Abstract
Artificial light at night (ALAN) is impacting sea turtle nesting around the globe by decreasing the nesting attempts, disorienting the sea turtle hatchlings while trying to find the sea, and disrupting hatchlings’ offshore migration. In the Mediterranean Sea, the shoreline of Kyparissia Bay is considered the most important nesting site for loggerhead sea turtles (Caretta caretta), with several thousand nests on an annual basis. The current study reports for the first time the exposure of the core 10 km of nesting area to ALAN pollution, evaluates the potential impact on sea turtle conservation, and discusses mitigation measures as coastal urbanization and touristic activity increase rapidly in the region. Our findings demonstrate that most of the core area was not impacted by ALAN pollution, although a specific region (Kalo Nero) was subjected to high illumination at night, leading to reduced sea turtle nests and potential threats to hatchlings.
1. Introduction
Artificial light at night (ALAN), the change in nocturnal light levels due to anthropogenic infrastructure, is considered a major threat to humans and several types of marine and terrestrial organisms [1,2,3]. More than 20% of our planet is exposed to this form of pollution, with an annual increase ranging from 2 to 10% [4].
In the coastal environment, more than 20% of our planet’s marine protected areas (MPAs) and general coastal areas are subject to ALAN pollution, a number that continues to increase due to increased coastal development [5,6,7]. This affects the organisms that use the coastal and marine habitat for foraging, nesting, resting, and reproduction (such as marine mammals, seabirds, fish, and sea turtles) [8]. Several studies have shown the impacts of ALAN on the behavior of organisms that use coastal habitats (e.g., [2,9,10,11,12,13]). The impacts for both marine and terrestrial organisms that inhabit the coastal environment discussed in these studies are most commonly fitness reduction, population dynamics, disorientation, behavior, increased risk of predation, and disruption of foraging and reproduction [14,15,16].
Sea turtles exhibit a complex life history, with distinct stages occurring in separate habitats and geographic regions on broad spatial scales [17]. In the weeks preceding the nesting season, adult males and females gather near breeding grounds to mate [18], after which males leave the area [19]. During the nesting season, females remain in near-shore waters and return to beaches near their natal sites to deposit eggs, often decades after hatching. Incubation typically lasts around two months, after which hatchlings emerge from the nest and engage in a brief “swimming frenzy” to reach deeper offshore waters [20]. The sea-finding process in hatchlings relies on visual cues, guiding them toward the brightest and lowest point on the horizon [21]. The overlap of the nesting season (May–September) with peak tourist activity in the Mediterranean poses considerable conservation challenges for sea turtles.
Sea turtles around the globe are subjected to several impacts by natural and/or indirect anthropogenic causes, such as the loss of nesting grounds from sea level rise and beach erosion, decreased hatchling success, storm severity, nest feminization due to higher temperatures, reduced food availability, and the expansion of diseases due to climate change [22,23,24,25,26,27,28,29]. Considering the anthropogenic impact, sea turtles are facing bycatch, habitat loss due to touristic development, boat collisions, touristic pressure, and plastic and microplastic pollution, among other threats [26,30,31,32,33,34,35,36,37]. Several studies have shown the negative impact of light pollution on hatchlings’ sea-finding orientation and the unwillingness of sea turtles to choose a highly illuminated nesting beach [10,38,39]. The most important negative effects of ALAN on sea turtles include the influence on nesting site selection, the decrease in nesting attempts, the increased predation risk for sea turtle hatchlings, the disruption of the sea-finding orientation of the hatchlings, the disturbance of hatchlings’ swimming behavior and offshore migration capacity, as well as the decreased reproductive success [21,39,40,41,42,43]. Moreover, as nighttime darkness becomes less common on nesting beaches, this could lead to increased nesting density, causing the destruction of other nests and an increase in hatchling predation and reduced fitness [44,45]. Changing the nesting location due to ALAN impacts may lead hatchlings to be born away from favourable oceanographic features and currents that assist their oceanic dispersal and conservation [46,47]. Thus, ALAN may critically undermine the successful recruitment of sea turtle populations [48].
In the current study, we investigated the exposure of sea turtle nests to ALAN in Kyparissia Bay, the most important nesting area in the Mediterranean for loggerhead sea turtles (Caretta caretta), based on the high number of nests recorded annually [49]. To achieve this, we analyzed the spatial distribution of sea turtle nests and their exposure to varying levels of light pollution within the 10 km area of the core nesting activity. The goal of our study was to achieve a deeper understanding of light pollution, its evolving trends, and potential future impacts during growing coastal development and tourism activities. Potential conservation measures aimed at safeguarding population recruitment under the influence of ALAN are discussed.
2. Methods
2.1. Study Site
Kyparissia Bay is an EU Natura 2000 site located on the western coast of central Peloponnese (Ionian Sea). Kyparissia Bay falls within the boundaries of three NATURA 2000 sites—one marine (GR2330008) and two terrestrial (GR2330005 and GR2550005)—managed under the authority of the Natural Environment and Climate Change Agency (NECCA) and the relevant Management Unit of the Strofylia Wetlands National Park and Protected Areas of Western Peloponnese (https://necca.gov.gr/en/protected-areas-management-units/, (accessed on 10 September 2025)) [50]. This area currently hosts the largest reproductive population across the Mediterranean for loggerhead sea turtles, with more than 7500 nests during 2024 [49]. The sandy coastline consists of about 44 km of almost continuous beach. Although sea turtles nest along the entire coast, the core nesting area is concentrated in the southernmost 10 km, which accounts for over 70% of all laid nests [49] (Figure 1).
Figure 1.
(a) A map of the Eastern Mediterranean region with (b) the location of the study area.
