Effect of Long-Term Immersion in Low-Salinity Seawater on Epoxy Resin Composites Filled with Marine Secondary Raw Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Composites
- A conventional epoxy resin, code RP 026 UV, with IPE 743 hardener, by Trias Chem (Polo di Torrile, Parma, Italy);
- A partially (30%) bio-based resin, code Super Sap CCR (Clear Casting Resin), with CCF (Clear Casting Fast) hardener, by Entropy Resins, Genoa, Italy.
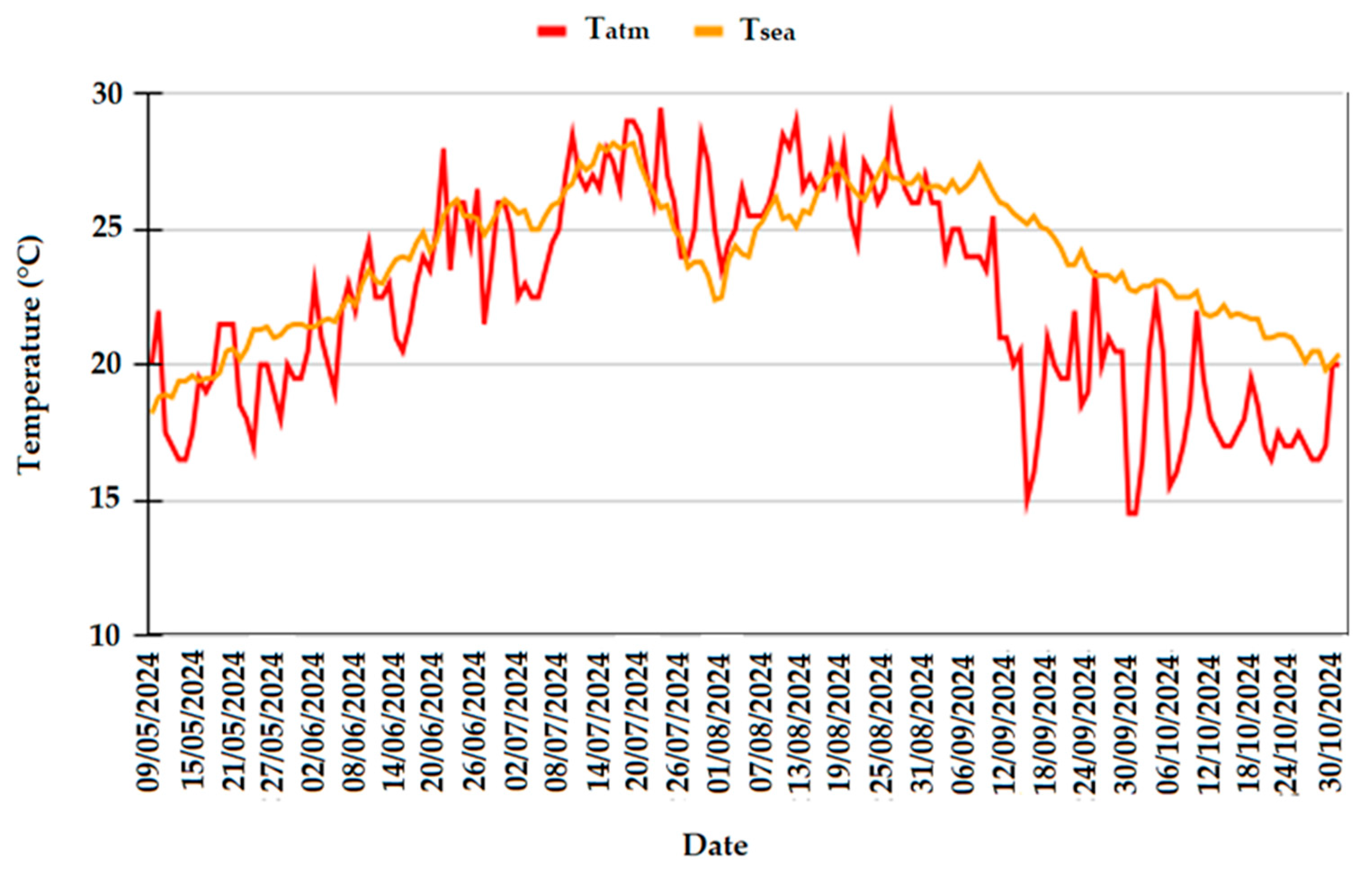
2.3. Environmental Conditions
- At open sea (coordinates 42.46°, 17.29°), approximately at 20 nautical miles by the mouth of the bay;
- Inside Kotor’s Bay, in front of the Institute of Marine Biology, at the immersion site of the specimens (coordinates 42.43604°, 18.76342°).
