Surface and Vertical Nutrient Profiles in the Northwestern Black Sea: Trends, Comparisons, and Sample Preservation Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analytical Methods
- -
- Surface Layer: A fixed depth (e.g., 1 m) within the upper mixed layer;
- -
- Extrema: Depths corresponding to the Upper Thermocline Limit, the maximum DO concentration, and the Subsurface Chlorophyll Maximum (SCM) (maximum fluorescence). These points capture the peak biological and physical boundaries controlling water column stability;
- -
- Water Masses: Depths within the core of the Cold Intermediate Layer (CIL), which acts as a dynamic reservoir influencing lateral transport of nutrients;
- -
- Biogeochemical Interfaces (σt): Specific isopycnal surfaces (σt) targeting redox and nutrient layers: 15.6, 15.8, 15.9, 16.0, 16.1, 16.2, and 16.5. These densities specifically encompass the base of the nitracline, the mitrate maximum layer, the phosphate minimum and maximum layers, and the suboxic/anoxic transition zones, which are critical areas for nitrogen and phosphorus cycling.
2.3. Rationale for Preservation Test: Pasteurization vs. Freezing
2.4. Preservation Test Procedure
2.5. Data Processing
3. Results
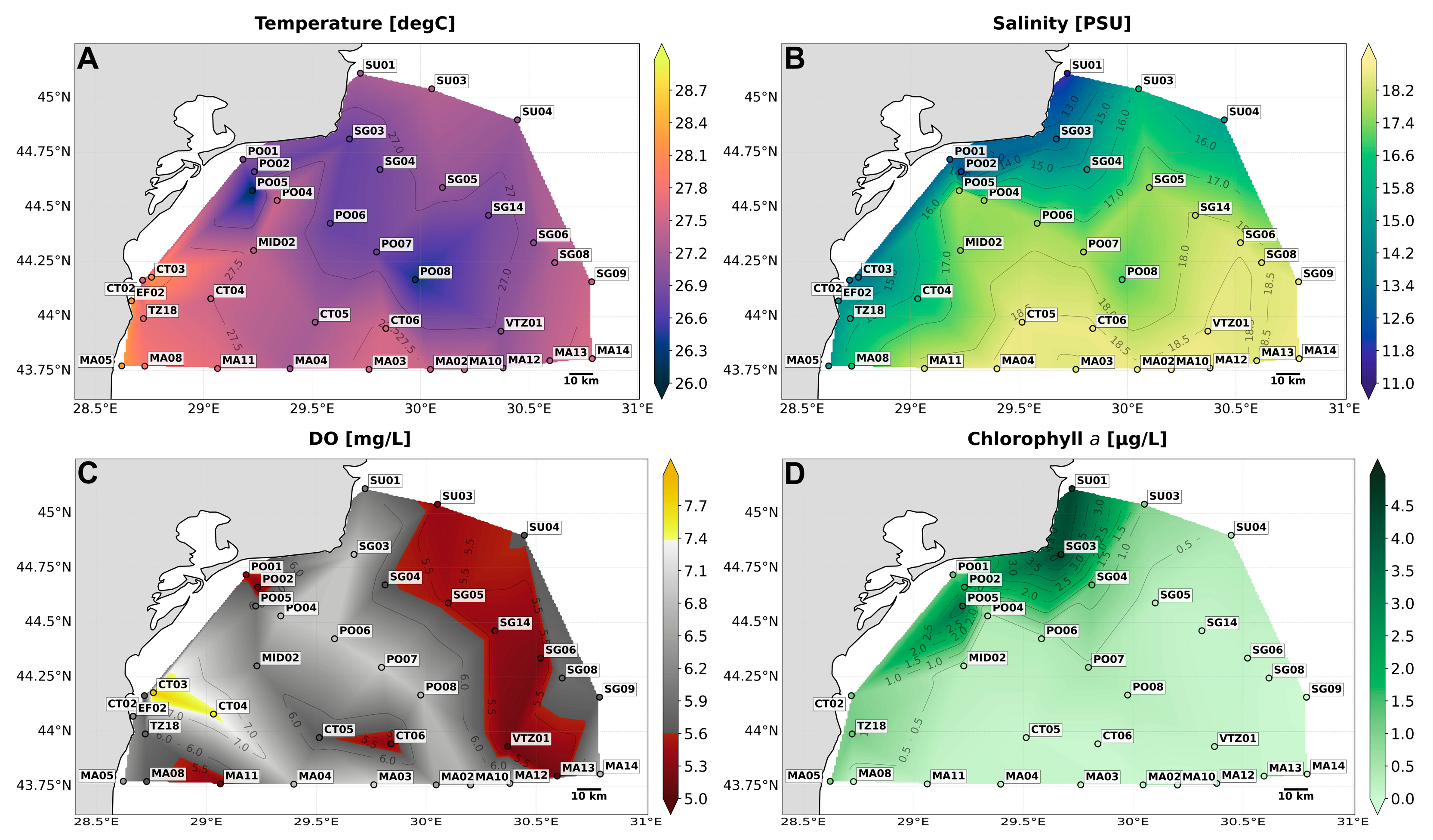
3.1. CTD Parameters (Temperature, Salinity, Dissolved Oxygen, and Chlorophyll)
3.1.1. Mangalia-East Transect
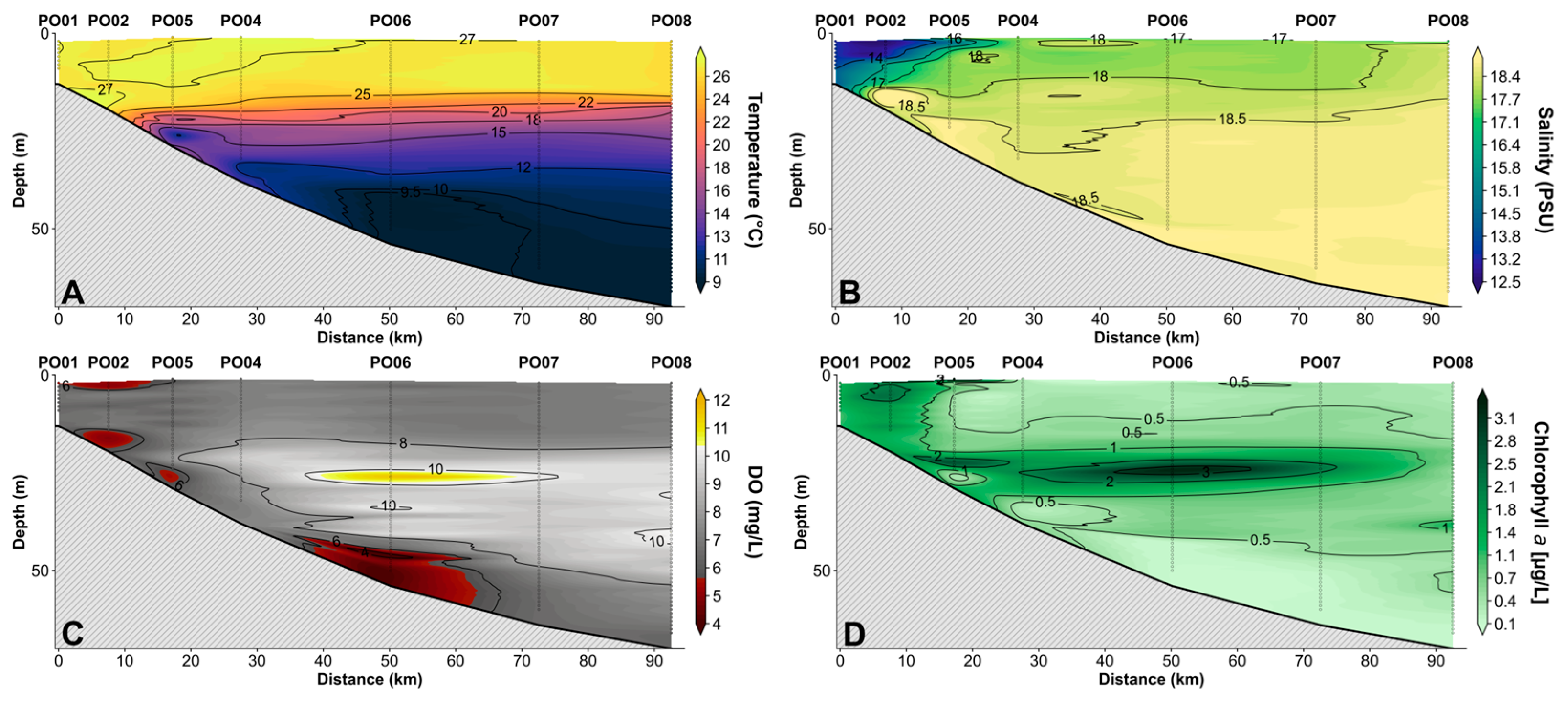
3.1.2. Portita South-East Transect
