Abstract
Microbial-Induced Calcium Carbonate Precipitation (MICP) is an environmentally friendly, efficient, and sustainable new soil reinforcement technology. For this study, Bacillus pasteurii were domesticated and cultured in a natural seawater environment with multiple gradients and used for coral reef calcareous sand reinforcement, comparing the mineral composition of the generated precipitates and the reinforcement strength under different domestication gradient conditions. The results revealed that, while the natural seawater environment inhibits the growth of Bacillus pasteurii, the gradient domestication method allows the bacteria to gradually adapt to the natural seawater environment. Notably, their shape becomes thin and long under the seawater environment. Furthermore, the MICP mineralisation reaction rate is faster in the natural seawater environment and, with an increase in the domestication gradient, the mineralisation reaction precipitates increased. At the same time, in the seawater environment, a small amount of mineral components were generated in addition to , such as , and the mineral content increased with an increase in the domestication gradient. When comparing the curing effect under different gradients in the natural seawater environment, it was found that the Bacillus pasteurii can effectively enhance the curing effect of the calcareous sand after multi-gradient domestication in the seawater environment, with the curing effect increasing with an increase in the domestication gradient. The results of this study provide new ideas for the application of MICP technology in seawater environments for the reinforcement of calcareous sand in the construction of South China Sea islands and reefs.
1. Introduction
In recent years, various countries have constructed a large number of artificial islands and reefs, as well as a series of defence and civil facilities in the relevant waters. Calcareous sand is a locally sourced building material used in the construction of island and reef projects in the South China Sea [1,2,3,4,5]. Calcareous sand is mainly deposited from the remains of marine organisms, such as coral and shells, and is mainly composed of calcium carbonate. It has an irregular morphology, angular appearance, high porosity, and is easy to break; thus, it is not conducive to directly use in engineering applications [6,7,8,9,10,11]. The traditional reinforcement methods mainly include physical tamping and vibratory ramming, using chemical cement mortar, etc., which have a high energy consumption, take a long time, are high-cost, and are prone to cause ecological and environmental pollution problems [12,13]. Microbial-Induced Calcium Carbonate Precipitation (MICP) is a new type of energy-saving, environmentally friendly, and sustainable soil reinforcement technology, utilising the accelerated decomposition of urea by urease-producing bacteria to produce and the mineralisation reaction of in the environment to form a calcium carbonate precipitate with a cementing effect. The calcium carbonate precipitate comprises sand grains cemented together, such that the loose soil body is bonded with a certain strength, thus improving the mechanical properties of the soil body, The reaction process is shown in Equations (1)–(5) [14,15,16,17,18]. MICP technology has the advantages of environmental protection, high efficiency, and sustainability, and it meets the requirements of island construction projects in the South China Sea [19,20].
Xiao et al. have investigated the particle fragmentation and compressibility behaviour of MICP-treated sand, and they found that MICP-treated specimens exhibited less particle fragmentation than untreated specimens, suggesting that MICP treatment can effectively inhibit particle fragmentation [21]. Xiao et al. have observed the kinetics of the MICP process through adjusting the concentration change of the calcium source in the cementing solution. Their results indicated the mechanism of bacterial diffusion and CaCO3 crystal growth, as well as its variation under different CaCl2 concentrations [22]. Wang et al. have investigated the effect of temperature on MICP-reinforced soils, and they showed that the effect of temperature on the growth of calcium carbonate in bio-reinforced soils was significant, where temperatures of 20–35 °C led to better curing of particles [23]. Safavizadeh et al., Al-Salloum et al., Liu et al., Mujah et al., Ghasemi et al., and Zhao et al. [24,25,26,27,28,29] have conducted research on the effects of various factors on the engineering properties of MICP-treated soils, and they have shown that various factors—including bacterial concentration, urease activity, cementation concentration, reaction time, and type of sand—have a significant effect on the MICP process and the engineering properties of bacterial and urease-treated sands. Numerous scholars, including Zheng Junjie, Liu Hanlong, and Liu Shiyu [30,31,32,33,34,35,36], have conducted in-depth studies on the mechanical properties of MICP-reinforced calcareous sand, and they have shown that the mechanical properties of calcareous sand after MICP treatment were significantly improved, indicating that this method can be applied for calcareous sand reinforcement on coral reefs.
As the main water resource on the islands and reefs in the South China Sea is natural seawater, scholars have begun to explore the effect of the MICP reinforcement of calcareous sand under seawater environments. Some scholars have conducted in-depth studies on the activity of micro-organisms in seawater environments; for example, Yu Zhenxing [37] and Dong Bowen et al. [38] have found that the seawater environment has an inhibitory effect on the growth of micro-organisms, and the activity of micro-organisms in the seawater environment was significantly weaker than that in a de-ionised water environment. Peng Jie et al. [15] and Ding Xunchen et al. [39] have found that a seawater environment inhibits the final production of calcium carbonate in the MICP process. Peng et al. [40] and Ou Yixi et al. [41] have discussed the effect on coral sand improvement in different water environments and found that the mechanical properties of the added solids in seawater environments decreased compared to those in freshwater environments. In addition, in order to improve the influence of the seawater environment on the microbial culture and the effect of calcareous sand consolidation, Xiao Yao et al. [42] have used the microbial domestication method to carry out an experimental study on a domestication culture of Bacillus pasteurii in an artificial seawater environment. They discussed the influence of microbial consolidation on the soil after domestication, and their results demonstrated that the adaptability of the domesticated bacteria to the seawater increased, effectively improving the reinforcement effect in calcareous sand, indicating that the microbial domestication method is not only a good solution to the problem of calcareous sand engineering, but also an effective solution to the considered problem. As such, the gradient domestication method of micro-organisms is considered to be feasible.
In summary, there have been few studies on the domestication of Bacillus pasteurii and the consolidation of calcareous sand in a seawater environment. Based on this, for this study, natural seawater was adopted to carry out a multi-gradient domestication culture test using Bacillus pasteurii, in order to explore the growth of Bacillus pasteurii before and after its domestication to natural seawater, as well as any morphological changes and its impact on the consolidation effect of calcareous sand. The research results provide new ideas for micro-organisms to improve calcareous sand in a seawater environment, which is of reference significance for the application of MICP in the fields of island reef construction and coastal protection, etc.