3. Results and Discussion
3.1. Preliminary Analysis on Posidonia oceanica Fibers
3.2. Biofouling Analysis of Waste-Based Structures in Kotor’s Bay
3.2.1. General Considerations
3.2.2. External Colonization Analysis
3.3. Analysis After Removal of Biofouling
- Weight measurement with respect to their initial (pre-immersion) weights to accurately assess whether the original material had been preserved;
- Shore D hardness tests to verify by a simple and reasonably accurate method if some strength had been retained, considering that other tests (e.g., flexural testing) would lead to unreliable results for the point-to-point variability of the sample properties;
- Macrophotographs and optical microscopy analysis to identify the surface differences sample by sample;
- Each sample was weighed before immersion (Starting Weight) and was weighed again after retrieval (Ending Weight). The biofouling material was then removed, and the percentage of weight gain was calculated. The obtained results (Figure 7) indicate a clear influence of material composition and exposure conditions on the absorption behavior. In general, specimens immersed at greater depths exhibited higher water uptake and biofouling accumulation compared to those positioned in the splashing zone, where prolonged dry periods and high solar radiation limited the moisture content and reduced the fouling effect to almost nothing. Among all samples, the highest weight increases were recorded for samples from Site 1, such as S1 (7.70%), T1 (7.67%), T2 (7.62%), A2 (7.42%), and BE1 (6.59%), suggesting that the siting of these materials made them more susceptible to water penetration and subsequent biological colonization. Conversely, samples from Site 3, such as T3 (0.09%), S3 (0.32%), BE3 (0.49%), and U3 (−0.04%), showed negligible variation, indicating higher resistance to water uptake and reduced fouling settlement. These results confirm that the water absorption capacity strongly depends on the polymer matrix type and the inclusion of natural fillers, which may alter the porosity and permeability of the composites. Furthermore, the synergy between the solidity of the structure and biofouling development highlights the relevance of surface properties in marine applications, where prolonged exposure can significantly affect both dimensional stability and long-term mechanical performance.
- Physical characterization, carried out by comparing the Shore D hardness of the samples and their weight variation (Δw) after a conditioning period, as from data reported in Table 5, was essential to observe the behavior of the composite. This allows ensuring its stability and durability and to understand how these materials react and degrade in response to environmental stimuli. From the calculation of the weight variation before and after of the samples, at all the sites, despite the loss of material and following the removal of bioconstructions, some increases in weight still emerged. Only at Site 3, where the samples had a lower if not quasi-absent degree of colonization by marine species, a decrease in the weight of the samples was evident. In measuring the material’s hardness, no evident differences between the samples were recorded. In some cases, namely Samples S at Site 1 and Site 2, and sample BE at Site 2, degradation did not allow for manual measurements to be taken on the front surface. There was a slight difference between the front surfaces, corresponding to the most degraded surfaces, and the rear ones, with a direct decrease in the hardness compared to the front. Samples S included 10% Posidonia, and their higher level of degradation was deemed to be due to the high salinity affecting the resin-confined lignocellulosic fibers when accessible to seawater [48]. It is also possible that for series T, with 15% Posidonia fibers, a higher level of adhesion with the matrix was obtained, which reduced the degradation effect.
