Experimental Study on CO2 Sequestration in Marine Environments During Hydrate Recovery by Depressurization Combined with Replacement
Abstract
1. Introduction
2. Materials and Methods
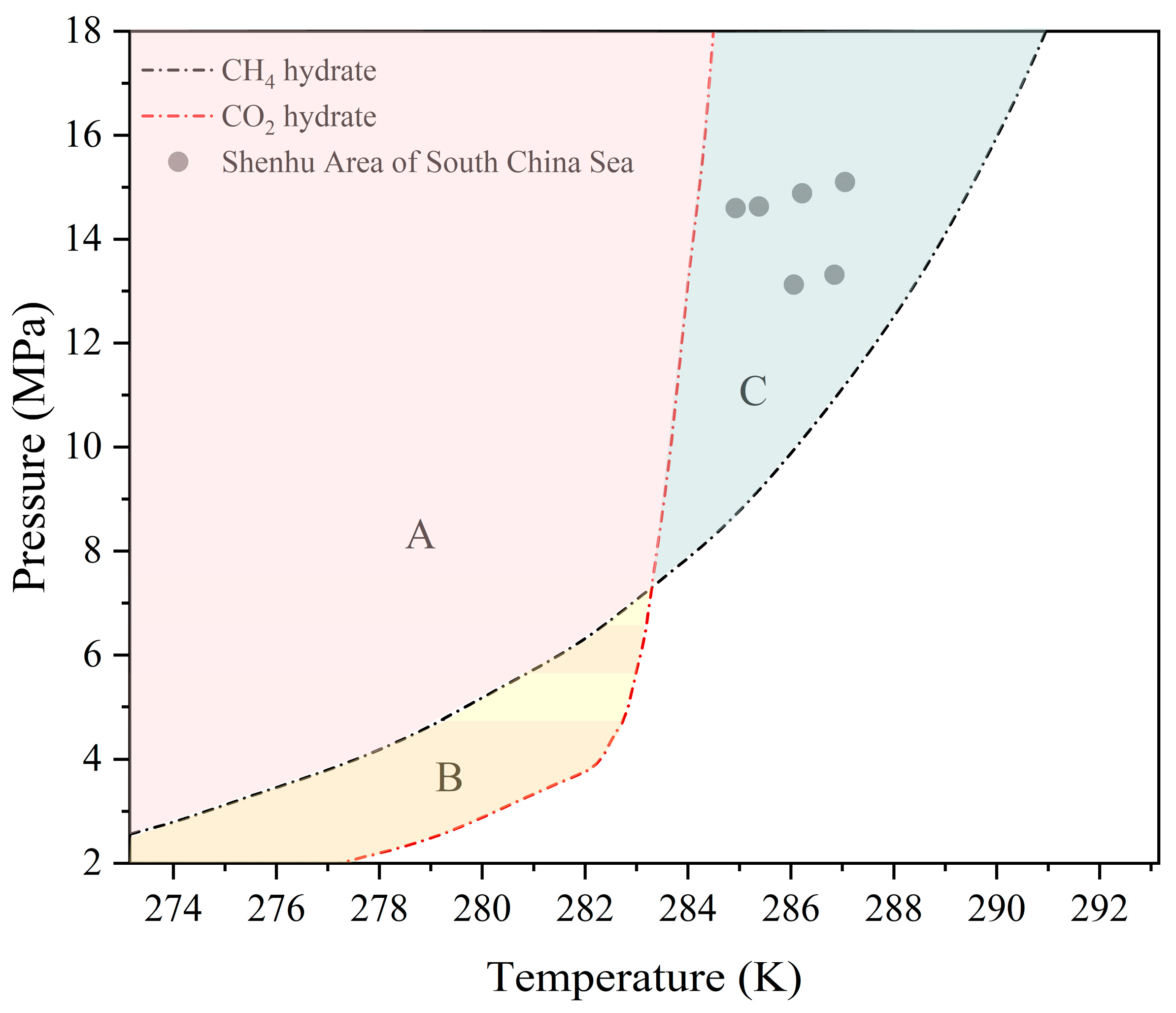
