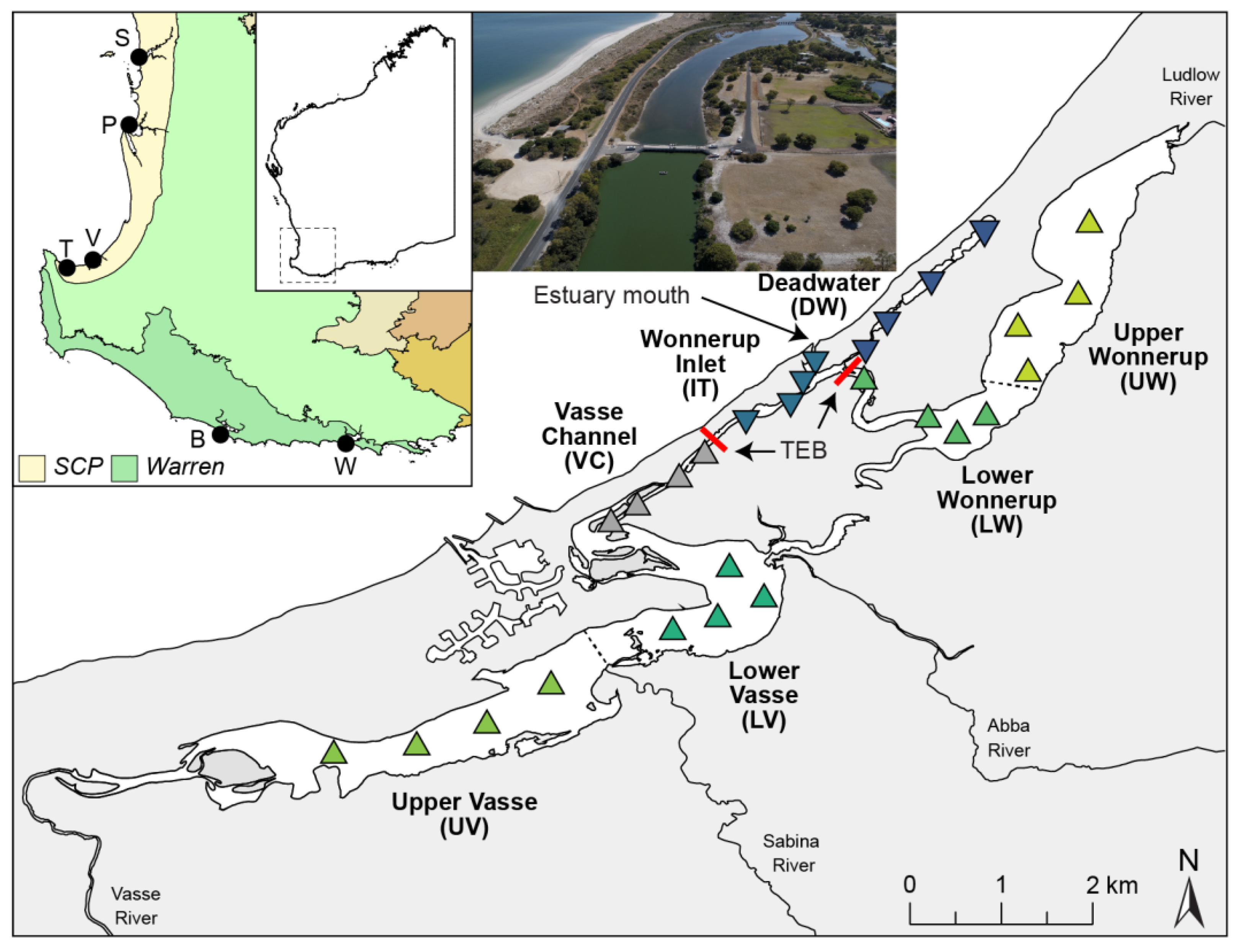
2.1. Study Site
2.2. Sample Collection and Processing
2.3. Data Analysis
2.3.1. Taxonomic Diversity and Composition
2.3.2. Functional Trait Analysis
2.3.3. Broader Comparisons of Faunal Composition
3. Results
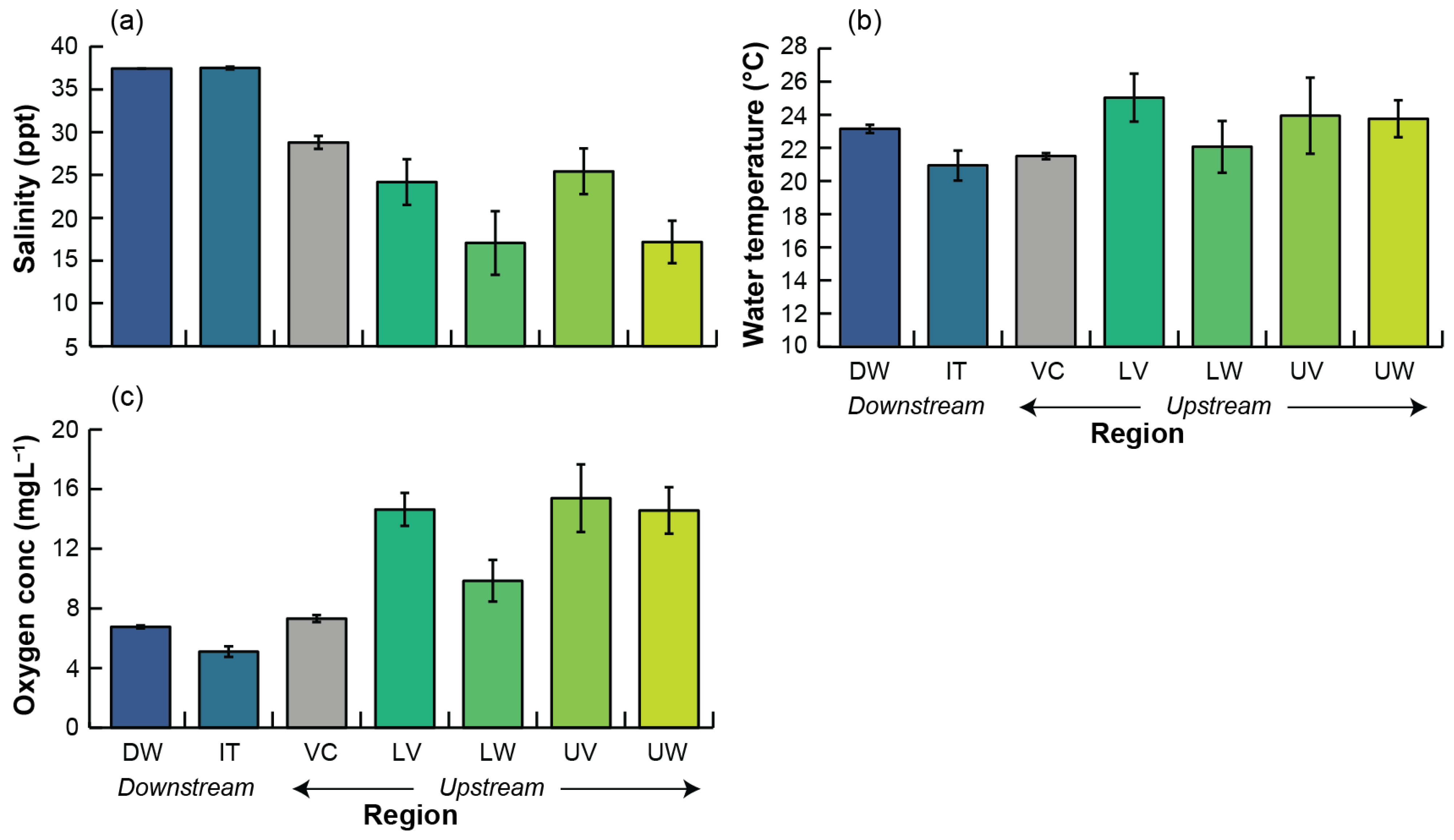
3.1. Environmental Conditions
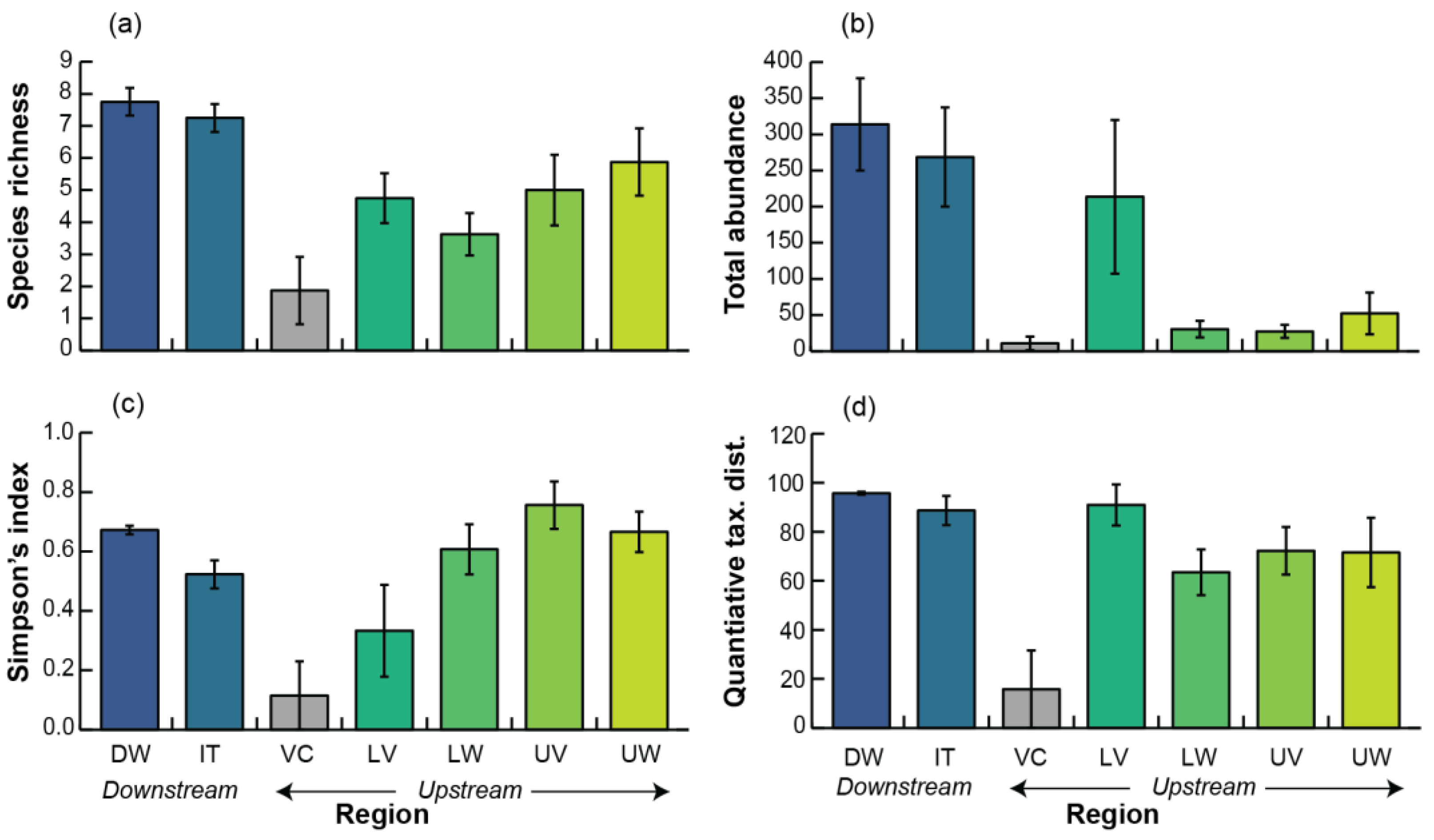
3.2. Faunal Community
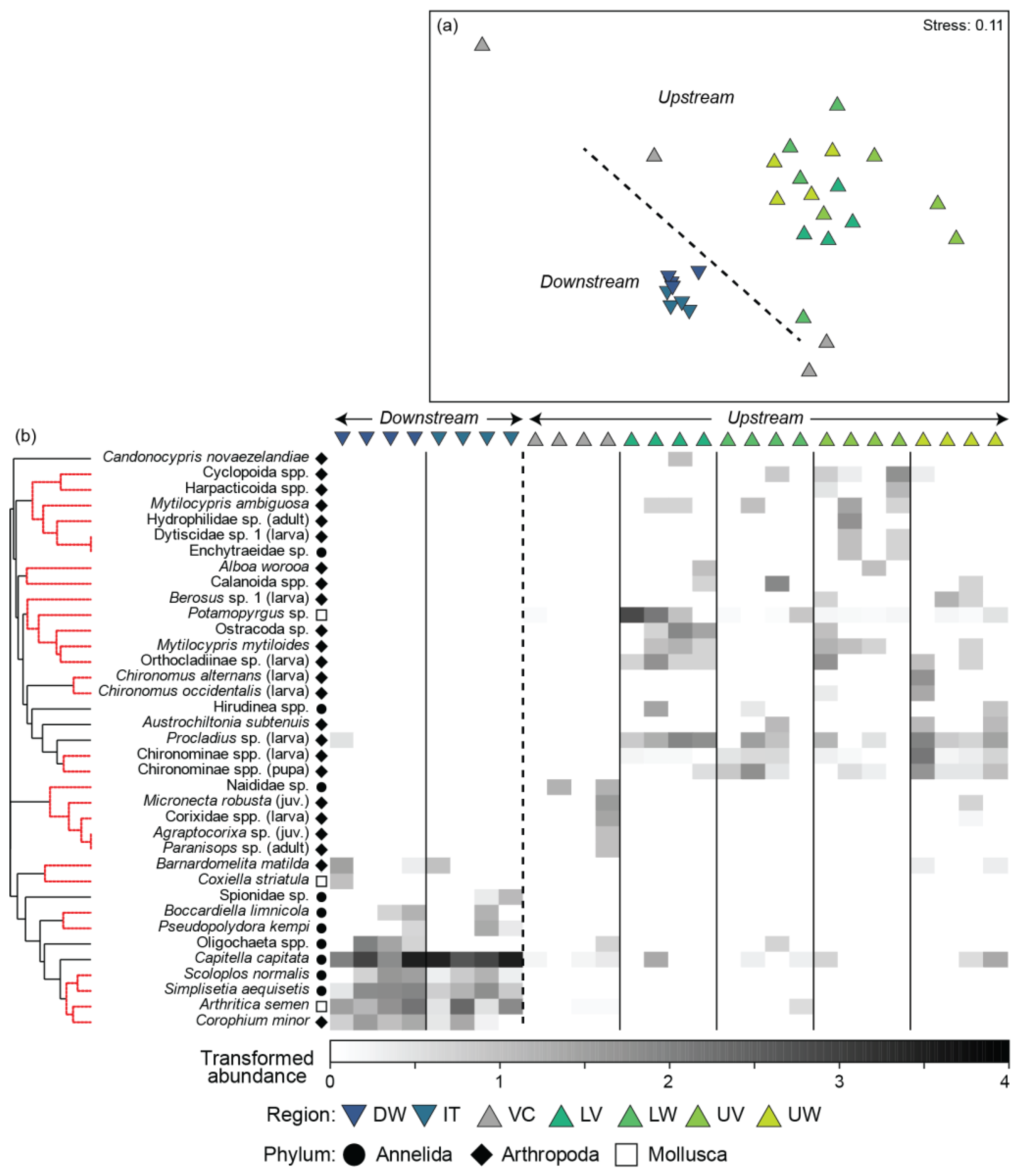
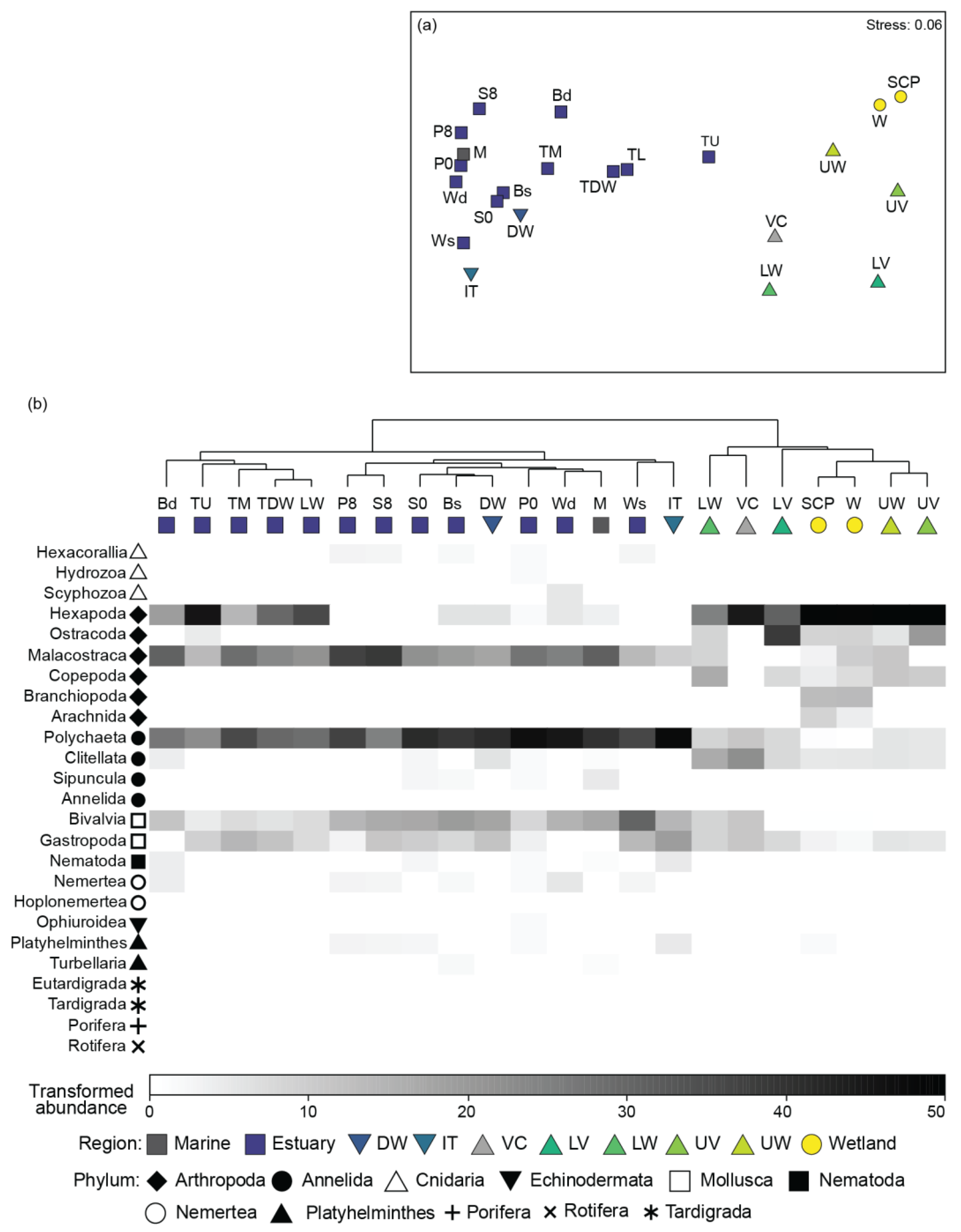
3.3. Taxonomic Composition
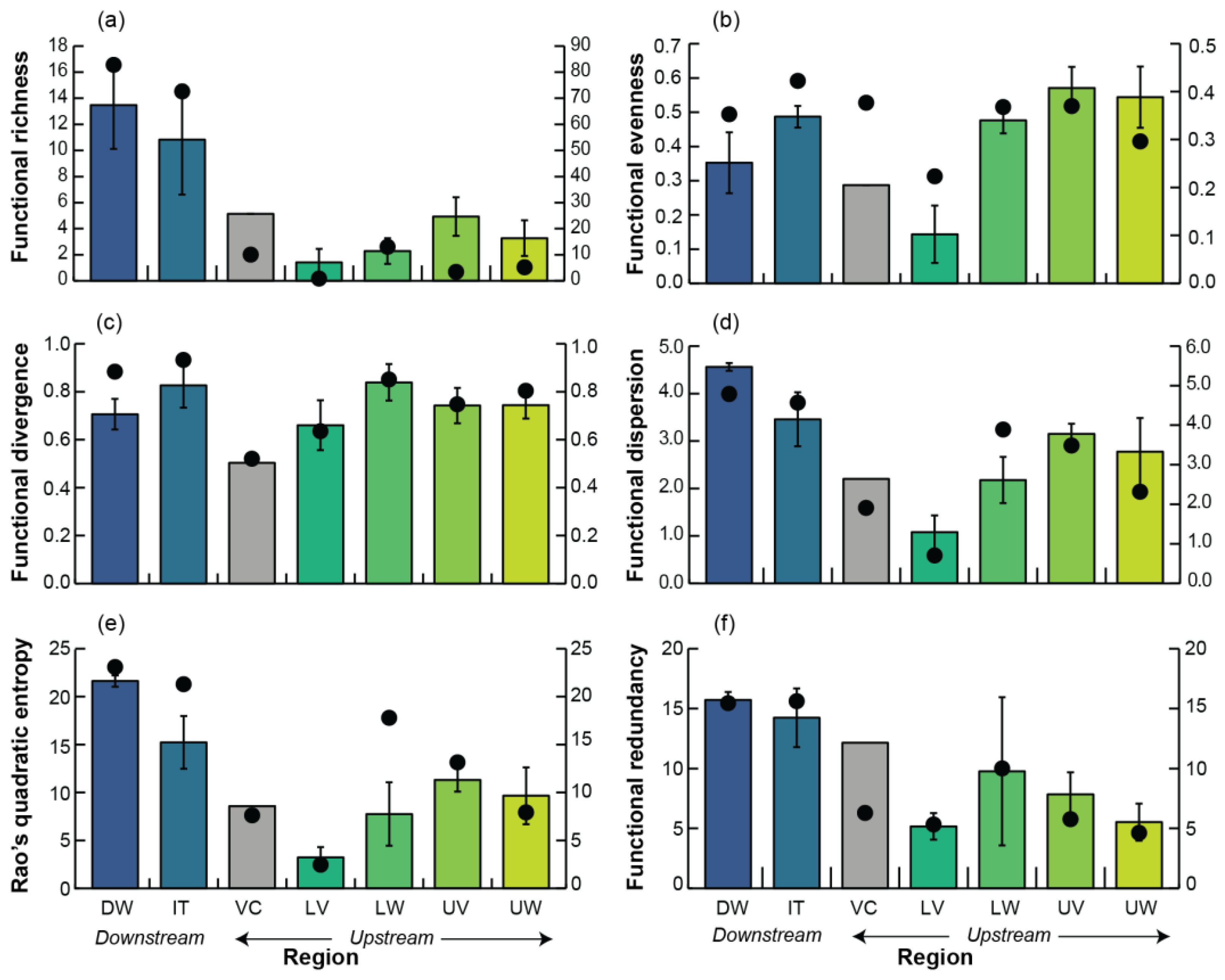
3.4. Functional Diversity
3.5. Broader Comparisons
4. Discussion
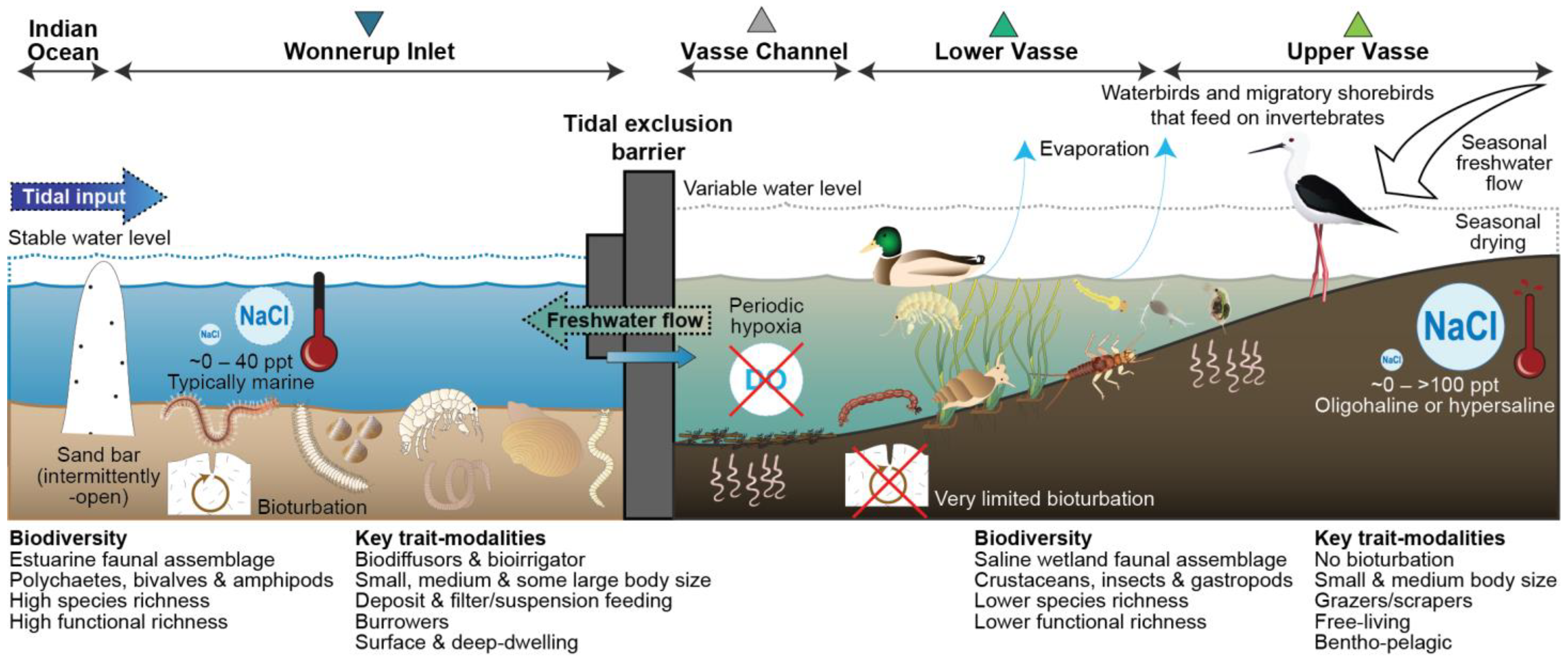
4.1. Environmental Conditions
4.2. Benthic Macroinvertebrate Fauna
4.2.1. Taxonomic Composition
4.2.2. Functional Diversity
4.3. Impacts of the Tidal Exclusion Barriers to Ecosystem Service Provision
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | P | Overall | DW | IT | Down | VC | LV | LW | UV | UW | Up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arthritica semen | M | 35.64 | 134.75 | 110.88 | 122.81 | 0.25 | 3.63 | 0.78 | |||
| Potamopyrgus sp. | M | 30.50 | 0.25 | 195.50 | 11.88 | 1.75 | 4.13 | 42.70 | |||
| Capitella ‘capitata’ (species complex) | An | 28.16 | 76.50 | 107.38 | 91.94 | 0.63 | 5.00 | 0.50 | 1.25 | 5.88 | 2.65 |
| Corophium minor | Ar | 10.86 | 55.75 | 20.25 | 38.00 | ||||||
| Simplisetia aequisetis | An | 6.55 | 29.38 | 16.50 | 22.94 | ||||||
| Chironominae spp. (larva) | Ar | 4.61 | 0.50 | 4.63 | 0.50 | 26.63 | 6.45 | ||||
| Chironominae spp. (pupa) | Ar | 1.73 | 0.38 | 5.50 | 0.50 | 5.75 | 2.43 | ||||
| Procladius sp. (larva) | Ar | 1.66 | 0.13 | 0.06 | 5.00 | 1.75 | 0.88 | 3.88 | 2.30 | ||
| Scoloplos normalis | An | 1.61 | 7.88 | 3.38 | 5.63 | ||||||
| Harpacticoida spp. | Ar | 1.07 | 7.50 | 1.50 | |||||||
| Corixidae spp. (larva) | Ar | 1.04 | 7.13 | 0.13 | 1.45 | ||||||
| Cyclopoida spp. | Ar | 0.80 | 0.88 | 4.63 | 0.13 | 1.13 | |||||
| Hydrochus sp. (adult) | Ar | 0.79 | 5.50 | 1.10 | |||||||
| Hirudinea spp. | An | 0.70 | 3.25 | 0.25 | 1.38 | 0.98 | |||||
| Barnardomelita matilda | Ar | 0.64 | 3.00 | 1.25 | 2.13 | 0.25 | 0.05 | ||||
| Hiatula biradiata | M | 0.43 | 1.63 | 1.38 | 1.50 | ||||||
| Nereididae sp. | An | 0.41 | 1.25 | 1.63 | 1.44 | ||||||
| Spionidae sp. | An | 0.36 | 2.50 | 1.25 | |||||||
| Orthocladiinae sp. (larva) | Ar | 0.32 | 1.13 | 0.75 | 0.38 | 0.45 | |||||
| Pseudopolydora kempi | An | 0.30 | 0.38 | 1.75 | 1.06 | ||||||
| Chironomus occidentalis (larva) | Ar | 0.27 | 0.13 | 1.75 | 0.38 | ||||||
| Oligochaeta spp. | An | 0.27 | 1.63 | 0.81 | 0.13 | 0.13 | 0.05 | ||||
| Ostracoda sp. | Ar | 0.25 | 1.50 | 0.25 | 0.35 | ||||||
| Mytilocypris mytiloides | Ar | 0.23 | 0.75 | 0.75 | 0.13 | 0.33 | |||||
| Agraptocorixa sp. (juv.) | Ar | 0.16 | 1.13 | 0.23 | |||||||