- After six months of composite conditioning and the mechanical removal of as many protruding bioconstruction structures as possible, one can observe from the comparison between the epoxy resin (samples A) and the eco-epoxy resin (samples BA) at the three sites that degradation occurred mainly at Site 2. In particular, sample A2 showed a high level of degradation, with erosion of the material and growth of Balanus sp. (barnacles), gastropods, mussels, Lithophyllum incrustans (coralline algae; a typical coralligenous outcrop of this marine area [49]), and algae. It is also clearly evident that the growth rate on the eco-epoxy resin was considerably lower, a difference that might depend on variations in porosities (Figure 8).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| CDW | Construction and demolition waste |
| Δw | Weight variation |
| PO | Posidonia oceanica |
| PSU | Practical salinity unit |
| SEM | Scanning electron microscope |
| Tatm | Atmospheric temperature |
| Tsea | Sea surface temperature |
| wa | Sample weight after sea conditioning |
| wb | Sample weight at fabrication |
Appendix A




References
- Baris-Camli, S.; Neser, G.; Sozen, A. Environmental marine degradation of pla/wood composite as an alternative sustainable boat building material. Pol. Marit. Res. 2024, 31, 127–134. [Google Scholar] [CrossRef]
- Hodul, J.; Mészárosová, L.; Drochytka, R. Recovery of industrial wastes as fillers in the epoxy thermosets for building application. Materials 2021, 14, 3490. [Google Scholar] [CrossRef]
- Ağcan, A.E.; Kartal, İ. A review of waste-derived fillers for enhancing the properties of epoxy resins. Int. J. Adhes. Adhesiv. 2025, 138, 103944. [Google Scholar] [CrossRef]
- Okpuwhara, R.O.; Oboirien, B.O.; Sadiku, E.R.; Ray, S.S.; Akinlabi, S.A. The use of ecofriendly recycled polymer composites in boat building. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Zilia, F.; Andreottola, F.G.; Orsi, L.; Parolini, M.; Bacenetti, J. Trash or treasure? A circular business model of recycling plasmix. Circ. Econ. 2024, 3, 100089. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, X.; Zhang, H.; Li, W.; Jiang, R.; Zhou, X. Review on formation of biofouling in the marine environment and functionalization of new marine antifouling coatings. J. Mater. Sci. 2022, 57, 18221–18242. [Google Scholar] [CrossRef]
- Muthukumar, T.; Aravinthan, A.; Lakshmi, K.; Venkatesan, R.; Vedaprakash, L.; Doble, M. Fouling and stability of polymers and composites in marine environment. Int. Biodeter. Biodegrad. 2011, 65, 276–284. [Google Scholar] [CrossRef]
- García Fernández, M.L.; Levy, I.K.; Salustro, D.; Negri, R.M.; Saleh Medina, L.M. Use of Lemon Peel Extract as Antimicrobial Supported on Eco-friendly Polyvinyl Alcohol/Polydimethylsiloxane Sponges. J. Polym. Environ. 2024, 32, 3179–3194. [Google Scholar] [CrossRef]
- Shilova, O.A.; Khalaman, V.V.; Komendantov, A.Y.; Kondratenko, Y.A.; Efimova, L.N.; Tsvetkova, I.N.; Kochina, T.A. Study of the process of biofouling of environmentally safe paint coatings in the natural conditions of the White Sea. Glass Phys. Chem. 2020, 46, 620–634. [Google Scholar] [CrossRef]
- Fragassa, C.; Mattiello, S.; Fronduti, M.; Del Gobbo, J.; Gagic, R.; Santulli, C. Prevention of biofouling due to water absorption of natural fiber composites in the aquatic environment: A critical review. J. Compos. Sci. 2024, 8, 532. [Google Scholar] [CrossRef]
- Rahman, F.; Ghazali, C.M.R.; Suriani, M.J.; Fitriadhy, A.; Mokhtar, N.A.; Aminnudin. Physical Properties Characterization of Ceramic Waste Particles Used as Filler in Boat Hull Production: A Proposed Study. In International Conference on Innovative Research; Springer Nature: Cham, Switzerland, 2023; pp. 134–144. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Madhu, S. Impact of brown algae particles on the mechanical properties of jute-reinforced polymeric composites for sustainable development. Results Eng. 2025, 25, 104548. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, S.; Lin, R.; Jin, H. Mechanical properties and marine durability of epoxy resin-modified binary geopolymer composites. Constr. Build. Mater. 2025, 474, 141135. [Google Scholar] [CrossRef]