2.2. UAV Survey
The authors performed a detailed Unmanned Aerial Vehicle (UAV) survey and mapping of the entire core nesting area of 10 km coastline to identify and record sea turtle nests (based on ARCHELON’s signs (the sea turtle protection society of Greece), cages, and poles on the beach). The survey occurred with the use of DJI’s Air2S drone, DJI, 2018, Shenzhen, China with 5.4 K photo capability (5472 × 3648 pixels) with GPS location embedded in each photo. The flight time was approximately 2.5 h from 09:30 a.m. to 12:00 p.m. on the 23 July 2025. A total of 178 photos were taken along the entire area at an altitude of 60 m with the camera at nadir (direct downward direction), which gave an approximate width of 120 m, a few meters of overlap so that we were able to put the images in the correct order, and allowed for the detection of sea turtle nests and other features in each photo. During the selected survey period, all human infrastructure and tourism activities were fully operational and at their peak. This approach aimed to capture an indication of the beach’s maximum potential illumination.
2.3. Light Pollution Measurements
The light meter survey was carried out to assess the level of the different lights at night. The measurements included the intensity of light coverage from artificial lights, using a portable digital light meter (iNGCO HETLU01, INGCO, 2006, Suzhou, China 0–200,000 Lux), with a sensitivity of 0.1 Lux (luminous flux per unit area), and a Garmin eTrex GPS, 2015, Olathe, KS, USA. Measurements below 0.1 Lux were recorded as 0 Lux owing to the sensitivity limitations of the light meter. Consequently, very low illumination levels may be present but remain undetectable with the instrumentation used. Given that hatchlings are oriented by using a visual ‘cone of acceptance’ from 0 to 30 degrees in the vertical level, light intensity at each sampling station was measured at an elevation of 15 degrees from the beach surface [21]. The light meter survey was conducted in the absence of cloud cover on a moonless night (24 July 2025) between 23:00 and 00:00 to eliminate interference from natural light sources and cloud albedo [51]. Light intensity was measured at stations placed at 10–40 m intervals near areas of intense illumination and at 100–400 m intervals in undeveloped coastal zones across the study area, based on the presence of light sources. Three replicate readings were obtained per station, and the average value was retained for analysis [8]. A total of 87 measurements were recorded at 29 stations, accompanied by the documentation of other beach characteristics such as backshore vegetation, presence of buildings, beach furniture, and light emission sources visible from the beach.
Furthermore, the authors collected and analyzed VIIRS/DMSP data (data from sensors onboard the satellites from the Defense Meteorology Satellite Program (DMSP) and the Visible Infrared Imaging Radiometer Suite (VIIRS)) from the study area over the past decade, to identify trends in time for the entire region and for selected areas, and to establish the reasoning for the sampling effort (source: https://www.lightpollutionmap.info, accessed on 9 September 2025) [52].
2.4. Data Analysis
The drone images were analyzed using MATLAB 2021a to detect and extract the location of sea turtle nests based on the signs of ARCHELON and other features [53]. It is important to acknowledge that this method offers only an approximation, rather than an exact count, of nest numbers and their spatial distribution. Thematic maps were created by integrating geospatial data on luminance, topography, and nest locations to depict high-exposure areas. Geographic information systems (GIS) software was used to analyze and visualize both sea turtle nesting patterns and artificial light pollution within the core 10 km nesting area of Kyparissia Bay. The spatial data layers generated during field surveys, including UAV-derived nest locations and ground-based measurements of light pollution, were processed and visualized using ArcGIS (Esri, ArcGIS Desktop: Release 10.4.1; Environmental Systems Research Institute: Redlands, CA, USA, 2015).
For the mapping of sea turtle nests, the GPS coordinates extracted from the UAV aerial images were georeferenced and overlaid on high-resolution satellite imagery (Figure 2). Kernel Density Estimation (KDE) was applied to visualize areas of high and low nesting density, facilitating the identification of critical nesting hotspots along the coastline (Figure 3) [54]. The Kernel Density tool in ArcMap 10.4.1 (Esri, Redlands, CA, USA) was used to calculate nest density. A default cell size of 10 m and a search radius of 100 m were applied to visualize spatial clustering patterns along the 10 km coastline.
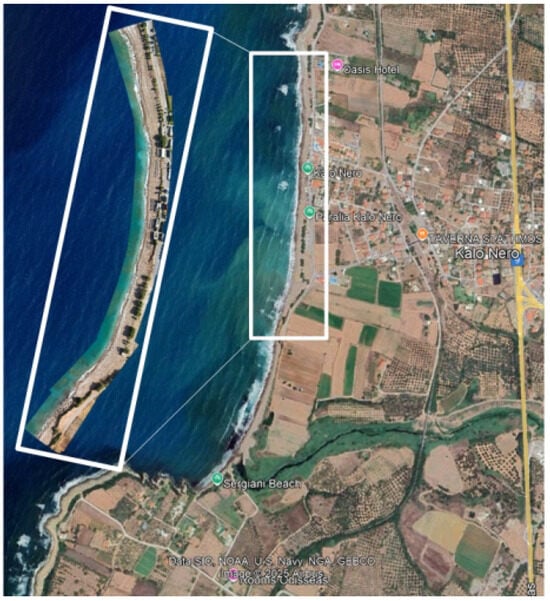
Figure 2.
Reconstruction of the coast of Kalo Nero, Greece, using images from the drone survey.
