Simple Fabrication and Biological Evaluation of Ulva australis-Derived Marine Carbon Dots with Anti-Inflammation, Anti-Oxidation, and Anti-Adipogenesis Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CDs Using U. australis and Other Marine Algae
2.3. Morphological Properties of MCDs from U. australis
2.4. Investigation of Optical Characteristics of MCDs from U. australis
2.5. Functional Bioactivity Assays of MCDs
2.5.1. Anti-Inflammatory Activity
2.5.2. Anti-Adipogenic Activity
2.5.3. Antioxidant Activity
2.6. Toxicity and Activity Test in Zebrafish
2.7. Statistical Analysis
3. Results and Discussion
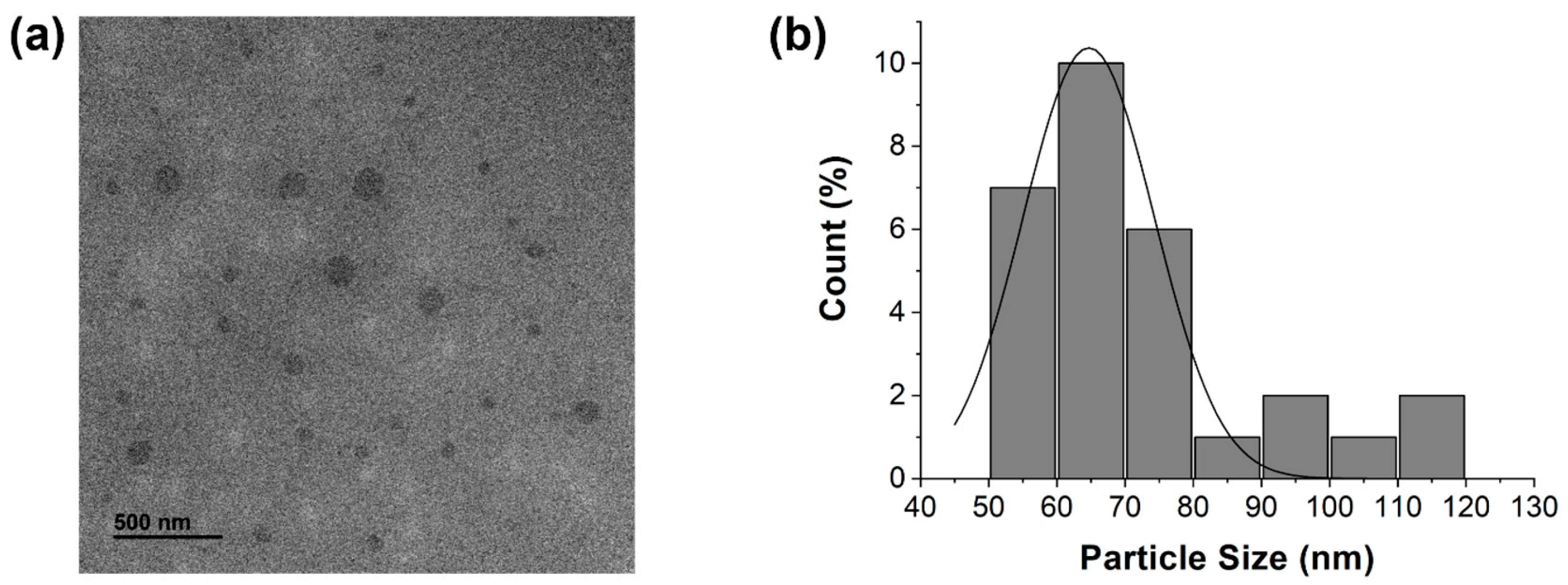
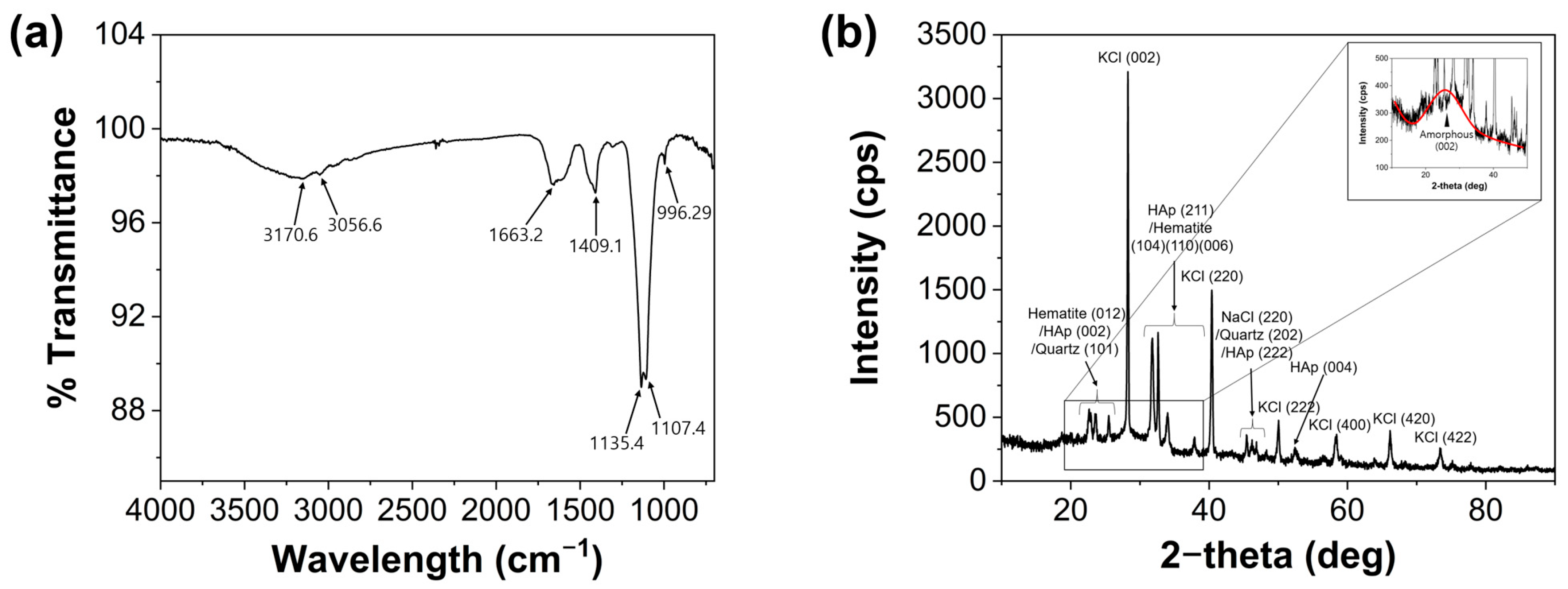
3.1. Morphological Properties of MCDs
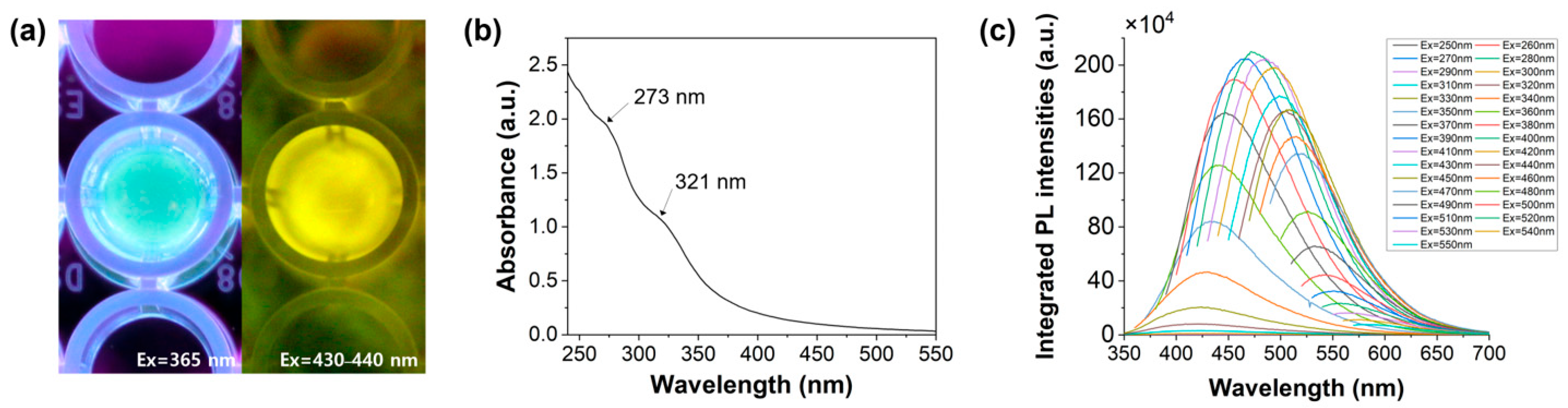
3.2. Optical Properties of MCDs
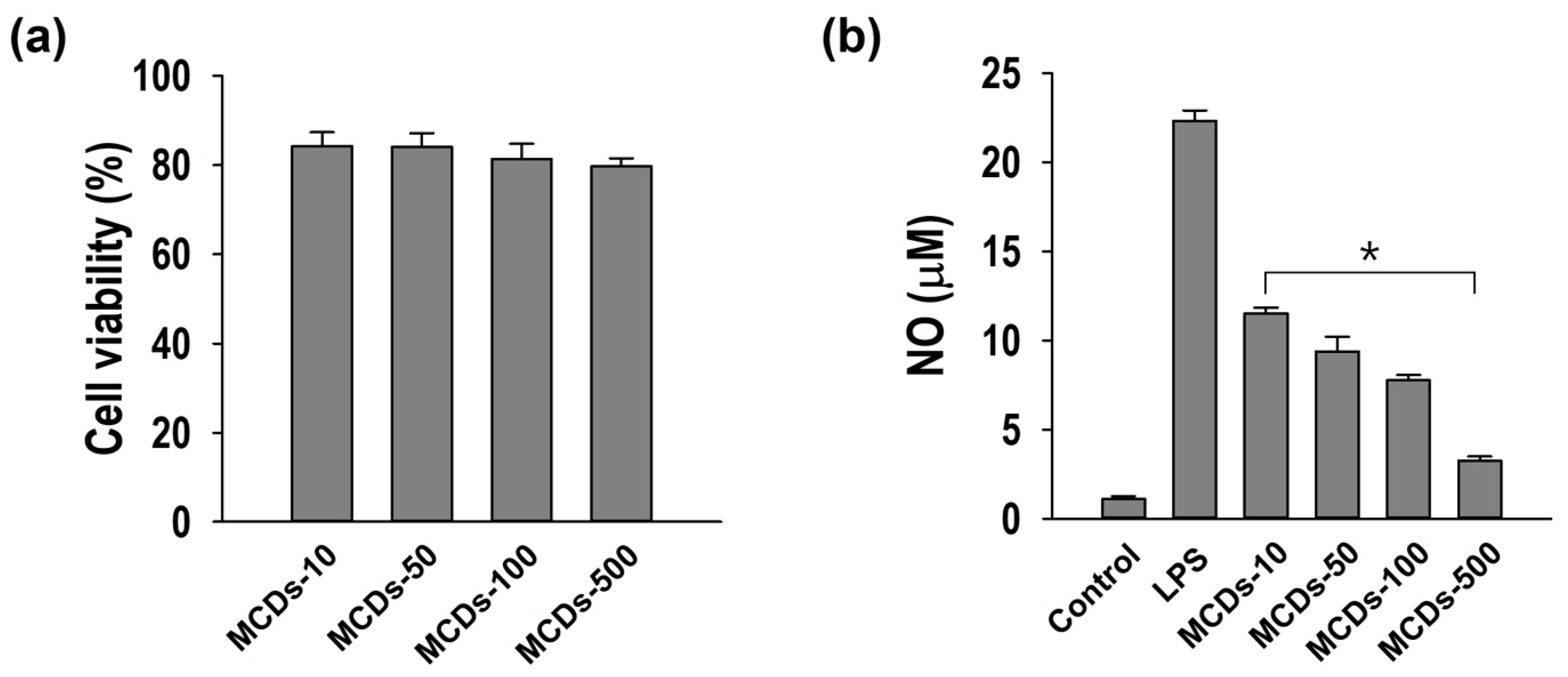
3.3. Anti-Inflammatory Activity of MCDs in RAW264.7 Macrophages
3.4. Anti-Adipogenic Effects of MCDs in 3T3-L1 Adipocytes
3.5. Inhibition of LPS-Induced ROS by MCDs
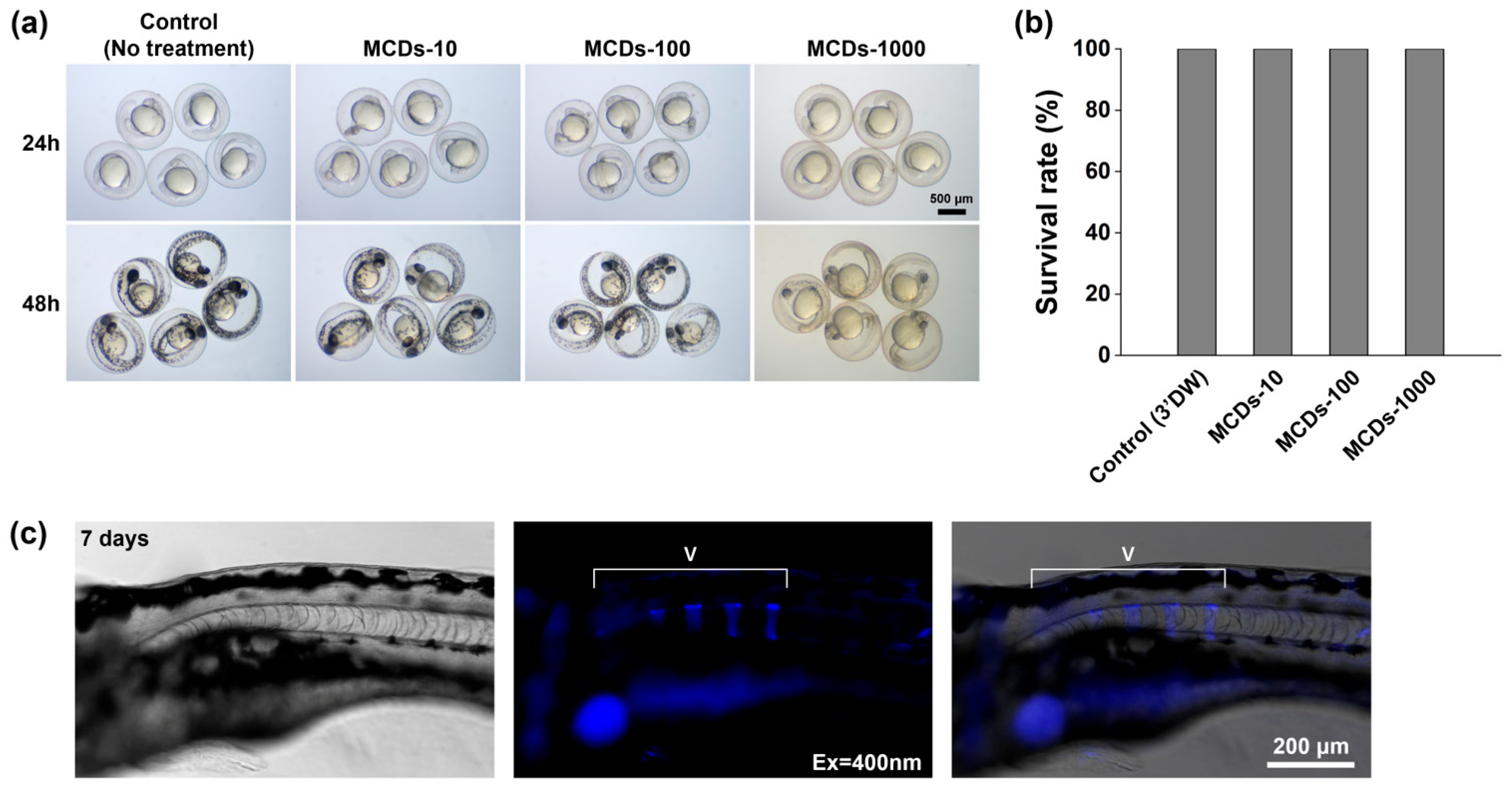
3.6. Toxicity and Activity of MCDs in Zebrafish
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDs | Carbon dots |
| DCFDA | 2′,7′-dichlorofluorescin diacetate |
| DMSO | Dimethyl sulfoxide |
| FT-IR | Fourier-transform infrared |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| HAp | Hydroxyapatite |
| IBMX | Dexamethasone, 3-Isobutyl-1-methylxanthine |
| MCDs | Marine carbon dots |
| LPS | Lipopolysaccharide |
| iNOS | Inducible nitric oxide synthase |
| PL | Photoluminescence |
| TEM | Transmission electron microscopy |
| ROS | Reactive oxygen species |
References