- Capretti, M.; Giammaria, V.; Santulli, C.; Boria, S.; Del Bianco, G. Use of bio-epoxies and their effect on the performance of polymer composites: A critical review. Polymers 2023, 15, 4733. [Google Scholar] [CrossRef]
- Karacor, B.; Ozcanli, M. The use of bioresin composites created with five different vegetable oils such as soybean oil, palm oil, rapeseed oil, cottonseed oil, linseed oil in the automotive industry. Polym. Compos. 2025, 46, 7091–7107. [Google Scholar] [CrossRef]
- Baley, C.; Davies, P.; Troalen, W.; Chamley, A.; Dinham-Price, I.; Marchandise, A.; Keryvin, V. Sustainable polymer composite marine structures: Developments and challenges. Prog. Mater. Sci. 2024, 145, 101307. [Google Scholar] [CrossRef]
- Pangallo, D.; Bučková, M.; Kraková, L.; Puškárová, A.; Šaková, N.; Grivalský, T.; Chovanová, K.; Zemánková, M. Biodeterioration of epoxy resin: A microbial survey through culture-independent and culture-dependent approaches. Environ. Microbiol. 2015, 17, 462–479. [Google Scholar] [CrossRef]
- Vizentin, G.; Vukelic, G. Marine environment induced failure of FRP composites used in maritime transport. Eng. Fail. Anal. 2022, 137, 106258. [Google Scholar] [CrossRef]
- Rudovica, V.; Rotter, A.; Gaudêncio, S.P.; Novoveská, L.; Akgül, F.; Akslen-Hoel, L.K.; Alexandrino, D.A.M.; Anne, O.; Arbidans, L.; Atanassova, M. Valorization of marine waste: Use of industrial by-products and beach wrack towards the production of high added-value products. Front. Mar. Sci. 2021, 8, 723333. [Google Scholar] [CrossRef]
- Santulli, C.; Fiore, V. Seawater aging and its degradation effects on the mechanical properties of biocomposites. In Biocomposites for Industrial Applications; Woodhead Publishing: Cambridge, UK, 2024; pp. 195–207. [Google Scholar] [CrossRef]
- Fragassa, C.; Latini, M.; Mattiello, S.; Pešić, A.; Santulli, C. Use of Adriatic Sea marine originated material in biocomposites with epoxy resin. Stud. Mar. 2024, 37, 24–32. [Google Scholar] [CrossRef]
- Simeone, S.; De Falco, G. Morphology and composition of beach-cast Posidonia oceanica litter on beaches with different exposures. Geomorphology 2012, 151, 224–233. [Google Scholar] [CrossRef]
- Fragassa, C.; Mattiello, S.; Latini, M.; Pešić, A.; Santulli, C. Saltwater Immersion Effects on Bio-Composites Reinforced with Seashell Powders. Appl. Compos. Mater. 2025, 32, 2067–2089. [Google Scholar] [CrossRef]
- Barthelat, F.; Rim, J.E.; Espinosa, H.D. A review on the structure and mechanical properties of mollusk shells–perspectives on synthetic biomimetic materials. In Applied Scanning Probe Methods XIII: Biomimetics and Industrial Applications; Bhushan, B., Fuchs, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 17–44. [Google Scholar] [CrossRef]
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. American Society for Standards and Materials: West Conshohocken, PA, USA, 2017.
- Johnson, R.D.J.; Arumugaprabu, V.; Rajasekar, E.; Santhosh, G.; Saravanakumar, M. Mechanical property studies on environmental friendly bio epoxy resin. Mater. Tod. Proc. 2018, 5, 6815–6820. [Google Scholar] [CrossRef]
- Mercator Ocean International. Available online: https://www.mercator-ocean.eu/ (accessed on 15 August 2025).
- Bellafiore, D.; Guarnieri, A.; Grilli, F.; Penna, P.; Bortoluzzi, G.; Giglio, F.; Pinardi, N. Study of the hydrodynamical processes in the Boka Kotorska Bay with a finite element model. Dyn. Atmos. Ocean. 2011, 52, 298–321. [Google Scholar] [CrossRef]
- Sukur, E.F.; Onal, G. Long-term salt-water durability of GNPs reinforced basalt-epoxy multiscale composites for marine applications. Tribol. Int. 2021, 158, 106910. [Google Scholar] [CrossRef]
- Fragassa, C.; Conticelli, F.; Francucci, B.; Seccacini, G.; Santulli, C. Biocomposites for marine applications: A review of friction, wear, and environmental degradation. J. Compos. Sci. 2025, 9, 331. [Google Scholar] [CrossRef]
- ASTM D2240-21; Standard Test Method for Rubber Property—Durometer Hardness. American Society for Standards and Materials: West Conshohocken, PA, USA, 2021.