3.1.3. Sfântu Gheorghe—South-East Transect
3.1.4. Characterization of Water Masses
- -
- Surface and Mixed Layer Waters (σθ < 14.3): This area is characterized by low and highly variable surface salinities (17 to 18.5 PSU) and low σθ values, primarily encompassing the seasonal thermocline and the upper CIL remnants. The high spread in salinity reflects the strong influence of river discharge (particularly the Danube) across the shelf;
- -
- Deep Saline Waters (σθ > 16.0): The dense, anoxic deep waters are tightly constrained to a high-salinity endmember (reaching ~22 PSU);
- -
- Permanent Halocline (σθ~14.3 to 16.0): This steep, diagonal feature connects the two masses, demonstrating the uniform relationship between increasing salinity and increasing density below the seasonal layer.
- -
- -
- Oxycline: This zone is defined by the steepest gradient and sharp drop in dissolved oxygen concentration as density increases. It spans the range DO maximum to 0.3–0.4 mg/L to the disappearance of measurable oxygen.
- -
- Suboxic Zone: The zone begins below the oxycline (with DO typically <0.3–0.4 mg/L) and extends to the onset of the anoxic zone;
- -
- Anoxic Zone: The deepest, densest waters (σθ > 16.0) are characterized by no measurable oxygen. This tight cluster of points confirms the presence of the deep, sulfide-rich anoxic layer below the suboxic zone across the deeper stations of the studied transects.
