Effects of Submarine Methane-Rich Fluids on Gas Hydrate Production During Depressurization
Abstract
1. Introduction

2. Simulation Methodology
2.1. Reservoir Background and Initial Conditions
2.2. Simulation Code
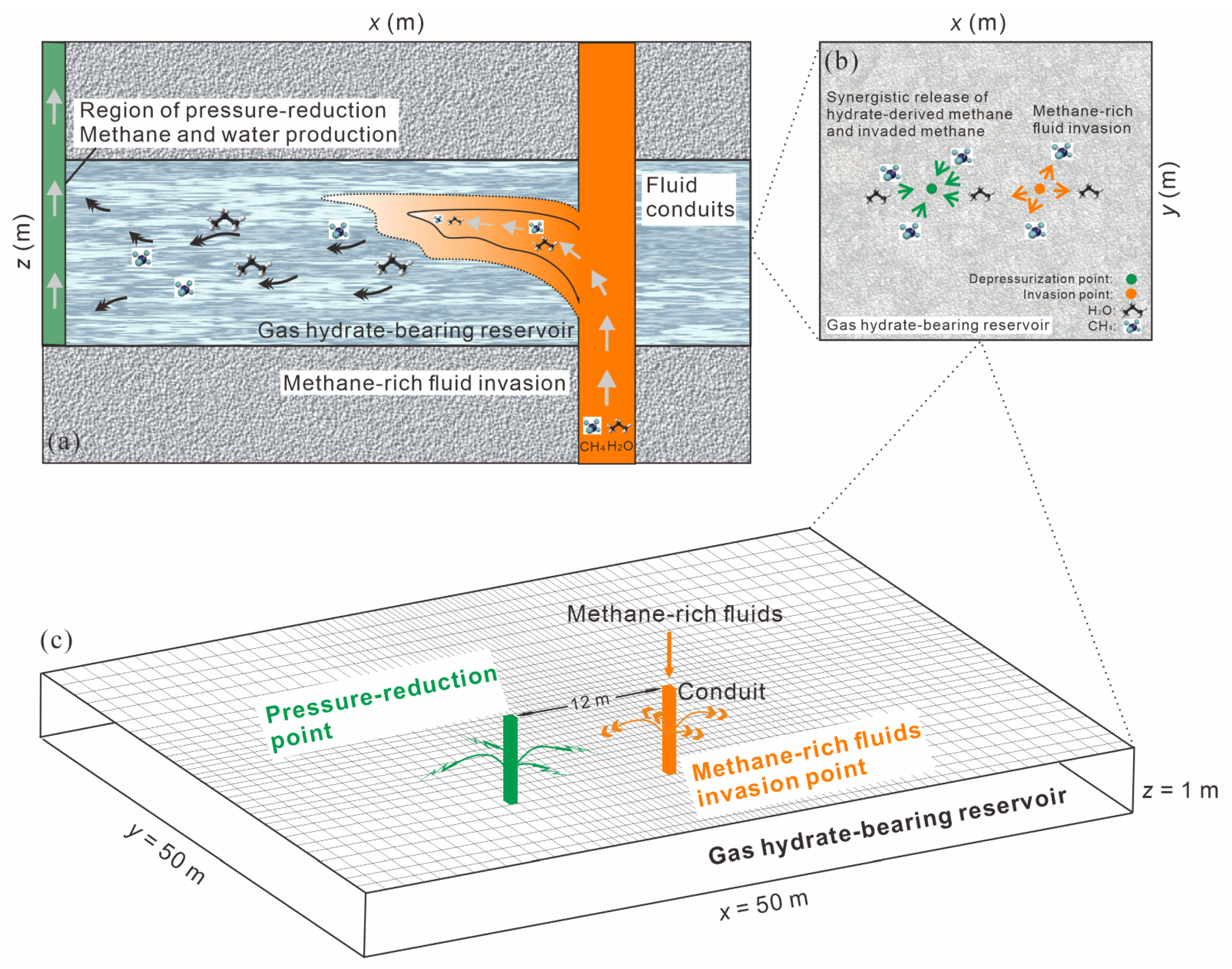
2.3. Model Construction, Fluid Invasion Flux and Depressurization Simulation
3. Results and Discussion
