Hydrology Modulates the Microplastics Composition and Transport Flux Across the River–Sea Interface in Zhanjiang Bay, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analysis Methods
2.3. Quality Assurance and Control
2.4. Diversity Index of MPs
2.5. Statistical Analysis
3. Results
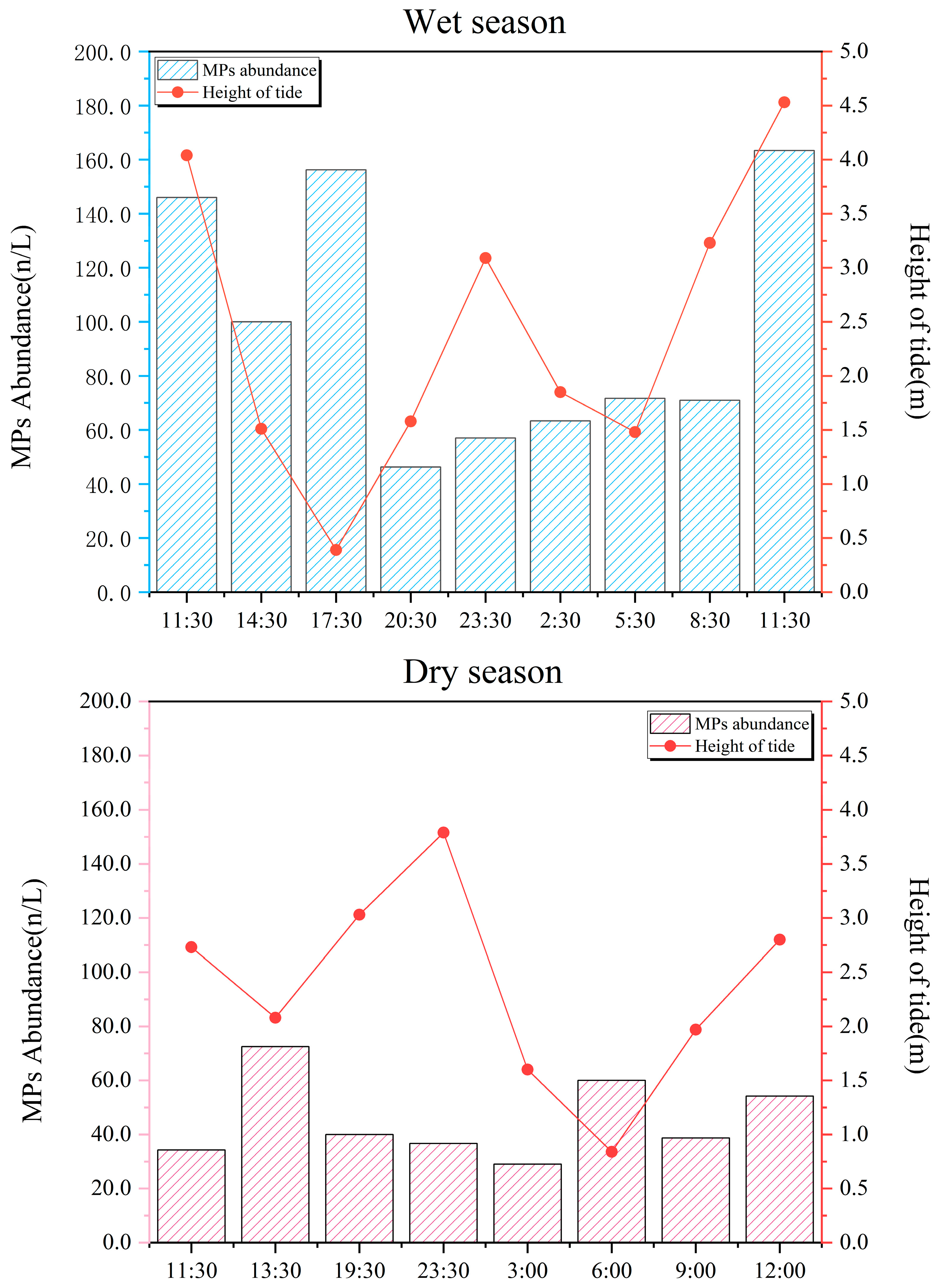
3.1. Hydrodynamic Variation in the Suixi River–Sea in ZJB
3.2. Hydrodynamics on MPs Abundance in the Suixi River–Sea in ZJB
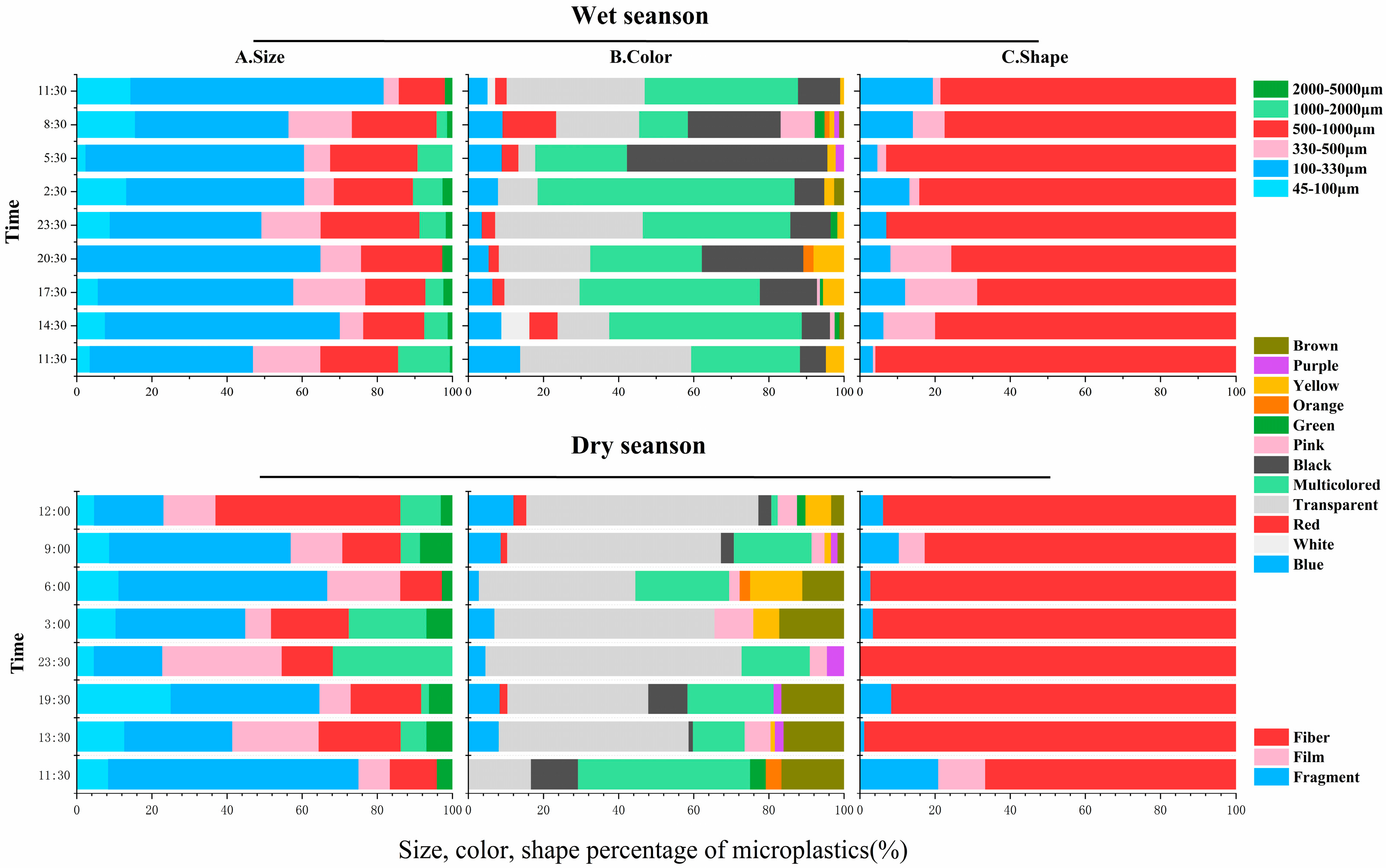
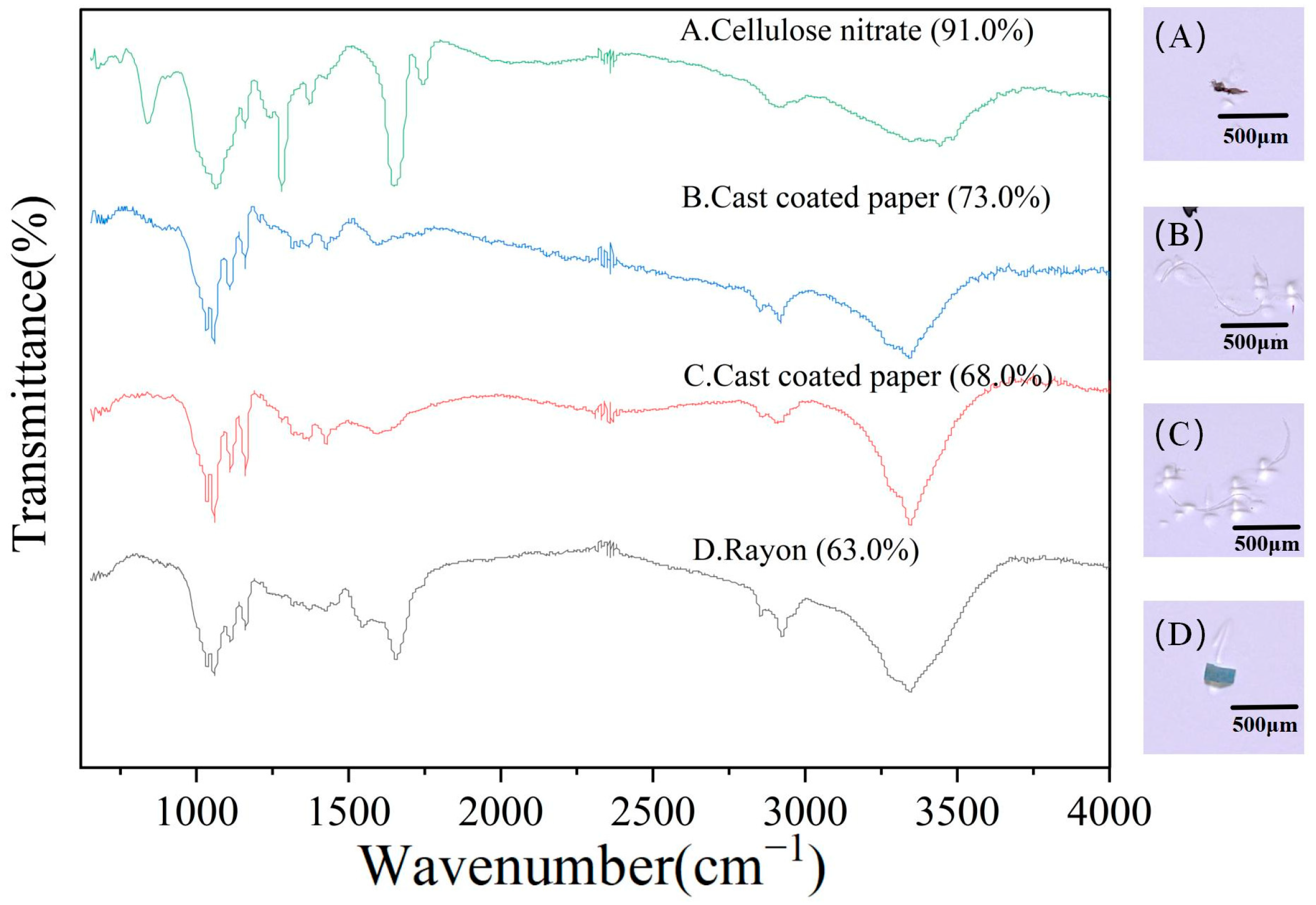
3.3. Hydrodynamics on MPs Composition in the Suixi River–Sea in ZJB
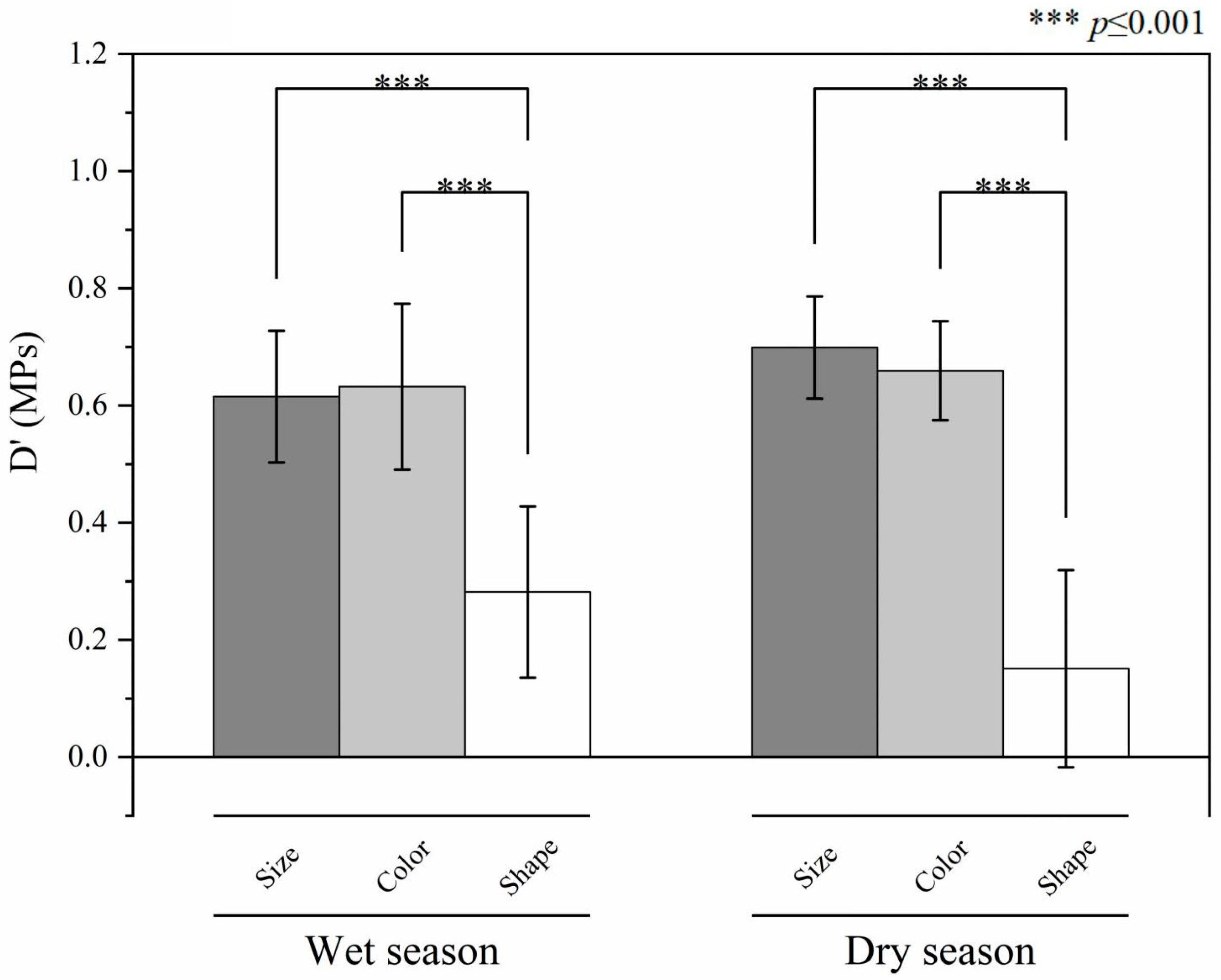
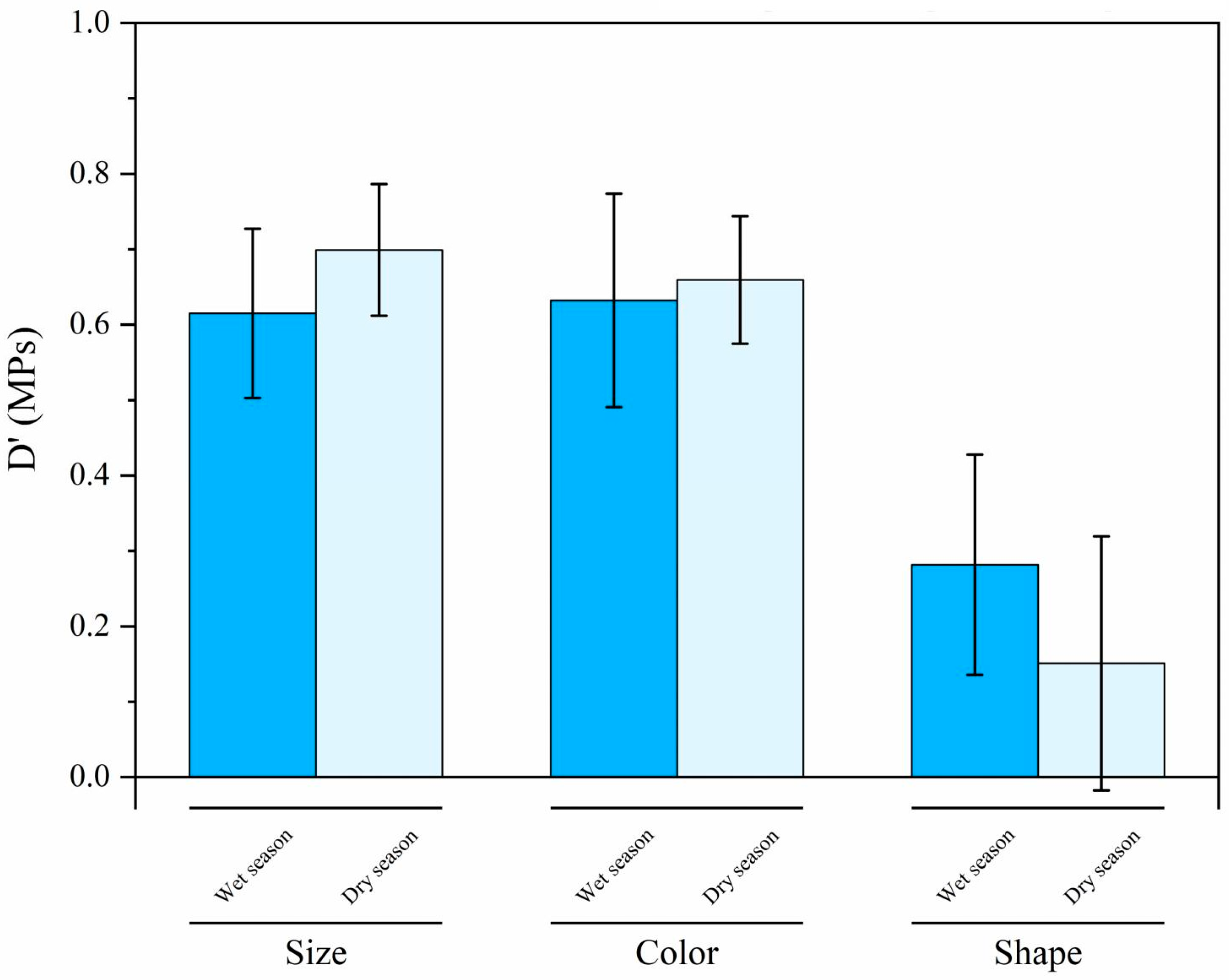
3.4. MPs on the Diversity of Dry and Wet Seasons in the River–Sea
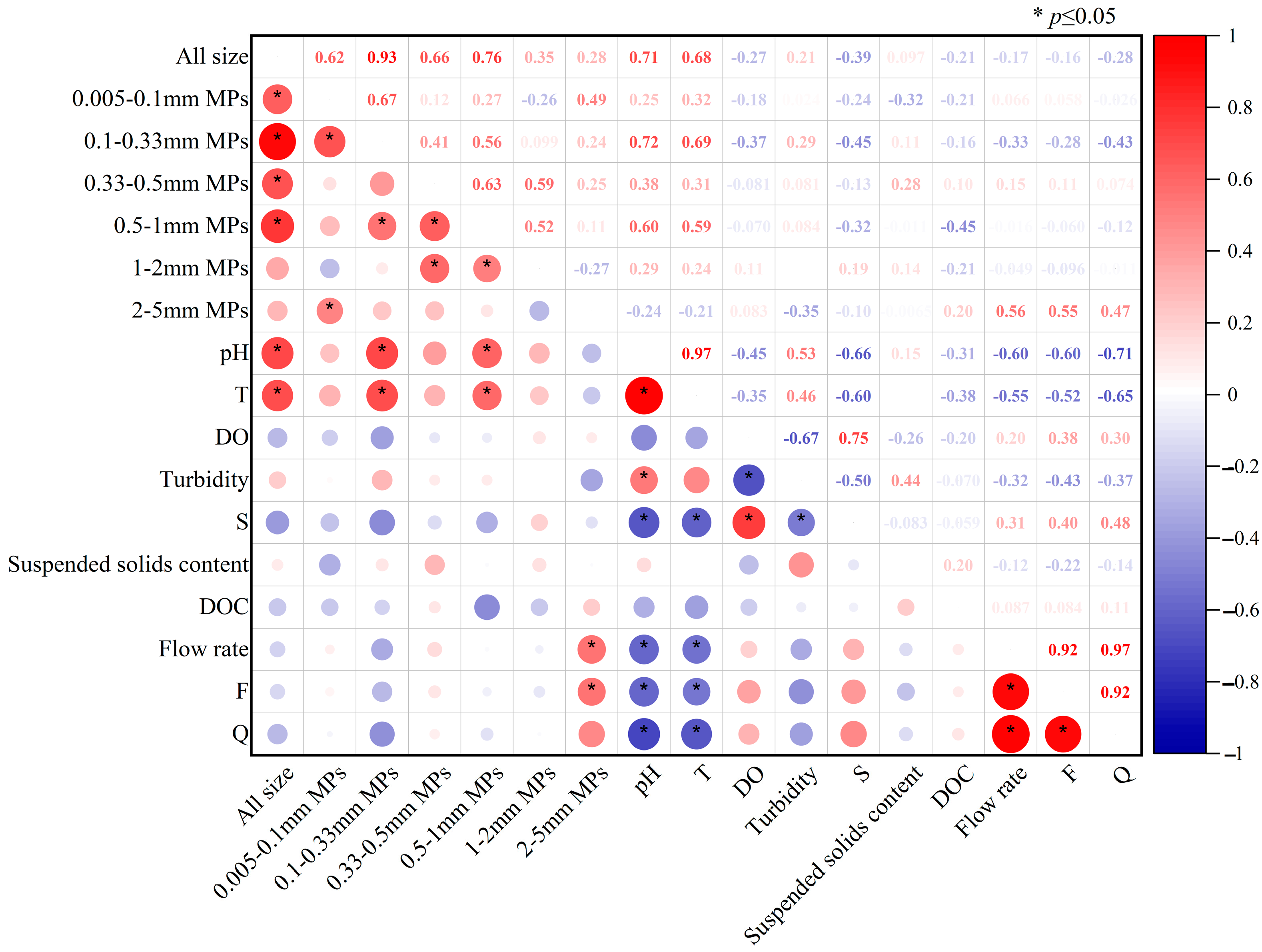
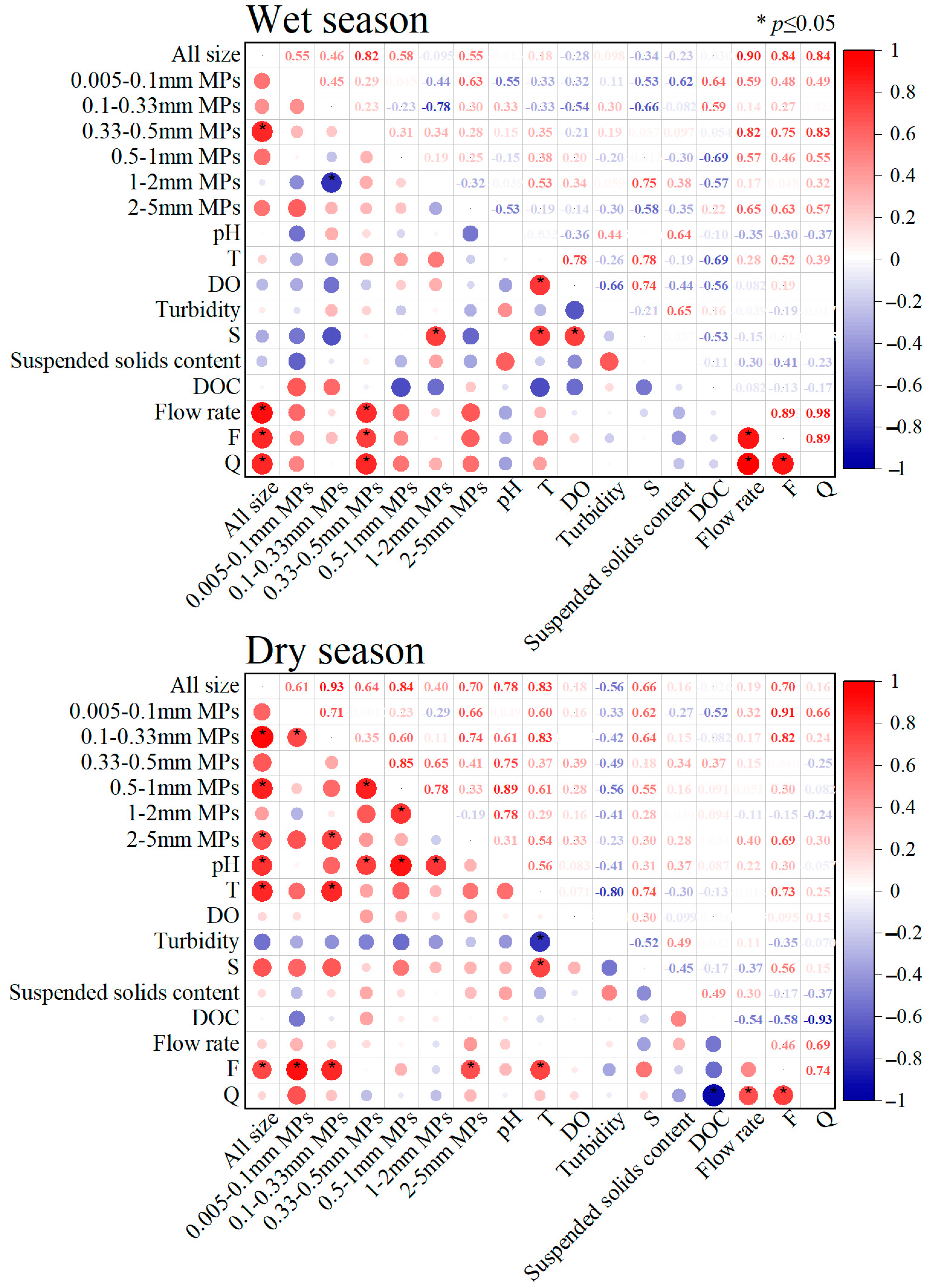
3.5. Relationships Between MPs of Different Sizes in the Suixi River–Sea as Influenced by Hydrodynamics and a Variety of Factors
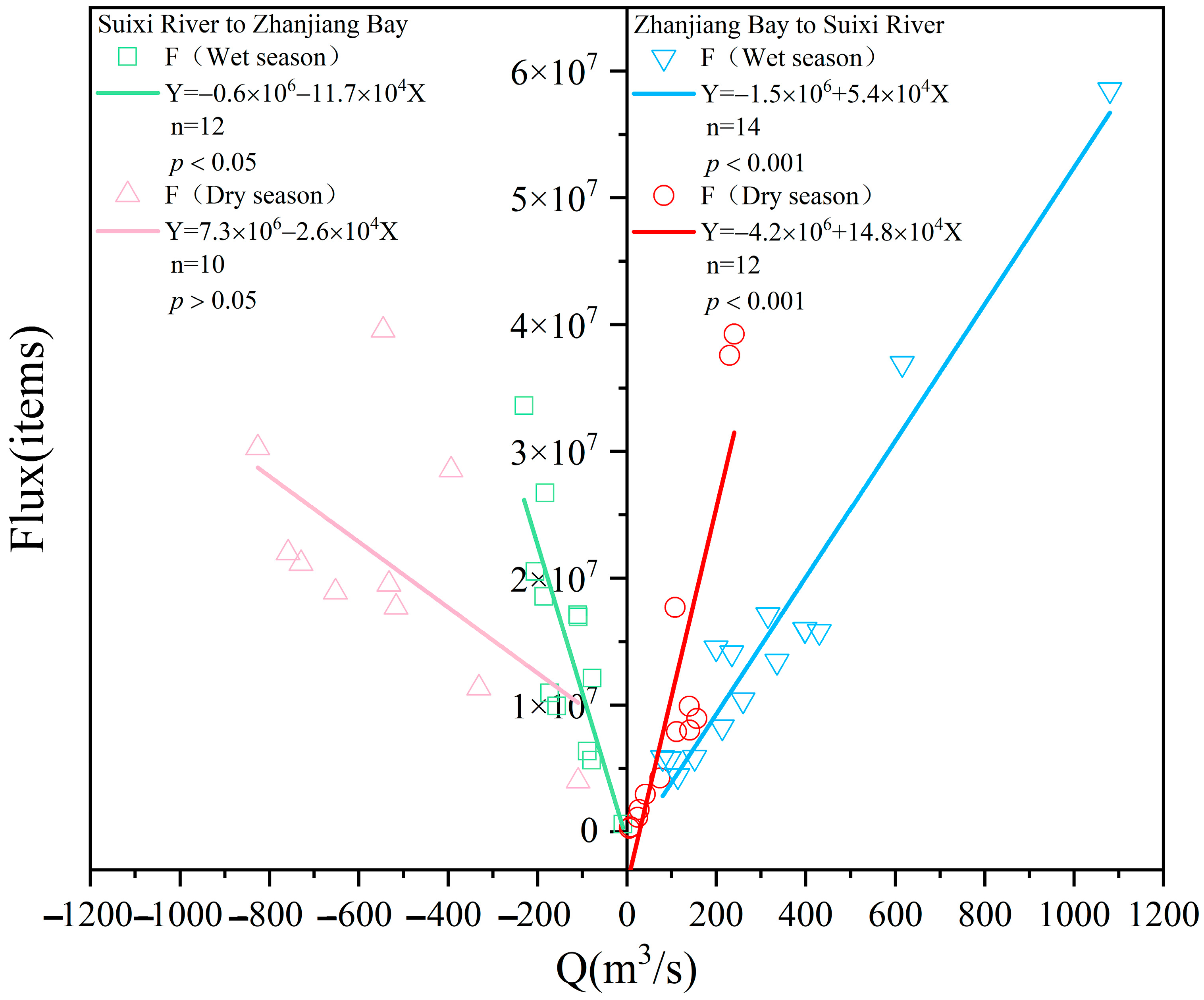
3.6. Hydrodynamic Regulation of MPs Flux into the River–Sea
4. Discussion
4.1. Levels of Microplastic Pollution in the Suixi River–Sea
4.2. Seasonal Variation in the of MPs in the River–Sea
4.3. Influence of Seasonal Variation and Tidal Dynamics on the Abundance and Flux of MPs in the River–Sea
4.4. Mitigation Strategies to Reduce the Accumulation of MPs in River–Sea Interface
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.R.; Thompson, J.T. Deposit-and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol. 2009, 368, 22–29. [Google Scholar] [CrossRef]
- Spear, L.B.; Ainley, D.G.; Ribic, C.A. Incidence of plastic in seabirds from the tropical pacific, 1984–1991: Relation with distribution of species, sex, age, season, year and body weight. Mar. Environ. Res. 1995, 40, 123–146. [Google Scholar] [CrossRef]
- Denuncio, P.; Bastida, R.; Dassis, M.; Giardino, G.; Gerpe, M.; Rodríguez, D. Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Mar. Pollut. Bull. 2011, 62, 1836–1841. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Lindsay, D.J.; Arias, A.H.; Nakano, H.; Jandang, S.; Isobe, A. Zooplankton as a suitable tool for microplastic research. Sci. Total Environ. 2023, 905, 167329. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Lebreton, L.C.; Van Der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Cordova, M.R.; Nurhati, I.S. Major sources and monthly variations in the release of land-derived marine debris from the Greater Jakarta area, Indonesia. Sci. Rep. 2019, 9, 18730. [Google Scholar] [CrossRef]
