Identification of Sex-Dependent Aroma Compounds in Gonads of Commercially Valuable Sea Urchins: Implications for Gastronomical Use of Paracentrotus lividus
Abstract
1. Introduction
2. Materials and Methods
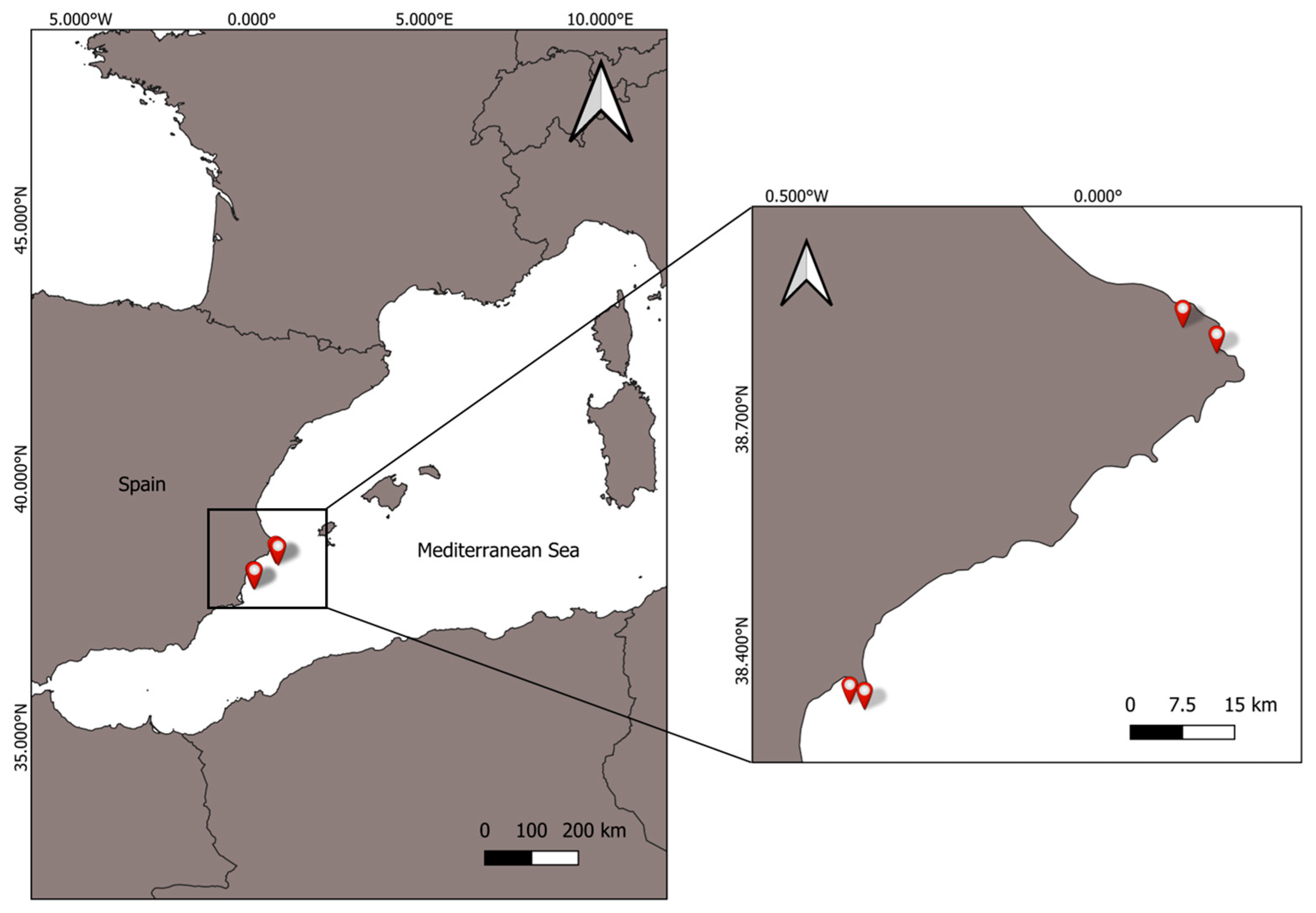
2.1. Sample Collection and Preparation
2.2. VOC Extraction and GC–MS Analysis
2.3. Data Processing and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, P.; Noble, C.; Hannon, C.; Stefansson, G.; Thórarinsdóttir, G.; Sloane, R.; Ziemer, N.; Lochead, J. Sea Urchin Fisheries, Management and Policy Review (Activity a4. 2.1 of the Urchin Project). 2016. Available online: https://urchinproject.com/wp-content/uploads/sites/3/2016/05/Report_18-2016.pdf (accessed on 12 November 2025).