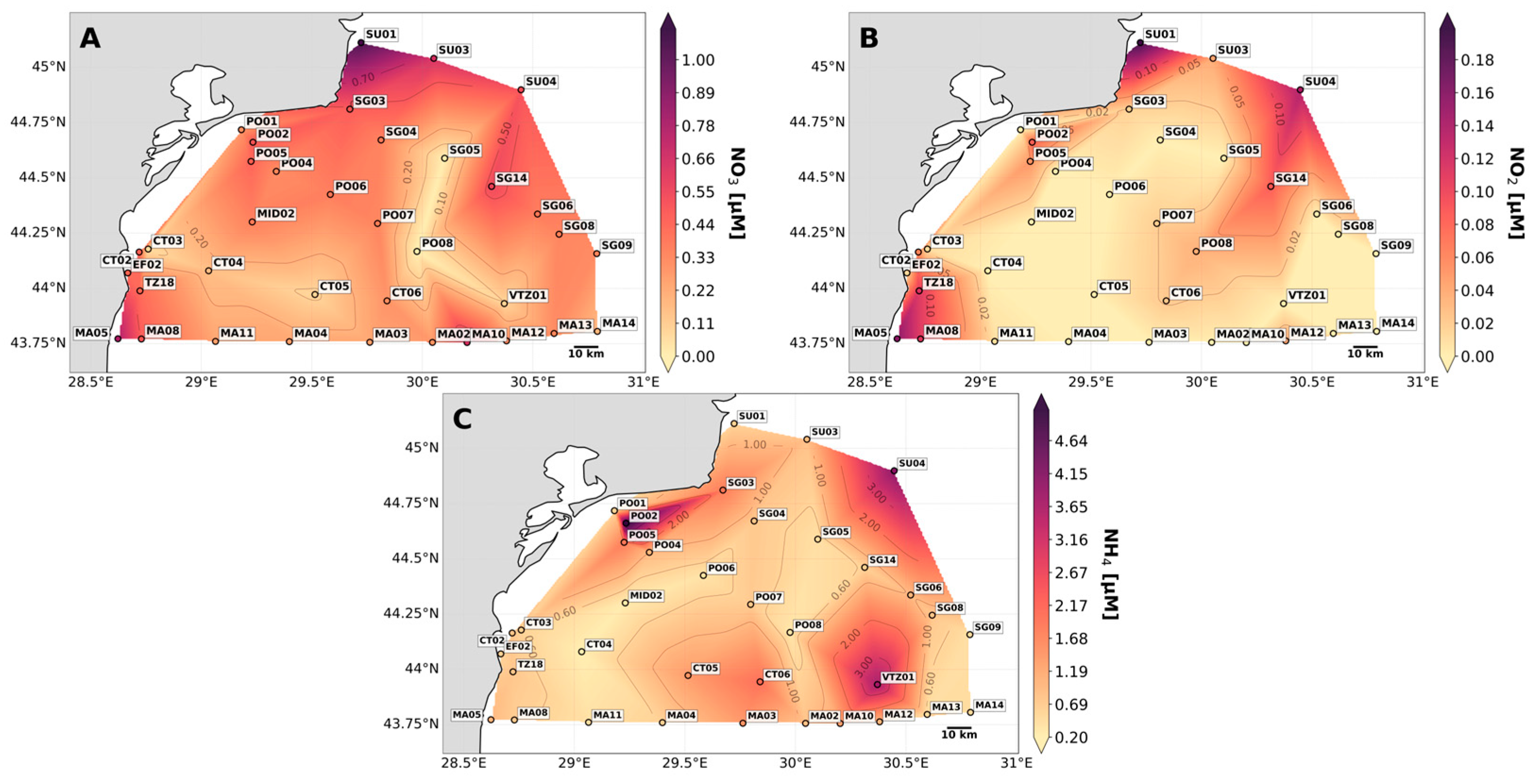
3.2. Nutrient Regime in the NW Black Sea Shelf
3.2.1. Surface Nutrients in the NW Black Sea Shelf
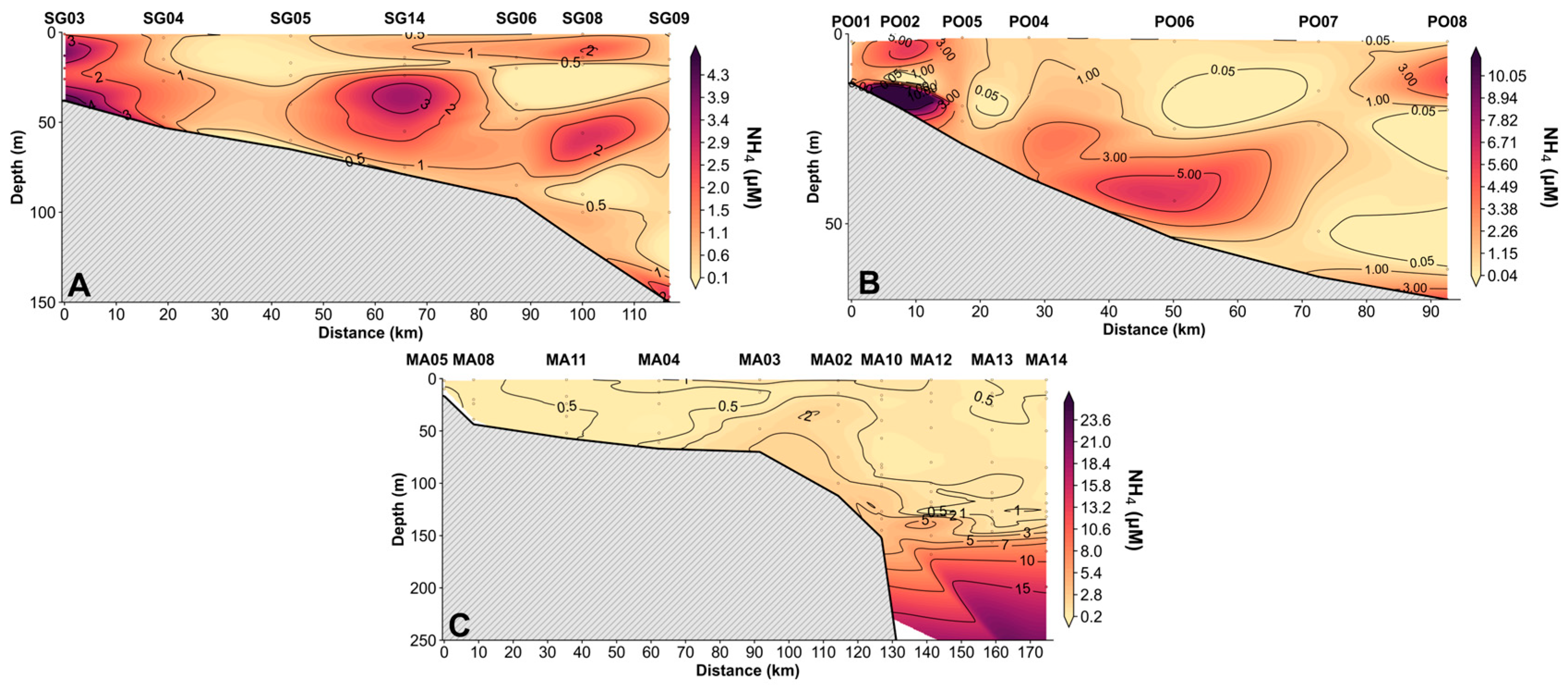
3.2.2. Vertical Distribution of Nutrients
3.3. Testing and Comparing the Preservation Methods
4. Discussion
4.1. Surface Nutrients
4.2. Vertical Profiles of Nutrients
4.3. Considerations on Nutrient Samples Preservation
- -
- Thawing Protocol for Silicate: Given that our analysis suggests overnight thawing may cause incomplete depolymerization, protocols for frozen samples containing silicate should implement a longer thawing period (e.g., at least 24 h at room temperature) to ensure the full dissolution of polymerized silicate species before analysis [82];
- -
- Silicate Measurement Accuracy (Alkalinization): For optimal accuracy, especially in samples where high concentration or polymerization is suspected, we recommend adopting the standard technique of alkalinization (e.g., pH adjustment pH > 10 with NaOH) prior to the colorimetric reaction, as detailed in established methodologies [83], to measure all forms of silica accurately;
- -
- Optimal Preservation Method: Considering the consistent stability maintained across PO4 and NOx in the pasteurized samples, we strongly recommend pasteurization over freezing as the superior long-term preservation method for comprehensive nutrient studies in matrices susceptible to microbial activity.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTD | Conductivity, Temperature and Depth Tool |
| CIL | Cold Intermediate Layer |
| NWBS | Northwestern Black Sea |
| ICDPR | International Commission for the Protection of the Danube River |
| WFD | Water Framework Directive |
| MSFD | Marine Strategy Framework Directive |
| MSP | Maritime Spatial Planning |
| EEZ | Romanian Exclusive Economic Zone |
| DO | Dissolved Oxygen |
| HDPE | High-density polyethylene |
| SFM | Subsurface Fluorescence Maximum |
| SST | Sea Surface Temperature |
| SSS | Sea Surface Salinity |
| O & G | Oil & Gas |
References
- Li, R.; Zhao, Y.; Zeng, X.; Mao, W. Analyzing the impact of coastal land use on seawater quality in the Bohai Rim of China. Mar. Dev. 2025, 3, 11. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Zhu, X.; Zheng, M. Effects of rainfall–runoff pollution on eutrophication in coastal zone: A case study in Shenzhen Bay, southern China. Hydrol. Res. 2019, 50, 1062–1075. [Google Scholar] [CrossRef]
- Zaitsev, Y. Recent Changes in the Trophic Structure of the Black Sea. Fish. Oceanogr. 1992, 1, 180–189. [Google Scholar] [CrossRef]
- Gomoiu, M.T. Some Remarks Concerning Actual State of the Danube River-Black Sea Ecological System, Danube Delta–Black Sea. Syst. Glob. Change Impact. 1996, 1, 31–34. [Google Scholar]
- Moncheva, S.; Gotsis-Skretas, O.; Pagou, K.; Krastev, A. Phytoplankton Blooms in Black Sea and Mediterranean Coastal Ecosystems Subjected to Anthropogenic Eutrophication: Similarities and Differences. Estuar. Coast. Shelf Sci. 2001, 53, 281–295. [Google Scholar] [CrossRef]
- Daskalov, G.M. Long-Term Changes in Fish Abundance and Environmental Indices in the Black Sea. Mar. Ecol. Prog. Ser. 2003, 255, 259–270. [Google Scholar] [CrossRef]
- Lazar, L.; Boicenco, L.; Marin, O.; Culcea, O.; Pantea, E.; Bișinicu, E.; Mihailov, M.E. Black Sea Eutrophication Dynamics from Causes to Effects. Cercet. Mar. 2018, 48, 100–117. [Google Scholar]
- Tan, I.; Atabay, H.; Evcen, A.; Kurt, G.; Taşkın, E.; Polat Beken, Ç. Integrated assessment of eutrophication in the southern Black Sea waters, using the Nested Environmental Status Assessment Tool. Mar. Poll. Bull. 2023, 195, 115424. [Google Scholar] [CrossRef] [PubMed]
- Alkan, A.; Serdar, S.; Fidan, D.; Akbas, U.; Zegin, B.; Kilic, M.B. Spatial, temporal, and vertical variability of nutrients in the Southeastern Black Sea. Chemosphere 2022, 302, 134809. [Google Scholar] [CrossRef] [PubMed]