2. Materials and Methods
In this study, according to the marine environment in which the island reef project is located, and in order to accurately simulate the actual environment of the island reef project, the test was conducted using natural seawater and calcareous sand collected in the field from the South China Sea islands. In order to promote the hydrolysis reaction of urea in the gelling solution, the experiments were carried out using Bacillus pasteurii, which is commonly used in MICP studies, and is a Gram-positive bacterium with the advantages of non-pathogenicity and high urease activity [43]. Specific information on the test materials is given below.
2.1. Test Materials
- (1)
- Natural seawater: the seawater used in the experiment was obtained from the sea near Dadonghai, Sanya City, with a salinity content of 3.6% and a pH value of 8.25. The ionic composition and concentration of the seawater are shown in Table 1 [44].
 Table 1. Ionic composition and concentration of seawater.
Table 1. Ionic composition and concentration of seawater.
- (2)
- Calcareous sand: The calcareous sand used in the test was obtained from an island in the South China Sea. The grain size of the calcareous sand was screened with 0.08 mm, 0.16 mm, and 0.315 mm mesh sieves, and calcareous sand with grain sizes of 0.08–0.16 and 0.16–0.315 mm was selected for the reinforcing sand column in the test. The basic parameters of calcareous sand are shown in Table 2.
 Table 2. Basic parameters of calcareous sand.
Table 2. Basic parameters of calcareous sand.
- (3)
- Bacteria cultivation and cementation solution: The bacteria selected for the test were Bacillus pasteurii (No. 337394, BeNa Culture Collection) originating from Guangdong Province Microbial Strain Preservation Centre. Bacteria were vacuum-dried in the form of freeze-dried powder and stored in ampoules, and needed to be activated. They were inoculated into a solid culture medium in a sterile environment on a vertical purification workbench, put into a bio-chemical incubator to be constant-temperature cultured for 36 h, and then subcultured for 2–3 generations after 36 h, as shown in Figure 1a, and stored in a refrigerator for refrigeration, so that bacteria could be directly added to the liquid culture medium when needed in the experiment. According to the needs of the simulation of the experimental environment, the liquid medium was divided into two kinds of de-ionised water and seawater liquid media: the de-ionised water medium used sodium hydroxide solution to adjust the pH to 7.3, and the seawater medium did not adjust the pH. After the configuration was completed and placed in the autoclave sterilisation at 121 °C for 30 min, the media required for the bacterial culture were obtained. The compositions of the culture media are shown in Table 3 [44]. The concentration of the cementation solution was 1 mol/L, which was composed of anhydrous calcium chloride and urea in the ratio of 1:1. The water environment of the cementation solution was set as natural seawater and de-ionised water. In order to prevent the interference of other micro-organisms in the water environment, the water used in the test was autoclaved.
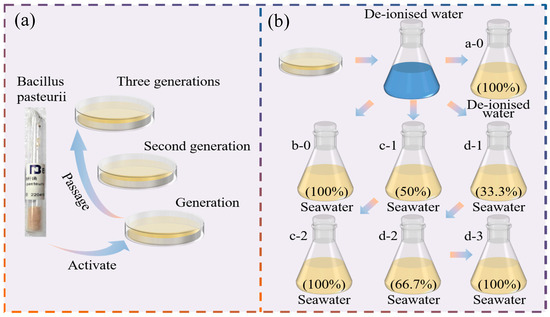 Figure 1. Bacteria cultivation process: (a) Activation and passaging of bacteria, and (b) the process of the domestication culture test with different seawater concentration gradients.
Figure 1. Bacteria cultivation process: (a) Activation and passaging of bacteria, and (b) the process of the domestication culture test with different seawater concentration gradients. Table 3. The compositions of the culture media.
Table 3. The compositions of the culture media.
2.2. Test Methods
Based on the research experience of related bacterial domestication [45,46], this paper adopted a multi-gradient seawater concentration domestication method to cultivate Bacillus pasteurii, in order to explore the growth status of the bacteria during the domestication cultivation process and the change of the bacterial morphology at the end of the domestication. Water solution tests and calcareous sand column tests were carried out for both undomesticated and domesticated bacteria, and the precipitation compositions and the microscopic morphology of the resulting minerals from the MICP reaction under natural seawater ambient conditions were investigated by water solution tests. An X-ray diffractometer (XRD) and a scanning electron microscope (SEM) were used to comprehensively analyse the mineral composition of the generated precipitates and the crystal form, and then to investigate the surface curing effect of the domesticated bacteria on calcareous sand.
- (1)
- Determination of bacterial concentration: A UV spectrophotometer model 752 N was used to measure the absorbance of the bacteria at 600 nm, and the concentration of the bacterial liquid was expressed according to OD600 [16]. The original blank medium without Bacillus pasteurii was used as a calibration solution during all measurements.
- (2)
- Measurement of urease activity: A conductivity meter model DDS-307 was used to determine the ability of the bacterial solution to hydrolyse urea. According to the research of Whiffin et al. [47], 5 mL of bacterial solution and 45 mL of urea solution (concentration of 1.1 mol/L) were measured and shaken well, and the changes in conductivity of the mixture solution of bacteria and urea were measured by a conductivity meter for 5 min continuously. Since the mixing of 5 mL of bacterial solution with 45 mL of urea solution diluted the bacterial solution by 10 times, the dilution factor should be multiplied when calculating the urease activity, i.e., the average conductivity change value in the measured 5 min was multiplied by the dilution factor of 10 times.
- (3)
- Observation of bacterial morphological changes: The observation of bacterial morphology was carried out using a Zeiss Axiolab 5 (Carl Zeiss (Shanghai) Management Co., Shanghai, China) research-grade microscope manufactured in Germany. The extracted bacterial solution at the end of each gradient of domestication, and the undomesticated bacterial solution, were treated with Gram stain solution, and the changes in their morphology were observed with a light microscope.
- (4)
- Determination of pH, Ca2+ concentration, and precipitate production in MICP water solutions: PH and Ca2+ concentration changes during the reaction of the water solutions were measured regularly using a pH meter manufactured by METTLER TOLEDO and a model PXS-270 ion meter manufactured by Lei-chi. Before each measurement, the instruments needed to be calibrated: the PXS-270 ion meter was calibrated with a standard solution, and the pH meter was calibrated with a pH standard buffer solution. After the end of the test, the aqueous solution precipitation was cleaned and soaked in de-ionised water for 48 h, then placed in an oven for drying, and its total mass was measured and the yield calculated using the weighing method.