- Wu, F.; Pennings, S.C.; Tong, C.; Xu, Y. Variation in microplastics composition at small spatial and temporal scales in a tidal flat of the Yangtze Estuary, China. Sci. Total Environ. 2020, 699, 134252. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Belay, B.S.; Moreno-Rodenas, A.; Kramer, M.; Franca, M.J. The key role of surface tension in the transport and quantification of plastic pollution in rivers. Water Res. 2022, 226, 119078. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Internicola, A.M.; Mason, R.A.; Kukulka, T. Observations and simulations of microplastic debris in a tide, wind, and freshwater-driven estuarine environment: The Delaware Bay. Environ. Sci. Technol. 2019, 53, 14204–14211. [Google Scholar] [CrossRef] [PubMed]
- Van Emmerik, T.; Mellink, Y.; Hauk, R.; Waldschläger, K.; Schreyers, L. Rivers as plastic reservoirs. Front. Water 2022, 3, 786936. [Google Scholar] [CrossRef]
- Schaafsma, M.; Turner, R.K. Valuation of coastal and marine ecosystem services: A literature review. In Coastal Zones Ecosystem Services: From Science to Values and Decision Making; Springer: Cham, Switzerland, 2015; pp. 103–125. [Google Scholar]
- Gray, A.D.; Wertz, H.; Leads, R.R.; Weinstein, J.E. Microplastic in two South Carolina Estuaries: Occurrence, distribution, and composition. Mar. Pollut. Bull. 2018, 128, 223–233. [Google Scholar] [CrossRef]
- Pazos, R.S.; Bauer, D.E.; Gómez, N. Microplastics integrating the coastal planktonic community in the inner zone of the Río de la Plata estuary (South America). Environ. Pollut. 2018, 243, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, T.; Zhu, L.; Xu, P.; Wang, X.; Gao, L.; Li, D. Analysis of suspended microplastics in the Changjiang Estuary: Implications for riverine plastic load to the ocean. Water Res. 2019, 161, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, S.; Wu, L.; Yang, Y.; Yu, X.; Liu, Q.; Liu, X.; Li, Y.; Wang, X. Vertical distribution and river-sea transport of microplastics with tidal fluctuation in a subtropical estuary, China. Sci. Total Environ. 2022, 822, 153603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Peng, C.; Zhang, J.; Zhang, J.; Chen, J.; Zhao, H. Long-term harmful algal blooms and nutrients patterns affected by climate change and anthropogenic pressures in the Zhanjiang Bay, China. Front. Mar. Sci. 2022, 9, 849819. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhang, P.; Li, Y.; Li, J.; Luo, X.; Xu, J.; Zhao, L. Seasonal phosphorus variation in coastal water affected by the land-based sources input in the eutrophic Zhanjiang Bay, China. Estuar. Coast. Shelf Sci. 2021, 252, 107277. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, S.; Zhang, J.; Zhong, H.; Wang, S.; Jian, Q. Seasonal distribution, composition, and inventory of plastic debris on the Yugang Park Beach in Zhanjiang Bay, South China Sea. Int. J. Environ. Res. Public Health 2022, 19, 4886. [Google Scholar] [CrossRef]
- Zhang, P.; Peng, C.H.; Zhang, J.B.; Zou, Z.B.; Shi, Y.Z.; Zhao, L.R.; Zhao, H. Spatiotemporal urea distribution, sources, and indication of DON bioavailability in Zhanjiang Bay, China. Water 2020, 12, 633. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Sun, C.; Cao, W.; Wang, M.; Jiang, F.; Ju, P. Comparative study of three sampling methods for microplastics analysis in seawater. Sci. Total Environ. 2021, 765, 144495. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, J.L.; Zhang, J.B.; Li, J.X.; Zhang, Y.C.; Li, Y.; Luo, X.Q. Spatiotemporal dissolved silicate variation, sources, and behavior in the eutrophic Zhanjiang Bay, China. Water 2020, 12, 3586. [Google Scholar] [CrossRef]
- Zhang, P.E.N.G.; Wei, L.R.; Lai, J.Y.; Dai, P.D.; Chen, Y.; Zhang, J.B. Concentration, composition and fluxes of land-based nitrogen and phosphorus source pollutants input into Zhanjiang Bay in Summer. J. Guangdong Ocean Univ. 2019, 39, 63–72. [Google Scholar]
- HJ/T 91-2002; Technical Specifications Requirements for Monitoring of Surface Water and Waste Water. Ministry of Environment Protection, People’s Republic of China, Standards Press of China: Beijing, China, 2002.
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wu, T.; Wang, X.; Song, Z.; Zong, C.; Wei, N.; Li, D. Consistent transport of terrestrial microplastics to the ocean through atmosphere. Environ. Sci. Technol. 2019, 53, 10612–10619. [Google Scholar] [CrossRef] [PubMed]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Li, R.L.; Yu, L.; Chai, M.; Wu, H.; Zhu, X. The Distribution, Characteristics and ecological risks of microplastics in the mangroves of Southern China. Sci. Total Environ. 2020, 708, 135025. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A. Microplastics in the environment: Challenges in analytical chemistry-A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Wang, S.; Jian, Q.; Zhang, P.; Zhang, J.; Zhao, L.; Liu, D.; Kang, X. Tracing land-based microplastic sources in coastal waters of Zhanjiang Bay, China: Spatiotemporal pattern, composition, and flux. Front. Mar. Sci. 2022, 9, 934707. [Google Scholar] [CrossRef]
- Huang, J.S.; Koongolla, J.B.; Li, H.X.; Lin, L.; Pan, Y.F.; Liu, S.; He, W.H.; Maharana, D.; Xu, X.R. Microplastic accumulation in fish from Zhanjiang mangrove wetland, South China. Sci. Total Environ. 2020, 708, 134839. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Xiao, X.; Effiong, K.; Xu, C.C.; Su, Z.N.; Hu, J.; Jiao, S.J.; Holmer, M. New Insights Into the Microplastic Enrichment in the Blue Carbon Ecosystem: Evidence From Seagrass Meadows and Mangrove Forests in Coastal South China Sea. Environ. Sci. Technol. 2021, 55, 4804–4812. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Alfonso, M.B.; Jandang, S.; Phinchan, N.; Chavanich, S.; Viyakarn, V.; Isobe, A. Influence of monsoon seasonality and tidal cycle on microplastics presence and distribution in the Upper Gulf of Thailand. Sci. Total Environ. 2024, 920, 170787. [Google Scholar] [CrossRef] [PubMed]
- Defontaine, S.; Sous, D.; Tesan, J.; Monperrus, M.; Lenoble, V.; Lanceleur, L. Microplastics in a salt-wedge estuary: Vertical structure and tidal dynamics. Mar. Pollut. Bull. 2020, 160, 111688. [Google Scholar] [CrossRef] [PubMed]
- Enfrin, M.; Lee, J.; Gibert, Y.; Basheer, F.; Kong, L.; Dumée, L.F. Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J. Hazard. Mater. 2020, 384, 121393. [Google Scholar] [CrossRef] [PubMed]
- Trindade, L.D.S.; Gloaguen, T.V.; Benevides, T.C.L.; Valentim, A.S.; Bomfim, M.R.; Santos, J.a.G. Microplastics in surface waters of tropical estuaries around a densely populated Brazilian bay. Environ. Pollut. 2023, 323, 121224. [Google Scholar] [CrossRef]
- Pasquier, G.; Doyen, P.; Chaïb, I.; Amara, R. Do tidal fluctuations affect microplastics distribution and composition in coastal waters? Mar. Pollut. Bull. 2024, 200, 116166. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Fu, D.; Wang, Z.; Gao, M.; Peng, L. Microplastics occurrence and spatial distribution in seawater and sediment of Haikou Bay in the northern South China Sea. Estuar. Coast. Shelf Sci. 2020, 239, 106757. [Google Scholar] [CrossRef]