| Calanoida spp. | Ar | 0.16 | 0.13 | 0.88 | 0.13 | 0.23 | |||||
| Mytilocypris ambiguosa | Ar | 0.16 | 0.25 | 0.25 | 0.63 | 0.23 | |||||
| Boccardiella limnicola | An | 0.13 | 0.50 | 0.38 | 0.44 | ||||||
| Bivalvia sp. | M | 0.11 | 0.50 | 0.25 | 0.38 | ||||||
| Chironomus alternans (larva) | Ar | 0.11 | 0.75 | 0.15 | |||||||
| Hydrophilidae sp. (adult) | Ar | 0.11 | 0.75 | 0.15 | |||||||
| Micronecta robusta (juv.) | Ar | 0.11 | 0.63 | 0.13 | 0.15 | ||||||
| Berosus sp. (larva) | Ar | 0.09 | 0.13 | 0.50 | 0.13 | ||||||
| Armandia intermedia | An | 0.05 | 0.13 | 0.25 | 0.19 | ||||||
| Austrochiltonia subtenuis | Ar | 0.05 | 0.13 | 0.25 | 0.08 | ||||||
| Dytiscidae sp. 1 (larva) | Ar | 0.05 | 0.38 | 0.08 | |||||||
| Enchytraeidae sp. | An | 0.05 | 0.38 | 0.08 | |||||||
| Alboa worooa | Ar | 0.04 | 0.13 | 0.13 | 0.05 | ||||||
| Gastropoda sp. 2 | M | 0.04 | 0.13 | 0.13 | 0.13 | ||||||
| Hydrophilidae sp. 2 (larva) | Ar | 0.04 | 0.25 | 0.05 | |||||||
| Megaporus sp. (larva) | Ar | 0.04 | 0.25 | 0.05 | |||||||
| Naididae sp. | An | 0.04 | 0.25 | 0.05 | |||||||
| Candonocypris novaezelandiae | Ar | 0.02 | 0.13 | 0.03 | |||||||
| Ceratopogonidae sp. (larva) | Ar | 0.02 | 0.13 | 0.03 | |||||||
| Coxiella striatula | M | 0.02 | 0.13 | 0.06 | |||||||
| Desdemona ornata | An | 0.02 | 0.13 | 0.06 | |||||||
| Gastropoda sp. 1 | M | 0.02 | 0.13 | 0.06 | |||||||
| Gastropoda sp. 3 | M | 0.02 | 0.13 | 0.06 | |||||||
| Mysida sp. | Ar | 0.02 | 0.13 | 0.06 | |||||||
| Nematoda spp. | N | 0.02 | 0.13 | 0.06 | |||||||
| Paranisops sp. (adult) | Ar | 0.02 | 0.13 | 0.03 | |||||||
| Planorbidae sp. | M | 0.02 | 0.13 | 0.06 | |||||||
| Platyhelminthes sp. | Pl | 0.02 | 0.13 | 0.06 | |||||||
| Polychaeta sp. | An | 0.02 | 0.13 | 0.06 | |||||||
| Number of taxa | 54 | 17 | 21 | 25 | 9 | 13 | 12 | 20 | 18 | 34 | |
| Total number of individuals | 130.93 | 313.75 | 268.75 | 291.25 | 10.50 | 213.63 | 30.38 | 27.25 | 52.25 | 66.80 |

| Metrics | Term | d.f. | Mean Squares | Pseudo-F | p |
|---|---|---|---|---|---|
| Species richness | Region | 6 | 16.6 | 6.04 | 0.004 |
| Residual | 21 | 2.8 | |||
| Total abundance | Region | 6 | 4.4 | 8.05 | 0.001 |
| Residual | 21 | 0.5 | |||
| Simpson’s index | Region | 6 | 0.2 | 6.19 | 0.002 |
| Residual | 21 | 0.0 | |||
| Quantitative taxonomic distinctness | Region | 6 | 2952.2 | 7.03 | 0.006 |
| Residual | 21 | 419.8 | |||
| Functional richness | Region | 5 | 3.68 | 4.89 | 0.007 |
| Residual | 18 | 2.8 | |||
| Functional evenness | Region | 5 | 0.8 | 4.74 | 0.009 |
| Residual | 18 | 0.2 | |||
| Functional divergence | Region | 5 | 0.2 | 0.80 | 0.555 |
| Residual | 18 | 0.2 | |||
| Functional dispersion | Region | 5 | 5.6 | 6.75 | 0.002 |
| Residual | 18 | 0.8 | |||
| Rao’s quadratic entropy | Region | 5 | 161.8 | 8.02 | <0.001 |