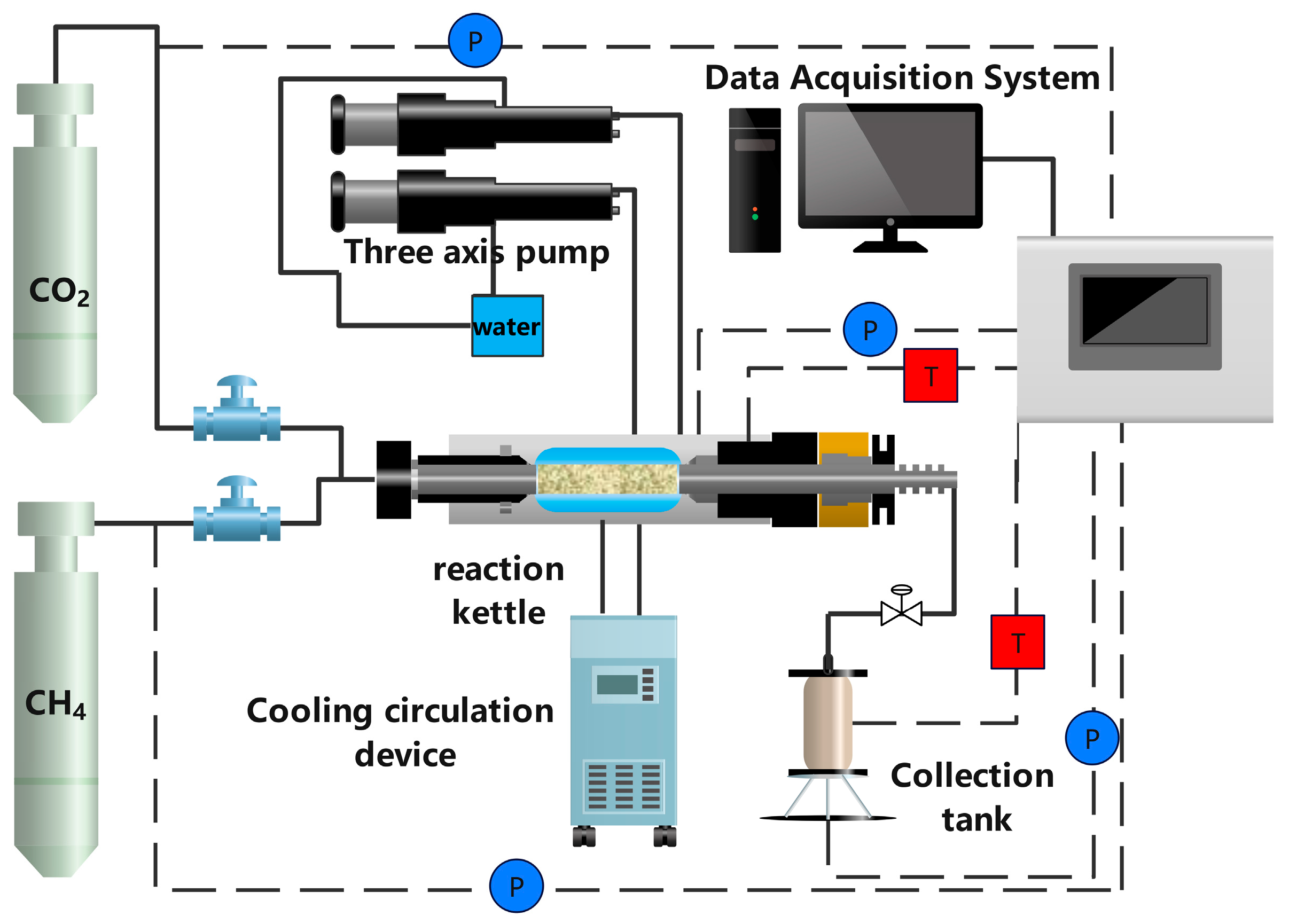
2.1. Experimental Materials and Apparatus
2.2. Experimental Procedures
2.2.1. Specimen Preparation and MH Formation
2.2.2. MH Replacement
2.2.3. Depressurization