- Naomi, R.; Teoh, S.H.; Embong, H.; Balan, S.S.; Othman, F.; Bahari, H.; Yazid, M.D. The role of oxidative stress and inflammation in obesity and its impact on cognitive impairments—A narrative review. Antioxidants 2023, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Meizlish, M.L.; Franklin, R.A.; Zhou, X.; Medzhitov, R. Tissue homeostasis and inflammation. Annu. Rev. Immunol. 2021, 39, 557–581. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.C.; Tareq, H.S.; Suggs, L.J. Inflammation: A matter of immune cell life and death. npj Biomed. Innov. 2025, 2, 7. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute inflammation and metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Kang, J.Y.; Sun, J.S.; Kim, K.W. Hypothalamic Inflammation and Obesity: A Mechanistic Review. Arch. Pharm. Res. 2019, 42, 379–392. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxabide Etxeberria, A.; Alhadi, A.A.; Masoomi Dezfooli, S.; Muhd Julkapli, N.B.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef]
- Samrot, A.V.; Singh, S.P.R.; Deenadhayalan, R.; Rajesh, V.V.; Padmanaban, S.; Radhakrishnan, K. Nanoparticles, a Double-Edged Sword with Oxidant as Well as Antioxidant Properties—A Review. Oxygen 2022, 2, 591–604. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Shah, S.T.; Chowdhury, Z.Z.; Simarani, K.; Basirun, W.J.; Badruddin, I.A.; Hussien, M.; Alrobei, H.; Kamangar, S. Nanoantioxidants: The Fourth Generation of Antioxidants—Recent Research Roadmap and Future Perspectives. Coatings 2022, 12, 1568. [Google Scholar] [CrossRef]