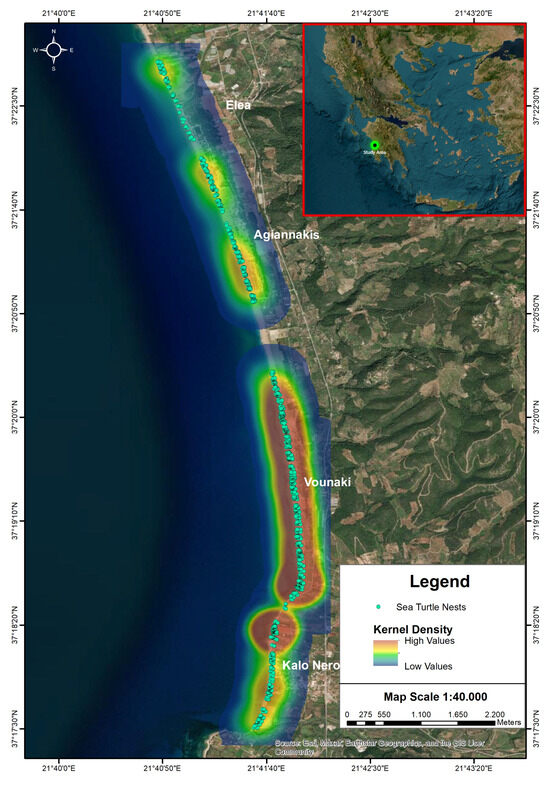
Figure 3.
Sea turtle nest distribution and kernel density in Kyparissia Bay, Greece. The map displays the spatial distribution of sea turtle nest approximations recorded during the study in the Kyparissia Bay. Kernel Density Estimation (KDE) is applied to visualize areas with high (red) and low (green) nest densities along the coastline. Key coastal locations such as Elea, Agiannakis, Vounaki, and Kalo Nero are indicated for reference.
Regarding light pollution, field measurements in lumens were georeferenced and interpolated using the Inverse Distance Weighting (IDW) method to visualize the spatial extent and intensity of artificial illumination across the study area [54]. The IDW method was selected for the interpolation of light pollution data because it provides a straightforward and reliable way to estimate illumination intensity across areas where direct measurements were not possible [55]. The default power parameter (p = 2) and a cell size of 10 m were applied, and the search radius was set to the 12 nearest points. IDW assumes that values measured closer to a given location exert a stronger influence than those farther away, making it particularly suitable for environmental studies with spatially clustered field data, such as light intensity measurements along a coastline. This approach ensures that the resulting spatial distribution map reflects the gradual decay of artificial light with distance from its sources, thereby producing a realistic visualization of illumination patterns [56,57]. Particular focus was given to the Kalo Nero settlement, where elevated levels of light pollution were recorded. An inset map was developed for this area to present the detailed spatial distribution of luminance values. Measurements for the rest of the coastline confirmed near-zero illumination. Additionally, spatial correlation analysis enabled the understanding of the relationship between light pollution and nesting patterns, supporting targeted management measures. The core nesting area was divided into five segments, each measuring 2000 m (Kalo Nero, Vounaki, Agiannakis, Elea, and the area between Kalo Nero and Vounaki), to evaluate whether the Lux intensity and the number of nests vary significantly in space. Then we performed a non-parametric Kruskal–Wallis test (followed by a pair-wise comparison) to check if the number of nests and light intensity levels differed significantly among different segments.
3. Results
The drone survey allowed the authors to analyze the entire coast between Kalo Nero and Elea in the Kyparissia Bay and evaluate the sea turtle nests and the location of lights that may pollute the nesting beach (Figure 2). Image analysis showed at least 2000 nests along the study area. The highest nest density was recorded on the beach sections adjacent to Kalo Nero and around Vounaki, along a stretch of approximately 2.5 km (Figure 3). Additional areas of moderate nest density were identified south of Agiannakis and Elea. According to the findings of the IDW method, light substantially affecting the nesting area was detected only on the coastline of the Kalo Nero village near the local restaurants and bars located above the nesting beach (Figure 4, Table 1). The nesting area most exposed to light pollution was located between sampling stations M2 and M15, with an average illumination level of 2 Lux (±0.8 SE, ranging between 0.1 and 11.1 Lux (Figure 5). The highest levels of light pollution were recorded at stations M13 and M3, in front of taverns, where the light sources were directly visible from the beach (Figure 4). In contrast, street lighting was not directly visible from the beach, as the road running parallel to the shore is elevated approximately 2–3 m above beach level, preventing direct line-of-sight exposure. Light intensity exhibited very low values for the rest of the study area (Table 1). There were significantly lower numbers of sea turtle nests in beach segments with higher light intensity (SS = 62.5, dF = 1, MS = 62.5, = 7.26, p = 0.0071 in the reported cases).

Figure 4.
A photo above the village of Kalo Nero at 23.00 as taken by the drone at an altitude of 50 m. The location and intensity of the light pollution measurements are shown on the left side of the image.

Table 1.
Light intensity measurements per sampling station along the coast of Kyparissia Bay, Greece. See Figure 5 for sampling station locations.
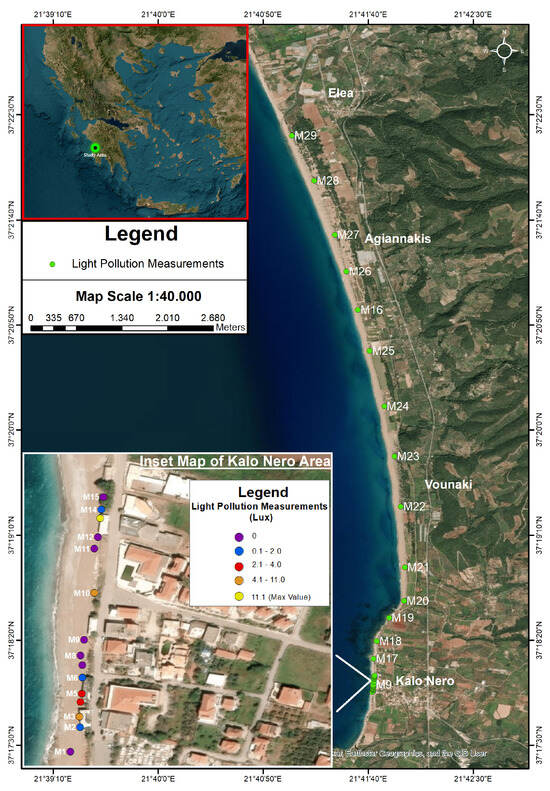
Figure 5.