- (5)
- Penetration resistance test: The fully automatic multifunctional penetrometer (model TKA-GRY-1F) produced by Nanjing TKA Technology Co., Ltd, Nanjing, China, was used. The test probe was selected as A type, the contact area was 0.3 cm2, the penetration rate was selected as 2 mm/min, the sampling frequency was 3 s, and the depth of penetration was 6 mm [48]. The test was carried out by direct penetration of the surface of the calcareous sand columns without stripping the surface of the film, to test the bearing capacity of the surface of the calcareous sand columns cured by domesticated bacteria.
- (6)
- SEM, EDS and XRD tests: The test samples were selected from the precipitates generated in the water solution, the collected samples were placed in an oven at 60 °C, and the samples were tested after drying. JSM-7610F PLUS Field Emission Scanning Electron Microscope (SEM) was used to collect microscopic images of the samples. The microscope was used to observe the microstructure of calcium carbonate precipitation induced by Bacillus pasteurii before and after domestication at an accelerating voltage of 10 kV, to identify the elements in the samples in conjunction with the EDS, and to comparatively analyse the changes in the composition of substances in the water solution of the MICP before and after the domestication of the Bacillus pasteurii. Before SEM, the samples were gold-plated with a gold-spraying instrument (i.e., an ion sputtering instrument), with the purpose of enhancing the electrical conductivity of the sand sample particles. Another part of the sample was taken and put into an agate mortar to be fully ground into powder, following which an X-ray diffraction test was carried out to determine the composition of the sample phase.
2.3. Test Protocols
2.3.1. Domestication of Bacillus pasteurii in Natural Seawater
In this study, multi-gradient seawater concentration domestication was adopted to study the culture of Bacillus pasteurii in a natural seawater environment. Different natural seawater concentrations were used to design three domestication modes—called the one-gradient, two-gradient, and three-gradient domestication modes—which were compared with de-ionised water to the culture of Bacillus pasteurii. The experimental scheme of domestication under different seawater concentration gradients is detailed in Table 4, and the process of domestication under different seawater concentration gradients is shown in Figure 1b.

Table 4.
Experimental programme for domestication of seawater with different gradients.
As the activation and passaging of Bacillus pasteurii were carried out on solid media, in order to ensure the consistency of bacterial concentration between the domesticated and undomesticated groups, sterilised de-ionised water was used to clean the bacteria on the solid medium, and a certain amount of bacterial solution was transferred to the liquid medium with a pipette. The conical flask was shaken well before sucking up the bacterial solution, in order to ensure that it was uniformly distributed. In addition, before the formal test was carried out, it was found that the bacterial concentration and urease activity were basically stable between 18–24 h by setting up a pre-test, where the time set for the domestication culture in this test was 24 h. The specific domestication process is as follows:
- (1)
- De-ionised water: Measure 2 mL of the bacterial solution and add it to a 200 mL conical flask (a-0) containing 100% de-ionised water, then put it into the shaker at a controlled temperature of 30 °C at 220 r/min and cultivate for 24 h. Then, the de-ionised water cultivation is completed, and the de-ionised water bacterial solution required for the test is obtained (A).
- (2)
- One-gradient domestication: Measure 2 mL of the bacterial solution and add it to a 200 mL conical flask (b-0) containing the 100% natural seawater domestication group medium, then put it into a shaker at a controlled temperature of 30 °C at 220 r/min, and then incubate for 24 h. Then, one-gradient domestication is completed, and the bacterial solution required for the one-gradient domestication test is obtained (B).
- (3)
- Two-gradient domestication: Measure 2 mL of the bacterial solution and add it to a 200 mL conical flask (c-1) containing the 50% natural seawater domestication group medium, and use culture conditions consistent with those for the first gradient domestication. Take the flask out after 24 h of incubation and set aside. Take 2 mL of bacterial solution from conical flask c-1 and add it to another 200 mL conical flask (c-2), which contains the 100% natural seawater domestication group medium, and incubate for another 24 h. Then, two-gradient domestication is completed, and the bacterial solution required for the two-gradient domestication test is obtained (C).
- (4)
- Three-gradient domestication: Measure 2 mL of the bacterial solution and add it to a 200 mL conical flask (d-1) containing the natural seawater domestication group medium with 33.3% seawater content, and then incubate for 24 h as described above. Then, take 2 mL of the bacterial solution from conical flask d-1 and add it to another 200 mL conical flask (d-2) containing the natural seawater domestication group medium with 66.7% seawater content, and incubate this flask for 24 h. Next, take 2 mL of the bacterial solution from conical flask d-2 and add it to a third 200 mL conical flask (d-3) containing the natural seawater domestication group medium with 100% seawater content, and then incubate flask d-3 for a further 24 h. Then, three-gradient domestication is completed, and the bacterial solution required for the three-gradient domestication test is obtained (D).
(Note: Natural seawater domestication of Bacillus pasteurii should be controlled at each stage of cultivation, otherwise the concentration of bacteria and urease activity will be affected.)
2.3.2. MICP Water Solution Test
The test was carried out in a 250 mL beaker, and 40 mL of bacterial solution and 160 mL of cementing solution were mixed thoroughly; the specific test protocol is detailed in Table 5.

Table 5.
MICP water solution test programme under different domestication methods.