- Garcia-Garcia, D.; Quiles-Carrillo, L.; Montanes, N.; Fombuena, V.; Balart, R. Manufacturing and characterization of composite fibreboards with Posidonia oceanica wastes with an environmentally-friendly binder from epoxy resin. Materials 2017, 11, 35. [Google Scholar] [CrossRef]
- Restaino, O.F.; Giosafatto, C.V.L.; Mirpoor, S.F.; Cammarota, M.; Hejazi, S.; Mariniello, L.; Schiraldi, C.; Porta, R. Sustainable exploitation of Posidonia oceanica sea balls (Egagropili): A Review. Int. J. Mol. Sci. 2023, 24, 7301. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, L.; Compère, P. The formation of aegagropiles from the Mediterranean seagrass Posidonia oceanica (L.) Delile (1813): Plant tissue sources and colonisation by melanised fungal mycelium. Mar. Biol. 2023, 170, 19. [Google Scholar] [CrossRef]
- Cennamo, P.; Caputo, P.; De Stefano, M.; Russo Ermolli, E.; Barone Lumaga, M.R. Epiphytic Diatom Communities on Sub-Fossil Leaves of Posidonia oceanica Delile in the Graeco-Roman Harbor of Neapolis: A Tool to Explore the Past. Am. J. Plant Sci. 2014, 5, 549–553. [Google Scholar] [CrossRef][Green Version]
- Rokbi, M.; Imad, A.; Herbelot, C.; Belouadah, Z. Fracture toughness of random short natural fibres polyester composites. Diff. Foundat. 2018, 18, 94–105. [Google Scholar] [CrossRef]
- Langer, M. Epiphytic foraminifera. Mar. Micropaleontol. 1993, 20, 235–265. [Google Scholar] [CrossRef]
- Kohn, T.; Rast, P.; Kallscheuer, N.; Wiegand, S.; Boedeker, C.; Jetten, M.S.M.; Jeske, O.; Vollmers, J.; Kaster, A.K.; Rohde, M.; et al. The Microbiome of Posidonia oceanica Seagrass Leaves Can Be Dominated by Planctomycetes. Front. Microbiol. 2020, 11, 1458. [Google Scholar] [CrossRef]
- Melelli, A.; Durand, S.; Arnould, O.; Richely, E.; Guessasma, S.; Jamme, F.; Beaugrand, J.; Bourmaud, A. Extensive investigation of the ultrastructure of kink-bands in flax fibres. Ind. Crops Prod. 2021, 164, 113368. [Google Scholar] [CrossRef]
- Hänninen, T.; Michud, A.; Hughes, M. Kink bands in bast fibres and their effects on mechanical properties. Plast. Rubber Compos. 2011, 40, 307–310. [Google Scholar] [CrossRef]
- Canessa, M.; Trainito, E.; Bavestrello, G.; Petović, S.; Đorđević, N.; Mačić, V. A large non-parasitic population of Savalia savaglia (Bertoloni, 1819) in the Boka Kotorska Bay (Montenegro). Sci. Rep. 2024, 14, 7785. [Google Scholar] [CrossRef]
- Relini, G. Le Metodologie per lo Studio del Fouling Nell’Indagine di Alcuni Ecosistemi Marini. Boll. Zool. 1977, 44, 97–112. [Google Scholar] [CrossRef]
- Bellomo, M.; Campisi, T.; Colajanni, S. Bio-Based Materials for the Improvement of “Zero Km” Human Quality Life. In Getting to Zero-Beyond Energy Transition Towards Carbon-Neutral Mediterranean Cities: Selected Papers from the World Renewable Energy Congress Med Green Forum 2024; Springer Nature: Cham, Switzerland, 2025; pp. 759–769. [Google Scholar] [CrossRef]