- Poulos, S.E. Water Masses of the Mediterranean Sea and Black Sea: An Overview. Water 2023, 15, 3194. [Google Scholar] [CrossRef]
- Pokazeev, K.; Sovga, E.; Chaplina, T. General Oceanographic Characteristics of the Black Sea. In Pollution in the Black Sea; Springer Oceanography; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Oguz, T.; Latun, V.S.; Latif, M.A.; Vladimirov, V.V.; Sur, H.I.; Makarov, A.A.; Ozsoy, E.; Kotovshchikov, B.B.; Eremeev, V.V.; Unluata, U. Circulation in the surface and intermediate layers of the Black Sea. Deep Sea Res. 1993, 40, 1597–1612. [Google Scholar] [CrossRef]
- Yakushev, E.V.; Chasovnikov, V.K.; Murray, J.W.; Pakhomova, S.V.; Podymov, O.I.; Stunzhas, P.A.; Kostianoy, A.G.; Kosarev, A.N. Vertical Hydrochemical Structure of the Black Sea. In The Black Sea Environment; The Handbook of Environmental Chemistry; Kostianoy, A.G., Kosarev, A.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-74291-3. [Google Scholar]
- Çokacar, T. Cold Intermediate Water Formation in the Black Sea Triggered by March 2022 Cold Intrusions. J. Mar. Sci. Eng. 2024, 12, 2027. [Google Scholar] [CrossRef]
- Oguz, T.; Dippner, J.W.; Kaymaz, Z. Climatic regulation of the Black Sea hydrometeorological and ecological properties at interannual-to-decadal time scales. J. Mar. Syst. 2006, 60, 235–254. [Google Scholar] [CrossRef]
- Konovalov, S.K.; Murray, J.W. Variations in the chemistry of the Black Sea on a time scale of decades (1960–1995). J. Mar. Syst. 2001, 31, 217–243. [Google Scholar] [CrossRef]
- Kordzadze, A.A.; Demetrashvili, D.I.; Surmava, A.A. Numerical Modeling of Hydrophysical Fields of the Black Sea under the Conditions of Alternation of Atmospheric Circulation Processes. Izv. Atmos. Ocean. Phys. 2008, 44, 213–224. [Google Scholar] [CrossRef]
- Akpinar, A.; Fach, B.A.; Oguz, T. Observing the subsurface thermal signature of the Black Sea cold intermediate layer with Argo profiling floats. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 124, 140–152. [Google Scholar] [CrossRef]
- Murray, J.; Top, Z.; Oszoy, E. Hydrographic properties and ventilation of the Black Sea. Deep Sea Res. Part A Oceanogr. Res. Pap. 1991, 38, S663–S689. [Google Scholar] [CrossRef]
- Capet, A.; Vandenbulcke, L.; Grégoire, M. A new intermittent regime of convective ventilation threatens the Black Sea oxygenation status. Biogeosciences 2020, 17, 6507–6525. [Google Scholar] [CrossRef]
- Murray, J.W.; Codispoti, L.A.; Friederich, G.E. Oxidation-reduction environments: The suboxic zone in the Black Sea. In Aquatic Chemistry: Interfacial and Interspecies Processes; Huang, C.P., O’Melia, C.R., Morgan, J.J., Eds.; ACS Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1995; p. 244. [Google Scholar]
- Codispoti, L.A.; Friederich, G.E.; Murray, J.W.; Sakamoto, C.M. Chemical variability in the Black Sea: Implications of continuous vertical profiles that penetrated the oxic/anoxic interface. Deep Sea Res. Part A Oceanogr. Res. Pap. 1991, 38, S691–S710. [Google Scholar] [CrossRef]
- Cociasu, A.; Dorogan, L.; Humborg, C.; Popa, L. Long-term ecological changes in the Romanian coastal waters of the Black Sea. Mar. Pollut. Bull. 1996, 32, 32–38. [Google Scholar] [CrossRef]
- Humborg, C.; Ittekkot, V.; Cociasu, A.; Bodungen, B. Effect of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature 1997, 386, 385–388. [Google Scholar] [CrossRef]
- Friedrich, J.; Dinkel, C.; Friedl, G.; Pimenov, N.; Wijsman, J.; Gomoiu, M.T.; Cociasu, A.; Popa, L.; Wehrli, B. Benthic nutrient cycling and diagenetic pathways in the north-western Black Sea. Estuar. Coast. Shelf Sci. 2002, 54, 369–383. [Google Scholar] [CrossRef]