2.3.3. MICP Sand Column Curing Test
A cylindrical hollow tube with a thickness of 3 mm, diameter of 45 mm, and height of 88 mm was used for the test moulds. When making samples, one end of the mould was wrapped with gauze and tape, and a layer of filter paper was put in the bottom to prevent the loss of calcareous sand particles. Then, a certain quantity of calcareous sand was weighed (the sand used in the test was cleaned and dried) and was loaded into the moulds four times. Each time it was poured into the mould, the mould was shaken, such that the calcareous sand was naturally and uniformly distributed in the moulds. Then, the last layer of the shaking was scraped with a steel ruler to flatten the surface, and the sample-making was considered to be complete at this time. When reinforcing, the calcareous sand was reinforced by manually dropping the bacterial liquid and cementing liquid. Finally, the reinforced calcareous sand specimens were put into an oven for drying in order to carry out the subsequent penetration test; the specific test protocols are given in Table 6. The test sand was divided into two groups according to particle size (0.08–0.16 and 0.16–0.315), and the bacterial injection was divided into primary and secondary bacterial injection methods. In the primary injection method, 10 mL of bacterial liquid was injected, the test sample was left for 12 h, and then 10 mL of cementing liquid was injected twice, with an injection interval of 24 h. In the secondary bacterial injection method, 5 mL of bacterial liquid was injected and, after an interval of 12 h, 10 mL of cementing liquid was injected, this being one round of injection, repeated in two rounds, with an interval of 24 h between each round.

Table 6.
Test protocols for calcareous sand consolidation under different domestication methods.
The penetration resistance test was conducted to study the bearing capacity of the Bacillus pasteurii tamed and untamed groups on the surface layer of the sand column after curing. During the test, the middle point was selected as the test point for each sand column, in order to reduce the test error. Each group of tests included two parallel samples, and the average value of the two points for each group of tests was taken to obtain the penetration depth–resistance change curve. The specific test process is depicted in Figure 2.
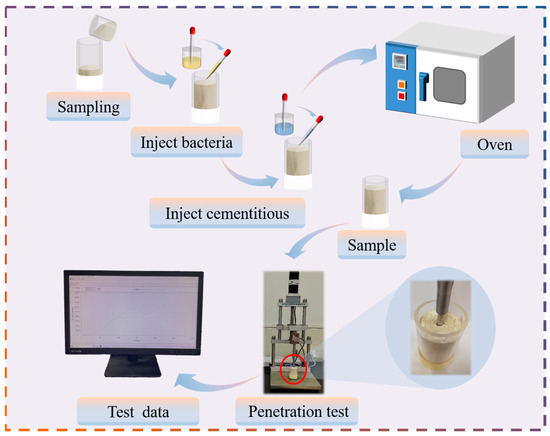
Figure 2.
Schematic diagram of MICP calcareous sand column reinforcement process.
3. Results and Analyses
3.1. Analysis of the Bacillus pasteurii Domestication Results
To investigate the effect of the domestication of Bacillus pasteurii using natural seawater on the bacterial growth, bacterial urease activity, and the final domestication morphology of the bacteria, the bacterial growth (OD600) and bacterial urease activity (UA) were monitored throughout the continuous incubation of the inoculated bacteria in a constant temperature shaker for 72 h. The differences in microbial concentration and activity under different domestication protocols varied significantly with time.
As can be seen from Figure 3a, ① in the process of the bacterial gradient domestication culture, the OD600 concentration value of the Bacillus pasteurii solution cultured in a natural seawater environment was always smaller than that of the Bacillus pasteurii solution cultured in a de-ionised water environment. This indicates that the natural seawater environment inhibits the growth of Bacillus pasteurii. ② The de-ionised water cultured bacteria at the beginning which entered into growth in a logarithmic phase, while the natural seawater culture showed a delayed growth phase, which became longer with an increase in the seawater concentration. The reason for this may be that the medium used for the activation and passaging of the bacteria was de-ionised water, which the Bacillus pasteurii had already adapted to, while the seawater environment is more complicated than the de-ionised water environment, such that the bacteria had to adapt to the environment after entering it, leading to a delay period. ③ The overall trend of bacterial liquid concentration was stable after 24 h of incubation. When the seawater gradient domestication tended to be stable, the concentration of the bacterial sap showed a gradual increase with an increase in the domestication gradient; that is, b < c-2 < d-3. In the domestication process for different concentrations in the same gradient, the concentration of the bacterial sap was lower than that under the previous seawater concentration when domestication tended to be stable; that is, c-1 < c-2 and d-1 < d-2 < d-3. The reason for this may be that in the process of gradient domestication, bacteria that not been expanded and cultured multiple times through concentration gradients had not well adapted to high-concentration seawater cultivation. Consequently, under conditions of elevated seawater concentration, the resultant increase in osmotic pressure and ionic strength could potentially cause structural damage to, or even the death of, a majority of the cells. In the process of gradient domestication, the observed trend was in agreement with conclusions drawn in the previous literature [42].
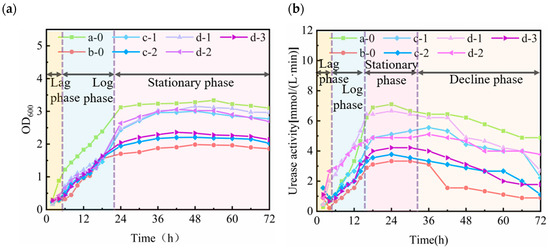
Figure 3.
Changes in growth of cultured micro-organisms under different domestication protocols: (a) OD600 values; (b) urease activity.
The following can be observed from Figure 3b: ① During the bacterial gradient domestication culture, the urease activity of Bacillus pasteurii increased with incubation time, growing rapidly in the first 18 h, remaining essentially stable in the 18–36 h interval, and then gradually decreasing. The one-gradient decay period was more pronounced during natural seawater gradient domestication. ② The urease activity of bacteria cultured in the de-ionised water environment was always higher than that in natural seawater. Comparing different seawater gradients, it was found that, the higher the seawater concentration, the greater the effect on the activity of the bacteria; and, the use of multiple gradients of seawater concentration to domesticate and cultivate Bacillus pasteurii better enabled the bacteria to transition from the de-ionised water environment to the seawater environment, which thus better protected the bacterial urease activity. ③ The bacterial urease activity at the end of the multi-gradient culture test with natural seawater under different domestication schemes also slightly differed: when the bacterial concentration tended to be stable, the bacterial urease activity of b was lower than that of c-2 and d-3, while c-2 and d-3 presented alternating urease activities due to the inconsistency in the time period that they arrived at their peaks. The bacterial urease activity did not differ much in general. Under the same gradient, the lower the concentration of natural seawater, the closer the bacterial urease activity was to that of de-ionised water. Before 12 h, a low concentration of natural seawater cultivation was conducive to promoting the bacterial urease activity in a certain period of time. In addition, when the bacteria entered the high concentration medium at the beginning, they showed a decline at first, followed with an increase up to the maximum value, from which it can be seen that the decreasing trend decreased with an increase in the gradient. This result indicates that the bacteria have a certain adaptability to natural seawater from low to high concentrations during the seawater gradient domestication process. Therefore, when cultivating bacteria, it is possible to increase the concentration properly according to the demand, in order to achieve the intended purpose.