- Gedda, G.; Sankaranarayanan, S.A.; Putta, C.L.; Gudimella, K.K.; Rengan, A.K.; Girma, W.M. Green Synthesis of Multi-Functional Carbon Dots from Medicinal Plant Leaves for Antimicrobial, Antioxidant, and Bioimaging Applications. Sci. Rep. 2023, 13, 6371. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Stagi, L. Carbon dots as oxidant–antioxidant nanomaterials, understanding the structure–properties relationship. A critical review. Nano Today 2023, 50, 101837. [Google Scholar] [CrossRef]
- Ji, Z.; Sheardy, A.; Zeng, Z.; Zhang, W.; Chevva, H.; Allado, K.; Yin, Z.; Wei, J. Tuning the functional groups on carbon nanodots and antioxidant studies. Molecules 2019, 24, 152. [Google Scholar] [CrossRef] [PubMed]
- Behi, M.; Gholami, L.; Naficy, S.; Palomba, S.; Dehghani, F. Carbon dots: A novel platform for biomedical applications. Nanoscale Adv. 2022, 4, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Lee, S.-T. Carbon dots: Advances in nanocarbons applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Rajesh, G.A.; John, V.L.; Santhosh, A.P.; Ambika, A.K.N.; Puthiyedath, V.T. Carbon Dots from Natural Sources for Biomedical Applications. Part. Part. Syst. Charact. 2022, 39, 2200017. [Google Scholar] [CrossRef]
- Kim, K.W.; Choi, T.-Y.; Kwon, Y.M.; Kim, J.Y.H. Simple Synthesis of Photoluminescent Carbon Dots from a Marine Polysaccharide Found in Shark Cartilage. Electron. J. Biotechnol. 2020, 47, 36–42. [Google Scholar] [CrossRef]
- Kim, K.W.; Kwon, Y.M.; Kim, S.Y.; Kim, J.Y.H. One-Pot Synthesis of UV-Protective Carbon Nanodots from Sea Cauliflower (Leathesia difformis). Electron. J. Biotechnol. 2022, 56, 22–30. [Google Scholar] [CrossRef]
- Lin, X.; Xiong, M.; Zhang, J.; He, C.; Ma, X.; Zhang, H.; Kuang, Y.; Yang, M.; Huang, Q. Carbon Dots Based on Natural Resources: Synthesis and Applications in Sensors. Microchem. J. 2020, 159, 105604. [Google Scholar] [CrossRef]