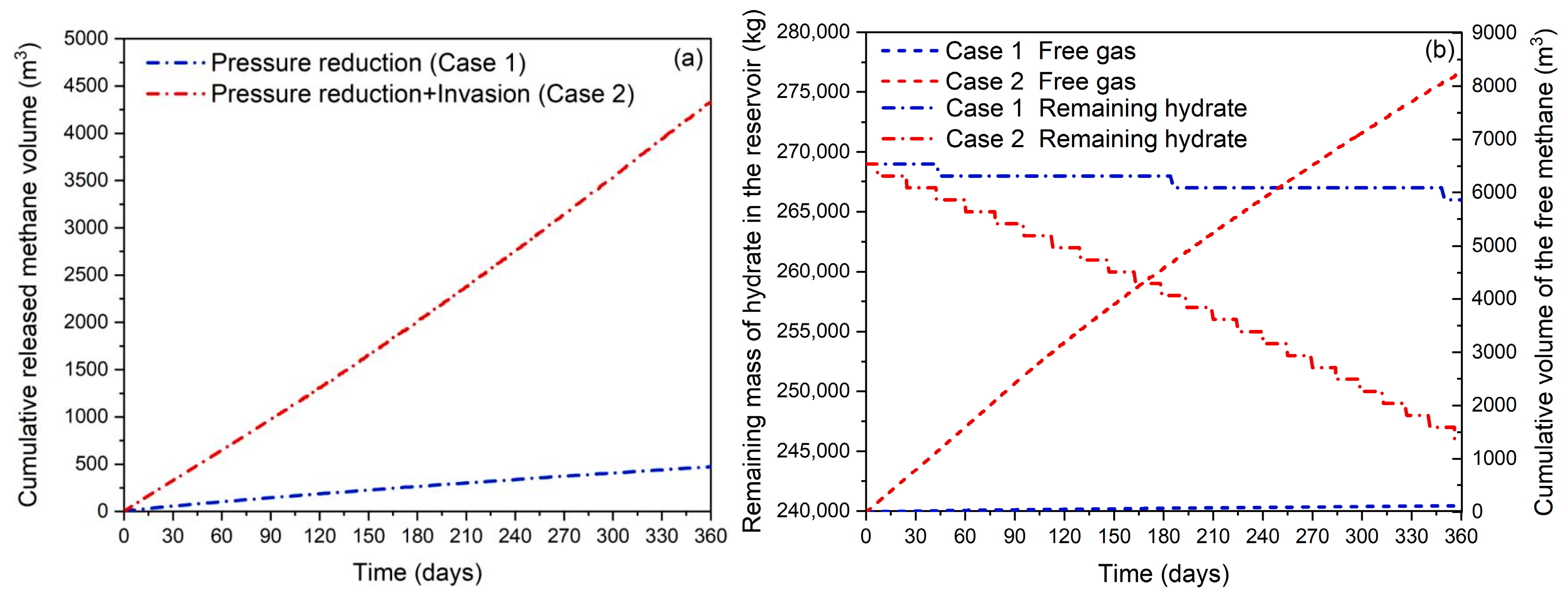
3.1. Effect of Methane-Rich Fluid Invasion
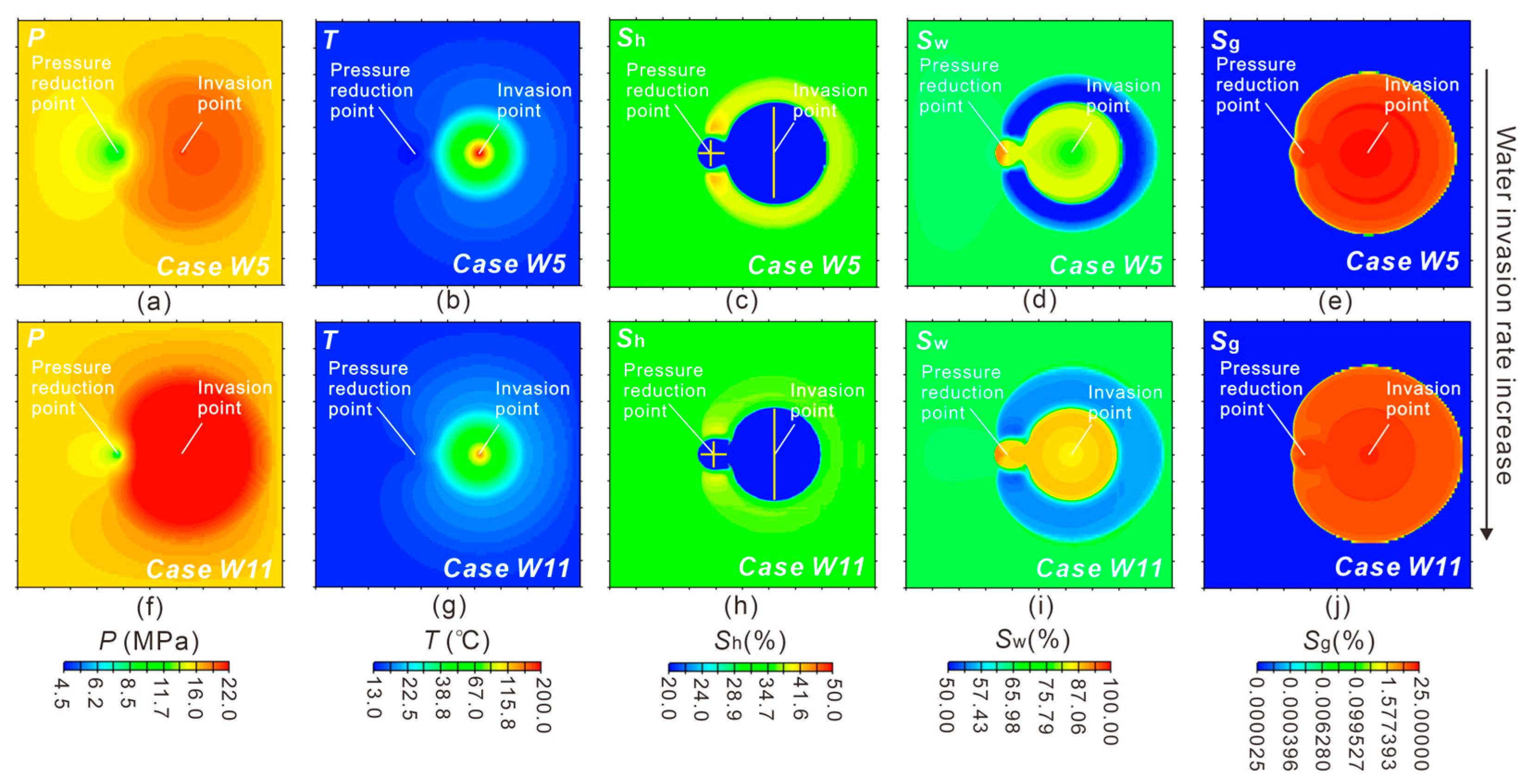
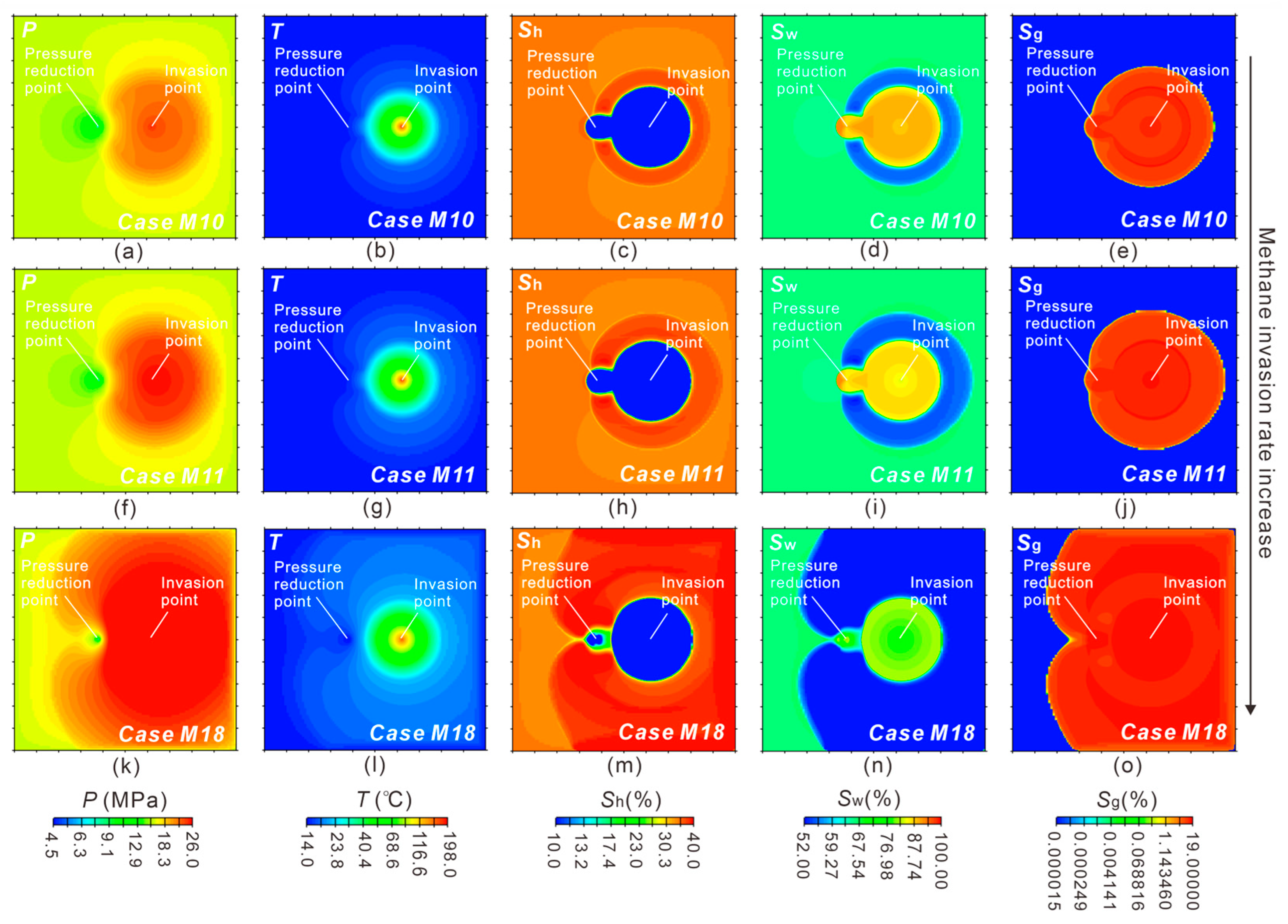
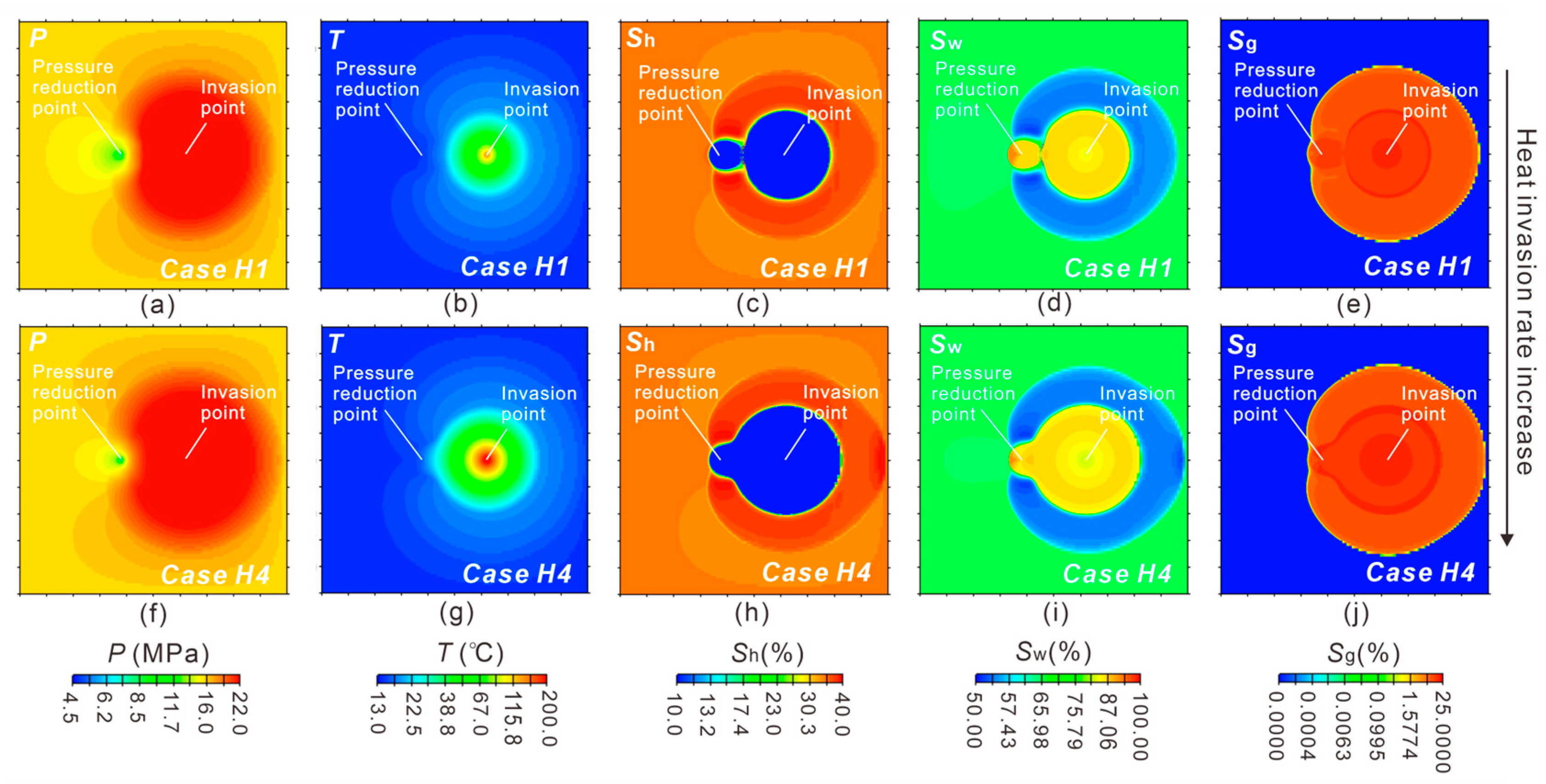
3.1.1. Reservoir Physical Fields Evolution
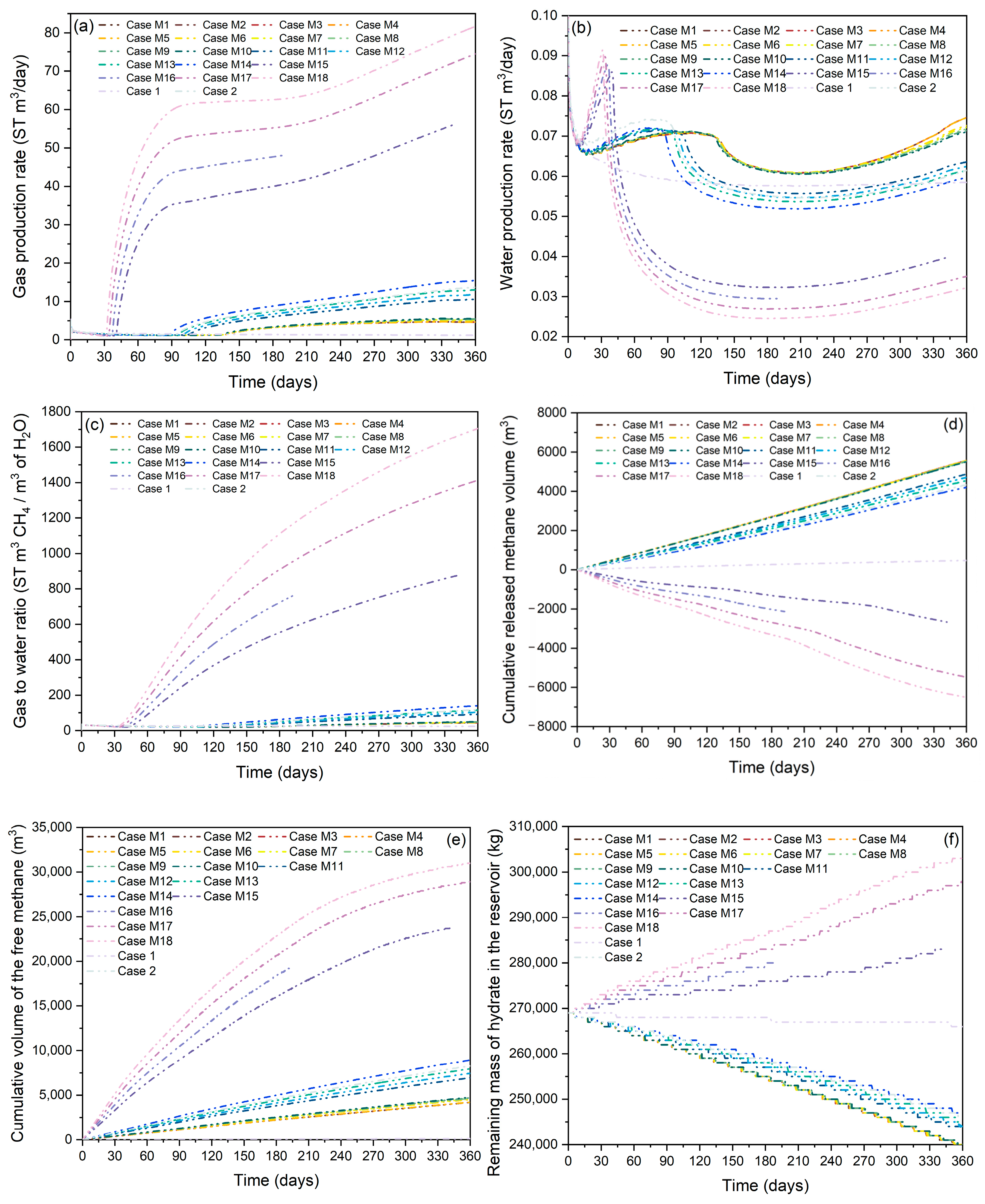
3.1.2. Gas Production Behavior