Measurements of light pollution along Kyparissia Bay. The map displays light pollution measurements (in Lux) along the coastline. The inset map focuses on the Kalo Nero area, where elevated light pollution values were recorded, whereas the remaining beach areas exhibit near-zero values (see Table 1).
Radiance data from 2014 to 2024 demonstrated that the Kalo Nero region has experienced levels of light pollution several times higher than those recorded in the Vounaki, Elea, and Agiannaki areas combined, throughout the year (Figure 6). Considering the average radiance measurements for the summer months over the past decade, the region has experienced a negligible decline in the light pollution trend, which is most probably associated with the low values observed between 2019 and 2021 (the COVID-19 pandemic epicenter). Comparing the summer months (June, July, and August), it was determined that July was the month of higher radiance levels for 2022–2024, justifying using this month in the current sampling efforts (Figure 7).
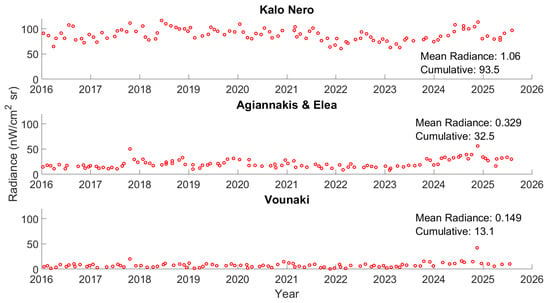
Figure 6.
The last decade’s monthly radiance measurements from the VIIRS/DMSP dataset for the three different subsections within the study area. Data were extracted from https://www.lightpollutionmap.info, (accessed on 10 September 2025).
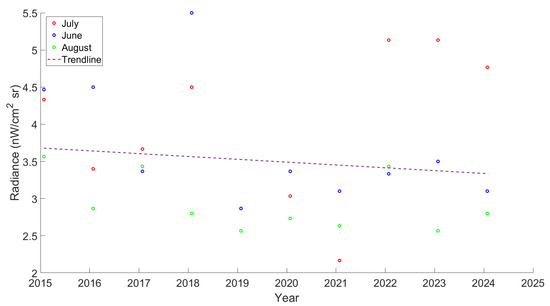
Figure 7.
Average measurements of radiance for the study area during the summer months from the VIIRS/DMSP dataset. Data were acquired from https://lighttrends.lightpollutionmap.info/, (accessed on 10 September 2025).
4. Discussion
The shoreline of Kyparissia Bay exhibits a high illumination only at a specific part of the nesting area, specifically the Kalo Nero (Figure 4). As shown in comparable studies (Table 2), the measured light intensity in Kyparissia (ranging from 0.01 to 11 Lux, with an average of 3.5 Lux) can be deemed as moderate locally in the Kalo Nero area and very low throughout the remainder of the study area. This level of illumination may have contributed to the reduced nesting density of sea turtles observed in Kalo Nero compared with that in adjacent beach segments. However, studies have shown that light intensity levels similar to those measured at Kalo Nero can cause hatchling disorientation and deter female adults from attempting to nest [8,10,58]. Besides light pollution, female adults may be driven away due to tourist activity, the occurrence of humans at the beach, and the presence of sunbeds and umbrellas [50].

Table 2.
ALAN measurements in sea turtle studies around the globe.
Apart from Kalo Nero, the rest of the 10 km nesting beach exhibited no measurable evidence of light pollution, thereby supporting suitable nesting conditions and facilitating an undisturbed sea-finding process for hatchlings. However, as noticed in the past 20 years, coastal development is increasing, and the areas near Vounaki, Agiannaki, and Elea are experiencing rapid urbanization. According to the local NGOs that protect and conserve the area, several houses and other infrastructure have been built over the past 2–3 years, which causes concerns regarding the possibility of experiencing higher levels of light pollution at night. Hence, there is an extremely important need to define the best practices for managing light pollution along the coast of Kyparissia Bay.
There are a number of methods used to prevent, manage, and mitigate ALAN pollution [64,65,66,67]. The most prominent ones are (i) keeping light sources at an appropriate distance from the beach to be practical but also not overilluminating the area with all lights consistently directed downward, (ii) removing unnecessary light sources, (iii) adjusting the light sources’ intensities to the necessary levels, (iv) using automated switches based on the movement of vehicles/people on the road at night, (v) replacing older types of light-emitting sources with newer and environmentally friendly ones (long wavelength lights), (vi) adjusting the direction of the beam at each one of the light sources near the nesting area to face away from the beach, (vii) designing the lighting of roads near the nesting beach to be targeted and not illuminating the nesting beach, (viii) finding ways to shield the nesting beach from the light sources (e.g., vegetation fence), (ix) the protection and establishment of sand dune systems and beach vegetation to reduce the impact of lights, and (x) raising awareness and informing the local owners of beach houses and tourism infrastructure about the best practices to mitigate ALAN impacts [10,52,66,68,69,70,71,72].