- Andrew, N.L.; Agatsuma, Y.; Ballesteros, E.; Bazhin, A.G.; Creaser, E.P.; Barnes, D.K.A.; Botsford, L.W.; Bradbury, A.; Campbell, A.; Dixon, J.D. Status and management of world sea urchin fisheries. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2002; pp. 351–438. [Google Scholar]
- Boudouresque, C.F.; Verlaque, M. Chapter 26—Paracentrotus lividus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 43, pp. 447–485. [Google Scholar]
- Rocha, F.; Baião, L.F.; Moutinho, S.; Reis, B.; Oliveira, A.; Arenas, F.; Maia, M.R.; Fonseca, A.J.; Pintado, M.; Valente, L.M. The effect of sex, season and gametogenic cycle on gonad yield, biochemical composition and quality traits of Paracentrotus lividus along the North Atlantic coast of Portugal. Sci. Rep. 2019, 9, 2994. [Google Scholar] [CrossRef] [PubMed]
- Ghisaura, S.; Loi, B.; Biosa, G.; Baroli, M.; Pagnozzi, D.; Roggio, T.; Uzzau, S.; Anedda, R.; Addis, M.F. Proteomic changes occurring along gonad maturation in the edible sea urchin Paracentrotus lividus. J. Proteom. 2016, 144, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M. On the relationship between marine plants and sea urchins. Oceanogr. Mar. Biol. Ann. Rev. 1975, 13, 213–286. [Google Scholar]
- Bulleri, F.; Benedetti-Cecchi, L.; Cinelli, F. Grazing by the sea urchins Arbacia lixula L. and Paracentrotus lividus Lam. in the Northwest Mediterranean. J. Exp. Mar. Biol. Ecol. 1999, 241, 81–95. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Bulleri, F.; Cinelli, F. Density dependent foraging of sea urchins in shallow subtidal reefs on the west coast of Italy (western Mediterranean). Mar. Ecol. Prog. Ser. 1998, 163, 203–211. [Google Scholar] [CrossRef]
- Bonaviri, C.; Vega Fernández, T.; Fanelli, G.; Badalamenti, F.; Gianguzza, P. Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean. Mar. Biol. 2011, 158, 2505–2513. [Google Scholar] [CrossRef]
- Boada, J.; Arthur, R.; Alonso, D.; Pagès, J.F.; Pessarrodona, A.; Oliva, S.; Ceccherelli, G.; Piazzi, L.; Romero, J.; Alcoverro, T. Immanent conditions determine imminent collapses: Nutrient regimes define the resilience of macroalgal communities. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162814. [Google Scholar] [CrossRef]