- Santucci, L.; Fernández-Severini, M.D.; Rimondino, G.N.; Colombo, C.V.; Prieto, G.; Forero-López, A.D.; Carol, E.S. Assessment of meso-and microplastics distribution in coastal sediments and waters at the middle estuary of the Rio De La Plata, Argentina (SW Atlantic Ocean). Sci. Total Environ. 2024, 914, 170026. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Yu, J.; Nur, A.A.U.; Banik, P.; Jolly, Y.N.; Al-Mamun, M.; Arai, T.; Albeshr, M.F. Microplastics in surface water from a mighty subtropical estuary: First observations on occurrence, characterization, and contamination assessment. Environ. Res. 2023, 226, 115594. [Google Scholar] [CrossRef] [PubMed]
- Taha, Z.D.; Amin, R.M.; Anuar, S.T.; Nasser, A.A.A.; Sohaimi, E.S. Microplastics in seawater and zooplankton: A case study from Terengganu estuary and offshore waters, Malaysia. Sci. Total Environ. 2021, 786, 147466. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Bai, M.; Wang, Y.; Wang, J.; Liu, K.; Zhu, L.; Zhang, F.; Wang, X.; Wu, T.; Zhang, Z.; et al. Dynamic signatures of microplastic distribution across the water column of Yangtze River Estuary: Complicated implication of tidal effects. Mar. Environ. Res. 2023, 188, 106005. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, W.; Yin, M.; Huang, P.; Ding, Y.; Ni, X.; Liu, H.; Wang, G.; Zheng, H.; Cai, M. Tributary inflows enhance the microplastic load in the estuary: A case from the Qiantang River. Mar. Pollut. Bull. 2020, 156, 111152. [Google Scholar] [CrossRef] [PubMed]
- Bikker, J.; Lawson, J.; Wilson, S.; Rochman, C.M. Microplastics and other anthropogenic particles in the surface waters of the Chesapeake Bay. Mar. Pollut. Bull. 2020, 156, 111257. [Google Scholar] [CrossRef]
- Gupta, P.; Saha, M.; Rathore, C.; Suneel, V.; Ray, D.; Naik, A.; Unnikrishnan, K.; Dhivya, M.; Daga, K. Spatial and seasonal variation of microplastics and possible sources in the estuarine system from central west coast of India. Environ. Pollut. 2021, 288, 117665. [Google Scholar] [CrossRef] [PubMed]
- Suteja, Y.; Atmadipoera, A.S.; Riani, E.; Nurjaya, I.W.; Nugroho, D.; Cordova, M.R. Spatial and temporal distribution of microplastic in surface water of tropical estuary: Case study in Benoa Bay, Bali, Indonesia. Mar. Pollut. Bull. 2021, 163, 111979. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Van Dinh, K.; Konestabo, H.S.; Borgå, K.; Hylland, K.; Macaulay, S.J.; Jackson, M.C.; Verheyen, J.; Stoks, R. Interactive effects of warming and pollutants on marine and freshwater invertebrates. Curr. Pollut. Rep. 2022, 8, 341–359. [Google Scholar] [CrossRef]