3.1.3. Free Methane Volume in the Reservoir and Residual Hydrate Mass
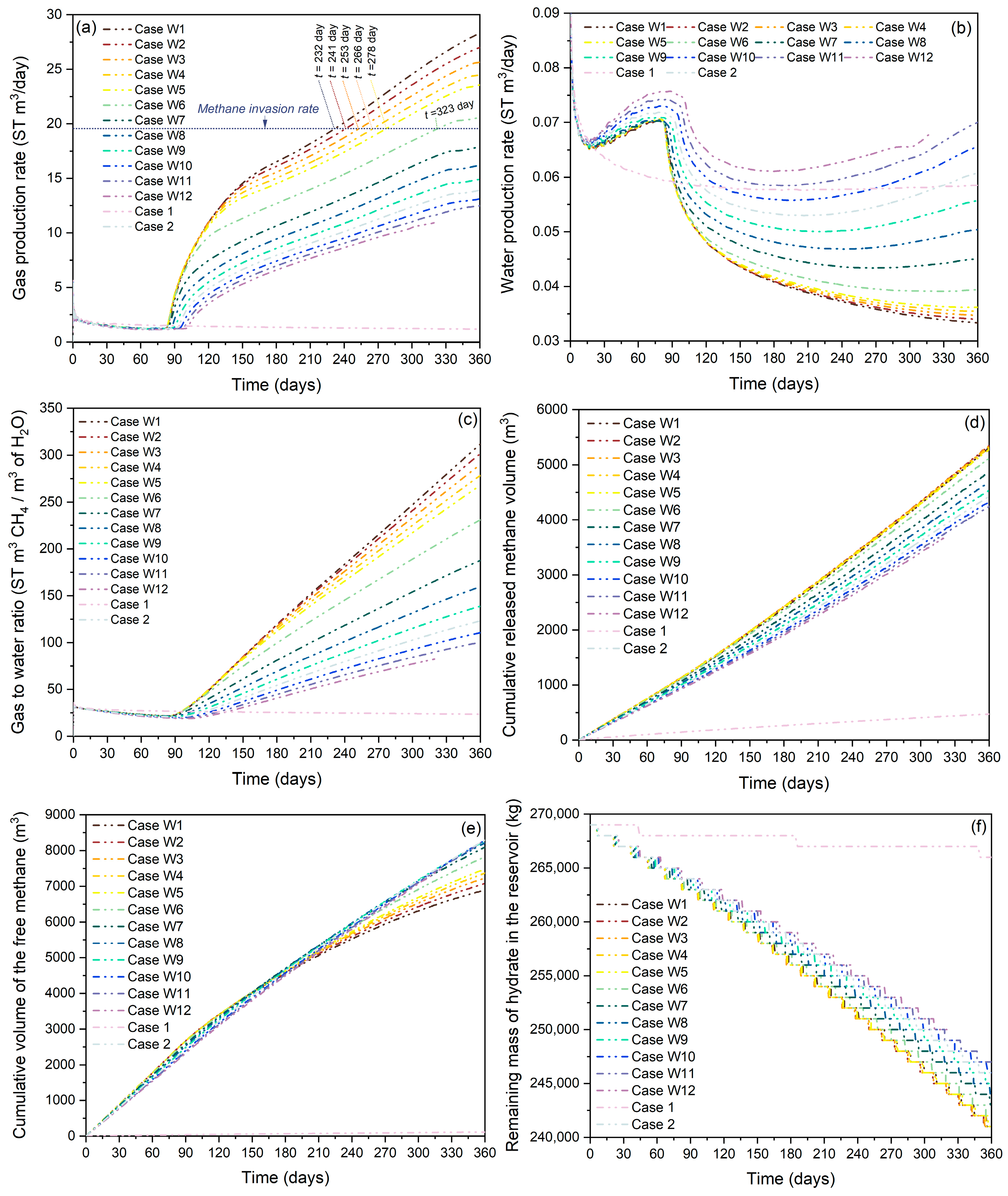
3.2. Effect of Methane-Rich Fluid Flux
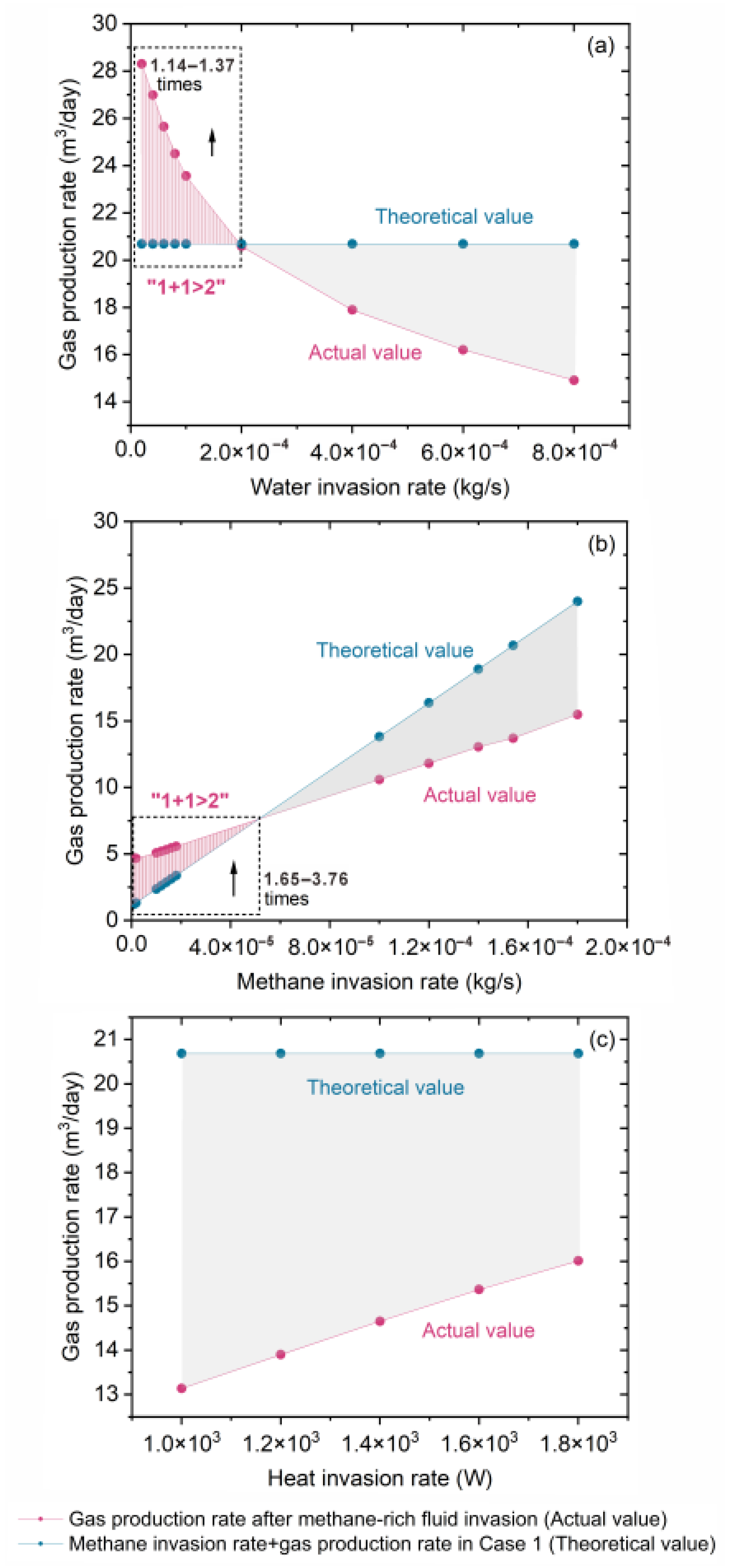
3.2.1. Effect of Water Invasion Rate
3.2.2. Effect of Methane Invasion Rate
3.2.3. Effect of Heat Supply Rate
3.3. Synergistic Enhancement of Gas Production by Methane-Rich Fluid Invasion (“1 + 1 > 2” Effect)
4. Conclusions and Outlook
- (1)
- The invasion of methane-rich fluid into gas hydrate systems exhibits a three-phase impact on gas production dynamics. In the initial stage, the invasion shows no significant effect. After a certain period, it begins to enhance hydrate dissociation but temporarily inhibits gas production. Under sustained invasion, it significantly promotes hydrate dissociation and increases gas production. Future studies should focus on quantifying the critical transition thresholds between the inhibition and promotion phases of gas production under field-scale conditions; this is essential for optimizing depressurization strategies and improving production efficiency.