- Oguz, T.; Velikova, V. Abrupt Transition of the Northwestern Black Sea Shelf Ecosystem from a Eutrophic to an Alternative Pristine State. Mar. Ecol. Prog. Ser. 2010, 405, 231–242. [Google Scholar] [CrossRef]
- Lazăr, L.; Boicenco, L.; Coatu, V.; Oros, A.; Tigănus, D.; Mihailov, M.E. Nutrient Levels and Eutrophication of the Romanian Black Sea Waters (2006–2011)—Assessment Related to the Marine Strategy Framework Directive Implementation. Cercet. Mar. 2013, 43, 162–173. [Google Scholar]
- Lazar, L.; Vlas, O.; Pantea, E.; Boicenco, L.; Marin, O.; Abaza, V.; Filimon, A.; Bisinicu, E. Black Sea Eutrophication Comparative Analysis of Intensity between Coastal and Offshore Waters. Sustainability 2024, 16, 5146. [Google Scholar] [CrossRef]
- Orekhova, N.A. Nutrients Dynamics in the Surface Waters of the Black Sea. Phys. Oceanogr. 2021, 28, 660–676. [Google Scholar] [CrossRef]
- Tan, I.; Atabay, H.; Aslan, E.; Mantıkcı, M.; Ergün Taşkın, E.; Kurt, G.; Polat Beken, C. Comparative assessment of eutrophication in the southern Black Sea using TRIX, BEAST, and NEAT. Reg. Stud. Mar. Sci. 2025, 91, 104538. [Google Scholar] [CrossRef]
- Lazar, L. ANEMONE Deliverable 2.1 “Impact of the Rivers on the Black Sea Ecosystem”; CD Press: Bucharest, Romania, 2021; p. 225. [Google Scholar]
- Lazar, L.; Boicenco, L.; Denga, Y. ANEMONE Deliverable 2.2 “Anthropogenic Pressures and Impacts on the Black Sea Coastal Ecosystem”; CD Press: Bucharest Romania, 2021; p. 167. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Koroleff, F. Determination of phosphorus. In Methods of Seawater Analysis, 2nd ed.; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Verlag Chemie: Hoboken, NJ, USA, 1983; pp. 125–142. [Google Scholar]
- Koroleff, F. On the Determination of Reactive Silicate in Natural Waters; CM 1971/C: 43; ICES: Toronto, ON, Canada, 1971. [Google Scholar]
- Bendschneider, K.; Robinson, R.J. A new spectrophotometric method for the determination of nitrite in sea water. J. Mar. Res. 1952, 11, 87–96. [Google Scholar]
- Mullin, J.B.; Riley, J.P. The Spectrophotometric Determination of Nitrate in Natural Waters, with Particular Reference to Sea-Water. Anal. Chim. Acta 1955, 12, 464–480. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Daniel, A.; Kerouel, R.; Aminot, A. Pasteurization: A reliable method for preservation of nutrients in seawater samples for inter-laboratory and field applications. Mar. Chem. 2012, 128–129, 57–63. [Google Scholar] [CrossRef]
- Becker, S.; Aoyama, M.; Woodward, E.M.S.; Bakker, K.; Coverly, S.; Mahaffey, C.; Tanhua, T. GO-SHIP Repeat Hydrography Nutrient Manual: The Precise and Accurate Determination of Dissolved Inorganic Nutrients in Seawater, Using Continuous Flow Analysis Methods. Front. Mar. Sci. 2020, 7, 581790. [Google Scholar] [CrossRef]
- Aminot, A.; Kérouel, R. Autoclaved seawater as a reference material for the determination of the nitrate and phosphate in the sea water. Anal. Chim. Acta 1991, 248, 277–283. [Google Scholar] [CrossRef]
- Aminot, A.; Kérouel, R. Assessment of heat treatment for nutrient preservation in seawater samples. Anal. Chim. Acta 1998, 351, 299–309. [Google Scholar] [CrossRef]
- Degobbis, D. On the storage of seawater samples for ammonia determination. Limnol. Oceanogr. 1973, 18, 146–150. [Google Scholar] [CrossRef]
- Rho, T.P.; Son, P.; Choi, S.H.; Kang, D.J. Cryogenic freezing: A reliable preservation method of samples for seawater nutrient analysis. Limnol. Oceanogr. 2022, 20, 543–552. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Thing, M.K.; Greene, C.A.; Hetland, R.D.; Zimmerle, H.M.; DiMarco, S.F. True Colors of Oceanography: Guidelines for Effective and Accurate Colormap Selection. Oceanog 2016, 29, 9–13. [Google Scholar] [CrossRef]
- Schlitzer, R. Ocean Data View. 2024. Available online: http://odv.awi.de (accessed on 10 September 2025).