- Cocito, S. Bioconstruction and biodiversity: Their mutual influence. Sci. Mar. 2004, 68 (Suppl. S1), 137–144. [Google Scholar] [CrossRef]
- Chemello, R. Marine bioconstructions in the Mediterranean Sea. A state of the art on the vermetid reef. Biol. Mar. Mediterr. 2009, 16, 2–18. [Google Scholar]
- Negra, O.; Zobele Lipparini, G. Gasteropodi, Bivalvi, Scafopodi. Preist. Alp. 2005, 40, 9–14. [Google Scholar]
- Dolenec, T.; Lojen, S.; Dolenec, M.; Lambaša, Ž.; Dobnikar, M.; Rogan, N. 15 N and 13 C Enrichment in Balanus perforatus: Tracers of Municipal Particulate Waste in the Murter Sea (Central Adriatic, Croatia). Acta Chim. Slov. 2006, 53, 469–476. [Google Scholar]
- Pistocchi, A.; Bleninger, T.; Dorati, C. Screening the hurdles to sea disposal of desalination brine around the Mediterranean. Desalination 2020, 491, 114570. [Google Scholar] [CrossRef]
- Ponti, M.; Falace, A.; Rindi, F.; Fava, F.; Kaleb, S.; Abbiati, M. Beta diversity patterns in northern Adriatic coralligenous outcrops. In Proceedings of the 2nd Mediterranean Symposium on the Conservation of Coralligenous and Other Calcareous Bio-Concretions, Portorož, Slovenia, 29–30 October 2014; Bouafif, C., Langar, H., Ouerghi, A., Eds.; pp. 147–152. [Google Scholar]
- Pardi, G.; Piazzi, L.; Balata, D.; Papi, I.; Cinelli, F.; Benedetti-Cecchi, L. Spatial variability of Posidonia oceanica (L.) Delile epiphytes around the mainland and the islands of Sicily (Mediterranean Sea). Mar. Ecol. 2006, 27, 397–403. [Google Scholar] [CrossRef]
- Costa, M.; Barrote, I.; Silva, J.; Olivé, I.; Alexandre, A.; Albano, S.; Santos, R. Epiphytes Modulate Posidonia oceanica Photosynthetic Production, Energetic Balance, Antioxidant Mechanisms, and Oxidative Damage. Front. Mar. Sci. 2015, 2, 111. [Google Scholar] [CrossRef]
- De Battisti, D.; Balestri, E.; Pardi, G.; Menicagli, V.; Lardicci, C. Substrate Type Influences the Structure of Epiphyte Communities and the Growth of Posidonia oceanica Seedlings. Front. Plant Sci. 2021, 12, 660658. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Al Fahdi, D.; Muthukrishnan, T. Short-term succession of marine microbial fouling communities and the identification of primary and secondary colonizers. Biofouling 2019, 35, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Waltz, G.T.; Hunsucker, K.Z.; Swain, G.; Wendt, D.E. Using encrusting bryozoan adhesion to evaluate the efficacy of fouling-release marine coatings. Biofouling 2020, 36, 1149–1158. [Google Scholar] [CrossRef]
- Xiong, C.; Huang, Y.; Jin, Z.; Wang, P.; Hu, Y. Influence of salt concentration and pH value on the degradation of epoxy resin used in FRP bars under simulated marine conditions. J. Build. Eng. 2024, 98, 111405. [Google Scholar] [CrossRef]
- Kamino, K. Mini-review: Barnacle adhesives and adhesion. Biofouling 2013, 29, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Petersen, D.S.; Gorb, S.N.; Heepe, L. The Influence of Material and Roughness on the Settlement and the Adhesive Strength of the Barnacle Balanus Improvisus in the Baltic Sea. Front Plant Sci. 2020, 7, 664. [Google Scholar] [CrossRef]