2.2.4. Secondary MH Replacement
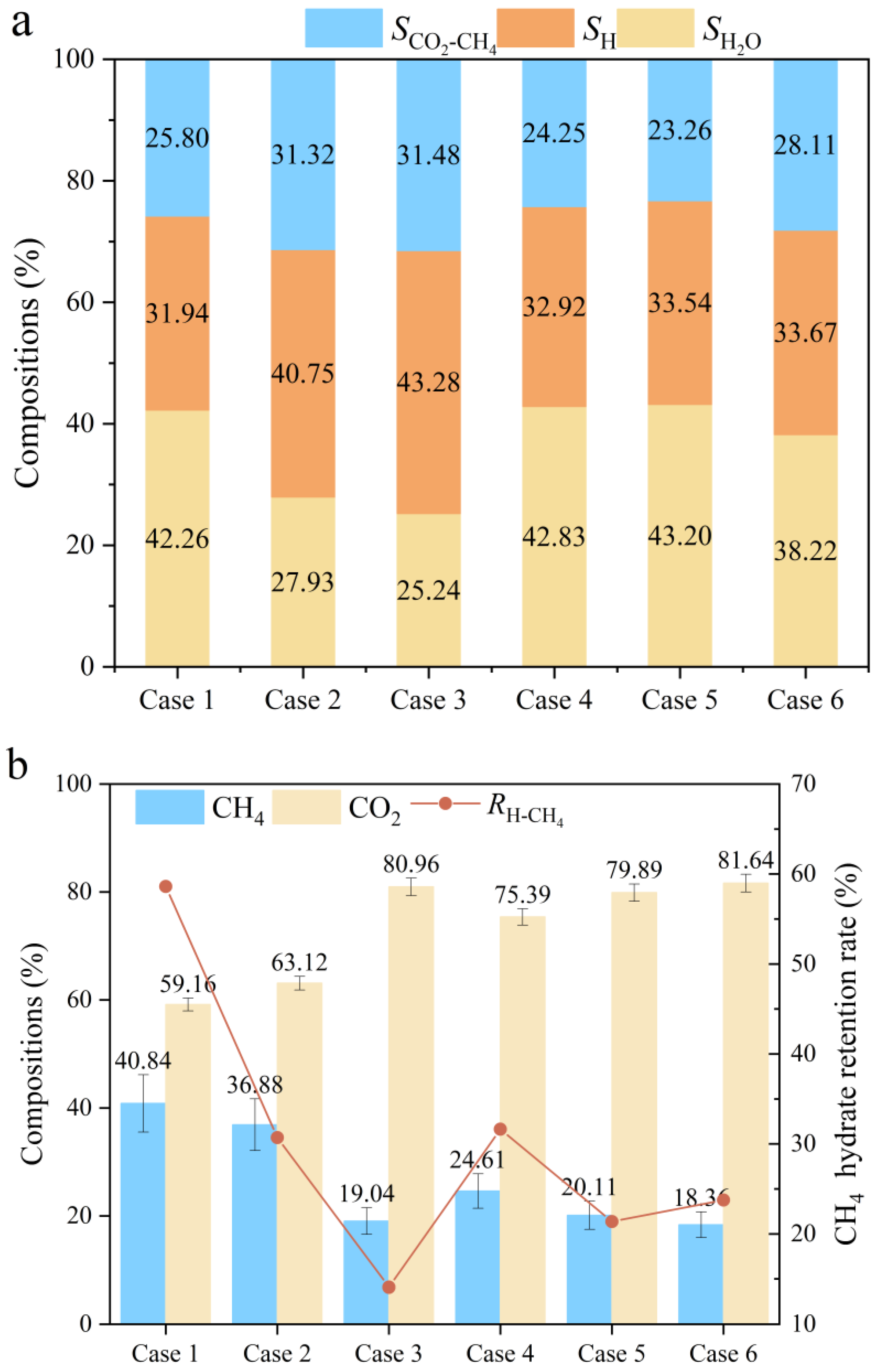
3. Results
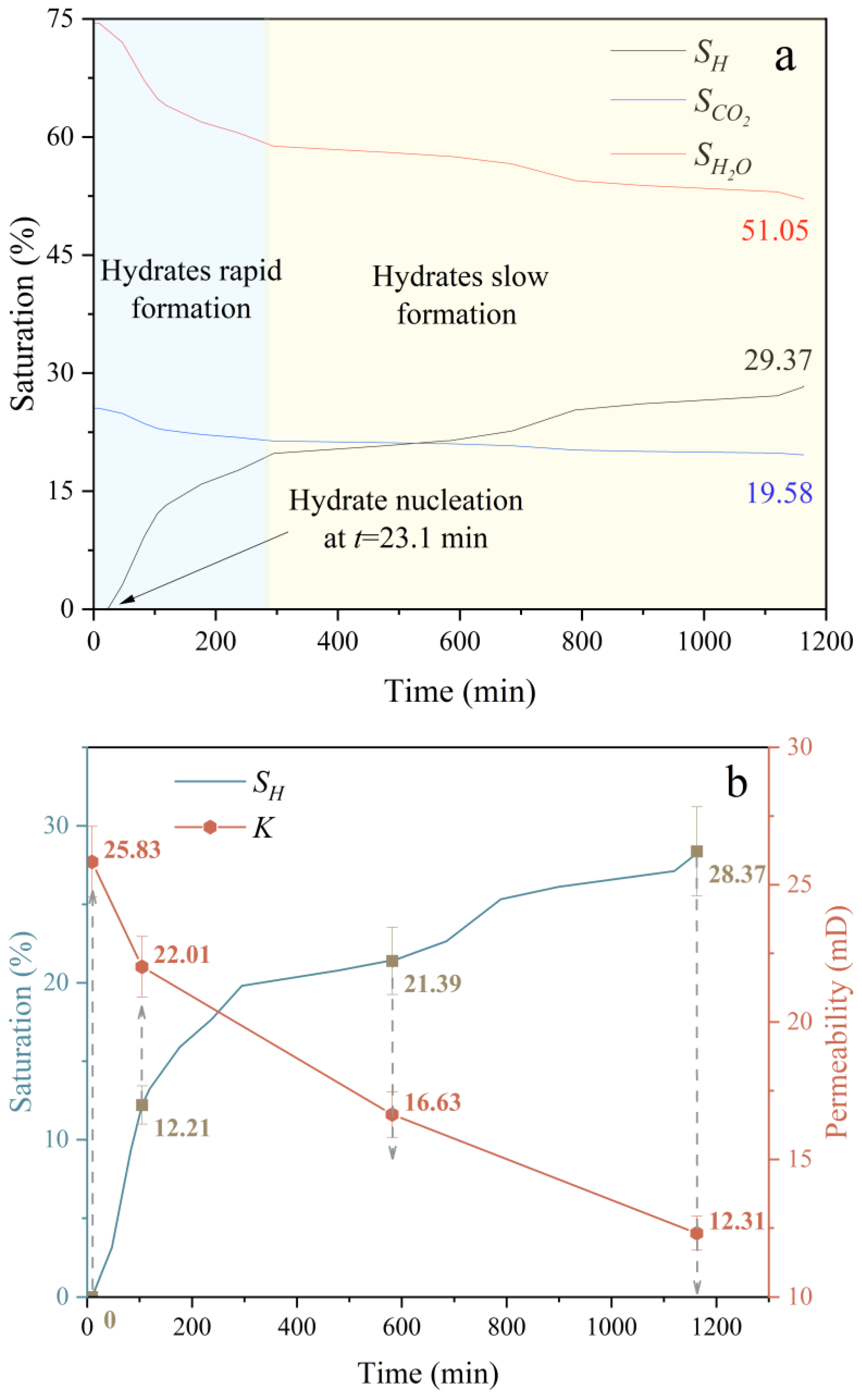
3.1. Hydrate Formation and Replacement
3.2. Depressurization
3.3. Mix-H Formation