- (2)
- Low water invasion and elevated heat supply enhance hydrate dissociation and promote gas production. In contrast, high methane flux reduces hydrate dissociation and increases external methane accumulation in reservoir pores. Notably, excessive methane input may lead to the formation of new hydrate exceeding the amount of dissociated hydrate in the reservoir. Therefore, in future field development site selection, priority should be given to fluid conduit-associated gas hydrate systems characterized by high methane flux, high heat flux, and low water invasion rates, as these conditions are more favorable for efficient and stable gas recovery.
- (3)
- When the actual gas production exceeds the theoretical value, it confirms that methane-rich fluid invasion enhances hydrate-reservoir gas production, exhibiting a synergistic “1 + 1 > 2” effect, particularly at low water or methane invasion rates. Controlled methane-rich fluid injection at optimally low rates may serve as an effective stimulation technique to enhance gas recovery while maintaining reservoir stability. Future studies should focus on the coupled effects of methane and water invasion under depressurization to determine optimal invasion rate combinations for maximizing the synergistic enhancement. Establishing quantitative criteria to identify the onset and intensity of this effect will be crucial for guiding real-time production control and intelligent operation strategies. Additionally, developing advanced monitoring and control technologies to regulate multiphase fluid fluxes, together with long-term assessments of reservoir stability and environmental safety, will support adaptive, high-efficiency, and environmentally sustainable gas production strategies in the SCS and other continental margins.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCS | South China Sea |
| H2O | Water |
| CH4 | Methane |
| MTD | Mass transport deposit |
| HAR | High amplitude reflection |
| GHSZ | Gas hydrate stability zone |
| BGHSZ | Base of gas hydrate stability zone |
| BSR | Bottom-simulating reflections |
| PRMB | Pearl River Mouth Basin |
| QDNB | Qiongdongnan Basin |
| FII | Fault |
| SW | Southwest |
| NE | Northeast |
References
- Wu, N.; Li, Y.; Wan, Y.; Sun, J.; Huang, L.; Mao, P. Prospect of Marine Natural Gas Hydrate Stimulation Theory and Technology System. Nat. Gas Ind. B 2021, 8, 173–187. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Yu, T.; Wu, Z.; Huang, L.; Wang, H.; Song, Y. Microstructure Evolution and Dynamic Permeability Anisotropy during Hydrate Dissociation in Sediment under Stress State. Energy 2023, 263, 126126. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Gas Hydrates-Geological Perspective and Global Change. Rev. Geophys. 1993, 31, 173–187. [Google Scholar] [CrossRef]
- Wu, P.; Dong, Z.; Huang, Z.; Zhi, Y.; Li, Y.; Song, Y. A Novel High-Pressure and Low-Temperature Ring Shear Apparatus for Large-Scale Deformation of Hydrate-Bearing Sediment. Energy Fuels 2025, 39, 18870–18879. [Google Scholar] [CrossRef]
- Li, X.-Y.; Feng, J.-C.; Li, X.-S.; Wang, Y.; Hu, H.-Q. Experimental Study of Methane Hydrate Formation and Decomposition in the Porous Medium with Different Thermal Conductivities and Grain Sizes. Appl. Energy 2022, 305, 117852. [Google Scholar] [CrossRef]
- Li, A.; Wu, N.; Li, Q.; Wang, Z.; Wan, Y.; Cai, F.; Sun, Z.; Feng, D. Methane Seepage Caused by Gas Hydrate Dissociation in the Mid-Okinawa Trough Since the Last Glacial Maximum. Geophys. Res. Lett. 2023, 50, e2023GL103375. [Google Scholar] [CrossRef]
- Phrampus, B.J.; Hornbach, M.J. Recent Changes to the Gulf Stream Causing Widespread Gas Hydrate Destabilization. Nature 2012, 490, 527–530. [Google Scholar] [CrossRef]
- Berndt, C.; Feseker, T.; Treude, T.; Krastel, S.; Liebetrau, V.; Niemann, H.; Bertics, V.J.; Dumke, I.; Dünnbier, K.; Ferré, B.; et al. Temporal Constraints on Hydrate-Controlled Methane Seepage off Svalbard. Science 2014, 343, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Skarke, A.; Ruppel, C.; Kodis, M.; Brothers, D.; Lobecker, E. Widespread Methane Leakage from the Sea Floor on the Northern US Atlantic Margin. Nat. Geosci. 2014, 7, 657–661. [Google Scholar] [CrossRef]
- Portnov, A.; Vadakkepuliyambatta, S.; Mienert, J.; Hubbard, A. Ice-Sheet-Driven Methane Storage and Release in the Arctic. Nat. Commun. 2016, 7, 10314. [Google Scholar] [CrossRef]
- Ruppel, C.D.; Kessler, J.D. The Interaction of Climate Change and Methane Hydrates. Rev. Geophys. 2017, 55, 126–168. [Google Scholar] [CrossRef]
- Kim, B.; Zhang, Y.G. Methane Hydrate Dissociation across the Oligocene–Miocene Boundary. Nat. Geosci. 2022, 15, 203–209. [Google Scholar] [CrossRef]