- Mant, R.C.; Moggridge, G.; Aldridge, D.C. Biofouling by bryozoans, Cordylophora and sponges in UK water treatment works. Water Sci. Technol. 2011, 63, 1815–1822. [Google Scholar] [CrossRef]
- Sacco Perasso, C.; Antonelli, F.; Calcinai, B.; Casoli, E.; Gravina, M.F.; Ricci, S. The bioerosion of submerged archeological artifacts in the mediterranean sea: An overview. Front. Mar. Sci. 2022, 9, 888731. [Google Scholar] [CrossRef]
- Joseph, T.M.; Unni, A.B.; Joshy, K.S.; Kar Mahapatra, D.; Haponiuk, J.; Thomas, S. Emerging bio-based polymers from lab to market: Current strategies, market dynamics and research trends. J. Carbon Res. 2023, 9, 30. [Google Scholar] [CrossRef]
- Nekhaev, I.O.; Krol, E.N. Hidden under ice and mud: Diversity of shell-bearing microgastropods in the eastern Arctic seas. Syst. Biodivers. 2020, 18, 794–809. [Google Scholar] [CrossRef]
- Anderson, M.J.; Underwood, A.J. Effects of gastropod grazers on recruitment and succession of an estuarine assemblage: A multivariate and univariate approach. Oecologia 1997, 109, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel Adhesion: Finding the Tricks Worth Mimicking. J. Adhes. 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Kreczak, H.; Willmott, A.J.; Baggaley, A.W. Subsurface dynamics of buoyant microplastics subject to algal biofouling. Limnol Oceanogr 2021, 66, 3287–3299. [Google Scholar] [CrossRef]
- Callow, M.; Callow, J. Marine biofouling: A sticky problem. Biologist 2002, 49, 1–5. [Google Scholar]
- Mieszkin, S.; Callow, M.E.; Callow, J.A. Interactions between microbial biofilms and marine fouling algae: A mini review. Biofouling 2013, 29, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
















| Seashell | Calcite (%) | Aragonite (%) |
|---|---|---|
| Mussel (Mytilus galloprovincialis) | 75.7 | 24.3 |
| Oyster (Ostrea edulis) | 98.5 | 1.5 |
| Clam (Ruditapes decussatus) | 1.1 | 98.9 |
| Category | Resin | Filler | Amount (%) |
|---|---|---|---|
| A | Epoxy | - | - |
| R | Epoxy | Posidonia | 5 |
| S | Epoxy | Posidonia | 10 |
| T | Epoxy | Posidonia | 15 |
| BA | Eco-Epoxy | - | - |
| BB | Eco-Epoxy | Mussels | 15 |
| BC | Eco-Epoxy | Oyster | 15 |
| BD | Eco-Epoxy | Clam | 15 |
| BE | Eco-Epoxy | Posidonia | 15 |
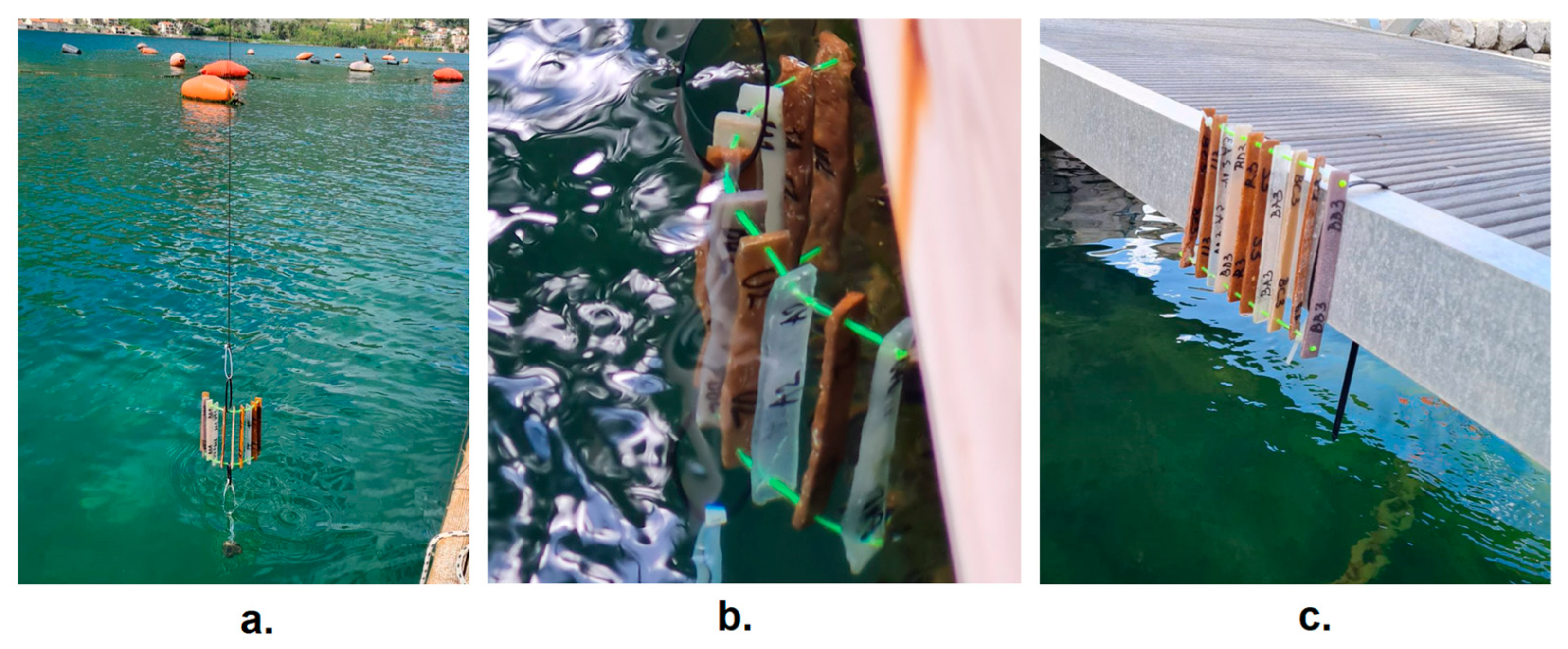
| Samples | Site | Location |
|---|---|---|
| A1, R1, S1, T1, BA1, BB1, BC1, BD1, BE1 | 1 | On the bottom of the sea |
| A2, R2, S2, T2, BA2, BB2, BC2, BD2, BE2 | 2 | Immersed just below the surface |
| A3, R3, S3, T3, BA3, BB3, BC3, BD3, BE3 | 3 | On the pier |
| Taxonomic Groups | Representative Species Observed |
|---|---|
| Algae | Sphacelaria sp. |
| Polysiphonia sp. | |
| Cladophora prolifera | |
| Enteromorpha sp. | |
| Hydrozoans | Eudendrium sp. |
| Worms (polychaetes) | Spirorbis spirorbis |