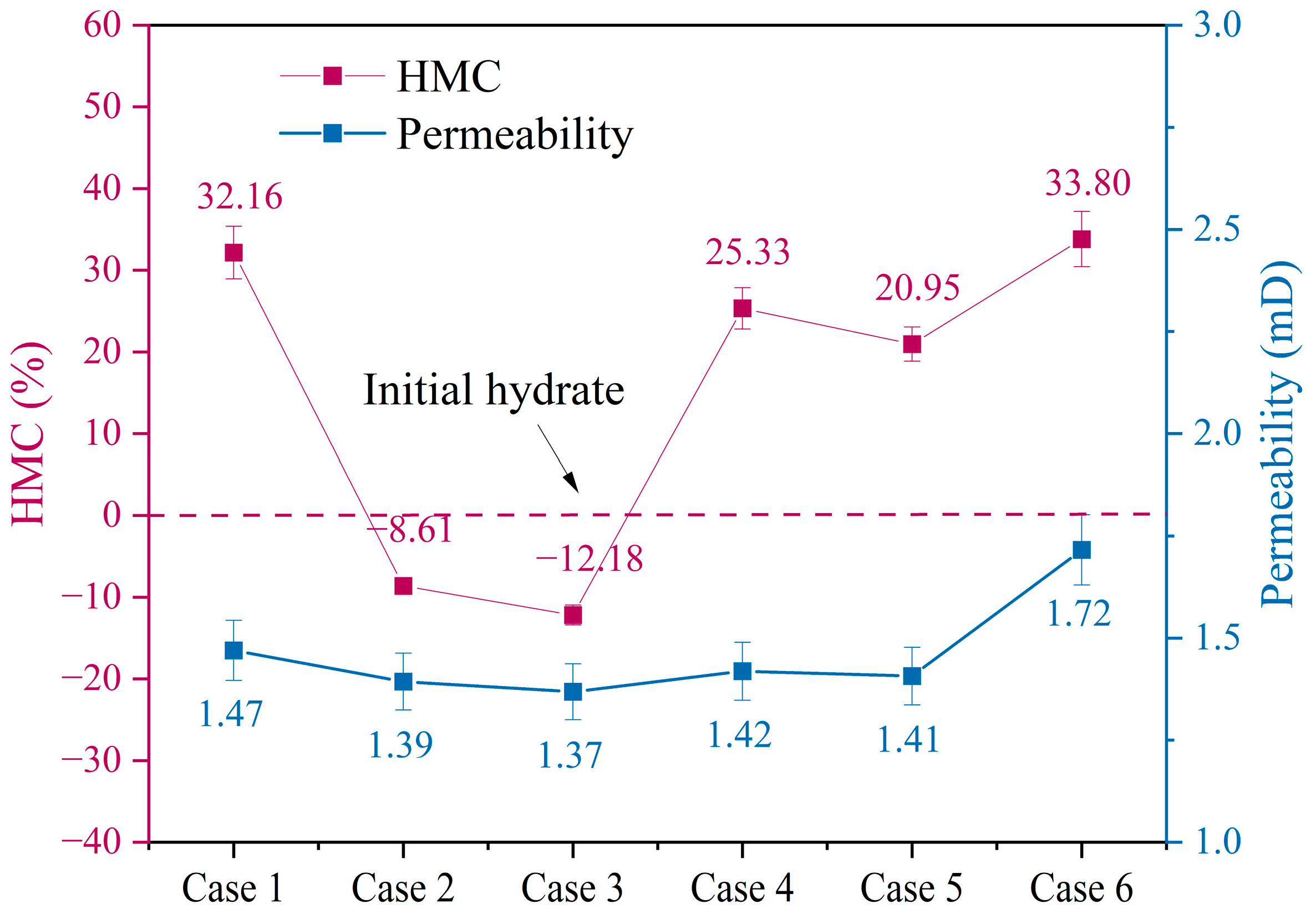
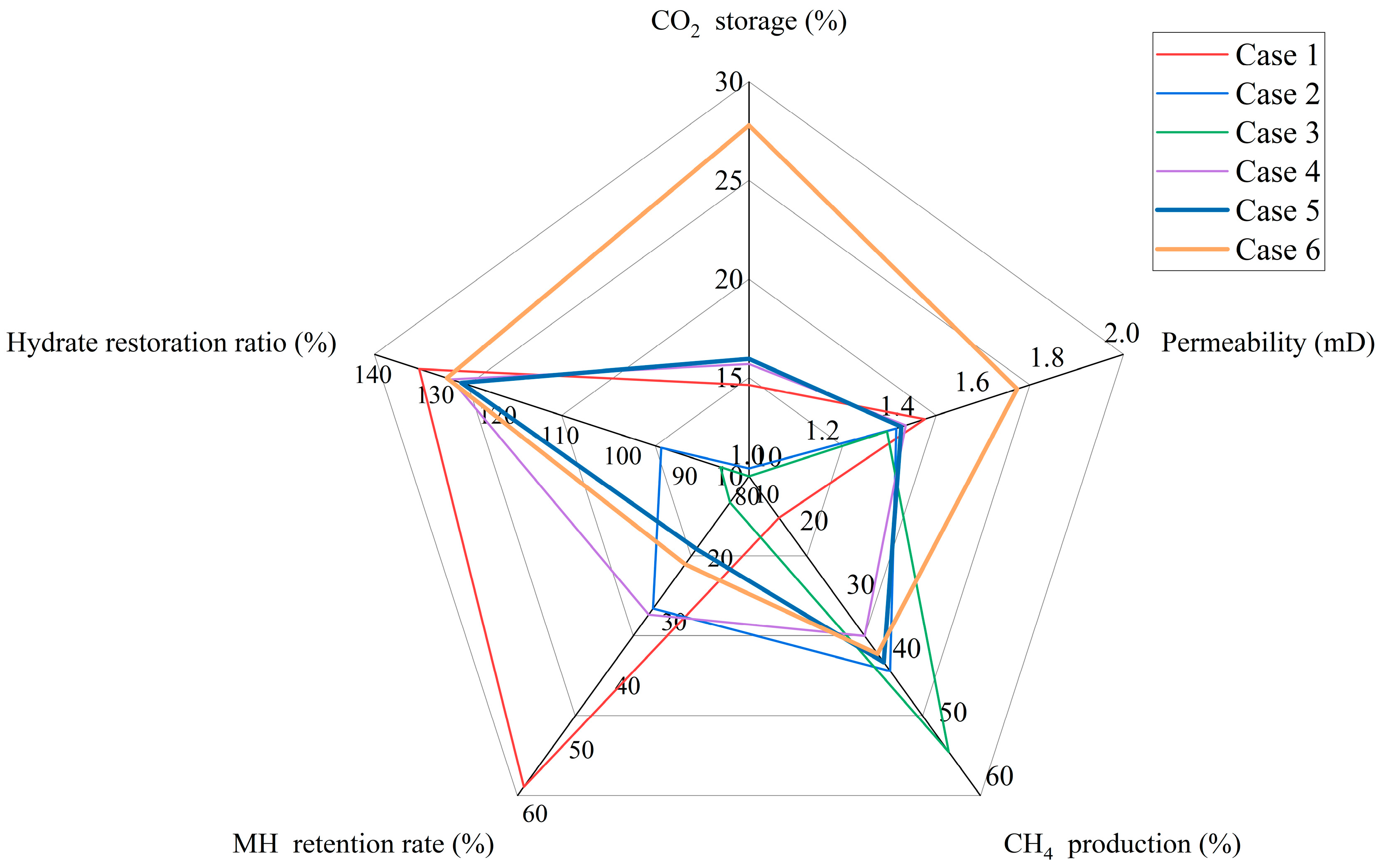
4. Discussion
5. Conclusions
- (1)
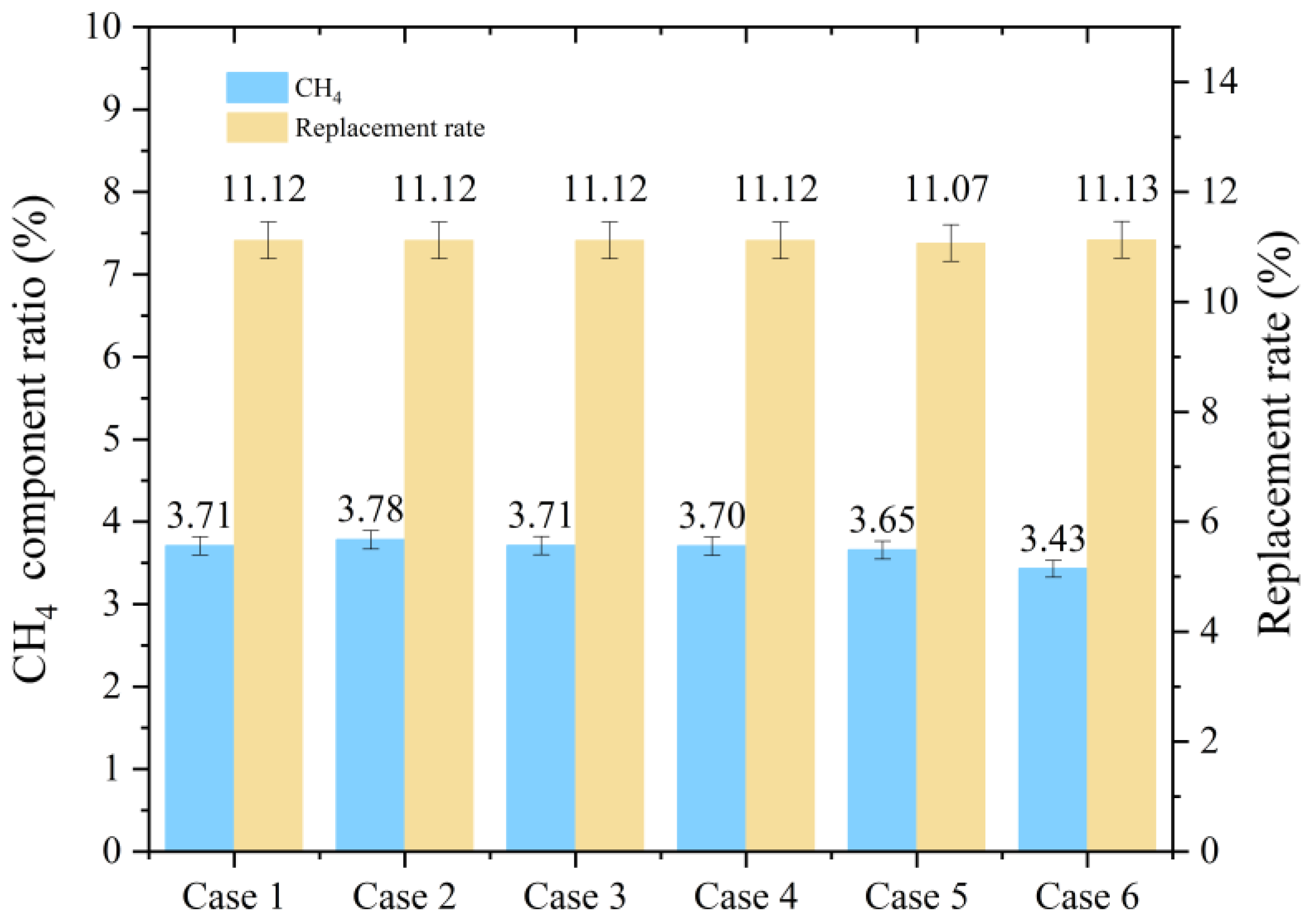
- In the seabed environment, the period during which hydrates form rapidly is very short (approximately 300 min). When injecting liquid carbon dioxide into the reservoir, a replacement rate of about 11.1% is insufficient to meet the requirements for extracting methane and sealing carbon dioxide.