Figure 4a–d shows the bacteria after the completed tests with de-ionised water and three gradient domestication cultures. Morphological observations using a microscope revealed that the length of Bacillus pasteurii cultured in a de-ionised water environment was 0.08–0.16 μm, while in 100% natural seawater concentration, the length of the bacteria cultured in the one-gradient was 0.10–0.17 μm, the length of the bacteria cultured in the two-gradient was 0.06–0.18 μm, and the length of the bacteria cultured in the three-gradient was 0.07–0.22 μm. The Bacillus pasteurii became elongated on the basis of the original with increased gradient domestication, and most of the domesticated bacteria became obviously longer and thinner, when compared with the undomesticated bacteria. Moreover, a small portion of the bacteria became shorter; the reason for this may be that the Bacillus pasteurii bacteria were in the process of splitting when observed after domestication.
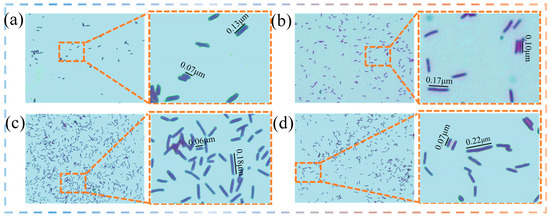
Figure 4.
Changes in microbial morphology under different domestication protocols: (a) de-ionised water bacterial solution A; (b) one-gradient domestication bacterial solution B; (c) two-gradient domestication bacterial solution C; (d) three-gradient domestication bacterial solution D.
3.2. Analysis of MICP Aqueous Solution Test
The degree of mineralisation under different domestication conditions was explored through measuring the pH, Ca2+ concentration, precipitate generation, and productivity of the solution during the mineralisation reaction of the MICP cementation solution. As can be seen from Figure 5a, the pH values under different water environments all increased and then decreased rapidly with time, and then gradually levelled off. This is due to the initial addition of bacteria to the cementing solution, with the bacteria promoting the hydrolysis of urea in the solution to produce a large amount of , thus increasing the pH value of the aqueous solution. Within 0.5 h, the in the solution and the cations in the solution had combined to generate a large number of precipitates. With the generation of precipitates, the pH value of the solution reduced to a stable level. Comparing the different test conditions, it can be seen that the pH of the de-ionised water environment was higher than that of the seawater environment and decreased slowly in the de-ionised water environment, which may be due to the high initial pH of the natural seawater environment. High pH values are conducive to the promotion of the hydrolysis of urea and the acceleration of the generation of precipitates, leading to the rapid decrease in pH under the seawater environment; in addition, with an increase in the domestication gradient, the pH tended to stabilise more quickly, indicating that the domesticated Bacillus pasteurii were more favourable for the promotion of urea hydrolysis and increased the reaction rate in the aqueous solution.
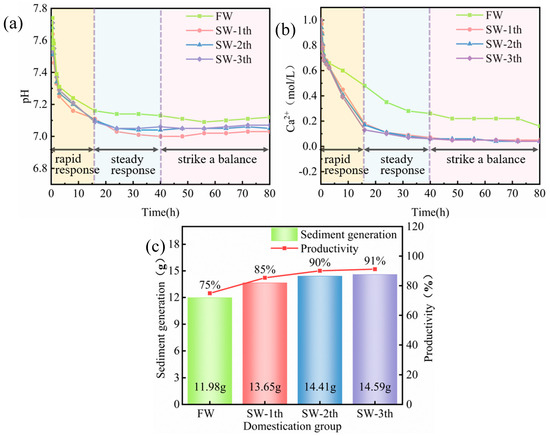
Figure 5.
Variation of MICP aqueous solution in: (a) pH; (b) Ca2+ concentration; (c) precipitate production vs. productivity.
Figure 5b shows that the Ca2+ concentration decreased and levelled off with increasing reaction time. The decrease rate of Ca2+ concentration in the de-ionised water environment was slower than that in the seawater environments, and the time to reach the equilibrium was obviously longer. With the gradient of domestication, the decrease in calcium ion concentration was accelerated, and there was not much difference in the change of Ca2+ concentration between the three domestication groups when the gradients were stable. The ionic change concentration under the three gradients was related to the activity of the bacteria, and the bacterial activity was improved, to a certain extent, after gradient domestication. The high activity of the bacteria was conducive to promoting the mineralisation reaction rate of the cementing solution, which led to a quick decrease in the Ca2+ concentration in the aqueous solution.
Figure 5c shows that the precipitates generated through the mineralisation reaction of the bacteria domesticated in natural seawater were higher in generation and productivity than those without domestication, indicating that the bacteria domesticated in natural seawater were conducive to promoting the mineralisation reaction in the cemented solution, resulting in an increase in the amount of precipitates generated. In addition, the presence of Ca2+ and Mg2+ in seawater provided more calcium and magnesium sources for the reaction environment, which combined with the in the environment to produce carbonate crystals, thus greatly improving the yield; this was consistent with the literature [44,49]. With an increase in gradient domestication, the precipitate production gradually increased, consistent with the experimental conclusions obtained by Xiao Yao et al. [42]. In this experiment, the domestication period was relatively short, which was conducive to the protection of the activity of Bacillus pasteurii. Compared with the precipitate production of the untamed group, the precipitate production through the mineralisation reaction was increased by 10.43%, 15.19%, and 16.31% after gradient domestication with the first, second, and third gradients, respectively, indicating that the bacteria adapted to the seawater and that their activity was enhanced gradually through gradient domestication.
3.3. Analysis of MICP Sand Sample Curing Test
To investigate the surface curing effect of Bacillus pasteurii using MICP technology for reinforcing calcareous sand columns before and after domestication, penetration tests were conducted on the sand columns. Through the penetration test, the relationship between penetration depth (penetration depth is the change in axial displacement) and penetration resistance could be obtained, reflecting the strength change in the surface layer of the sand column at different depths from the surface downwards, thus indicating the effect of the MICP technology on the surface curing of calcareous sand columns.