| Residual | 18 | 20.2 | |||
| Functional redundancy | Region | 5 | 78.4 | 2.28 | 0.082 |
| Residual | 18 | 34.3 | |||
| Community-level weighted means | Region | 6 | 4715.2 | 8.82 | <0.001 |
| Residual | 21 | 534.9 | |||
| Species composition | Region | 6 | 8961.6 | 3.78 | 0.001 |
| Residual | 21 | 2368.7 |
References
- McLusky, D.S.; Elliott, M. The Estuarine Ecosystem: Ecology, Threats and Management, 3rd ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Tweedley, J.R.; Warwick, R.M.; Potter, I.C. The contrasting ecology of temperate macrotidal and microtidal estuaries. Oceanogr. Mar. Biol. Annu. Rev. 2016, 54, 73–172. [Google Scholar] [CrossRef]
- Sheaves, M.; Baker, R.; Nagelkerken, I.; Connolly, R.M. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuaries Coasts 2015, 38, 401–414. [Google Scholar]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2010, 81, 169–193. [Google Scholar] [CrossRef]
- Pennifold, M.; Davis, J. Macrofauna and nutrient cycling in the Swan River Estuary, Western Australia: Experimental results. Hydrol. Process. 2001, 15, 2537–2553. [Google Scholar] [CrossRef]
- Potter, I.C.; Warwick, R.M.; Hall, N.G.; Tweedley, J.R. The physico-chemical characteristics, biota and fisheries of estuaries. In Freshwater Fisheries Ecology; Craig, J., Ed.; Wiley-Blackwell: Chichester, UK, 2015; pp. 48–79. [Google Scholar]
- Wilson, J.G. Productivity, fisheries and aquaculture in temperate estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 953–967. [Google Scholar] [CrossRef]
- Kennish, M.J. Estuaries: Anthropogenic Impacts. In Encyclopedia of Coastal Science; Finkl, C.W., Makowski, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–9. [Google Scholar]
- Jung, N.W.; Lee, G.-h.; Dellapenna, T.M.; Jung, Y.; Jo, T.-C.; Chang, J.; Figueroa, S.M. Economic Development Drives Massive Global Estuarine Loss in the Anthropocene. Earth’s Futur. 2024, 12, e2023EF003691. [Google Scholar] [CrossRef]
- Bice, C.M.; Furst, D.; Lamontagne, S.; Oliver, R.L.; Zampatti, B.P.; Revill, A.S. The Influence of Freshwater Discharge on Productivity, Microbiota Community Structure and Trophic Dynamics in the Murray Estuary: Evidence of Freshwater Derived Trophic Subsidy in the Sandy Sprat; Goyder Institute for Water Research: Adelaide, Australia, 2015. [Google Scholar]
- Burt, N.; Rees, A. Guidelines for the Assessment and Planning of Estuarine Barrages; Thomas Telford Publishing: London, UK, 2001. [Google Scholar]
- Mooyaart, L.; Jonkman, S.; de Vries, P.; Van der Toorn, A.; van Ledden, M. Storm surge barrier: Overview and design considerations. Coast. Eng. Proc. 2014, 1, 45. [Google Scholar]
- Mooyaart, L.F.; Jonkman, S.N. Overview and design considerations of storm surge barriers. J. Waterw. Port Coast. Ocean Eng. 2017, 143, 06017001. [Google Scholar]