- Xiang, S.; Tan, M. Carbon Dots Derived from Natural Sources and Their Biological and Environmental Impacts. Environ. Sci. Nano 2022, 9, 3206–3225. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kim, H.-R.; Yeo, M.-H.; Kim, S.C.; Hyun, H.-B.; Ham, Y.-M.; Jung, Y.-H.; Kim, H.-S.; Chang, K.-S. Anti-obesity potential of Sargassum horneri and Ulva australis extracts: Study in vitro and in vivo. Appl. Sci. 2023, 13, 8951. [Google Scholar] [CrossRef]
- Li, G.-L.; Wang, R.-R.; Hou, Y.-X.; Guo, W.-J.; Wang, G.-B.; Liu, K.; Liu, Y.; Wu, W. Sterols from the green alga Ulva australis and their human recombinant aldose reductase iinhibitory activities. Mar. Drugs 2017, 15, 299. [Google Scholar] [CrossRef] [PubMed]
- Trentin, R.; Custódio, L.; Rodrigues, M.J.; Moschin, E.; Sciuto, K.; da Silva, J.P.; Moro, I. Biotechnological potential of the invasive alga Ulva australis collected in the Venice Lagoon (Italy). Algal Res. 2023, 69, 102969. [Google Scholar] [CrossRef]
- Lee, H.; Kim, G.; Depuydt, S.; Shin, K.; Han, T.; Park, J. Metal toxicity across different thallus sections of the green macroalga, Ulva australis. Toxics 2023, 11, 548. [Google Scholar] [CrossRef]
- Wu, X. Guidelines for anti-inflammatory assays in RAW264.7 cells. Food Saf. Health 2025, 3, 128–136. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef]
- Truskewycz, A.; Yin, H.; Halberg, N.; Lai, D.T.H.; Ball, A.S.; Truong, V.K.; Rybicka, A.M.; Cole, I. Carbon dot therapeutic platforms: Administration, distribution, metabolism, excretion, toxicity, and therapeutic potential. Small 2022, 18, e2106342. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Carbon dots: Classification, properties, synthesis, characterization, and applications in health care—An updated review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef]