Visually, a thin white layer was cemented on the surface of the calcareous sand columns through the curing with bacteria in the seawater environment, whereas no white deposit was observed on the calcareous sand columns cured with bacteria in the de-ionised water environment (see the two red circles in Figure 6a). Comparing the damage patterns under the two curing conditions, it was found that the fine-grained sand columns cured under the de-ionised water environment had a better overall integrity without any obvious ring-breaking fracture, whereas the surface of the sand columns cured in the seawater environment ruptured and the surface of the sand columns presented a thin layer. Comparing the thickness of the calcareous sand under the two curing conditions, it was found that the de-ionised water environment-cured calcareous sand could form a sand layer with a certain thickness cemented on the surface of the sand column, while the seawater environment-cured calcareous sand formed a thin layer.
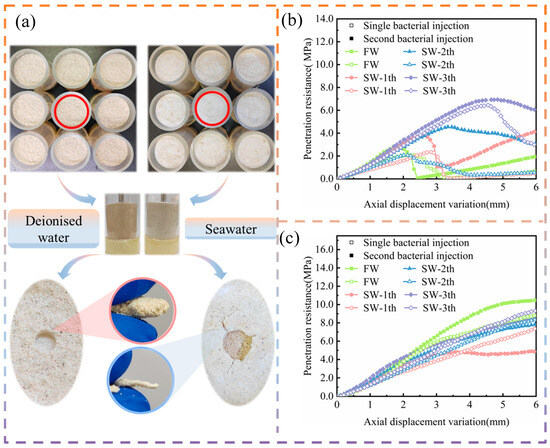
Figure 6.
Analysis of penetration test results: (a) sedimentation and destruction characteristics of consolidated calcareous sand under different gradient domestication schemes; (b) different bacterial injection methods for curing calcareous sand under coarse-grained conditions; and (c) different bacterial injection methods for curing calcareous sand under fine-grained conditions.
From the results of the penetration test, as shown in Figure 6b,c, the penetration resistance of the coarse-size calcareous sand plus solid increased with an increase in the penetration depth, then decreased rapidly and increased again. When the penetrometer probe continued to penetrate into the sand column, the penetration resistance continued to increase, after the force borne by the surface of the sand column reached the maximum value, and the hard layer on the surface of the sand column broke, resulting in the penetration resistance to rise and then decrease. When continuing to penetrate, the compactness of the sand increased, the confining pressure increased, and the penetration resistance continued to rise. The fine-grained calcareous sand column presented resistance to penetration with an increase in the depth of penetration, while the coarse-grained sand column presented a different change curve. It is possible that, on the one hand, the effect of bacterially reinforced fine-grained calcareous sand is better than that of coarse particles while, on the other hand, the penetrometer probe continued to penetrate into the sand column, and the compactness of the sand at a certain depth had already reached the value of the force borne by the surface. When the penetrometer probe continued to penetrate deeper into the sand column, the compactness of the sand gradually increased and the penetration resistance also gradually increased. From the perspective of the mode of bacterial injection, it was found that the secondary bacterial injection mode was conducive to improving the curing effect of a coarse grain size. The penetration resistance of the coarse-grained calcareous sand under the secondary bacterial injection was significantly improved. In addition, with an increase in the domestication gradient, the penetration resistance increased; however, the effect of the secondary bacterial injection mode on the fine-grained calcareous sand was not significant.
In terms of the curing effect of calcareous sand with different grain sizes, in the coarse-grained sand, the curing effect of domesticated bacteria was superior to that of undomesticated bacteria, while the curing effect in the fine grains was completely opposite to that of the coarse-grained calcareous sand. In coarse calcareous sand particles, the porosity is high, which is conducive to bacterial penetration into the interior of the sand column, promoting the mineralisation reaction inside and on the surface of the sand column; however, in fine calcareous sand, the gaps between the particles are small, and when reinforced, the undomesticated Bacillus pasteurii is not conducive to the downward infiltration into the sand column, resulting in the concentration of the bacteria on the surface of the sand column. Thus, it is gathered and cemented on the surface of the calcareous sand and generates calcium carbonate precipitation on the surface of calcareous sand, leading to the formation of a hard shell on the sand column’s surface. In addition, no matter whether reinforcing coarse or fine calcareous sand, it was found that the effect of the domesticated bacteria cementing calcareous sands was better with an increase in gradient domestication. This indicates that the use of domesticated Bacillus pasteurii is conducive to improving the curing effect of the calcareous sand, especially in the process of curing fine-grained calcareous sand. With an increase in the gradient of domestication, the volume of the Bacillus pasteurii was relatively reduced, which is conducive to their downward infiltration, leading to the solidification of the interior of the calcareous sand, more than just that aggregated on the surface, such that the effect of curing is further improved.
It can be seen that Bacillus pasteurii of the multi-gradient domestication for the curing of calcareous sand can improve the bearing capacity of the surface of the sand column with an increase in the gradient of domestication, a greater bearing capacity of the surface of the sand column, and a greater penetration resistance of the surface of the sand column were also observed. In addition, different bacterial injection and cementation methods presented differing effects when considering the same grain size, the secondary injection mode improved the bearing capacity on the surface of the coarse-grained sand columns, and domesticated and undomesticated bacteria reinforced different particle sizes of calcareous sand, which had different effects, and which can be used as reference for future research.
3.4. Microstructural Characterisation
To investigate the reinforcing effect of domesticated Bacillus pasteurii on calcareous sand, SEM imaging processing and EDS elemental determination were carried out, combined with XRD spectroscopic testing and quantitative mineral analysis. It can be observed that the precipitate crystals generated by the untamed group, as shown in Figure 7a–d, were predominantly spherical in shape, with each crystal being a different size, forming a spherical-shaped morphology from one or more spheres joined together. The crystal shapes of the precipitates generated by the domesticated groups were spherical and rhombic (mainly rhombic), arranged as multiple irregular rhombic shapes tightly aggregated together to form a cluster structure in a layer-by-layer stacked manner, resulting in a cluster-like structure. The number of spheres gradually reduced with an increase in the domestication gradient.