- Guarnieri, G.; Bevilacqua, S.; Vignes, F.; Fraschetti, S. Grazer removal and nutrient enrichment as recovery enhancers for overexploited rocky subtidal habitats. Oecologia 2014, 175, 959–970. [Google Scholar] [CrossRef]
- Ouréns, R.; Naya, I.; Freire, J. Mismatch between biological, exploitation, and governance scales and ineffective management of sea urchin (Paracentrotus lividus) fisheries in Galicia. Mar. Policy 2015, 51, 13–20. [Google Scholar] [CrossRef]
- Phillips, K.; Niimi, J.; Hamid, N.; Silcock, P.; Bremer, P. Sensory and volatile analysis of sea urchin roe from different geographical regions in New Zealand. LWT-Food Sci. Technol. 2010, 43, 202–213. [Google Scholar] [CrossRef]
- Paredes, E.; Costas, D. Non-lethal sex identification of sea urchins: Method and advantages. Lab Anim. 2020, 49, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, J.; Sun, Z.; Liu, B.; Zhao, C.; Chang, Y. Identification of Sex-Specific Markers Through 2b-RAD Sequencing in the Sea Urchin (Mesocentrotus nudus). Front. Genet. 2021, 12, 717538. [Google Scholar] [CrossRef] [PubMed]
- Betancourt-Arango, J.P.; Villaroel-Solis, E.E.; Fiscal-Ladino, J.A.; Taborda-Ocampo, G. Volatilomics: An emerging discipline within Omics Sciences—A systematic review. F1000Research 2024, 13, 991. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Cheli, F.; Chiofalo, V. Fusion of electronic nose, electronic tongue and computer vision for animal source food authentication and quality assessment—A review. J. Food Eng. 2017, 210, 62–75. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; López-Pedrouso, M.; Gagaoua, M.; Lorenzo, J.M. Foodomics in meat quality. Curr. Opin. Food Sci. 2021, 38, 79–85. [Google Scholar] [CrossRef]
- Brattoli, M.; De Gennaro, G.; De Pinto, V.; Demarinis Loiotile, A.; Lovascio, S.; Penza, M. Odour Detection Methods: Olfactometry and Chemical Sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef]
- Wang, X.; Rogers, K.M.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC–MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef]
- Acquaticci, L.; Angeloni, S.; Baldassarri, C.; Sagratini, G.; Vittori, S.; Torregiani, E.; Petrelli, R.; Caprioli, G. A new HS-SPME-GC-MS analytical method to identify and quantify compounds responsible for changes in the volatile profile in five types of meat products during aerobic storage at 4 °C. Food Res. Int. 2024, 187, 114398. [Google Scholar] [CrossRef]