In the case of the Kyparissia Bay, most of the aforementioned management methods are urgently needed near the Kalo Nero where the higher intensity of light pollution was recorded, while the increasing urbanization of the surrounding areas of Vounaki and Agiannakis shows that a management plan is needed for the protection and conservation of sea turtle nesting in the most important nesting area of the entire Mediterranean region. Continuous monitoring efforts for the occurrence of and increase in ALAN in this area are necessary, and local authorities should urgently take further measures to protect sea turtles in Kalo Nero.
Considering the limitations of the present study, the short sampling time frame (one summer) does not reflect variations in nesting behavior or how it was affected by light pollution over time. However, it does provide a reference for the exposure of sea turtles to ALAN pollution and can be used as a preliminary dataset for assessing its impact in the future. Additionally, the drone survey implemented in this study cannot detect all nests due to obstacles such as vegetation or human artifacts on the beaches. Nevertheless, this shortcoming applies to the entire coastline and does not affect the comparison between the different locations. It is also important to state that the nesting behavior is also affected by other factors, such as erosion, anthropogenic activities, and climate change, among others, and light pollution is one important parameter, but it does not solely affect sea turtle nesting in the region. Future research on the effects of ALAN in this critical rookery for the Mediterranean population of Caretta caretta should take into account changes in beach erosion, climate, and exploitation, and encompass additional aspects, including mapping and identifying visible light sources along the horizon, assessing night-glow sources, and quantifying hatchling mis–disorientation levels [8,41,70]. Further studies on the number of non-nesting emergences (i.e., false crawls) at the study area are necessary to assess the specific impact of these factors on nesting females. Furthermore, the Kalo Nero area’s hatchling disorientation should be evaluated in future studies, in order to evaluate the direct impact of light pollution.
5. Conclusions
In the current study, we assessed ALAN pollution along the coast of Kyparissa Bay, the most important sea turtle nesting area in the entire Mediterranean region. Our findings demonstrated that most nests are located away from the highly polluted area of Kalo Nero, a village with high anthropogenic pressure due to the presence of restaurants and bars above the nesting beach. The Kalo Nero nesting beach is subjected to severe illumination at night that reaches up to 11.1 Lux, which may disrupt the sea-finding orientation of sea turtle hatchlings and discourage nesting attempts for female adults. Concerning other locations, the increasing urbanization of the region has the potential to cause ALAN impacts in the entire nesting area, which holds several thousand nests each year and is essential to the species’ conservation and sustainability. Preserving the natural coastal environment of the Kyparissia Bay and ensuring the mitigation of ALAN pollution should be considered as a priority for local authorities and the management agency of the nesting beaches. In the future, monitoring the level of ALAN pollution and its impact on sea turtles is crucial for the protection of the species and the entire marine ecosystem.
Author Contributions
N.S.: Conceptualization, Methodology, Software, Resources, Investigation, Data Curation, Writing—Original Draft, Visualization, Formal analysis, Project administration. M.Z.V.: Data Curation, Write, Review and Editing. C.D.: Data Curation, Write, Review and Editing. O.N.: Data Curation. G.M.: Supervision, Methodology, Data Curation, Write, Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davies, T.W.; Smyth, T. Why artificial light at night should be a focus for global change research in the 21st century. Glob. Change Biol. 2018, 24, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Gaston, S.; Bennie, J.; Hopkins, J. Benefits and costs of artificial nighttime lighting of the environment. Environ. Rev. 2015, 23, 14–23. [Google Scholar] [CrossRef]
- Marangoni, L.F.; Davies, T.; Smyth, T.; Rodríguez, A.; Hamann, M.; Duarte, C.; Pendoley, K.; Berge, J.; Maggi, E.; Levy, O. Impacts of artificial light at night in marine ecosystems—A review. Glob. Change Biol. 2022, 28, 5346–5367. [Google Scholar] [CrossRef]
- Bará, S.; Castro-Torres, J.J. Diverging evolution of light pollution indicators: Can the globe at night and VIIRS-DNB measurements be reconciled? J. Quant. Spectrosc. Radiat. Transf. 2025, 335, 109378. [Google Scholar] [CrossRef]