- Wojtal-Frankiewicz, A. The effects of global warming on Daphnia spp. population dynamics: A review. Aquat. Ecol. 2011, 46, 37–53. [Google Scholar] [CrossRef]
- Malli, A.; Corella-Puertas, E.; Hajjar, C.; Boulay, A.M. Transport mechanisms and fate of microplastics in estuarine compartments: A review. Mar. Pollut. Bull. 2022, 177, 113553. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.C.; DeCastro, M.; Gago, J.; Ribeiro, A.S.; Des, M.; Gómez-Gesteira, J.L.; Dias, J.M.; Gomez-Gesteira, M. Modelling the distribution of microplastics released by wastewater treatment plants in Ria de Vigo (NW Iberian Peninsula). Mar. Pollut. Bull. 2021, 166, 112227. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, L.; Dai, Y.; Sun, H.; Liu, C. Behavior of microplastics in inland waters: Aggregation, settlement, and transport. Bull. Environ. Contam. Toxicol. 2021, 107, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Deng, Y.; Wang, R.; Ma, E.; Wang, J.; Bai, J.; Wu, J.; Zhou, Y. Microplastics in the surface water of small-scale estuaries in Shanghai. Mar. Pollut. Bull. 2019, 149, 110569. [Google Scholar] [CrossRef] [PubMed]
- Kooi, M.; Reisser, J.; Slat, B.; Ferrari, F.F.; Schmid, M.S.; Cunsolo, S.; Brambini, R.; Noble, K.; Sirks, L.A.; Linders, T.E.; et al. The effect of particle properties on the depth profile of buoyant plastics in the ocean. Sci. Rep. 2016, 6, 33882. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Quik, J.T.; Sun, M.; Koelmans, A.A. Fate of nano-and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Ustabasi, G.S.; Baysal, A. Bacterial interactions of microplastics extracted from toothpaste under controlled conditions and the influence of seawater. Sci. Total Environ. 2020, 703, 135024. [Google Scholar] [CrossRef] [PubMed]
- Stead, J.L.; Cundy, A.B.; Hudson, M.D.; Thompson, C.E.; Williams, I.D.; Russell, A.E.; Pabortsava, K. Identification of tidal trapping of microplastics in a temperate salt marsh system using sea surface microlayer sampling. Sci. Rep. 2020, 10, 14147. [Google Scholar] [CrossRef] [PubMed]