- Tréhu, A.M.; Long, P.E.; Torres, M.E.; Bohrmann, G.; Rack, F.R.; Collett, T.S.; Goldberg, D.S.; Milkov, A.V.; Riedel, M.; Schultheiss, P.; et al. Three-Dimensional Distribution of Gas Hydrate beneath Southern Hydrate Ridge: Constraints from ODP Leg 204. Earth Planet. Sci. Lett. 2004, 222, 845–862. [Google Scholar] [CrossRef]
- Collett, T.S.; Lee, M.W.; Zyrianova, M.V.; Mrozewski, S.A.; Guerin, G.; Cook, A.E.; Goldberg, D.S. Gulf of Mexico Gas Hydrate Joint Industry Project Leg II Logging-While-Drilling Data Acquisition and Analysis. Mar. Pet. Geol. 2012, 34, 41–61. [Google Scholar] [CrossRef]
- Ishizu, K.; Oda, A.; Goto, T.; Kasaya, T.; Watanabe, T.; Machiyama, H. Electrical Resistivity Tomography Combined with Seismic Data Estimates Heterogeneous Distribution of Near-Seafloor Concentrated Gas Hydrates within Gas Chimneys. Sci. Rep. 2024, 14, 15045. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.A.; Kastner, M.; Jannasch, H.; Robertson, G.; Weinstein, Y. Dynamic Fluid Flow and Chemical Fluxes Associated with a Seafloor Gas Hydrate Deposit on the Northern Gulf of Mexico Slope. Earth Planet. Sci. Lett. 2008, 270, 95–105. [Google Scholar] [CrossRef]
- Liu, J.; Haeckel, M.; Rutqvist, J.; Wang, S.; Yan, W. The Mechanism of Methane Gas Migration Through the Gas Hydrate Stability Zone: Insights From Numerical Simulations. J. Geophys. Res. Solid. Earth 2019, 124, 4399–4427. [Google Scholar] [CrossRef]
- Flemings, P.B.; Liu, X.; Winters, W.J. Critical Pressure and Multiphase Flow in Blake Ridge Gas Hydrates. Geology 2003, 31, 1057. [Google Scholar] [CrossRef]
- Liu, X.; Flemings, P.B. Dynamic Multiphase Flow Model of Hydrate Formation in Marine Sediments. J. Geophys. Res. 2007, 112, b03101. [Google Scholar] [CrossRef]
- Dugan, B.; Flemings, P.B. Overpressure and Fluid Flow in the New Jersey Continental Slope: Implications for Slope Failure and Cold Seeps. Science 2000, 289, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.J.; Yang, J.; Ireland, M.T.; Berndt, C.; Maqueda, M.Á.M.; Huuse, M. Long-Distance Migration and Venting of Methane from the Base of the Hydrate Stability Zone. Nat. Geosci. 2024, 17, 32–37. [Google Scholar] [CrossRef]
- You, K.; Flemings, P.B. Methane Hydrate Formation in Thick Sandstones by Free Gas Flow. J. Geophys. Res. Solid Earth 2018, 123, 4582–4600. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, L.; Zhang, J.; Shang, S.; Lu, H. Numerical Investigation on Environmental Effect Associated with Gas-Hydrate Exploitation. Geoenergy Sci. Eng. 2023, 227, 211857. [Google Scholar] [CrossRef]
- Shi, K.; Wei, K.; Jiang, Z.; Fan, Q.; Li, Q.; Leng, S.; Zhou, Y.; Zhang, L.; Liu, Y.; Zhao, J.; et al. Optimization of Energy Efficiency in Gas Production from Hydrates Assisted by Geothermal Energy Enriched in the Deep Gas. Int. J. Heat Mass Transf. 2024, 234, 126122. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, J.; Su, P.; Meng, M.; Huang, W.; Liu, P.; Yuan, S.; Ji, C. Dynamic Accumulation of a High-Grade Gas Hydrate System: Insights from the Trial Production Gas Hydrate Reservoir in the Shenhu Area, Northern South China Sea. Front. Mar. Sci. 2024, 11, 1418716. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, T.; Kuang, Z.; Cheng, C.; Xiong, P.; Chen, Y. Accumulation characteristics of gas hydrate-shallow gas symbiotic system in Qiongdongnan Basin. Earth Sci. 2022, 47, 1619–1634. [Google Scholar] [CrossRef]
- Wang, J.; Li, A.; Wang, L.H.; Wu, S.; Li, Q. Submarine Fluid Flow System Feeding Methane Emission in the Northern South China Sea. Basin Res. 2024, 36, e12839. [Google Scholar] [CrossRef]
- Wu, S.; Sun, J.; Li, Q.; Ma, Y.; Lüdmann, T. Potential on Joint Development of Three-Gas Reservoirs in the Qiongdongnan Basin. Innov. Geosci. 2024, 2, 100065. [Google Scholar] [CrossRef]
- Wu, S.; Wu, S.; Sun, J.; Li, Q.; Chen, J.; Chen, Y.; Zhou, X.; Khan, U. The Spatial Coupling of Fluid Pathways with Gas Hydrates and Shallow Gas Reservoirs: A Case Study in the Qiongdongnan Basin, South China Sea. J. Mar. Sci. Eng. 2024, 12, 659. [Google Scholar] [CrossRef]
- Hustoft, S.; Mienert, J.; Bünz, S.; Nouzé, H. High-Resolution 3D-Seismic Data Indicate Focussed Fluid Migration Pathways above Polygonal Fault Systems of the Mid-Norwegian Margin. Mar. Geol. 2007, 245, 89–106. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Wang, H.; Yin, S.; Wan, X.; Chen, J.; Hernández-Molina, F.J. Flow Conditions of the Quaternary Deep-Water Current Reconstructed by Sediment Waves in the Northeastern South China Sea. Mar. Geol. 2024, 477, 107414. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, J.; Wei, J.; Lu, J.; Su, P.; Lin, L.; Huang, W.; Guo, Y.; Deng, W.; Yang, X.; et al. Geological and Geophysical Features of and Controls on Occurrence and Accumulation of Gas Hydrates in the First Offshore Gas-Hydrate Production Test Region in the Shenhu Area, Northern South China Sea. Mar. Pet. Geol. 2020, 114, 104191. [Google Scholar] [CrossRef]