- (2)
- Decreasing the depressurization pressure can increase gas production. However, an excessively large depressurization pressure will cause the PDR to reach 76.40%, which is not conducive to the continuous progress of the project. Increasing the depressurization pressure and extending the depressurization pressure time can effectively increase the gas production rate and protect the reservoir permeability.
- (3)
- Lowering the depressurization pressure will reduce the final hydrate saturation to 25.24%. Extending the reduction time can achieve a final hydrate saturation of 43.20%. A greater final hydrate saturation means higher reservoir stability and more sealed carbon dioxide.
- (4)
- Using the method of depressurization coupled with injecting CO2 essentially increases the reduction time, enabling the average reservoir permeability to reach 1.72 millidarcies and the CO2 storage rate to reach 27.7%. At the same time, it avoids the repeated implementation of pressure reduction and CO2 injection, reducing the complexity of the project.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NGH | Natural gas hydrate |
| MH | Methane hydrate |
| HDP | Hydrate depressurization pressure (MPa) |
| HS | Hydrate saturation (%) |
| P | Reactor pressure (MPa) |
| SH | Hydrate saturation (%) |
| Mix-H | Mixed hydrate |
| Smix-H | Mix-H saturation (%) |
| nH | Consumption of CH4 (mol) |
| MHC | Hydrate molar quantity change ratio (%) |
| MH | Molar mass (g/mol) |
| ρH | Density (g/mol) |
| φ | Porosity |
| K0 | Permeability of the sample (%) |
| RET | MH retention rate (%) |
| RES | Hydrate restoration ratio (%) |
| PDR | Permeability damage rate (%) |
References
- Zantye, M.S.; Arora, A.; Hasan, M.M.F. Renewable-integrated flexible carbon capture: A synergistic path forward to clean energy future. Energy Environ. Sci. 2021, 14, 3986–4008. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; He, G.; Wang, H.; Zhang, X.; Lin, J.; Qi, Y.; Liang, X. Challenges and opportunities for carbon neutrality in China. Nat. Rev. Earth Environ. 2022, 3, 141–155. [Google Scholar] [CrossRef]
- Raza, A.; Mahmoud, M.; Arif, M.; Alafnan, S. Predictive Power of Theoretical Adsorption Models for Gases (H2, CO2 and CH4) in Overmature Kerogen. Arab. J. Sci. Eng. 2023, 48, 16319–16327. [Google Scholar] [CrossRef]
- Janjua, A.N.; Mahmoud, M.; Patil, S.; Raza, A.; Alafnan, S.; Kamal, M.S. Selectivity of Adsorption Isotherms based on Fluid and Rock Types: Insights into Carbon Sequestration. In Proceedings of the Fourth EAGE/AAPG Hydrocarbon Seals Workshop 2024, Al Khobar, Saudi Arabia, 13–15 May 2024; Volume 2024, pp. 1–15. [Google Scholar] [CrossRef]
- Glatz, G.; Alafnan, S.; Raza, A.; Mahmoud, M. Multicomponent Gas Adsorption Behavior of Kerogen: A Molecular Investigation. Energy Fuels 2022, 36, 6695–6710. [Google Scholar] [CrossRef]
- Raza, A. Theoretical Adsorption Models for the Methane–Shale System. In Proceedings of the Middle East Oil, Gas and Geosciences Show (MEOS GEO), Manama, Bahrain, 16 September 2025; p. D021S040R002. [Google Scholar]
- Glatz, G.; Alafnan, S.; Gholami, R.; Raza, A.; Mahmoud, M.; Al-Azani, K.; Awotunde, A. Effect of kerogen maturity on the adsorption capacity of CO2 and CH4: A molecular investigation. Fuel 2022, 327, 125188. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Q.; Li, S.; Li, X.; Ye, J.; Zhang, Y.; Ye, H.; Kuang, Y.; Zheng, Y. Electrical Resistivity Tomography Methods and Technical Research for Hydrate-Based Carbon Sequestration. J. Mar. Sci. Eng. 2025, 13, 1205. [Google Scholar] [CrossRef]
- Zhang, W.; Su, P.; Wang, J.; Liang, Q. Advances in Marine Gas Hydrate Exploration and Discovery. J. Mar. Sci. Eng. 2025, 13, 1689. [Google Scholar] [CrossRef]
- Seo, Y.-j.; Park, S.; Kang, H.; Ahn, Y.-H.; Lim, D.; Kim, S.-J.; Lee, J.; Lee, J.Y.; Ahn, T.; Seo, Y.; et al. Isostructural and cage-specific replacement occurring in sII hydrate with external CO2/N2 gas and its implications for natural gas production and CO2 storage. Appl. Energy 2016, 178, 579–586. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Long, Z.; Wen, H.; Fan, S.; Liang, D. Effect of CO2 Thickeners on CH4-CO2 Replacement in Hydrate-Bearing Sediment. J. Mar. Sci. Eng. 2024, 12, 1861. [Google Scholar] [CrossRef]