- Piehl, S.; Atwood, E.C.; Bochow, M.; Imhof, H.K.; Franke, J.; Siegert, F.; Laforsch, C. Can water constituents be used as proxy to map microplastic dispersal within transitional and coastal waters? Front. Environ. Sci. 2020, 8, 92. [Google Scholar] [CrossRef]
- Qian, J.; Tang, S.; Wang, P.; Lu, B.; Li, K.W.; He, X. From source to sink: Review and prospects of microplastics in wetland ecosystems. Sci. Total Environ. 2021, 758, 143633. [Google Scholar] [CrossRef]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.T.; Wang, G.; Yue, W.; Kong, X.; Zhu, J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci. Total Environ. 2020, 707, 135601. [Google Scholar] [CrossRef] [PubMed]
- Waldschläger, K.; Schüttrumpf, H. Effects of particle properties on the settling and rise velocities of microplastics in freshwater under laboratory conditions. Environ. Sci. Technol. 2019, 53, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Maghsodian, Z.; Sanati, A.M.; Tahmasebi, S.; Shahriari, M.H.; Ramavandi, B. Study of microplastics pollution in sediments and organisms inmangrove forests: A review. Environ. Res. 2022, 208, 112725. [Google Scholar] [CrossRef] [PubMed]
- Aliabad, M.K.; Nassiri, M.; Kor, K. Microplastics in the surface sea waters of Chabahar Bay, Gulf of Oman (Makran coasts). Mar. Pollut. Bull. 2019, 143, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Peng, G.; Su, L.; Gao, Y.; Gao, L.; Li, D. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef]
- Oben Mbeng, L.; David, M.; Ntyanm Ondo, S. Classification Study of Ingested Plastic Particles in Marine Organisms using Electron Microscope: A Case Study of Cameroon Beaches. Int. J. Adv. Sci. Res. Eng. (IJASRE) 2021, 7, 85–92. [Google Scholar]
- Parvin, F.; Jannat, S.; Tareq, S.M. Abundance, characteristics and variation of microplastics in different freshwater fish species from Bangladesh. Sci. Total Environ. 2021, 784, 147137. [Google Scholar] [CrossRef] [PubMed]
- Pol, W.; Żmijewska, A.; Stasińska, E.; Zieliński, P. Spatial–temporal distribution of microplastics in lowland rivers flowing through two cities (Ne Poland). Water Air Soil Pollut. 2022, 233, 140. [Google Scholar] [CrossRef]
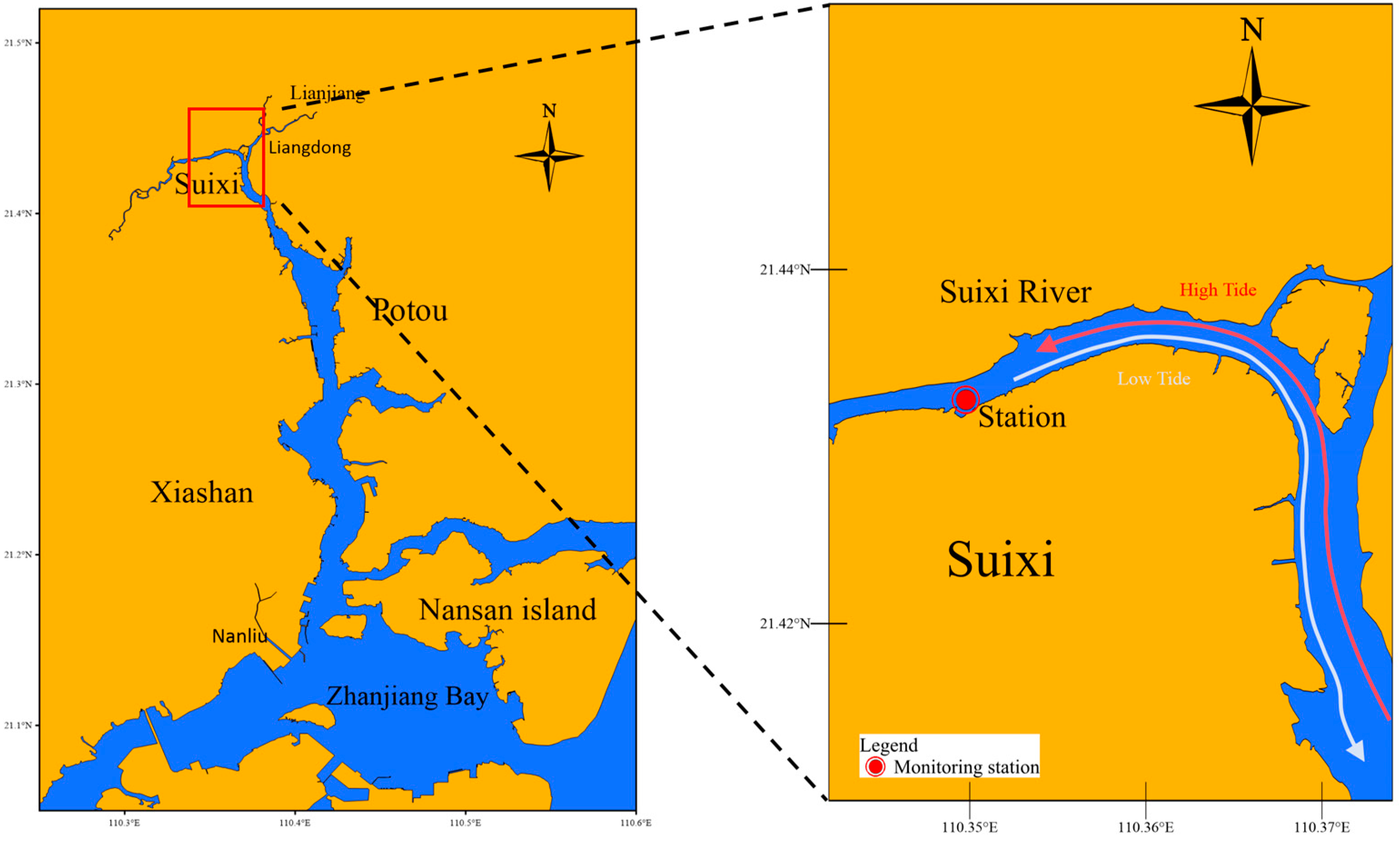


| Season | Dry Season | Wet Season | ||
|---|---|---|---|---|
| Average Value | Range | Average Value | Range | |
| Temperature (°C) | 19.3 ± 0.8 | 18.1–20.1 | 29.5 ± 0.8 | 28.4–30.7 |
| Salinity (PSU) | 4.2 ± 2.7 | 0.7–9.6 | 0.6 ± 0.6 | 0.1–1.9 |
| Flow velocity (cm/s) | 36.9 ± 20.4 | 10.8–65.6 | 43.6 ± 22.1 | 3.3–75.4 |