- Bice, C.M.; Huisman, J.; Kimball, M.E.; Mallen-Cooper, M.; Zampatti, B.P.; Gillanders, B.M. Tidal barriers and fish—Impacts and remediation in the face of increasing demand for freshwater and climate change. Estuar. Coast. Shelf Sci. 2023, 289, 108376. [Google Scholar] [CrossRef]
- Environment Agency. The Thames Barrier. Available online: https://www.gov.uk/guidance/the-thames-barrier (accessed on 6 February 2025).
- Orton, P.; Ralston, D.; van Prooijen, B.; Secor, D.; Ganju, N.; Chen, Z.; Fernald, S.; Brooks, B.; Marcell, K. Increased Utilization of Storm Surge Barriers: A Research Agenda on Estuary Impacts. Earth’s Futur. 2023, 11, e2022EF002991. [Google Scholar] [CrossRef]
- Louters, T.; Berg, J.H.; Mulder, J.P.M. Geomorphological changes of the Oosterschelde tidal system during and after the implementation of the Delta project. J. Coast. Res. 1998, 14, 1134–1151. [Google Scholar]
- Nienhuis, P.H.; Smaal, A.C. The Oosterschelde estuary, a case-study of a changing ecosystem: An introduction. Hydrobiologia 1994, 282, 1–14. [Google Scholar] [CrossRef]
- Leentvaar, J.; Nijboer, S.M. Ecological Impacts of the Construction of Dams in an Estuary. Water Sci. Technol. 1986, 18, 181–191. [Google Scholar] [CrossRef]
- Roshni, K.; Renjithkumar, C.R.; Raghavan, R.; Ranjeet, K. Fish distribution and assemblage structure in a hydrologically fragmented tropical estuary on the south-west coast of India. Reg. Stud. Mar. Sci. 2021, 43, 101693. [Google Scholar] [CrossRef]
- Gordon, J.; Arbeider, M.; Scott, D.; Wilson, S.M.; Moore, J.W. When the Tides Don’t Turn: Floodgates and Hypoxic Zones in the Lower Fraser River, British Columbia, Canada. Estuaries Coasts 2015, 38, 2337–2344. [Google Scholar] [CrossRef]
- Yen, N.T.M.; Vanreusel, A.; Lins, L.; Thai, T.T.; Nara Bezerra, T.; Quang, N.X. The Effect of a Dam Construction on Subtidal Nematode Communities in the Ba Lai Estuary, Vietnam. Diversity 2020, 12, 137. [Google Scholar] [CrossRef]
- Beatty, S.J.; Tweedley, J.R.; Cottingham, A.; Ryan, T.; Williams, J.; Lynch, K.; Morgan, D.L. Entrapment of an estuarine fish associated with a coastal surge barrier can increase the risk of mass mortalities. Ecol. Eng. 2018, 122, 229–240. [Google Scholar] [CrossRef]
- Torio, D.D.; Chmura, G.L. Assessing Coastal Squeeze of Tidal Wetlands. J. Coast. Res. 2013, 29, 1049–1061. [Google Scholar] [CrossRef]
- Rillahan, C.B.; Alcott, D.; Castro-Santos, T.; He, P. Activity Patterns of Anadromous Fish below a Tide Gate: Observations from High-Resolution Imaging Sonar. Mar. Coast. Fish. 2021, 13, 200–212. [Google Scholar] [CrossRef]
- Yoon, J.-D.; Jang, M.-H.; Jo, H.-B.; Jeong, K.-S.; Kim, G.-Y.; Joo, G.-J. Changes of fish assemblages after construction of an estuary barrage in the lower Nakdong River, South Korea. Limnology 2016, 17, 183–197. [Google Scholar] [CrossRef]