- Mu, L.; Zhou, Z.; Tan, Q.; Li, X.; Zhao, H.; Cui, Q. Experimental investigation on the methane storage by forming sH hydrate. J. Energy Storage 2024, 77, 109940. [Google Scholar] [CrossRef]
- Li, B.; Xu, T.; Zhang, G.; Guo, W.; Liu, H.; Wang, Q.; Qu, L.; Sun, Y. An experimental study on gas production from fracture-filled hydrate by CO2 and CO2/N2 replacement. Energy Convers. Manag. 2018, 165, 738–747. [Google Scholar] [CrossRef]
- Liu, J.; Kong, L.; Zhao, Y.; Sang, S.; Niu, G.; Wang, X.; Zhou, C. Test research progress on mechanical and physical properties of hydrate-bearing sediments. Int. J. Hydrogen Energy 2024, 53, 562–581. [Google Scholar] [CrossRef]
- Tupsakhare, S.S.; Castaldi, M.J. Efficiency enhancements in methane recovery from natural gas hydrates using injection of CO2/N2 gas mixture simulating in-situ combustion. Appl. Energy 2019, 236, 825–836. [Google Scholar] [CrossRef]
- Salamatin, A.N.; Falenty, A.; Kuhs, W.F. Diffusion Model for Gas Replacement in an Isostructural CH4-CO2 Hydrate System. J. Phys. Chem. C 2017, 121, 17603–17616. [Google Scholar] [CrossRef]
- Niu, M.; Yin, Z.; Sun, Y.; Fang, W.; Chen, G.; Chen, D. CH4 hydrate production coupled with CO2 sequestration and hydrate restoration employing depressurization assisted by CO2-N2 injection at marine conditions. Chem. Eng. J. 2023, 456, 140981. [Google Scholar] [CrossRef]
- Niu, M.; Yao, Y.; Yin, Z.; Liu, K.; Bian, P.; Zi, M.; Chen, D. Synergistic CH4 hydrate recovery and CO2 storage by coupling depressurization with CO2/N2 injection: A pilot-scale investigation. Chem. Eng. J. 2023, 475, 146216. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Song, Y.; Zhu, Z.; Yang, L.; Tian, Y.; Wang, J.; Yang, M.; Zhang, Y. Experimental Study on a Novel Way of Methane Hydrates Recovery: Combining CO2 Replacement and Depressurization. Energy Procedia 2014, 61, 75–79. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, L.; Linga, P. Effect of wellbore design on the production behaviour of methane hydrate-bearing sediments induced by depressurization. Appl. Energy 2019, 254, 113635. [Google Scholar] [CrossRef]
- Choi, W.; Mok, J.; Lee, J.; Lee, Y.; Lee, J.; Sum, A.K.; Seo, Y. Effective CH4 production and novel CO2 storage through depressurization-assisted replacement in natural gas hydrate-bearing sediment. Appl. Energy 2022, 326, 119971. [Google Scholar] [CrossRef]
- Lee, Y.; Deusner, C.; Kossel, E.; Choi, W.; Seo, Y.; Haeckel, M. Influence of CH4 hydrate exploitation using depressurization and replacement methods on mechanical strength of hydrate-bearing sediment. Appl. Energy 2020, 277, 115569. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.; Dong, B.; Li, M.; Yang, L.; Dong, H.; Zhang, L.; Zhao, J.; Song, Y. Pressure oscillation controlled CH4/CO2 replacement in methane hydrates: CH4 recovery, CO2 storage, and their characteristics. Chem. Eng. J. 2021, 425, 129709. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Huang, Y.; Xu, Z.; Liu, X.; Jiang, S.; You, X.; Wang, Y.; Zhong, X.; Chen, C. Research progress on hydrate replacement mechanism and enhancement methods: A review. Gas Sci. Eng. 2025, 139, 205631. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Zi, M.; Ye, H.; Chen, D. The role of phase saturation in depressurization and CO2 storage in natural gas hydrate reservoirs: Insights from a pilot-scale study. Chem. Eng. J. 2024, 499, 156444. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Sun, Y.; Chen, D. Significance of well placement and degree of hydrate reserve recovery for synergistic CH4 recovery and CO2 storage in marine heterogeneous hydrate-bearing sediments. Geoenergy Sci. Eng. 2024, 242, 213290. [Google Scholar] [CrossRef]
- Yao, Y.; Niu, M.; Zi, M.; Ye, H.; Duan, J.; Chen, D. CO2/CH4 hydrate formation from CO2/CH4 liquid mixtures: A key to CO2 sequestration in marine depleted hydrate and shallow gas reservoirs via liquid CO2 injection. Chem. Eng. J. 2025, 509, 161169. [Google Scholar] [CrossRef]
- Yao, Y.; Zi, M.; Niu, M.; Ye, H.; Duan, J.; Chen, D. Investigation of scaling criteria and multiscale experiments for hydrate-based CO2 sequestration in marine sediment and depleted NGH reservoir via CO2/N2 injection. Chem. Eng. Sci. 2025, 316, 121912. [Google Scholar] [CrossRef]