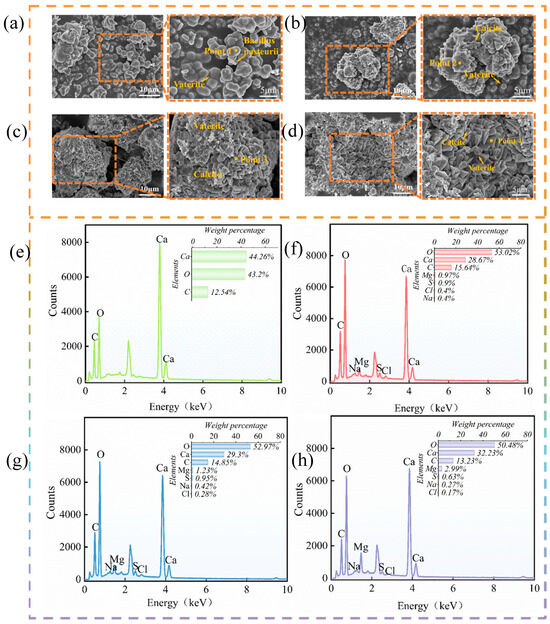
Figure 7.
SEM and EDS maps of precipitates: (a) undomesticated group; (b) one-gradient domesticated group; (c) two-gradient domesticated group; (d) three-gradient domestication group. (e) Point 1; (f) point 2; (g) point 3; (h) point 4.
Combined with the energy spectrum analysis, as shown in Figure 7e–h, the elements generated by the undomesticated group were Ca, C, and O, indicating that the main component of generation from the water solution was CaCO3.the elements generated in the seawater environment included Ca, C, O, Mg, S, Na, and Cl. Compared with the de-ionised water environment, Mg, S, Na, and Cl were observed (the elements Na and Cl are likely to have been generated by seawater attached to the unwashed surface). The elemental content suggests that at least CaCO3 and MgCO3 were generated in the water solution precipitates, as well as possibly calcium sulphate hemihydrate [38]. Under the different domestication gradients, the content of each element was not consistent, especially the element Mg, which increased with the gradient, suggesting that the content of MgCO3 in the precipitate also increased, as the domesticated bacteria promoted the formation of magnesium salts. In addition, the cured strength of formed MgCO3 is slightly higher than that of formed CaCO3 [50], which may also explain the calcareous sand reinforcement results obtained with the domesticated Bacillus pasteurii. This may also be the reason why the surface of calcareous sand reinforced by domesticated Bacillus pasteurii improved as the gradient increased. The experimental results showed that the domesticated Bacillus pasteurii was conducive for inducing carbonate precipitation and increased the amount of carbonate precipitation, which was also conducive to the reinforcement of the calcareous sand.
Figure 8 shows the XRD spectroscopic testing and quantitative mineral analysis results for the precipitates generated by the different domestication groups. Overall, the water solution precipitates generated by both domesticated and undomesticated groups were carbonate crystals, and both contained calcite crystal phases. The carbonate crystals of the undomesticated group consisted of two crystalline phases: calcite and vaterite. In the domesticated groups, the carbonate crystals consisted of both calcite and magnesium calcite phases. Using standard cards to compare the precipitates’ crystals, it was found that the spectral peaks of the two water environments were very different, as the two phases of the undomesticated group presented distinct peaks, while the two phases of the domesticated group presented superimposed peaks, which could be attributed to the fact that the high magnesium calcite content in the domesticated group was a combination of calcium and magnesium carbonates, while the standard cards contained calcium carbonate in both phases, which resulted in the superimposition of the two phases obtained under the seawater environments [49,51]. In addition, quantitative analysis revealed that the content of each crystalline phase in the undomesticated group was 77.4% calcite and 22.6% vaterite whereas, in the domesticated group, the content of each crystalline phase varied with the different domestication gradients; in particular, the content of high-magnesium calcite increased with an increase in the domestication gradient, which is in agreement with the results obtained from the above EDS elemental content analysis. Combined with SEM, it was found that no calcite was observed in the undomesticated group; however, a large amount of the calcite crystal phase was detected with XRD, which may be due to the fact that low quantities of precipitates used for SEM measurements did not select a fraction containing both crystalline phases. In the domestication groups, the presence of the vaterite crystal phase in the sample was observed with SEM, but not observed XRD, which may be due to the fact that the magnesium ions can promote the disappearance of vaterite when they reach a certain concentration, and the remaining content of vaterite was also low in the precipitate [52]. In summary, all of the above detection methods were a means of detection, the selection of samples for detection and the expression of the characteristic diffraction peaks of the material composition of the samples affected the test results, and there was a certain degree of error in the detection results [53].
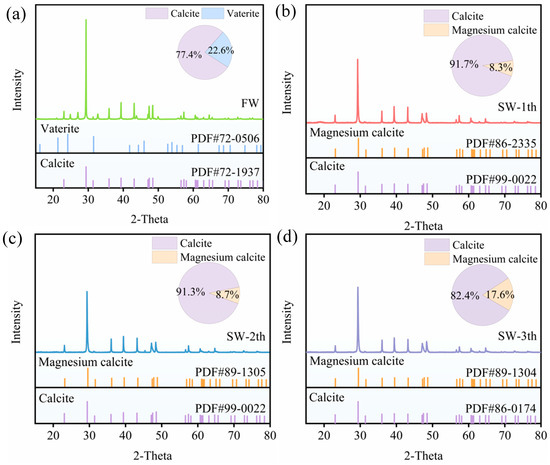
Figure 8.
XRD images of precipitates: (a) undomesticated group; (b) one-gradient domesticated group; (c) two-gradient domesticated group; and (d) three-gradient domesticated group.
4. Conclusions
In this paper, Bacillus pasteurii was domesticated by multi-gradient seawater concentrations and a series of tests were carried out on the domesticated bacteria, combined with microscopic test methods to analyse the effect of the domesticated bacteria in curing calcareous sand. The main conclusions are as follows:
- (1)
- The comparison of the growth of Bacillus pasteurii in seawater and de-ionised water environments revealed that seawater environments inhibit the growth of Bacillus pasteurii, with a delay period observed for the growth of bacteria in the seawater environments. The gradient domestication method allowed the bacteria to gradually adapt to the seawater environment, improving the growth and urease activity of the bacteria. With the three-domestication gradient, the Bacillus pasteurii could better adapt to the natural seawater environment, their morphology became thinner and longer, and their size increased from 0.07–0.13 μm to 0.07–0.22 μm.