- Yoon, J.-D.; Kim, J.-H.; Park, S.-H.; Kim, E.; Jang, M.-H. Impact of estuary barrage construction on fish assemblages in the lower part of a river and the role of fishways as a passage. Ocean Sci. J. 2017, 52, 147–164. [Google Scholar] [CrossRef]
- Addicoat, R.; Tweedley, J.R.; Ryan, T.; Cottingham, A.; Morgan, D.L.; Lynch, K.; Beatty, S.J. Determining the fine-scale movement of an estuarine fish through a tidal-exclusion barrier improves the understanding of mass fish mortality risk. Estuar. Coast. Shelf Sci. 2025, 313, 109085. [Google Scholar] [CrossRef]
- Jannah, M.R.; Saha, D.; Bappy, M.M.M.; Nur, A.-A.U.; Banik, P.; Albeshr, M.F.; Arai, T.; Hossain, M.B. Macrobenthos community responses to tidal barrier in a sub-tropical river estuary: Insights for coastal management. Reg. Stud. Mar. Sci. 2024, 79, 103842. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, M.C.; Suh, K.I.; Kim, D.G. Impact of artificial barriers on benthic macroinvertebrate functional diversity in estuarine ecosystems. Entomol. Res. 2024, 54, e12728. [Google Scholar] [CrossRef]
- Nicholas, W.L.; Bird, A.F.; Beech, T.A.; Stewart, A.C. The nematode fauna of the Murray River estuary, South Australia; the effects of the barrages across its mouth. Hydrobiologia 1992, 234, 87–101. [Google Scholar] [CrossRef]
- Bernier, N.B.; Hemer, M.; Mori, N.; Appendini, C.M.; Breivik, O.; de Camargo, R.; Casas-Prat, M.; Duong, T.M.; Haigh, I.D.; Howard, T.; et al. Storm surges and extreme sea levels: Review, establishment of model intercomparison and coordination of surge climate projection efforts (SurgeMIP). Weather. Clim. Extrem. 2024, 45, 100689. [Google Scholar] [CrossRef]
- Lane, J.A.; Hardcastle, K.A.; Tregonning, R.J.; Holtfreter, S. Management of the Vasse-Wonnerup Wetland System in Relation to Sudden, Mass Fish Deaths; Vasse Estuary Technical Working Group: Busselton, Australia, 1997; p. 55. [Google Scholar]
- Lane, J.A.K.; Clarke, A.G.; Winchcombe, Y.C. Depth, Salinity and Temperature Profiling of Vasse-Wonnerup Wetlands in 1998–2000; Western Australian Department of Environment and Conservation: Busselton, Australia, 2011; p. 73. [Google Scholar]
- Marillier, B. Reconnecting Rivers Flowing to the Vasse Estuary; Water Science Technical Series, report no. 81, Water Science Branch; Department of Water and Environmental Regulation: Perth, Australia, 2018. [Google Scholar]
- Tweedley, J.R.; Beatty, S.J.; Cottingham, A.; Morgan, D.L.; Lynch, K.; Lymbery, A.J. Spatial and temporal changes in the fish fauna of a low-inflow estuary following a mass mortality event and natural and artificial bar breaches. Coasts 2024, 4, 366–391. [Google Scholar] [CrossRef]