- Ye, H.; Chen, J.; Yao, Y.; Dong, P.; Chen, D.; Niu, M.; Duan, J.; Wu, X.; Li, D.; Jiang, Y.; et al. How injecting CO2 enhances marine natural gas hydrate exploitation: Review and prospect. Renew. Sustain. Energy Rev. 2025, 222, 116011. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, X.; Luan, H.; Wu, X.; Wang, C.; Shan, Q.; Cheng, X. Numerical simulation on natural gas hydrate depressurization production considering sediment compression effects. Energy 2024, 301, 131745. [Google Scholar] [CrossRef]
- Shankar, U.; Riedel, M. Assessment of gas hydrate saturation in marine sediments from resistivity and compressional-wave velocity log measurements in the Mahanadi Basin, India. Mar. Pet. Geol. 2014, 58, 265–277. [Google Scholar] [CrossRef]
- Liu, C.; Ye, Y.; Meng, Q.; He, X.; Lu, H.; Zhang, J.; Liu, J.; Yang, S. The Characteristics of Gas Hydrates Recovered from Shenhu Area in the South China Sea. Mar. Geol. 2012, 307–310, 22–27. [Google Scholar] [CrossRef]
- Ning, F.; Liang, J.; Wu, N.; Zhu, Y.; Wu, S.; Liu, C.; Wei, C.; Wang, D.; Zhang, Z.; Xu, M. Reservoir characteristics of natural gas hydrates in China. Nat. Gas Ind. 2020, 40, 1–24. [Google Scholar]
- Zhang, C.; Zhao, J.; Yang, D.; Gao, Q. Permeability Evolution in Hydrate-Bearing Sediments during Hydrate Recovery by Depressurization Combined with Replacement. Energy Fuels 2025, 39, 9451–9462. [Google Scholar] [CrossRef]
- You, C.-Y.; Chen, Z.-Y.; Li, X.-S.; Xu, C.-G.; Peng, H.; Ji, H.-F. Liquid CO2-CH4 Hydrate Replacement Reaction above 281.15 K: Implication for CH4 Recovery and CO2 Sequestration in Marine Environments. Energy Fuels 2024, 38, 10813–10825. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Zhang, K.; Wang, L.; Ma, S.; Liu, Z.; Zhao, Z.; Wu, P.; Wu, Z. Experimental study on the impact of effective stress and cyclic loading on permeability of methane hydrate clayey sediments. Gas Sci. Eng. 2024, 124, 205282. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]







| Title 1 | Title 2 | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|---|
| Pend MPa | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | |
| Formation | Tend K | 285.15 | |||||
| Final SH % | 29.37 | 31.40 | 30.82 | 30.77 | 30.50 | 29.77 | |
| 1st Replacement | Pend MPa | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 |
| 1st Replacement | Final SMix-H % | 30.62 | 31.23 | 30.65 | 30.60 | 30.34 | 29.60 |
| 1st Depressurization | Pend MPa | 7.00 | 6.00 | 5.00 | 7.00 | 7.00 | 7.00 |
| 1st Depressurization | Final SMix-H % | 24.70 | 21.61 | 18.99 | 22.42 | 20.86 | 38.22 |
| 2nd Replacement | Pend MPa | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | |
| 2nd Replacement | Final SMix-H % | 39.82 | 36.63 | 35.09 | 41.36 | 38.29 | |
| 2nd Depressurization | Pend MPa | 7.00 | 6.00 | 5.00 | 7.00 | 7.00 | |
| 2nd Depressurization | Final SMix-H % | 34.64 | 26.89 | 14.30 | 31.87 | 31.75 | |
| 3rd Replacement | Pend MPa | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | |
| 3rd Replacement | Final SMix-H % | 42.26 | 27.93 | 25.24 | 42.83 | 43.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhao, J.; Yang, D.; Gao, Q. Experimental Study on CO2 Sequestration in Marine Environments During Hydrate Recovery by Depressurization Combined with Replacement. J. Mar. Sci. Eng. 2025, 13, 1977. https://doi.org/10.3390/jmse13101977
Zhang C, Zhao J, Yang D, Gao Q. Experimental Study on CO2 Sequestration in Marine Environments During Hydrate Recovery by Depressurization Combined with Replacement. Journal of Marine Science and Engineering. 2025; 13(10):1977. https://doi.org/10.3390/jmse13101977
Chicago/Turabian StyleZhang, Chi, Jianzhong Zhao, Dong Yang, and Qiang Gao. 2025. "Experimental Study on CO2 Sequestration in Marine Environments During Hydrate Recovery by Depressurization Combined with Replacement" Journal of Marine Science and Engineering 13, no. 10: 1977. https://doi.org/10.3390/jmse13101977
APA StyleZhang, C., Zhao, J., Yang, D., & Gao, Q. (2025). Experimental Study on CO2 Sequestration in Marine Environments During Hydrate Recovery by Depressurization Combined with Replacement. Journal of Marine Science and Engineering, 13(10), 1977. https://doi.org/10.3390/jmse13101977