- Manzoor, S.; Dar, A.H.; Dash, K.K.; Singh, S.; Choudhury, M.D.; Nayak, B. Carbon dots: A novel photoluminescent nanomaterial for multifaceted applications. Appl. Food Res. 2023, 3, 100263. [Google Scholar] [CrossRef]
- Yuan, F.; Li, X.; Li, Y.; Fan, L.; Yang, S. Engineering Carbon Dots for Photonics and Optoelectronics. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent Carbon Dots and Their Sensing Applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Zavidovskiy, I.A.; Streletskiy, O.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Savchenko, N.F.; Tatarintsev, A.A.; Pavlikov, A.V. Highly Selective Polyene-Polyyne Resistive Gas Sensors: Response Tuning by Low-Energy Ion Irradiation. J. Compos. Sci. 2023, 7, 156. [Google Scholar] [CrossRef]
- Ozyurt, D.; Al Kobaisi, M.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Deng, H.; Yao, S.; Wang, Y. Regulatory synthesis and characterization of hydroxyapatite nanocrystals by a microwave-assisted hydrothermal method. Ceram. Int. 2020, 46, 2185–2193. [Google Scholar] [CrossRef]
- Ismail, S.N.; Ali, E.; Alwan, B.J.; Abd, A.N. Potassium Chloride Nanoparticles: Synthesis, Characterization, and Study the Antimicrobial Applications. Macromol. Symp. 2022, 401, 2100312. [Google Scholar] [CrossRef]