- Lam-Gordillo, O.; Baring, R.; Dittmann, S. Taxonomic and Functional Patterns of Benthic Communities in Southern Temperate Tidal Flats. Front. Mar. Sci. 2021, 8, 723749. [Google Scholar] [CrossRef]
- Lane, J.A.K.; Clarke, A.G.; Pearson, G.B. Waterbirds of the Vasse-Wonnerup Wetlands in 1998–2000 and Some Comparisons with Earlier Data; Western Australian Department of Environment and Conservation: Busselton, Australia, 2007; p. 51. [Google Scholar]
- Wetland Research & Management. Ecological Character Description: Vasse-Wonnerup Wetlands Ramsar Site South-West Western Australia; Wetland Research & Management: Perth, Australia, 2007; p. 319. [Google Scholar]
- Department of Water and Environmental Regulation. Sediments of the Vasse Estuary Exit Channel: A Study of the Characteristics and Feasibility of Removing Sediments; Department of Water and Environmental Regulation: Perth, Australia, 2019; p. 72. [Google Scholar]
- DWER. Water Information Reporting. Available online: http://wir.water.wa.gov.au/Pages/Water-Information-Reporting.aspx (accessed on 7 February 2025).
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015; p. 296. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3 ed.; PRIMER-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Clarke, K.R.; Chapman, M.G.; Somerfield, P.J.; Needham, H.R. Dispersion-based weighting of species counts in assemblage analyses. Mar. Ecol. Prog. Ser. 2006, 320, 11–27. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar]
- Clarke, K.R.; Tweedley, J.R.; Valesini, F.J. Simple shade plots aid better long-term choices of data pre-treatment in multivariate assemblage studies. J. Mar. Biol. Assoc. U. K. 2014, 94, 1–16. [Google Scholar] [CrossRef]
- Lam-Gordillo, O.; Baring, R.; Dittmann, S. Establishing the South Australian Macrobenthic Traits (SAMT) database: A trait classification for functional assessments. Ecol. Evol. 2020, 10, 14372–14387. [Google Scholar] [CrossRef]
- Kefford, B.J.; Botwe, P.K.; Brooks, A.J.; Kunz, S.; Marchant, R.; Maxwell, S.; Metzeling, L.; Schäfer, R.B.; Thompson, R.M. An integrated database of stream macroinvertebrate traits for Australia: Concept and application. Ecol. Indic. 2020, 114, 106280. [Google Scholar] [CrossRef]
- Phillips, N.; Smith, B. New Zealand Freshwater Macroinvertebrate Trait Database; National Institute of Water & Atmospheric Research: Hamilton, New Zealand, 2018. [Google Scholar]
- Odume, O.N.; Akamagwuna, F.C.; Ntloko, P.; Dallas, H.F.; Nnadozie, C.F.; Barber-James, H.M. A trait database for southern African freshwater invertebrates. Afr. J. Aquat. Sci. 2023, 48, 64–70. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, P. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology; 2014. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 15 August 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 15 August 2024).