- Epping, R.; O’Sullivan, M.; Higgins, S.; Cremonesi, M.; Voilley, A.; Cayot, N. Changes in Tuber melanosporum Aroma during Storage: SPME-GC-MS Versus Sensory. Foods 2024, 13, 837. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Vittori, S.; Caprioli, G. An Overview on Truffle Aroma and Main Volatile Compounds. Molecules 2020, 25, 5948. [Google Scholar] [CrossRef]
- Gloaguen, Y.; Riu-Boni, M.; Stark, T.; Schieberle, P. Characterization of the Volatilome of Tuber canaliculatum by HS-SPME-GC-MS. ACS Omega 2022, 7, 26534–26544. [Google Scholar] [CrossRef]
- Brundu, G.; Cannavacciuolo, A.; Nannini, M.; Somma, E.; Munari, M.; Zupo, V.; Farina, S. Development of an efficient, noninvasive method for identifying gender year-round in the sea urchin Paracentrotus lividus. Aquaculture 2023, 564, 739082. [Google Scholar] [CrossRef]
- Takagi, S.; Sato, Y.; Kokubun, A.; Inomata, E.; Agatsuma, Y. Odor-active compounds from the gonads of Mesocentrotus nudus sea urchins fed Saccharina japonica kelp. PLoS ONE 2020, 15, e0231673. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal Chim Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Peng, S.L.; Bi, Y.M.; Shan, P.; Hu, X.Y. A New Method Combining LDA and PLS for Dimension Reduction. PLoS ONE 2014, 9, e96944. [Google Scholar] [CrossRef]
- Wen, Z.C.; Sun, T.; Geng, X.; Liu, M.H. Discrimination of Pressed and Extracted Camellia Oils by Vis/NIR Spectra Combined with UVE-PLS-LDA. Spectrosc. Spectr. Anal. 2013, 33, 2354–2358. [Google Scholar] [CrossRef]
- Niimi, J.; Leus, M.; Silcock, P.; Hamid, N.; Bremer, P. Characterisation of odour active volatile compounds of New Zealand sea urchin (Evechinus chloroticus) roe using GC-O-FSCM. Food Chem. 2010, 121, 601–607. [Google Scholar] [CrossRef]
- Su, T.; Zhang, G.; Zhao, Y.; Zhang, J.; Shang, X.; Shen, Q.; Hua, S.; Zhu, X. The Biosynthesis of 1-octen-3-ol by a Multifunctional Fatty Acid Dioxygenase and Hydroperoxide Lyase in Agaricus bisporus. J. Fungi 2022, 8, 827. [Google Scholar] [CrossRef]
- Morawicki, R.O.; Beelman, R.B. Study of the Biosynthesis of 1-Octen-3-ol Using a Crude Homogenate of Agaricus bisporus in a Bioreactor. J. Food Sci. 2008, 73, C135–C139. [Google Scholar] [CrossRef]