- Pulgar, J.; Zeballos, D.; Vargas, J.; Aldana, M.; Manriquez, P.H.; Manriquez, K.; Quijón, P.A.; Widdicombe, S.; Anguita, C.; Quintanilla, D.; et al. Endogenous cycles, activity patterns and energy expenditure of an intertidal fish is modified by artificial light pollution at night (ALAN). Environ. Pollut. 2019, 244, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, A.; Hagenaars, G.; Ranasinghe, R.; Baart, F.; Donchyts, G.; Aarninkhof, S. The state of the world’s beaches. Sci. Rep. 2018, 8, 6641. [Google Scholar] [CrossRef]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. Stemming the tide of light pollution encroaching into marine protected areas. Conserv. Lett. 2016, 9, 164–171. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Fournari-Konstantinidou, I.; Sourbès, L.; Koutsoubas, D.; Mazaris, A.D. Reduction of sea turtle population recruitment caused by nightlight: Evidence from the Mediterranean region. Ocean Coast. Manag. 2018, 153, 108–115. [Google Scholar] [CrossRef]
- Rich, C.; Longcore, T. Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Di Bari, D.; Tiberti, C.; Mazzei, E.; Pagli, D. Light Pollution and Sea Turtles Nest-Site Selection. Is it Possible a Practical Management of the Problem? Eur. J. Sustain. Dev. 2023, 12, 35. [Google Scholar] [CrossRef]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 2014, 12, 347–355. [Google Scholar] [CrossRef]
- Becker, A.; Whitfield, A.K.; Cowley, P.D.; Järnegren, J.; Næsje, T.F. Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J. Appl. Ecol. 2013, 50, 43–50. [Google Scholar] [CrossRef]
- Luarte, T.; Bonta, C.; Silva-Rodriguez, E.; Quijón, P.; Miranda, C.; Farias, A.; Duarte, C. Light pollution reduces activity, food consumption and growth rates in a sandy beach invertebrate. Environ. Pollut. 2016, 218, 1147–1153. [Google Scholar] [CrossRef]
- Perry, G.; Fisher, R.N. Night lights and reptiles: Observed and potential effects. In Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006; pp. 169–191. [Google Scholar]
- Berge, J.; Geoffroy, M.; Daase, M.; Cottier, F.; Priou, P.; Cohen, J.H.; Johnsen, G.; McKee, D.; Kostakis, I.; Renaud, P.E.; et al. Artificial light during the polar night disrupts Arctic fish and zooplankton behaviour down to 200 m depth. Commun. Biol. 2020, 3, 102. [Google Scholar] [CrossRef]
- Gaston, K.J.; Ackermann, S.; Bennie, J.; Cox, D.T.; Phillips, B.B.; Sánchez de Miguel, A.; Sanders, D. Pervasiveness of biological impacts of artificial light at night. Integr. Comp. Biol. 2021, 61, 1098–1110. [Google Scholar] [CrossRef]
- Reece, J.S.; Castoe, T.A.; Parkinson, C.L. Historical perspectives on population genetics and conservation of three marine turtle species. Conserv. Genet. 2005, 6, 235–251. [Google Scholar] [CrossRef]
- Schofield, G.; Lilley, M.K.; Bishop, C.M.; Brown, P.; Katselidis, K.A.; Dimopoulos, P.; Pantis, J.D.; Hays, G.C. Conservation hotspots: Implications of intense spatial area use by breeding male and female loggerheads at the Mediterranean’s largest rookery. Endanger. Species Res. 2009, 10, 191–202. [Google Scholar] [CrossRef]
- Schofield, G.; Dimadi, A.; Fossette, S.; Katselidis, K.A.; Koutsoubas, D.; Lilley, M.K.; Luckman, A.; Pantis, J.D.; Karagouni, A.D.; Hays, G.C. Satellite tracking large numbers of individuals to infer population level dispersal and core areas for the protection of an endangered species. Divers. Distrib. 2013, 19, 834–844. [Google Scholar] [CrossRef]
- Bolten, A.B.; Lutz, P.; Musick, J.; Wyneken, J. Variation in sea turtle life history patterns: Neritic vs. oceanic developmental stages. Biol. Sea Turtles 2003, 2, 243–257. [Google Scholar]
- Limpus, C.; Kamrowski, R.L. Ocean-finding in marine turtles: The importance of low horizon elevation as an orientation cue. Behaviour 2013, 150, 863–893. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Karditsa, A.; Almpanidou, V.; Anastasatou, M.; Petrakis, S.; Poulos, S.; Koutsoubas, D.; Sourbes, L.; Mazaris, A.D. Sea level rise threatens critical nesting sites of charismatic marine turtles in the Mediterranean. Reg. Environ. Chang. 2022, 22, 56. [Google Scholar] [CrossRef]
- Pike, D.A. Forecasting the viability of sea turtle eggs in a warming world. Glob. Change Biol. 2014, 20, 7–15. [Google Scholar] [CrossRef]
- Salmon, M.; Hamann, M.; Wyneken, J.; Schauble, C. Early swimming activity of hatchling flatback sea turtles Natator depressus: A test of the ‘predation risk’hypothesis. Endanger. Species Res. 2009, 9, 41–47. [Google Scholar] [CrossRef]
- Polovina, J.J.; Balazs, G.H.; Howell, E.A.; Parker, D.M.; Seki, M.P.; Dutton, P.H. Forage and migration habitat of loggerhead (Caretta caretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish. Oceanogr. 2004, 13, 36–51. [Google Scholar] [CrossRef]
- Simantiris, N. The impact of climate change on sea turtles: Current knowledge, scientometrics, and mitigation strategies. Sci. Total Environ. 2024, 923, 171354. [Google Scholar] [CrossRef] [PubMed]
- Patrício, A.R.; Varela, M.R.; Barbosa, C.; Broderick, A.C.; Catry, P.; Hawkes, L.A.; Regalla, A.; Godley, B.J. Climate change resilience of a globally important sea turtle nesting population. Glob. Change Biol. 2019, 25, 522–535. [Google Scholar] [CrossRef]
- Hays, G.C.; Laloë, J.O.; Seminoff, J.A. Status, trends and conservation of global sea turtle populations. Nat. Rev. Biodivers. 2025, 1, 119–133. [Google Scholar] [CrossRef]