| Tide level (m) | 2.4 ± 0.9 | 0.8–3.8 | 2.4 ± 1.3 | 0.9–4.5 |
| Area | Sampling Time | Abundance (n/m3) | Mesh (μm) | Main Size (mm) | References |
|---|---|---|---|---|---|
| Si Chang Island (Thailand) | August 2022, October–November 2022, March–April 2023 | 17.58 ± 21.68 | 212 | 1–1.1 | [43] |
| The Adour Estuary (France) | June 2019, September 2019 | 1.13 | 300 | <2 | [44] |
| The Liane River (France) | March 2022 | 0.76 ± 0.26 | 300 | <1 | [47] |
| Pearl River Estuary (China) | December 2017 | 8902 | 50 | <0.5 | [48] |
| Haikou Bay (China) | June 2018 | 0.44 ± 0.21 | 333 | 1–1.9 | [49] |
| The Rio De La Plata (Argentina) | March 2021 | 14,170 ± 5500 | 0.45 | <1.5 | [50] |
| The Meghna (Bangladesh) | March-April 2022 | 128.89 ± 67.94 | 300 | <0.5 | [51] |
| Terengganu Estuary (Malaysia) | August 2018, September 2018 | 421.8 ± 110 | 20 | 0.36 ± 0.23 | [52] |
| Yangtze River Estuary (China) | July 2017, October 2017, January 2018, May 2018 | 83.95 ± 19.5 | 300 | <1 | [53] |
| The Qiantang River (China) | June 2018, November 2018 | 1183 ± 269 | 100 | <5 | [54] |
| The Chesapeake Bay (United States) | 2015 | 0.16 ± 0.287 | 330 | 0.5–1 | [55] |
| Suixi River Estuary (China) | December 2022, August 2023 | 91,099 | 0.45 | <1 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, P.; Lu, J.; Chen, Y.; Zhang, J. Hydrology Modulates the Microplastics Composition and Transport Flux Across the River–Sea Interface in Zhanjiang Bay, China. J. Mar. Sci. Eng. 2025, 13, 428. https://doi.org/10.3390/jmse13030428
Chen X, Zhang P, Lu J, Chen Y, Zhang J. Hydrology Modulates the Microplastics Composition and Transport Flux Across the River–Sea Interface in Zhanjiang Bay, China. Journal of Marine Science and Engineering. 2025; 13(3):428. https://doi.org/10.3390/jmse13030428
Chicago/Turabian StyleChen, Xiaoqing, Peng Zhang, Jing Lu, Yuanting Chen, and Jibiao Zhang. 2025. "Hydrology Modulates the Microplastics Composition and Transport Flux Across the River–Sea Interface in Zhanjiang Bay, China" Journal of Marine Science and Engineering 13, no. 3: 428. https://doi.org/10.3390/jmse13030428
APA StyleChen, X., Zhang, P., Lu, J., Chen, Y., & Zhang, J. (2025). Hydrology Modulates the Microplastics Composition and Transport Flux Across the River–Sea Interface in Zhanjiang Bay, China. Journal of Marine Science and Engineering, 13(3), 428. https://doi.org/10.3390/jmse13030428