- Damodar, D.; Kumar, S.K.; Martha, S.K.; Deshpande, A.S. Nitrogen-doped graphene-like carbon nanosheets from commercial glue: Morphology, phase evolution and Li-ion battery performance. Dalton Trans. 2018, 47, 12218–12227. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Javed, S.; Javed, H.M.A.; Jamshaid, M.; Ali, U.; Akram, M.A. Systematic Investigation of Structural, Morphological, Thermal, Optoelectronic, and Magnetic Properties of High-Purity Hematite/Magnetite Nanoparticles for Optoelectronics. Nanomaterials 2022, 12, 1635. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Hu, Y.; He, J.; Tian, M.; Zhou, J.; Zhou, Q.; Chen, S.; Chen, D.; Chen, P.; et al. Novel insights into the hydroxylation behaviors of α-quartz (101) surface and its effects on the adsorption of sodium oleate. Minerals 2019, 9, 450. [Google Scholar] [CrossRef]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-derived carbon dots and their applications. Energy Environ. Mater. 2019, 2, 172–192. [Google Scholar] [CrossRef]
- Quraishi, S.; Plappert, S.; Ungerer, B.; Taupe, P.; Gindl-Altmutter, W.; Liebner, F. Preparation and Characterization of Bacterial Cellulose-Carbon Dot Hybrid Nanopaper for Potential Sensing Applications. Appl. Sci. 2019, 9, 107. [Google Scholar] [CrossRef]
- Farahani, A.; Farahani, A.; Kashfi, K.; Ghasemi, A. Inducible nitric oxide synthase (iNOS): More than an inducible enzyme? Rethinking the classification of NOS isoforms. Pharmacol. Res. 2025, 216, 107781. [Google Scholar] [CrossRef]
- Kumar, M.; Chinnathambi, S.; Bakhori, N.; Abu, N.; Etezadi, F.; Thangavel, V.; Packwood, D.; Sivaniah, E.; Pandian, G.N. Biomass-derived carbon dots as fluorescent quantum probes to visualize and modulate inflammation. Sci. Rep. 2024, 14, 12665. [Google Scholar] [CrossRef]