- Sato, Y.; Takagi, S.; Inomata, E.; Agatsuma, Y. Odor-active volatile compounds from the gonads of the sea urchin Mesocentrotus nudus in the wild in Miyagi Prefecture, Tohoku, Japan. Food Nutr. Sci. 2019, 10, 860–875. [Google Scholar] [CrossRef]
- Rodríguez-Bernaldo de Quirós, A.; López-Hernández, J.; González-Castro, M.J.; de la Cruz-García, C.; Simal-Lozano, J. Comparison of volatile components in fresh and canned sea urchin (Paracentrotus lividus, Lamarck) gonads by GC–MS using dynamic headspace sampling and microwave desorption. Eur. Food Res. Technol. 2001, 212, 643–647. [Google Scholar] [CrossRef]
- Hughes, A.D.; Kelly, M.S.; Barnes, D.K.A.; Catarino, A.I.; Black, K.D. The dual functions of sea urchin gonads are reflected in the temporal variations of their biochemistry. Mar. Biol. 2006, 148, 789–798. [Google Scholar] [CrossRef]
- Anedda, R.; Siliani, S.; Melis, R.; Loi, B.; Baroli, M. Lipid metabolism of sea urchin Paracentrotus lividus in two contrasting natural habitats. Sci. Rep. 2021, 11, 14174. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, D.; Shen, Y.; Gao, H.; Yao, X. Mapping gaseous dimethylamine, trimethylamine, ammonia, and their particulate counterparts in marine atmospheres of China’s marginal seas—Part 2: Spatiotemporal heterogeneity, causes, and hypothesis. Atmos. Chem. Phys. 2022, 22, 1515–1528. [Google Scholar] [CrossRef]
- Arafa, S.; Chouaibi, M.; Sadok, S.; El Abed, A. The influence of season on the gonad index and biochemical composition of the sea urchin Paracentrotus lividus from the Golf of Tunis. Sci. World J. 2012, 2012, 815935. [Google Scholar] [CrossRef]
- Machado, A.M.; Fernández-Boo, S.; Nande, M.; Pinto, R.; Costas, B.; Castro, L.F.C. The male and female gonad transcriptome of the edible sea urchin, Paracentrotus lividus: Identification of sex-related and lipid biosynthesis genes. Aquac. Rep. 2022, 22, 100936. [Google Scholar] [CrossRef]
- Martínez, A.; Abanto, M.; Días, N.B.; Olate, P.; Pérez Nuñez, I.; Díaz, R.; Sepúlveda, N.; Paz, E.A.; Quiñones, J. Recent Trends in Food Quality and Authentication: The Role of Omics Technologies in Dairy and Meat Production. Int. J. Mol. Sci. 2025, 26, 4405. [Google Scholar] [CrossRef]