- Simantiris, N. Sea turtles swim in warmer and saltier waters than 30 years ago. Estuar. Coast. Shelf Sci. 2025, 323, 109384. [Google Scholar] [CrossRef]
- Casale, P.; Broderick, A.C.; Camiñas, J.A.; Cardona, L.; Carreras, C.; Demetropoulos, A.; Fuller, W.J.; Godley, B.J.; Hochscheid, S.; Kaska, Y.; et al. Mediterranean sea turtles: Current knowledge and priorities for conservation and research. Endanger. Species Res. 2018, 36, 229–267. [Google Scholar] [CrossRef]
- Simantiris, N. Single-use plastic or paper products? A dilemma that requires societal change. Clean. Waste Syst. 2024, 7, 100128. [Google Scholar] [CrossRef]
- Ostiategui-Francia, P.; Usategui-Martín, A.; Liria-Loza, A. Microplastics presence in sea turtles. In Fate and Impact of Microplastics in Marine Ecosystems; Elsevier: Amsterdam, The Netherlands, 2016; pp. 34–35. [Google Scholar]
- Papafitsoros, K.; Adam, L.; Schofield, G. A social media-based framework for quantifying temporal changes to wildlife viewing intensity. Ecol. Model. 2023, 476, 110223. [Google Scholar] [CrossRef]
- Solomando, A.; Pujol, F.; Sureda, A.; Pinya, S. Ingestion and characterization of plastic debris by loggerhead sea turtle, Caretta caretta, in the Balearic Islands. Sci. Total Environ. 2022, 826, 154159. [Google Scholar] [CrossRef]
- Fuentes, M.M.; Meletis, Z.A.; Wildermann, N.E.; Ware, M. Conservation interventions to reduce vessel strikes on sea turtles: A case study in Florida. Mar. Policy 2021, 128, 104471. [Google Scholar] [CrossRef]
- Simantiris, N.; Dimitriadis, C.; Xirouchakis, S.; Voulgaris, M.D.; Beka, E.; Vardaki, M.Z.; Karris, G. Combining methods for detection of bycatch hotspot areas of marine megafauna species in and around critical rookeries and foraging areas. Mar. Environ. Res. 2025, 210, 107299. [Google Scholar] [CrossRef]
- Simantiris, N.; Vardaki, M.Z.; Kourkoumelis, N.; Avlonitis, M.; Theocharis, A. Microplastics in the Mediterranean and elsewhere in coastal seas. In Treatise on Estuarine and Coastal Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Windle, A.E.; Hooley, D.S.; Johnston, D.W. Robotic vehicles enable high-resolution light pollution sampling of sea turtle nesting beaches. Front. Mar. Sci. 2018, 5, 493. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, H.; Huang, Y. Association between nighttime artificial light pollution and sea turtle nest density along Florida coast: A geospatial study using VIIRS remote sensing data. Environ. Pollut. 2018, 239, 30–42. [Google Scholar] [CrossRef]
- Price, J.T.; Drye, B.; Domangue, R.J.; Paladino, F.V. Exploring the role of artificial lighting in loggerhead turtle (Caretta caretta) nest-site selection and hatchling disorientation. Herpetol. Conserv. Biol. 2018, 13, 415–422. [Google Scholar]
- Berry, M.; Booth, D.T.; Limpus, C.J. Artificial lighting and disrupted sea-finding behaviour in hatchling loggerhead turtles (Caretta caretta) on the Woongarra coast, south-east Queensland, Australia. Aust. J. Zool. 2013, 61, 137–145. [Google Scholar] [CrossRef]
- Silva, E.; Marco, A.; da Graça, J.; Pérez, H.; Abella, E.; Patino-Martinez, J.; Martins, S.; Almeida, C. Light pollution affects nesting behavior of loggerhead turtles and predation risk of nests and hatchlings. J. Photochem. Photobiol. B Biol. 2017, 173, 240–249. [Google Scholar] [CrossRef]
- Truscott, Z.; Booth, D.T.; Limpus, C.J. The effect of on-shore light pollution on sea-turtle hatchlings commencing their off-shore swim. Wildl. Res. 2017, 44, 127–134. [Google Scholar] [CrossRef]
- Pilcher, N.; Enderby, S.; Stringell, T.; Bateman, L. Nearshore turtle hatchling distribution and predation. In Sea Turtles of the Indo-Pacific; ASEAN Academic Press: London, UK, 2000; pp. 151–166. [Google Scholar]
- Wyneken, J.; Salmon, M.; Fisher, L.; Weege, S. Managing relocated sea turtle nests in open-beach hatcheries. Lessons in hatchery design and implementation in Hillsboro Beach, Broward County, Florida. In Proceedings of the 19 th Annual Sea Turtle Symposium H. Kalb and T. Wibbels (Compilers). US Department of Commerce, NOAA Technical Memorandum NMFS-SEFSC-443, South Padre Island, TX, USA, 2–6 March 1999. [Google Scholar]
- Putman, N.F.; Bane, J.M.; Lohmann, K.J. Sea turtle nesting distributions and oceanographic constraints on hatchling migration. Proc. R. Soc. B Biol. Sci. 2010, 277, 3631–3637. [Google Scholar] [CrossRef]
- Hamann, M.; Grech, A.; Wolanski, E.; Lambrechts, J. Modelling the fate of marine turtle hatchlings. Ecol. Model. 2011, 222, 1515–1521. [Google Scholar] [CrossRef]
- Lorne, J.K.; Salmon, M. Effects of exposure to artificial lighting on orientation of hatchling sea turtles on the beach and in the ocean. Endanger. Species Res. 2007, 3, 23–30. [Google Scholar] [CrossRef]
- Margaritoulis, D.; Rees, A.F.; Riggall, T.E. Exponential Increase in a Loggerhead Sea Turtle Nesting Population: Investigating the Role of Multi-decadal Nest Protection in Kyparissia Bay, Greece. Zool. Stud. 2025, 64, 29. [Google Scholar]