- Habelreeh, H.H.; Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Maillard reaction-derived S-doped carbon dots promotes downregulation of PPARγ, C/EBPα, and SREBP-1 genes in-vitro. Molecules 2024, 29, 2008. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.W.; Oh, G.-W.; Jung, S.-H.; Ko, S.-C.; Kim, J.-Y.; Yang, D.; Jo, D.-M.; Lee, D.-S.; Choi, G. Simple Fabrication and Biological Evaluation of Ulva australis-Derived Marine Carbon Dots with Anti-Inflammation, Anti-Oxidation, and Anti-Adipogenesis Features. J. Mar. Sci. Eng. 2025, 13, 1878. https://doi.org/10.3390/jmse13101878
Kim KW, Oh G-W, Jung S-H, Ko S-C, Kim J-Y, Yang D, Jo D-M, Lee D-S, Choi G. Simple Fabrication and Biological Evaluation of Ulva australis-Derived Marine Carbon Dots with Anti-Inflammation, Anti-Oxidation, and Anti-Adipogenesis Features. Journal of Marine Science and Engineering. 2025; 13(10):1878. https://doi.org/10.3390/jmse13101878
Chicago/Turabian StyleKim, Kyung Woo, Gun-Woo Oh, Seung-Hyun Jung, Seok-Chun Ko, Ji-Yul Kim, Dongwoo Yang, Du-Min Jo, Dae-Sung Lee, and Grace Choi. 2025. "Simple Fabrication and Biological Evaluation of Ulva australis-Derived Marine Carbon Dots with Anti-Inflammation, Anti-Oxidation, and Anti-Adipogenesis Features" Journal of Marine Science and Engineering 13, no. 10: 1878. https://doi.org/10.3390/jmse13101878
APA StyleKim, K. W., Oh, G.-W., Jung, S.-H., Ko, S.-C., Kim, J.-Y., Yang, D., Jo, D.-M., Lee, D.-S., & Choi, G. (2025). Simple Fabrication and Biological Evaluation of Ulva australis-Derived Marine Carbon Dots with Anti-Inflammation, Anti-Oxidation, and Anti-Adipogenesis Features. Journal of Marine Science and Engineering, 13(10), 1878. https://doi.org/10.3390/jmse13101878