- Holmes, R.M.; Aminot, A.; Kerouel, R.; Hooker, B.A.; Peterson, B.J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 1999, 56, 1801–1808. [Google Scholar] [CrossRef]
- Cociasu, A.; Popa, L. Significant changes in Danube nutrient loads and their impact on the Romanian Black Sea coastal waters. Cercet. Mar. 2004, 35, 43–68. [Google Scholar]
- Ragueneau, O.; Lancelot, C.; Egorov, V.; Roubeix, V.; Schrimm, M.; Dandois, J.M. Biogeochemical transformations of inorganic nutrients in the mixing zone between the Danube River and the north-western Black Sea. Mar. Ecol. Prog. Ser. 2002, 238, 17–30. [Google Scholar] [CrossRef]
- Lazar, L.; Spanu, A.; Boicenco, L.; Oros, A.; Damir, N.; Bisinicu, E.; Abaza, V.; Filimon, A.; Harcota, G.; Marin, O.; et al. Methodology for prioritizing marine environmental pressures under various management scenarios in the Black Sea. Front. Mar. Sci. 2024, 11, 1388877. [Google Scholar] [CrossRef]
- Dragomir, A.; Vuta, L.; Petrescu, M.; Stoian, V. Water quality in the coastal zone of the Black Sea and the phenomenon of massive algae development. Sci. Bull. Politeh. Univ. Timişoara 2014, 59, 2. [Google Scholar]
- Trapp-Müller, G.; Aller, R.C.; Sluijs, A.; Middelburg, J.J. Silicate Weathering and Diagenetic Reaction Balances in Deltaic Muds. Am. J. Sci. 2025, 325, 10. [Google Scholar] [CrossRef]
- Cociasu, A.; Lazar, L.; Varga, E.; Vasiliu, D. Recent data concerning evolution of the eutrophication level indicators in Romanian seawater. J. Environ. Prot. Ecol. 2009, 10, 701–731. [Google Scholar]
- Lee, J.; An, S. High ammonium recycling in an anthropogenically altered Yeongsan River Estuary, South Korea. Front. Mar. Sci. 2022, 9, 1017434. [Google Scholar] [CrossRef]
- Yunev, O.A.; Carstensen, J.; Moncheva, S.; Khaliulin, A.; Aeligrtebjerg, G.; Nixon, S. Nutrient and phytoplankton trends on the western Black Sea shelf in response to cultural eutrophication and climate changes. Estuar. Coast. Shelf Sci. 2007, 74, 63–76. [Google Scholar] [CrossRef]
- Ristea, E.; Bisinicu, E.; Lavric, V.; Parvulescu, O.C.; Lazar, L. A Long-Term Perspective of Seasonal Shifts in Nutrient Dynamics and Eutrophication in the Romanian Black Sea Coast. Sustainability 2025, 17, 1090. [Google Scholar] [CrossRef]
- Varenik, A.V.; Myslina, M.A.; Tarasevich, D.V. Atmospheric Input of Silica in Crimea and Factors Affecting it. Ecol. Saf. Coast. Shelf Zones Sea 2023, 1, 77–90. [Google Scholar] [CrossRef]
- Pakhomova, S.; Vinogradova, E.; Yakusheva, E.; Zatsepin, A.; Shtereva, G.; Chasovnikov, V.; Podymov, O. Interannual variability of the Black Sea Proper oxygen and nutrients regime: The role of climatic and anthropogenic forcing. Estuar. Coast. Shelf Sci. 2014, 140, 134–145. [Google Scholar] [CrossRef]
- Yakushev, E.V.; Chasovnikov, V.K.; Debolskaya, E.I.; Egorov, A.V.; Makkaveev, P.N.; Pakhomova, S.V.; Podymov, O.I.; Yakubenko, V.G. The northeastern Black Sea redox zone: Hydrochemical structure and its temporal variability. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 1769–1786. [Google Scholar] [CrossRef]
- Murray, J.W.; Jannasch, H.W.; Honjo, S.; Anderson, R.F.; Reeburgh, W.S.; Top, Z.; Friederich, G.E.; Codispoti, L.A.; Izdar, E. Unexpected changes in the oxic/anoxic interface in the Black Sea. Nature 1989, 338, 411–413. [Google Scholar] [CrossRef]
- Kondratev, S.I.; Khoruzhii, D.S. Vertical Distribution of Phosphates in the Black Sea Based on the Expeditionary Data, 2016–2019. Phys. Oceanogr. 2021, 28, 538–548. [Google Scholar] [CrossRef]
- Yakushev, E.V.; Lukashev, Y.F.; Chasovnikov, V.K.; Chzhu, V.P. Modern notion of the vertical hydrochemical structure of the Black Sea redox zone. In Complex Investigation of the Northeastern Black Sea; Zatsepin, A.G., Flint, M.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; p. 119. [Google Scholar]
- Basturk, O.; Saydam, C.; Salihoglu, I.; Eremeev, L.V.; Konovalov, S.K.; Stoyanov, A.; Dimitrov, A.; Cociasu, A.; Dorogan, L.; Altabet, M. Vertical variations in the principle chemical properties of the Black Sea in the autumn of 1991. Mar. Chem. 1994, 45, 149–165. [Google Scholar] [CrossRef]