| Janua heterostropha | |
| Anelida 1 | |
| Anelida 2 | |
| Molluscs | Mytilus galloprovincialis |
| Arthropods | Perforatus perforatus |
| Amphibalanus eburneus | |
| Bryozoans | Cryptosula pallasiana |
| Site 1 (2 m Depth) | Site 2 (0.5 m Depth) | Site 3 (Splashing Zone) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Series | Hardness (Front) | Hardness (Rear) | Δw (%) | Hardness (Front) | Hardness (Rear) | Δw (%) | Hardness (Front) | Hardness (Rear) | Δw (%) |
| A | 78.2 ± 2.4 | 74.2 ± 3.8 | 3.09 | 73.4 ± 4.4 | 72.0 ± 4.9 | 5.44 | 80.0 ± 0.7 | 77.6 ± 3.4 | 0.93 |
| R | 76.0 ± 2.0 | 75.2 ± 5.8 | 0.74 | 77.2 ± 2.6 | 76.2 ± 4.0 | 1.77 | 74.2 ± 3.7 | 73.0 ± 2.1 | −0.08 |
| S | - * | 75.2 ± 6.1 | 0.58 | - * | 76.6 ± 3.9 | 3.04 | 76.0 ± 4.3 | 79.2 ± 3.1 | −0.49 |
| T | 76.0 ± 3.2 | 74.4 ± 4.5 | 1.12 | 75.0 ± 3.2 | 77.2 ± 1.6 | 4.28 | 77.4 ± 2.1 | 77.6 ± 1.8 | −0.70 |
| BA | 76.2 ± 0.8 | 76.2 ± 1.6 | 1.11 | 76.6 ± 1.9 | 75.4 ± 0.9 | 1.26 | 77.6 ± 2.4 | 77.8 ± 2.5 | 0.64 |
| BB | 78.8 ± 3.8 | 79.0 ± 1.0 | 1.23 | 76.4 ± 4.3 | 76.2 ± 2.2 | 3.13 | 74.0 ± 2.5 | 78.8 ± 3.3 | 0.48 |
| BC | 77.2 ± 2.7 | 77.8 ± 2.4 | 0.60 | 76.8 ± 2.5 | 74.8 ± 3.1 | 2.51 | 77.0 ± 2.9 | 75.8 ± 3.8 | 0.54 |
| BD | 83.0 ± 0.7 | 74.6 ± 4.0 | 0.19 | 80.4 ± 1.5 | 77.8 ± 1.3 | 2.10 | 82.6 ± 1.1 | 77.6 ± 1.1 | 0.34 |
| BE | 75.2 ± 1.8 | 75.0 ± 2.4 | 0.11 | - * | 75.6 ± 1.1 | 1.74 | 73.2 ± 2.5 | 75.4 ± 3.8 | −0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicentini, G.; Santulli, C.; Mattiello, S.; Matassa, R.; Nikolić, D.; Petovic, S.; Pesic, A.; Gagic, R.; Felici, A.; Fragassa, C. Effect of Long-Term Immersion in Low-Salinity Seawater on Epoxy Resin Composites Filled with Marine Secondary Raw Materials. J. Mar. Sci. Eng. 2025, 13, 1985. https://doi.org/10.3390/jmse13101985
Vicentini G, Santulli C, Mattiello S, Matassa R, Nikolić D, Petovic S, Pesic A, Gagic R, Felici A, Fragassa C. Effect of Long-Term Immersion in Low-Salinity Seawater on Epoxy Resin Composites Filled with Marine Secondary Raw Materials. Journal of Marine Science and Engineering. 2025; 13(10):1985. https://doi.org/10.3390/jmse13101985
Chicago/Turabian StyleVicentini, Greta, Carlo Santulli, Sara Mattiello, Roberto Matassa, Danilo Nikolić, Slavica Petovic, Ana Pesic, Radmila Gagic, Alberto Felici, and Cristiano Fragassa. 2025. "Effect of Long-Term Immersion in Low-Salinity Seawater on Epoxy Resin Composites Filled with Marine Secondary Raw Materials" Journal of Marine Science and Engineering 13, no. 10: 1985. https://doi.org/10.3390/jmse13101985
APA StyleVicentini, G., Santulli, C., Mattiello, S., Matassa, R., Nikolić, D., Petovic, S., Pesic, A., Gagic, R., Felici, A., & Fragassa, C. (2025). Effect of Long-Term Immersion in Low-Salinity Seawater on Epoxy Resin Composites Filled with Marine Secondary Raw Materials. Journal of Marine Science and Engineering, 13(10), 1985. https://doi.org/10.3390/jmse13101985