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Kuebbing, S.E.; Maynard, D.S.; Bradford, M.A. Linking functional diversity and ecosystem processes: A framework for using functional diversity metrics to predict the ecosystem impact of functionally unique species. J. Ecol. 2018, 106, 687–698. [Google Scholar] [CrossRef]
- van der Linden, P.; Patrício, J.; Marchini, A.; Cid, N.; Neto, J.M.; Marques, J.C. A biological trait approach to assess the functional composition of subtidal benthic communities in an estuarine ecosystem. Ecol. Indic. 2012, 20, 121–133. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Cottingham, A.; Krispyn, K.N.; Beatty, S.J. Influence of Bar Opening on the Fish Fauna of Toby Inlet; Murdoch University: Perth, Australia, 2018; p. 36. [Google Scholar]
- Wildsmith, M.D.; Rose, T.H.; Potter, I.C.; Warwick, R.M.; Clarke, K.R. Benthic macroinvertebrates as indicators of environmental deterioration in a large microtidal estuary. Mar. Pollut. Bull. 2011, 62, 525–538. [Google Scholar]
- Wildsmith, M.D.; Rose, T.H.; Potter, I.C.; Warwick, R.M.; Clarke, K.R.; Valesini, F.J. Changes in the benthic macroinvertebrate fauna of a large microtidal estuary following extreme modifications aimed at reducing eutrophication. Mar. Pollut. Bull. 2009, 58, 1250–1262. [Google Scholar]
- Tweedley, J.R.; Warwick, R.M.; Valesini, F.J.; Platell, M.E.; Potter, I.C. The use of benthic macroinvertebrates to establish a benchmark for evaluating the environmental quality of microtidal, temperate southern hemisphere estuaries. Mar. Pollut. Bull. 2012, 64, 1210–1221. [Google Scholar] [CrossRef]
- Platell, M.E.; Potter, I.C. Influence of water depth, season, habitat and estuary location on the macrobenthic fauna of a seasonally closed estuary. J. Mar. Biol. Assoc. U. K. 1996, 76, 1–21. [Google Scholar]
- Horwitz, P.; Rogan, R.; Halse, S.; Davis, J.; Sommer, B. Wetland invertebrate richness and endemism on the Swan Coastal Plain, Western Australia. Mar. Freshw. Res. 2009, 60, 1006–1020. [Google Scholar] [CrossRef]
- Trayler, K.M.; Davis, J.A.; Horwitz, P.; Morgan, D.L. Aquatic fauna of the Warren bioregion, south-west Western Australia: Does reservation guarantee preservation? J. R. Soc. West. Aust. 1996, 79, 281–291. [Google Scholar]
- Wildsmith, M.D.; Potter, I.C.; Valesini, F.J.; Platell, M.E. Do the assemblages of the benthic macroinvertebrates in nearshore waters of Western Australia vary among habitat types, zones and seasons? J. Mar. Biol. Assoc. U. K. 2005, 85, 217–232. [Google Scholar] [CrossRef]
- Johnston, S.G.; Slavich, P.G.; Hirst, P. The impact of controlled tidal exchange on drainage water quality in acid sulphate soil backswamps. Agric. Water Manag. 2005, 73, 87–111. [Google Scholar] [CrossRef]
- Nel, M.; Adams, J.B.; Human, L.R.D.; Nunes, M.; Van Niekerk, L.; Lemley, D.A. Ineffective artificial mouth-breaching practices and altered hydrology confound eutrophic symptoms in a temporarily closed estuary. Mar. Freshw. Res. 2023, 74, 1519–1535. [Google Scholar] [CrossRef]
- Becker, A.; Laurenson, L.J.B.; Bishop, K. Artificial mouth opening fosters anoxic conditions that kill small estuarine fish. Estuar. Coast. Shelf Sci. 2009, 82, 566–572. [Google Scholar] [CrossRef]
- Hallett, C.S.; Hobday, A.J.; Tweedley, J.R.; Thompson, P.A.; McMahon, K.; Valesini, F.J. Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region. Reg. Environ. Change 2018, 18, 1357–1373. [Google Scholar] [CrossRef]
- Bourman, R.P.; Murray-Wallace, C.V.; Wilson, C.; Mosley, L.; Tibby, J.; Ryan, D.D.; De Carli, E.D.; Tulley, A.; Belperio, A.P.; Haynes, D.; et al. Holocene freshwater history of the Lower River Murray and its terminal lakes, Alexandrina and Albert, South Australia, and its relevance to contemporary environmental management. Aust. J. Earth Sci. 2022, 69, 605–629. [Google Scholar] [CrossRef]
- Chiew, F.H.S.; Hale, J.; Joehnk, K.D.; Reid, M.A.; Webster, I.T. Independent Review of Lower Lakes Science Informing Water Management; Report for the Murray-Darling Basin Authority: Canberra, Australia, 2020; p. 79. [Google Scholar]
- Ysebaert, T.; Meire, P.; Maes, D.; Buijs, J. The benthic macrofauna along the estuarine gradient of the Schelde Estuary. Neth. J. Aquat. Ecol. 1993, 27, 327–341. [Google Scholar]
- Jones, A.; Watson-Russell, C.; Murray, A. Spatial patterns in the macrobenthic communities of the Hawkesbury Estuary, New South Wales. Mar. Freshw. Res. 1986, 37, 521–543. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in estuarine ecology—A review of the Remane diagram with a suggested revised model for estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Ritter, A.F.; Wasson, K.; Lonhart, S.I.; Preisler, R.K.; Woolfolk, A.; Griffith, K.A.; Connors, S.; Heiman, K.W. Ecological Signatures of Anthropogenically Altered Tidal Exchange in Estuarine Ecosystems. Estuaries Coasts 2008, 31, 554–571. [Google Scholar] [CrossRef]
- Van Diggelen, A.D.; Montagna, P.A. Is Salinity Variability a Benthic Disturbance in Estuaries? Estuaries Coasts 2016, 39, 967–980. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Dittmann, S.R.; Whitfield, A.K.; Withers, K.; Hoeksema, S.D.; Potter, I.C. Hypersalinity: Global distribution, causes, and present and future effects on the biota of estuaries and lagoons. In Coasts and Estuaries; Wolanski, E., Day, J.W., Elliott, M., Ramachandran, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 523–546. [Google Scholar]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Hallett, C.S.; Warwick, R.M.; Clarke, K.R.; Potter, I.C. The hypoxia that developed in a microtidal estuary following an extreme storm produced dramatic changes in the benthos. Mar. Freshw. Res. 2016, 67, 327–341. [Google Scholar] [CrossRef]
- Glasby, C.J.; Erséus, C.; Martin, P. Annelids in Extreme Aquatic Environments: Diversity, Adaptations and Evolution. Diversity 2021, 13, 98. [Google Scholar] [CrossRef]
- Popham, E.J. Respiration of Corixidae (Hemiptera-Heteroptera). Nature 1959, 183, 914. [Google Scholar] [CrossRef]
- Popham, E.J. On the respiration of aquatic Hemiptera Heteroptera with special reference to the Corixidae. Proc. Zool. Soc. Lond. 1960, 135, 209–242. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Warwick, R.M.; Potter, I.C. Can biotic indicators distinguish between natural and anthropogenic environmental stress in estuaries? J. Sea Res. 2015, 102, 10–21. [Google Scholar]
- Hale, S.S.; Hughes, M.M.; Buffum, H.W. Historical Trends of Benthic Invertebrate Biodiversity Spanning 182 Years in a Southern New England Estuary. Estuaries Coasts 2018, 41, 1525–1538. [Google Scholar] [CrossRef]
- Dittmann, S.; Baring, R.; Baggalley, S.; Cantin, A.; Earl, J.; Gannon, R.; Keuning, J.; Mayo, A.; Navong, N.; Nelson, M.; et al. Drought and flood effects on macrobenthic communities in the estuary of Australia’s largest river system. Estuar. Coast. Shelf Sci. 2015, 165, 36–51. [Google Scholar] [CrossRef]
- Davis, J.A.; Rosich, R.S.; Bradely, J.S.; Growns, J.E.; Schmidt, L.G.; Cheal, F. Wetland classification on the basis of water quality and invertebrate community data. In Wetlands of the Swan Coastal Plain; Water Authority of Western Australia and Environmental Protection Authority: Perth, Australia, 1993; Volume 6, p. 242. [Google Scholar]
- Rose, T.H.; Tweedley, J.R.; Warwick, R.M.; Potter, I.C. Influences of microtidal regime and eutrophication on estuarine zooplankton. Estuar. Coast. Shelf Sci. 2020, 238, 106689. [Google Scholar] [CrossRef]
- Attrill, M.J.; Rundle, S.D. Ecotone or Ecocline: Ecological Boundaries in Estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 929–936. [Google Scholar] [CrossRef]
- Walker, K.F.; Corbin, T.A.; Cummings, C.R.; Geddes, M.C.; Goonan, P.M.; Kokkin, M.J.; Lester, R.E.; Madden, C.P.; McEvoy, P.K.; Whiterod, N.; et al. Freshwater Macro-Invertebrates. In Natural History of the Coorong, Lower Lakes, and Murray Mouth Region (Yarluwar-Ruwe); Mosley, L., Ye, Q., Shepherd, S., Hemming, S., Fitzpatrick, R., Eds.; University of Adelaide Press: Adelaide, Australia, 2019; pp. 349–370. [Google Scholar]