- Konovalov, S.K.; Tugrul, S.; Basturk, O.; Salihoglu, I. Spatial isopycnal analysis of the main pycnocline chemistry of the Black Sea: Seasonal and interannual variations. In Sensitivity to Change: Black Sea, Baltic Sea and North Sea; NATO ASI, 2/27; Ozsoy, Ž.E., Mikaelyan, A., Eds.; Kluwer Academic Publishing: Dordrecht, The Netherlands, 1997; pp. 197–210. [Google Scholar]
- Murray, J.; Yakushev, E. The Suboxic Transition Zone in the Black Sea. In Past and Present Water Column Anoxia; Nato Science Series: IV: Earth and Environmental Sciences; Neretin, L., Ed.; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Mousing, E.A.; Adjou, M.; Ellegaard, M. Evidence of intensified biogenic silica recycling in the Black Sea after 1970. Estuar. Coast. Shelf Sci. 2015, 164, 335–339. [Google Scholar] [CrossRef]
- Tugrul, S.; Murray, J.W.; Friederich, G.E.; Salihoğlu, I. Spatial and temporal variability in the chemical properties of the oxic and suboxic layers of the Black Sea. J. Mar. Syst. 2014, 135, 29–43. [Google Scholar] [CrossRef]
- Gundersen, K.; Jannasch, H.W.; Murray, J.W.; Codispoti, L.A.; Taylor, K.E. Dinitrogen production from the nitracline to the anoxic waters in the Black Sea. Mar. Chem. 1998, 60, 1–13. [Google Scholar]
- Murray, J.W.; Jannasch, H.W.; Honjo, S.; Anderson, R.F.; Reeburgh, W.S.; Top, Z.; Friederich, G.E.; Codispoti, L.A.; Izdar, E. Physical and chemical properties of the Black Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 1995, 42, 209–239. [Google Scholar]
- Cannaby, H.; Fach, B.A.; Arkin, S.S.; Salihoglu, B. Climatic controls on biophysical interactions in the Black Sea under present day conditions and a potential future (A1B) climate scenario. J. Mar. Syst. 2015, 141, 149–166. [Google Scholar] [CrossRef]
- Kondratev, S.I.; Varenik, A.V.; Orekhova, N.A. Inorganic Forms of Nitrogen in the Deep Part of the Black Sea Based on the Expeditionary Data, 2016–2019. Phys. Oceanogr. 2023, 30, 186–201. [Google Scholar]
- Vaccaro, R.F.; Ryther, J.H. Marine phytoplankton and the distribution of nitrite in the sea. ICES J. Mar. Sci. 1960, 25, 260–271. [Google Scholar] [CrossRef]
- Dore, J.E.; Karl, D.M. Nitrite distributions and dynamics at station ALOHA. Deep Sea Res. Part II Top. Stud. Oceanogr. 1996, 43, 385–402. [Google Scholar] [CrossRef]
- Kremling, K.; Wenck, A. On the Storage of Dissolved Inorganic Phosphate, Nitrate and Reactive Silicate in Atlantic Ocean Water Samples; Verlag Paul Parey: Singhofen, Germany, 1986; ISSN 0341-6836. [Google Scholar]
- Aminot, A.; Kérouel, R. Dosage Automatique des Nutriments Dans les Eaux Marines; Edition Quae: Versailles, France, 2007. [Google Scholar]
- Pujo-Pay, M.; Raimbault, P. Improvement of the wet-oxidation procedure for simultaneous determination of particulate organic nitrogen and phosphorus collected on filters. Mar. Ecol. Prog. 1994, 105, 203–207. [Google Scholar] [CrossRef]
- Kirkwood, D.S. Stability of solutions of nutrient salts during storage. Mar. Chem. 1992, 38, 151–164. [Google Scholar] [CrossRef]
- Kattner, G. Storage of dissolved inorganic nutrients in seawater: Poisoning with mercuric chloride. Mar. Chem. 1999, 67, 61–66. [Google Scholar] [CrossRef]
- Kirkwood, D. Nutrients: Practical Notes on Their Determination in Sea Water; Techniques in Marine Environmental Sciences; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1996. [Google Scholar]
- Becker, S.; Aoyama, M.; Woodward, E.M.S.; Bakker, K.; Coverly, S.; Mahaffey, C.; Tanhua, T. GO-SHIP Repeat Hydrography Nutrient Manual: The precise and accurate determination of dissolved inorganic nutrients in seawater, using Continuous Flow Analysis methods. In GO-SHIP Repeat Hydrography Manual: A Collection of Expert Reports and Guidelines; Version 1.1; GO-SHIP Program and SCOR: Brest, France, 2019; p. 56. [Google Scholar] [CrossRef]
- Aminot, A.; Kérouel, R. Pasteurization as an alternative method for preservation of nitrate and nitrite in seawater samples. Mar. Chem. 1997, 61, 203–208. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada Bulletin: Ottawa, ON, Canada, 1972; p. 167.