- Cozzolino, D. The role of vibrational spectroscopy as a tool to assess economically motivated fraud and counterfeit issues in agricultural products and foods. Anal. Methods 2015, 7, 9390–9400. [Google Scholar] [CrossRef]
- Banton, M.I.; Bus, J.S.; Collins, J.J.; Delzell, E.; Gelbke, H.P.; Kester, J.E.; Moore, M.M.; Waites, R.; Sarang, S.S. Evaluation of potential health effects associated with occupational and environmental exposure to styrene—An update. J. Toxicol. Environ. Health Part B 2019, 22, 1–130. [Google Scholar] [CrossRef]
- Choi, H.; Hwang, U.-K.; Lee, M.; Kim, Y.-J.; Han, T. Evaluating Toxic Interactions of Polystyrene Microplastics with Hazardous and Noxious Substances Using the Early Life Stages of the Marine Bivalve Crassostrea gigas. Nanomaterials 2025, 15, 349. [Google Scholar] [CrossRef]
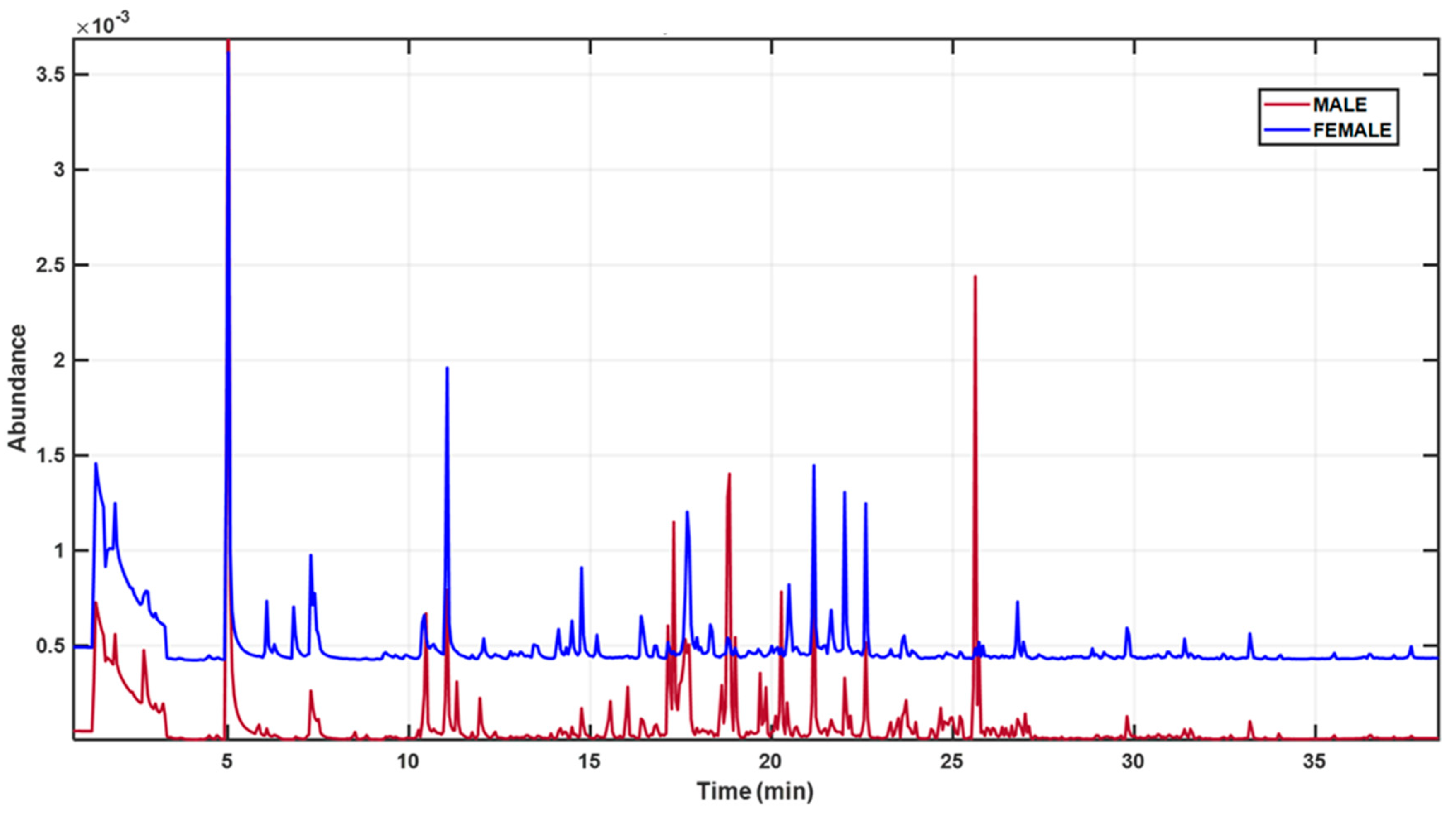
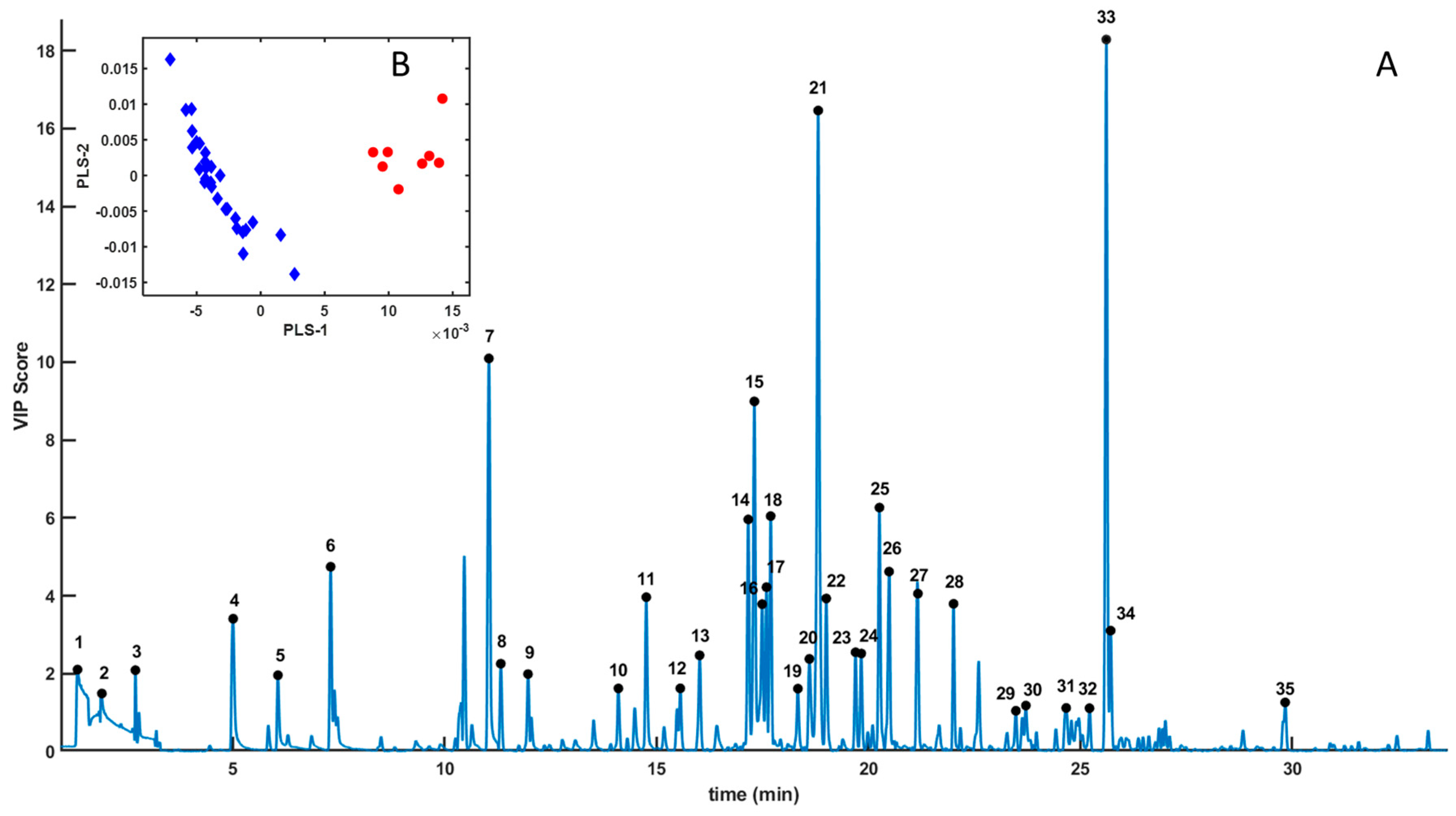

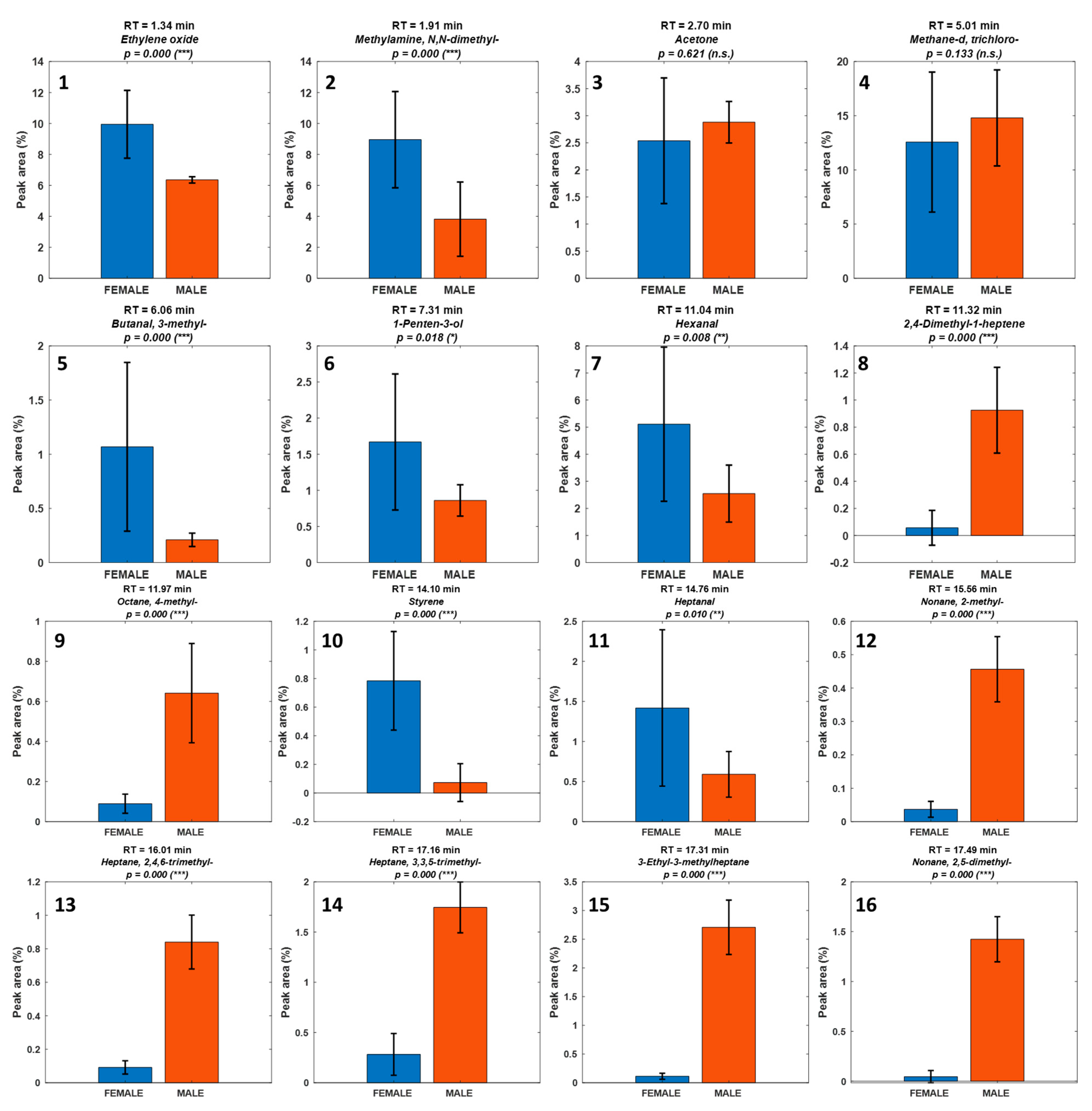
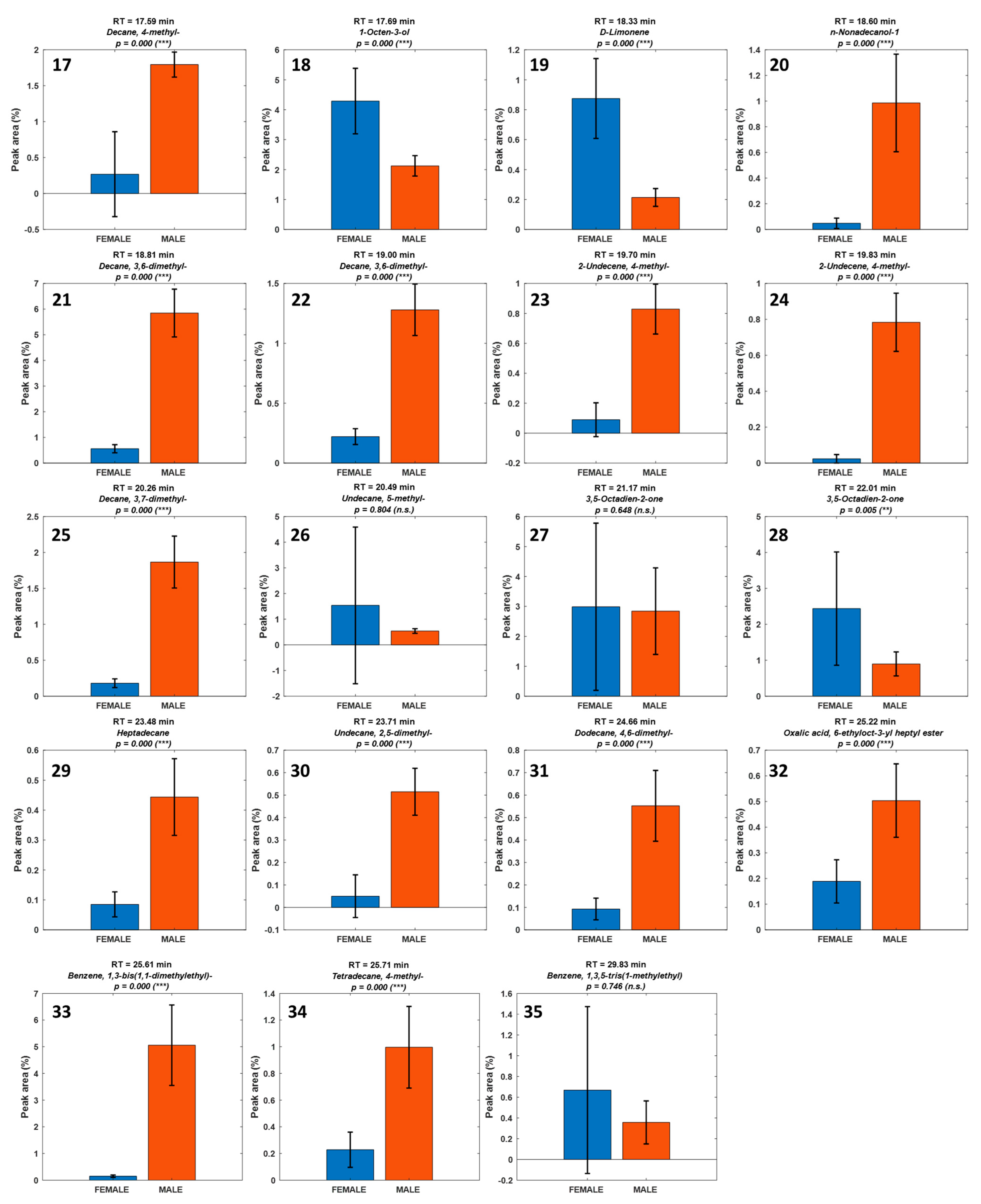
| Number | RT (min) | Compound | Chemical Class | SourcPutative Source e |
|---|---|---|---|---|
| 1 | 1.34 | Ethylene oxide | Epoxide | Environmental/artifact |
| 2 | 1.91 | N,N-Dimethyl-methylamine | Amine | Biogenic amine/degradation |
| 3 | 2.70 | Acetone | Ketone | Uncertain |
| 4 | 5.01 | Trichloro-methane-d | Halogenated solvent | Environmental/artifact |
| 5 | 6.06 | 3-Methyl-butanal | Aldehyde | Biogenic (PUFA oxidation, LOX/HPL) |
| 6 | 7.31 | 1-Penten-3-ol | Alcohol | Biogenic (PUFA oxidation, LOX/HPL) |
| 7 | 11.04 | Hexanal | Aldehyde | Biogenic (PUFA oxidation, LOX/HPL) |
| 8 | 11.32 | 2,4-Dimethyl-1-heptene | Alkene | Biogenic (low odor impact) |
| 9 | 11.97 | 4-Methyl-octane | Other | Uncertain |
| 10 | 14.10 | Styrene | Aromatic hydrocarbon | Environmental/artifact |
| 11 | 14.76 | Heptanal | Alkane | Biogenic (low odor impact) |
| 12 | 15.56 | 2-Methyl-nonane | Aromatic hydrocarbon | Environmental (possible packaging/ambient) |
| 13 | 16.01 | 2,4,6-Trimethyl-heptane | Alkane | Biogenic (low odor impact) |
| 14 | 17.16 | 3,3,5-Trimethyl-heptane | Alkane | Biogenic (low odor impact) |
| 15 | 17.31 | 3-Ethyl-3-methylheptane | Monoterpene | Diet-derived (macroalgae)/biogenic |
| 16 | 17.49 | 2,5-Dimethyl-nonane | Alkane | Biogenic (low odor impact) |