- Simantiris, N.; Andreanidou, K.; Sampson, G. Over 30 years of monitoring and implementing the Bern Convention’s recommendations for the protection of Mediterranean sea turtles. Mar. Policy 2024, 168, 106319. [Google Scholar] [CrossRef]
- Kamrowski, R.L.; Limpus, C.; Pendoley, K.; Hamann, M. Influence of industrial light pollution on the sea-finding behaviour of flatback turtle hatchlings. Wildl. Res. 2015, 41, 421–434. [Google Scholar] [CrossRef]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef]
- Papazekou, M.; Kyprioti, A.; Chatzimentor, A.; Dimitriadis, C.; Vallianos, N.; Mazaris, A.D. Advancing Sea Turtle Monitoring at Nesting and Near Shore Habitats with UAVs, Data Loggers, and State of the Art Technologies. Diversity 2024, 16, 153. [Google Scholar] [CrossRef]
- Malaperdas, G. Practical Methods of GIS for Archaeologists: Spatial Division in a Large Area. SSRG Int. J. Geoinform. Geol. Sci. (SSRG-IJGGS) 2019, 6, 1–6. [Google Scholar]
- Tomczak, M. Spatial interpolation and its uncertainty using automated anisotropic inverse distance weighting (IDW)-cross-validation/jackknife approach. J. Geogr. Inf. Decis. Anal. 1998, 2, 18–30. [Google Scholar]
- Setianto, A.; Triandini, T. Comparison of kriging and inverse distance weighted (IDW) interpolation methods in lineament extraction and analysis. J. Appl. Geol. 2013, 5. [Google Scholar] [CrossRef]
- Malaperdas, G.; Panagopoulos, N. Mapping shoreline changes over the years: The case study of Navarino bay, Pylos, Messenia, Greece. World J. Geomat. Geosci. 2021, 1, 28–35. [Google Scholar] [CrossRef]
- Vandersteen, J.; Kark, S.; Sorrell, K.; Levin, N. Quantifying the impact of light pollution on sea turtle nesting using ground-based imagery. Remote Sens. 2020, 12, 1785. [Google Scholar] [CrossRef]
- Yen, C.H.; Chan, Y.T.; Peng, Y.C.; Chang, K.H.; Cheng, I.J. The effect of light pollution on the sea finding behavior of green turtle hatchlings on Lanyu Island, Taiwan. Zool. Stud. 2023, 62, e47. [Google Scholar] [PubMed]
- Cruz, L.M.; Shillinger, G.L.; Robinson, N.J.; Tomillo, P.S.; Paladino, F.V. Effect of light intensity and wavelength on the in-water orientation of olive ridley turtle hatchlings. J. Exp. Mar. Biol. Ecol. 2018, 505, 52–56. [Google Scholar] [CrossRef]
- Witherington, B.E.; Bjorndal, K.A. Influences of artificial lighting on the seaward orientation of hatchling loggerhead turtles Caretta caretta. Biol. Conserv. 1991, 55, 139–149. [Google Scholar] [CrossRef]
- Magyar, T. The impact of artificial lights and anthropogenic noise on loggerheads (Caretta caretta) and green turtles (Chelonia mydas), assessed at index nesting beaches in Turkey and Mexico. Ph.D. Thesis, Universitäts-und Landesbibliothek Bonn, Bonn, Germany, 2009. [Google Scholar]
- Flores Turcios, M. Relación entre la Intensidad Lumínica de Fuentes de luz Artificial y la Densidad de Huellas de Anidación de Tortuga Parlama (Lepidochelys olivacea) en el Litoral Costero de la Playa de Hawaii, Santa Rosa, Guatemala. Bachelor’s Thesis, Universidad del Valle de Guatemala, Guatemala City, Guatemala, 2021. [Google Scholar]
- Witherington, B.E.; Martin, R.E. Understanding, assessing, and resolving light-pollution problems on sea turtle nesting beaches. In Florida Marine Research Institute Technical Report; FWRI: St. Petersburg, FL, USA, 2000; pp. 2–73. [Google Scholar]
- Lopez, G.G.; Saliés, E.d.C.; Lara, P.H.; Tognin, F.; Marcovaldi, M.A.; Serafini, T.Z. Coastal development at sea turtles nesting ground: Efforts to establish a tool for supporting conservation and coastal management in northeastern Brazil. Ocean Coast. Manag. 2015, 116, 270–276. [Google Scholar] [CrossRef]
- Pendoley, K.; Kamrowski, R.L. Sea-finding in marine turtle hatchlings: What is an appropriate exclusion zone to limit disruptive impacts of industrial light at night? J. Nat. Conserv. 2016, 30, 1–11. [Google Scholar] [CrossRef]
- Arthur, K.; Whiting, S.; Pendoley, K. Indian Ocean Turtle Newsletter-Issue 32. Department of Agriculture, Australia. Available online: https://www.iotn.org/wp-content/uploads/2020/08/IOTN-32.pdf (accessed on 10 September 2025).
- Falchi, F.; Cinzano, P.; Elvidge, C.D.; Keith, D.M.; Haim, A. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manag. 2011, 92, 2714–2722. [Google Scholar] [CrossRef]
- Gaston, K.J.; Davies, T.W.; Bennie, J.; Hopkins, J. Reducing the ecological consequences of night-time light pollution: Options and developments. J. Appl. Ecol. 2012, 49, 1256–1266. [Google Scholar] [CrossRef]
- Kamrowski, R.L.; Limpus, C.; Jones, R.; Anderson, S.; Hamann, M. Temporal changes in artificial light exposure of marine turtle nesting areas. Glob. Change Biol. 2014, 20, 2437–2449. [Google Scholar] [CrossRef]
- Bourgeois, S.; Gilot-Fromont, E.; Viallefont, A.; Boussamba, F.; Deem, S.L. Influence of artificial lights, logs and erosion on leatherback sea turtle hatchling orientation at Pongara National Park, Gabon. Biol. Conserv. 2009, 142, 85–93. [Google Scholar] [CrossRef]
- Salmon, M. Artificial night lighting and sea turtles. Biologist 2003, 50, 163–168. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).