| Sample Station | Date (dd/mm/yyyy) /Time (UTC) | Longitude (°) | Latitude (°) | Water Depth (m) |
|---|---|---|---|---|
| SU01 | 26 July 2024/12:30 | 29.7225 | 45.1125 | 22 |
| SU03 | 26 July 2024/09:50 | 30.0517 | 45.0411 | 34 |
| SU04 | 26 July 2024/05:45 | 30.4464 | 44.8989 | 52 |
| SG03 | 26 July 2024/04:50 | 29.6719 | 44.8111 | 38 |
| SG04 | 27 July 2024/05:15 | 29.8131 | 44.6714 | 53 |
| SG05 | 27 July 2024/07:50 | 30.1008 | 44.5889 | 65 |
| SG06 | 25 July 2024/14:30 | 30.5208 | 44.3364 | 92.5 |
| SG08 | 25 July 2024/11:20 | 30.6183 | 44.2450 | 118 |
| SG09 | 25 July 2024/09:10 | 30.7889 | 44.1572 | 150 |
| SG14 | 27 July 2024/10:00 | 30.3131 | 44.4614 | 79 |
| PO01 | 29 July 2024/08:35 | 29.1811 | 44.7181 | 13 |
| PO02 | 29 July 2024/05:50 | 29.2336 | 44.6614 | 19.5 |
| PO08 | 27 July 2024/14:10 | 29.9755 | 44.1671 | 70 |
| PO04 | 28 July 2024/13:50 | 29.3392 | 44.5294 | 38 |
| PO05 | 29 July 2024/05:10 | 29.2250 | 44.5747 | 29 |
| PO06 | 28 July 2024/08:30 | 29.5836 | 44.4250 | 54 |
| PO07 | 28 July 2024/05:10 | 29.7973 | 44.2938 | 64 |
| CT02 | 29 July 2024/13:35 | 28.7189 | 44.1644 | 27 |
| CT05 | 22 July 2024/06:02 | 29.5142 | 43.9722 | 64 |
| CT06 | 22 July 2024/12:14 | 29.8400 | 43.9436 | 70 |
| CT03 | 20 July 2024/12:45 | 28.7597 | 44.1780 | 27 |
| CT04 | 20 July 2024/15:50 | 29.0331 | 44.0797 | 46 |
| MID02 | 28 July 2024/11:10 | 29.2303 | 44.3006 | 45 |
| EF02 | 21 July 2024/05:20 | 28.6672 | 44.0700 | 16.5 |
| TZ18 | 21 July 2024/06:55 | 28.7225 | 43.9889 | 33.1 |
| MA02 | 23 July 2024/05:30 | 30.0456 | 43.7556 | 112 |
| MA03 | 22 July 2024/14:40 | 29.7622 | 43.7561 | 70 |
| MA04 | 22 July 2024/05:10 | 29.3983 | 43.7589 | 67 |
| MA05 | 21 July 2024/10:50 | 28.6228 | 43.7719 | 16.6 |
| MA08 | 21 July 2024/12:50 | 28.7289 | 43.7708 | 43.7 |
| MA10 | 23 July 2024/09:40 | 30.2022 | 43.7550 | 152 |
| MA11 | 21 July 2024/16:30 | 29.0642 | 43.7600 | 57 |
| MA12 | 24 July 2024/06:05 | 30.3806 | 43.7625 | 484 |
| MA13 | 24 July 2024/09:40 | 30.5957 | 43.7959 | 887 |
| MA14 | 24 July 2024/12:35 | 30.7917 | 43.8053 | 1116 |
| VTZ01 | 23 July 2024/15:25 | 30.3705 | 43.9313 | 621 |
| Variables | Temp. [°C] | Salinity [PSU] | DO [mg/L] | Chl. [µg/L] | PO4 [µM] | SiO4 [µM] | NO3 [µM] | NO2 [µM] | NH4 [µM] |
|---|---|---|---|---|---|---|---|---|---|
| Temp. [°C] | 1 | 0.128 | −0.070 | −0.397 | −0.069 | −0.213 | 0.224 | 0.201 | −0.247 |
| Salinity [PSU] | 0.128 | 1 | 0.203 | −0.721 | −0.670 | −0.530 | −0.584 | −0.623 | −0.265 |
| DO [mg/L] | −0.070 | 0.203 | 1 | 0.003 | −0.077 | −0.149 | 0.005 | −0.129 | −0.314 |
| Chl [µg/L] | −0.397 | −0.721 | 0.003 | 1 | 0.737 | 0.473 | 0.570 | 0.455 | 0.181 |
| PO4 [µM] | −0.069 | −0.670 | −0.077 | 0.737 | 1 | 0.397 | 0.638 | 0.545 | 0.392 |
| SiO4 [µM] | −0.213 | −0.530 | −0.149 | 0.473 | 0.397 | 1 | 0.169 | 0.036 | 0.068 |
| NO3 [µM] | 0.224 | −0.584 | 0.005 | 0.570 | 0.638 | 0.169 | 1 | 0.709 | 0.078 |
| NO2 [µM] | 0.201 | −0.623 | −0.129 | 0.455 | 0.545 | 0.036 | 0.709 | 1 | 0.240 |
| NH4 [µM] | −0.247 | −0.265 | −0.314 | 0.181 | 0.392 | 0.068 | 0.078 | 0.240 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliu, D.; Bucșe, A.; Rădulescu, F.; Fediuc, F.; Balan, S. Surface and Vertical Nutrient Profiles in the Northwestern Black Sea: Trends, Comparisons, and Sample Preservation Assessment. J. Mar. Sci. Eng. 2025, 13, 2178. https://doi.org/10.3390/jmse13112178
Vasiliu D, Bucșe A, Rădulescu F, Fediuc F, Balan S. Surface and Vertical Nutrient Profiles in the Northwestern Black Sea: Trends, Comparisons, and Sample Preservation Assessment. Journal of Marine Science and Engineering. 2025; 13(11):2178. https://doi.org/10.3390/jmse13112178
Chicago/Turabian StyleVasiliu, Dan, Andra Bucșe, Florina Rădulescu, Florentina Fediuc, and Sorin Balan. 2025. "Surface and Vertical Nutrient Profiles in the Northwestern Black Sea: Trends, Comparisons, and Sample Preservation Assessment" Journal of Marine Science and Engineering 13, no. 11: 2178. https://doi.org/10.3390/jmse13112178
APA StyleVasiliu, D., Bucșe, A., Rădulescu, F., Fediuc, F., & Balan, S. (2025). Surface and Vertical Nutrient Profiles in the Northwestern Black Sea: Trends, Comparisons, and Sample Preservation Assessment. Journal of Marine Science and Engineering, 13(11), 2178. https://doi.org/10.3390/jmse13112178