- Mao, P.; Wan, Y.; Sun, J.; Li, Y.; Hu, G.; Ning, F.; Wu, N. Numerical Study of Gas Production from Fine-Grained Hydrate Reservoirs Using a Multilateral Horizontal Well System. Appl. Energy 2021, 301, 117450. [Google Scholar] [CrossRef]
- Moridis, G. User’s Manual of the TOUGH+ Core Code v1.5: A General-Purpose Simulator of Non-Isothermal Flow and Transport Through Porous and Fractured Media; LBNL-6871E; LBL Publications: Berkeley, CA, USA, 2014; p. 1165988. [Google Scholar]
- Yin, Z.; Zhang, S.; Koh, S.; Linga, P. Estimation of the Thermal Conductivity of a Heterogeneous CH4-Hydrate Bearing Sample Based on Particle Swarm Optimization. Appl. Energy 2020, 271, 115229. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A Closed-Form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, X.-S.; Chen, Z.-Y.; Li, Q.-P.; He, J. Dynamic Competitive Co-Production Behaviors between Natural Gas Hydrate and Higher-Pressure Shallow Gas under Wellbore Interference: An Experimental Study. Appl. Energy 2025, 384, 125474. [Google Scholar] [CrossRef]
- Yu, J.; Zhuo, L.; Chen, Y.; Sun, W.; Liu, Y. Production Performance Analysis of Class II Hydrate-Bearing Layers Based on an Analytic Aquifer Model. Front. Energy Res. 2021, 9, 702456. [Google Scholar] [CrossRef]
- Yang, L.; Falenty, A.; Chaouachi, M.; Haberthür, D.; Kuhs, W.F. Synchrotron X-Ray Computed Microtomography Study on Gas Hydrate Decomposition in a Sedimentary Matrix. Geochem. Geophys. Geosyst. 2016, 17, 3717–3732. [Google Scholar] [CrossRef]
- He, Y.; Liang, J.; Shi, W.; Kuang, Z.; Deng, W.; Wang, R.; Xu, L.; Du, H. Influencing factors and accumulation modes of gas hydrate in south low uplift and its surrounding area of Qiongdongnan Basin. Earth Sci. 2022, 47, 1711. [Google Scholar] [CrossRef]





| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Reservoir basic parameters | |||
| Permeability/m2 | 2.20 × 10−16 | Seawater salinity/wt% | 3.05 |
| Porosity/% | 34.5 | Seawater density/(kg∙m3) | 1035 |
| Hydrate saturation/% | 34.0 | Thermal conductivity (wet)/(W∙m−1∙°C) | 2.917 |
| Particle density/(kg∙m−3) | 2700 | Thermal conductivity (dry)/(W∙m−1∙°C) | 1.000 |
| Specific heat capacity/(J∙kg−1∙C−1) | 1000 | ||
| Relative permeability model [34] | |||
| KrA = (SA*)n | KrG = (SG*)nG | ||
| SA* = (SA − SirA)/(1 − SirA) | SG* = (SG − SirG)/(1 − SirA) | ||
| SirA | 0.3 | nG | 3 |
| SirG | 0.03 | n | 5 |
| Capillary pressure model [36] | |||
| Pcap = −P0 [(S*)−1/λ − 1]1−λ | S* = (SA − SirA)/(SmxA − SirA) | ||
| P0/Pa | 105 | SmxA | 1.00 |
| λ | 0.45 | SirA | 0.50 |
| Pressure reduction point parameters | |||
| Permeability/m2 | 5.0 × 10−9 | Capillary pressure | 0 |
| Porosity/% | 100 | Area/m2 | 0.01 |
| Invasion point parameters | |||
| Porosity/% | 100 | Relative permeability in conduit | krA = SA, krG = SG |
| Capillary pressure | 0 | Area/m2 | 0.01 |
| Gas composition | 100% CH4 | ||
| Case ID | Water Invasion Rate (kg/s) | Case ID | Methane Invasion Rate (kg/s) | Case ID | Heat Supply Rate (W) |
|---|---|---|---|---|---|
| W1 | 2.00 × 10−5 | M1 | 1.00 × 10−6 | H1 | 1.00 × 103 |
| W2 | 4.00 × 10−5 | M2 | 1.20 × 10−6 | 2 | 1.20 × 103 |
| W3 | 6.00 × 10−5 | M3 | 1.40 × 10−6 | H2 | 1.40 × 103 |
| W4 | 8.00 × 10−5 | M4 | 1.60 × 10−6 | H3 | 1.60 × 103 |
| W5 | 1.00 × 10−4 | M5 | 1.80 × 10−6 | H4 | 1.80 × 103 |
| W6 | 2.00 × 10−4 | M6 | 1.00 × 10−5 | H5 | 1.00 × 102 |
| W7 | 4.00 × 10−4 | M7 | 1.20 × 10−5 | H6 | 1.20 × 102 |
| W8 | 6.00 × 10−4 | M8 | 1.40 × 10−5 | H7 | 1.40 × 102 |
| W9 | 8.00 × 10−4 | M9 | 1.60 × 10−5 | H8 | 1.60 × 102 |
| 2 | 1.00 × 10−3 | M10 | 1.80 × 10−5 | H9 | 1.80 × 102 |
| W10 | 1.20 × 10−3 | M11 | 1.00 × 10−4 | H10 | 1.00 × 101 |
| W11 | 1.40 × 10−3 | M12 | 1.20 × 10−4 | H11 | 1.20 × 101 |
| W12 | 1.60 × 10−3 | M13 | 1.40 × 10−4 | H12 | 1.40 × 101 |
| 2 | 1.54 × 10−4 | H13 | 1.60 × 101 | ||
| M14 | 1.80 × 10−4 | ||||
| M15 | 1.00 × 10−3 | ||||
| M16 | 1.20 × 10−3 | ||||
| M17 | 1.40 × 10−3 | ||||
| M18 | 1.60 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, P.; Lu, W.; Wan, Y.; Wu, N. Effects of Submarine Methane-Rich Fluids on Gas Hydrate Production During Depressurization. J. Mar. Sci. Eng. 2025, 13, 2166. https://doi.org/10.3390/jmse13112166
Mao P, Lu W, Wan Y, Wu N. Effects of Submarine Methane-Rich Fluids on Gas Hydrate Production During Depressurization. Journal of Marine Science and Engineering. 2025; 13(11):2166. https://doi.org/10.3390/jmse13112166
Chicago/Turabian StyleMao, Peixiao, Wanjun Lu, Yizhao Wan, and Nengyou Wu. 2025. "Effects of Submarine Methane-Rich Fluids on Gas Hydrate Production During Depressurization" Journal of Marine Science and Engineering 13, no. 11: 2166. https://doi.org/10.3390/jmse13112166
APA StyleMao, P., Lu, W., Wan, Y., & Wu, N. (2025). Effects of Submarine Methane-Rich Fluids on Gas Hydrate Production During Depressurization. Journal of Marine Science and Engineering, 13(11), 2166. https://doi.org/10.3390/jmse13112166