| 17 | 17.59 | 4-Methyl-decane | Alkane | Biogenic (low odor impact) |
| 18 | 17.69 | 1-Octen-3-ol | Aldehyde | Biogenic (PUFA oxidation, LOX/HPL) |
| 19 | 18.33 | D-Limonene | Alkane | Biogenic (low odor impact) |
| 20 | 18.60 | n-Nonadecanol-1 | Alkane | Biogenic (low odor impact) |
| 21 | 18.81 | 3,6-Dimethyl-decane | Alkane | Biogenic (low odor impact) |
| 22 | 19.00 | 3,6-Dimethyl-decane | Alkene | Biogenic (low odor impact) |
| 23 | 19.70 | 4-Methyl-2-undecene | Alkene | Biogenic (low odor impact) |
| 24 | 19.83 | 4-Methyl-2-undecene | Alkane | Biogenic (low odor impact) |
| 25 | 20.26 | 3,7-Dimethyl-decane | Ketone | Biogenic (PUFA oxidation, LOX/HPL) |
| 26 | 20.49 | 5-Methyl-undecane | Ketone | Biogenic (PUFA oxidation, LOX/HPL) |
| 27 | 21.17 | 3,5-Octadien-2-one | Alkane | Biogenic (low odor impact) |
| 28 | 22.01 | 3,5-Octadien-2-one | Alkane | Biogenic (low odor impact) |
| 29 | 23.48 | Heptadecane | Alkane | Biogenic (low odor impact) |
| 30 | 23.71 | 2,5-Dimethyl-undecane | Alkane | Biogenic (low odor impact) |
| 31 | 24.66 | 4,6-Dimethyl-dodecane | Alkane | Biogenic (low odor impact) |
| 32 | 25.22 | 6-Ethyloct-3-yl heptyl ester oxalic acid | Ester | Uncertain (low-volatility ester unlikely in HS-SPME; keep tentative) |
| 33 | 25.61 | 1,3-Bis(1,1-dimethylethyl)-benzene | Aromatic hydrocarbon | Environmental/artifact |
| 34 | 25.71 | 4-Methyl-tetradecane | Alkane | Biogenic (low odor impact) |
| 35 | 29.83 | 1,3,5-Tris(1-methylethyl) benzene | Aromatic hydrocarbon | Environmental/artifact |
| Attribute | Female Gonads | Male Gonads |
|---|---|---|
| Dominant Chemical Families | Terpenes, Aldehydes, Alcohols, Ketones, Amines | Saturated and Branched Hydrocarbons, Long-chain Alcohols, Aromatic Hydrocarbons |
| Key Compounds | D-Limonene, Hexanal, Heptanal, 1-Penten-3-ol, 1-Octen-3-ol, 3-Methyl-butanal | 3,6-Dimethyldecane, 3-Ethyl-3-methylheptane, Heptadecane, n-Nonadecanol-1 |
| Sensory Attributes | Sweet, fruity, citrus, green-herbaceous, earthy-mushroom, marine freshness | Neutral, mild, smooth; negligible aroma intensity |
| Aromatic Complexity | High—Presence of low-threshold oxygenated volatiles and terpenes | Low—Dominance of high-threshold saturated hydrocarbons |