- Dye, A.H.; Barros, F. Spatial patterns of macrofaunal assemblages in intermittently closed/open coastal lakes in New South Wales, Australia. Estuar. Coast. Shelf Sci. 2005, 64, 357–371. [Google Scholar]
- Teske, P.R.; Wooldridge, T.H. A comparison of the macrobenthic faunas of permanently open and temporarily open/closed South African estuaries. Hydrobiologia 2001, 464, 227–243. [Google Scholar]
- Loiseau, V.; Gendreau, Y.; Calosi, P.; Cusson, M. Freshwater input significantly reduces specific and functional diversity of small subarctic estuaries. Estuar. Coast. Shelf Sci. 2024, 305, 108856. [Google Scholar] [CrossRef]
- Morais, G.C.; Gusmao, J.B.; Oliveira, V.M.; Lana, P. Macrobenthic functional trait diversity at multiple scales along a subtropical estuarine gradient. Mar. Ecol. Prog. Ser. 2019, 624, 23–37. [Google Scholar]
- Elliott, M.; Quintino, V. The Estuarine Quality Paradox, Environmental Homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. Pollut. Bull. 2007, 54, 640–645. [Google Scholar] [CrossRef]
- Cronin-O’Reilly, S.; Krispyn, K.N.; Maus, C.; Standish, R.J.; Loneragan, N.R.; Tweedley, J.R. Empirical evidence of alternative stable states in an estuary. Sci. Total Environ. 2024, 954, 176356. [Google Scholar] [CrossRef] [PubMed]
- McCallum, R.; Eyre, B.; Hyndes, G.; McMahon, K.; Oakes, J.M.; Wells, N.S. Importance of internal dissolved organic nitrogen loading and cycling in a small and heavily modified coastal lagoon. Biogeochemistry 2021, 155, 237–261. [Google Scholar] [CrossRef]
- Department of Water and Environmental Regulation. Year on the Vasse-Wonnerup Wetlands: An Ecological Snapshot March 2017—January 2018; Department of Water and Environmental Regulation: Busselton, Australia, 2019; p. 20. [Google Scholar]
- Welsh, D.T. It’s a dirty job but someone has to do it: The role of marine benthic macrofauna in organic matter turnover and nutrient recycling to the water column. Chem. Ecol. 2003, 19, 321–342. [Google Scholar] [CrossRef]
- Lam-Gordillo, O.; Huang, J.; Barceló, A.; Kent, J.; Mosley, L.M.; Welsh, D.T.; Simpson, S.L.; Dittmann, S. Restoration of benthic macrofauna promotes biogeochemical remediation of hostile sediments; An in situ transplantation experiment in a eutrophic estuarine-hypersaline lagoon system. Sci. Total Environ. 2022, 833, 155201. [Google Scholar] [CrossRef]
- Aller, R.C. Bioturbation and remineralization of sedimentary organic matter: Effects of redox oscillation. Chem. Geol. 1994, 114, 331–345. [Google Scholar] [CrossRef]
- Kristensen, E.; Holmer, M. Decomposition of plant materials in marine sediment exposed to different electron acceptors (O2, NO3−, and SO42−), with emphasis on substrate origin, degradation kinetics, and the role of bioturbation. Geochim. Cosmochim. Acta 2001, 65, 419–433. [Google Scholar] [CrossRef]
- Cronin-O’Reilly, S.; Wells, N.S.; McCallum, R.; Hallett, C.S.; Tweedley, J.R.; Valesini, F.J.; Eyre, B.D. Chronically stressed benthic macroinvertebrate communities exhibit limited effects on ecosystem function in a microtidal estuary. Mar. Ecol. Prog. Ser. 2022, 701, 1–16. [Google Scholar]
- Gao, J.; Zhi, Y.; Huang, Y.; Shi, S.; Tan, Q.; Wang, C.; Han, L.; Yao, J. Effects of benthic bioturbation on anammox in nitrogen removal at the sediment-water interface in eutrophic surface waters. Water Res. 2023, 243, 120287. [Google Scholar] [CrossRef]
- Laverock, B.; Gilbert, J.A.; Tait, K.; Osborn, A.M.; Widdicombe, S. Bioturbation: Impact on the marine nitrogen cycle. Biochem. Soc. Trans. 2011, 39, 315–320. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Keleher, J.; Cottingham, A.; Beatty, S.J.; Lymbery, A.J. The Fish Fauna of the Vasse-Wonnerup and the Impact of a Substantial Fish Kill Event; Murdoch University: Perth, Australia, 2014; p. 113. [Google Scholar]
- Collazo, J.A.; Dawn, A.O.H.; Kelly, C.A. Accessible Habitat for Shorebirds: Factors Influencing Its Availability and Conservation Implications. Waterbirds Int. J. Waterbird Biol. 2002, 25, 13–24. [Google Scholar]
- Aarif, K.M.; Zouhar, J.; Musilova, Z.; Musil, P.; Nefla, A.; Muzaffar, S.B.; Rubeena, K.A. Bill Length of Non-breeding Shorebirds Influences the Water Depth Preferences for Foraging in the West Coast of India. Ecol. Evol. 2024, 14, e70396. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cai, Y.; Li, B.; Chen, J. Managing Wetland Habitats for Waterbirds: An International Perspective. Wetlands 2010, 30, 15–27. [Google Scholar] [CrossRef]
- Schaffer-Smith, D.; Swenson, J.J.; Reiter, M.E.; Isola, J.E. Quantifying shorebird habitat in managed wetlands by modeling shallow water depth dynamics. Ecol. Appl. 2018, 28, 1534–1545. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Chambers, J.M.; Potter, I.C. The Benthic Macroinvertebrate Faunas of the Vasse-Wonnerup Estuary as Indicators of Environmental Degradation; Report for the South West Catchments Council; Murdoch University: Perth, Australia, 2011; p. 41. [Google Scholar]
- Krispyn, K.N.; Loneragan, N.R.; Whitfield, A.K.; Tweedley, J.R. Salted mullet: Protracted occurrence of Mugil cephalus under extreme hypersaline conditions. Estuar. Coast. Shelf Sci. 2021, 261, 107533. [Google Scholar] [CrossRef]
- Michael, T.C.; Costello, D.M.; Fitzgibbon, A.S.; Kinsman-Costello, L.E. Invertebrate activities in wetland sediments influence oxygen and nutrient dynamics at the sediment-water interface. Wetlands 2023, 43, 96. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saha, G.K.; Aditya, G. Macroinvertebrates as engineers for bioturbation in freshwater ecosystem. Environ. Sci. Pollut. Res. 2022, 29, 64447–64468. [Google Scholar] [CrossRef]
- Ganglo, C.; Manfrin, A.; Mendoza-Lera, C.; Lorke, A. Effects of chironomid larvae density and mosquito biocide on methane and carbon dioxide dynamics in freshwater sediments. PLoS ONE 2024, 19, e0301913. [Google Scholar] [CrossRef]
- Mosley, L.M.; Bourman, B.; Muller, K.; Tibby, J. Comment on Finlayson et al. ‘Continuing the discussion about ecological futures for the lower Murray river (Australia) in the Anthropocene’. Mar. Freshw. Res. 2022, 73, 573–577. [Google Scholar] [CrossRef]



| Trait | Modality | Acronym |
|---|---|---|
| Bioturbation mode | Biodiffusor | Bdiff |
| Bioirrigator | Birrig | |
| Surface modifier | Surmo | |
| No bioturbation | Nbio | |
| Body size | Large (>20 mm) | Lar |
| Medium (5–20 mm) | Med | |
| Small (0.5–5 mm) | Sma | |
| Feeding mode | Deposit feeder | Defe |
| Filter/Suspension feeder | Fisus | |
| Grazer/Scraper | Graz/Sc | |
| Omnivore | On | |
| Predator | Pred | |
| Scavenger/Opportunist | Scav | |
| Sub-surface deposit feeder | Ssdefe | |
| Living habit | Burrower | Burr |
| Free-living/Surface crawler | Free | |
| Tube dwelling | Tubdw | |
| Attached/Sessile | Att/S | |
| Sediment position | Bentho-pelagic | Be-pel |
| Deeper than 3 cm | Deep | |
| Surface shallow <3 cm | Surfsh | |
| Attached | Att |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cronin-O’Reilly, S.; Cottingham, A.; Kalnejais, L.H.; Lynch, K.; Tweedley, J.R. Tidal Exclusion Barriers Fragment an Invertebrate Community into Taxonomically and Functionally Distinct Estuarine and Wetland Assemblages. J. Mar. Sci. Eng. 2025, 13, 635. https://doi.org/10.3390/jmse13040635
Cronin-O’Reilly S, Cottingham A, Kalnejais LH, Lynch K, Tweedley JR. Tidal Exclusion Barriers Fragment an Invertebrate Community into Taxonomically and Functionally Distinct Estuarine and Wetland Assemblages. Journal of Marine Science and Engineering. 2025; 13(4):635. https://doi.org/10.3390/jmse13040635
Chicago/Turabian StyleCronin-O’Reilly, Sorcha, Alan Cottingham, Linda H. Kalnejais, Kath Lynch, and James R. Tweedley. 2025. "Tidal Exclusion Barriers Fragment an Invertebrate Community into Taxonomically and Functionally Distinct Estuarine and Wetland Assemblages" Journal of Marine Science and Engineering 13, no. 4: 635. https://doi.org/10.3390/jmse13040635
APA StyleCronin-O’Reilly, S., Cottingham, A., Kalnejais, L. H., Lynch, K., & Tweedley, J. R. (2025). Tidal Exclusion Barriers Fragment an Invertebrate Community into Taxonomically and Functionally Distinct Estuarine and Wetland Assemblages. Journal of Marine Science and Engineering, 13(4), 635. https://doi.org/10.3390/jmse13040635