| Lipid Metabolic Activity Indicator | High lipid turnover and oxidative activity associated with reproductive phase | Stable lipid matrix, lower oxidative activity |
| Dietary Influence Markers | Presence of diet-derived terpenoids (e.g., D-limonene) | Minimal or absent; no terpenoid accumulation |
| Spoilage/Degradation Markers | Controlled levels of amines (e.g., N,N-dimethyl-methylamine) linked to freshness perception | Not detected; no significant spoilage-related VOCs |
| Environmental Contamination Indicators | Styrene (low levels, potential packaging or environmental marker) | 1,3-Bis(1,1-dimethylethyl)-benzene (potential environmental marker) |
| Organoleptic Profile Summary | Rich, sweet, fruity-marine aroma with complex aromatic bouquet | Mild, bland, structurally neutral flavor, lacking fruity or marine aromatic complexity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibanco-Cañete, R.; Carbonell-Garzón, E.; Sanchez-Jerez, P.; Marhuenda Egea, F.C. Identification of Sex-Dependent Aroma Compounds in Gonads of Commercially Valuable Sea Urchins: Implications for Gastronomical Use of Paracentrotus lividus. J. Mar. Sci. Eng. 2025, 13, 2160. https://doi.org/10.3390/jmse13112160
Ibanco-Cañete R, Carbonell-Garzón E, Sanchez-Jerez P, Marhuenda Egea FC. Identification of Sex-Dependent Aroma Compounds in Gonads of Commercially Valuable Sea Urchins: Implications for Gastronomical Use of Paracentrotus lividus. Journal of Marine Science and Engineering. 2025; 13(11):2160. https://doi.org/10.3390/jmse13112160
Chicago/Turabian StyleIbanco-Cañete, Ricardo, Estela Carbonell-Garzón, Pablo Sanchez-Jerez, and Frutos C. Marhuenda Egea. 2025. "Identification of Sex-Dependent Aroma Compounds in Gonads of Commercially Valuable Sea Urchins: Implications for Gastronomical Use of Paracentrotus lividus" Journal of Marine Science and Engineering 13, no. 11: 2160. https://doi.org/10.3390/jmse13112160
APA StyleIbanco-Cañete, R., Carbonell-Garzón, E., Sanchez-Jerez, P., & Marhuenda Egea, F. C. (2025). Identification of Sex-Dependent Aroma Compounds in Gonads of Commercially Valuable Sea Urchins: Implications for Gastronomical Use of Paracentrotus lividus. Journal of Marine Science and Engineering, 13(11), 2160. https://doi.org/10.3390/jmse13112160