- (2)
- The MICP water solution test demonstrated that the MICP mineralisation reaction rate was faster in the natural seawater environment, yielding more precipitates from the mineralisation reaction than in the de-ionised water environment. Comparing the generation of precipitates under different gradients, it was found that the generation of The MICP water solution test precipitates increased by 10.43% after the bacteria were domesticated in the one-gradient, 15.19% after the two-gradient, and 16.31% after the three-gradient (which was obviously higher than the first and second gradients). The comparison of the mineral compositions revealed that the generated calcite content was high in the natural seawater environment, and there were small amounts of and other minerals in the generated product in addition to . Furthermore, with an increase in the domestication gradient, the content of in the precipitates increased and the mineral content increased.
- (3)
- According to the results of the penetration resistance tests, conducted in order to compare the curing effect on calcareous sand under different gradients, it was found that the reinforcement of calcareous sand with Bacillus pasteurii after multi-gradient domestication in a seawater environment effectively enhanced the curing effect. The curing effect was more obvious and the penetration resistance gradually increased with an increase in the domestication gradient. Comparing the consolidation effect on calcareous sand with coarse and fine grain sizes, it was found that the surface curing effect on the sand column of fine-grained calcareous sand was better than that for the coarse-grained calcareous sand specimen. In addition, different bacterial injection cementation methods presented different effects when considering the same grain size, where the secondary bacterial injection mode improved the surface bearing capacity of the coarse-grained sand column.
5. Discussion
In this study, it was confirmed that the cultivation of Bacillus pasteurii by using multi-gradient seawater concentration domestication can make it gradually adapt to the seawater environment, and the concentration and activity of the bacteria were improved. The domesticated bacteria can achieve a better curing effect for the reinforcement of calcareous sand. This indicates that the MICP curing technology can directly cure calcareous sand in a seawater environment, which is greener and more environmentally friendly than the traditional concrete curing method. In addition, the micro-organisms used in the MICP technology are present in the natural island environment, which has less impact on the ecological environment of the island and is more suitable for the application to the island. However, this study was performed indoors and with fewer types of particle size settings. In the future, engineering practices could consider a few points: (1) tropical islands, coral reefs, and coastal areas of beaches are all in environments with high temperatures and humidity, and further research is needed to study the effects of high temperatures and humidity on Bacillus pasteurii; (2) calcareous sands are sand bodies formed by the deposition of biological debris from the death of reef-building coral colonies and fragmentation and refinement by various ocean dynamics. There are differences in the particle gradation and compaction of sand formed under various ocean dynamics, as well as differences in its porosity and its distribution, which are directly related to the morphology and size of the reinforcing bacteria which have been domesticated by natural seawater. Therefore, in engineering practice, the applicability of particle gradation and particle size should be taken into account, so as to obtain a more suitable reinforcement method for calcareous sands in the actual environment.
Author Contributions
Writing—review and editing, resources, data curation, project administration, and funding acquisition, Z.W.; investigation, data curation, and writing—original draft preparation, W.C.; formal analysis, writing—review and editing, Z.T.; investigation, X.C.; visualisation, X.D. and Y.X.; supervision, W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 42162024, 41602322, 42107213), the Hainan Provincial Natural Science Foundation of China (Grant No. 421RC592), a major Science and Technology Plan Project of Yazhou Bay Innovation Research Institute of Hainan Tropical Ocean College (Grant No. 2022CXYZD003), and a student innovation project of Yazhou Bay Innovation Institute of Hainan Tropical Ocean University (Grant No. 2022CXYXSCXXM06).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data used during the study are available from the first author and corresponding authors by request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xiao, P.; Lan, H.L.; Shi, J.Q.; He, X.; Chu, J.; Xiao, Y. Dynamic responses of calcareous foundation reinforced by microbially induced calcite precipitation. Chin. J. Geotech. Eng. 2023, 45, 1303–1313. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.S.; Yin, L.Y.; Liu, B.; Lv, C.; Wang, D.L.; Pan, X.H.; Wang, H.L.; Shi, B. Experimental study on surface erosion resistances and me MICP-FR-treated calcareous sand. Chin. J. Geotech. Eng. 2021, 43, 1941–1949. [Google Scholar] [CrossRef]
- Yang, S.M.; Peng, J.; Wen, Z.L.; Liu, Z.M.; Leng, M.; Xu, P.X. Applicaation of concentrated seawater as calcium source solution in sand reinforcement using MICP. Rock Soil Mech. 2021, 42, 746–754. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, J.; Li, N.; Wang, X.; Wu, Y.; Guo, S. Experimental study on the mechanical behavior and particle breakage characteristics of hydraulic filled coral sand on a coral reef island in the South China Sea. Rock Soil Mech. 2021, 41, 1. [Google Scholar]
- Li, Y.J.; Guo, Z.; Li, Y.L.; Rui, S.J.; Zhu, Y.Q. Advances in MICP reinforcement technology used in island engineering. Chin. J. Eng. 2023, 45, 819–832. [Google Scholar] [CrossRef]
- Wang, C.G.; Shu, S.Z.; Xiao, Y.; Lu, D.C.; Liu, H.L. Fractional-order bounding surface model considering breakage of calcareous sand. Chin. J. Geotech. Eng. 2023, 45, 1162–1170. [Google Scholar] [CrossRef]
- Yang, S.; Liu, W. Research on Unconstrained Compressive Strength and Microstructure of Calcareous Sand with Curing Agent. J. Mar. Sci. Eng. 2019, 7, 294. [Google Scholar] [CrossRef]
- Yu, X.; Pan, X. Seawater based bio-cementation for calcareous sand improvement in marine environment. Mar. Georesour. Geotechnol. 2023, 41, 949–958. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, H.; Xiao, Y.; Stuedlein, A.W.; Evans, T.M. Liquefaction resistance of bio-cemented calcareous sand. Soil Dyn. Earthq. Eng. 2018, 107, 9–19. [Google Scholar] [CrossRef]
- Shen, G.; Liu, S.; He, Y.; Pan, M.; Yu, J.; Cai, Y. Reinforcement of Calcareous Sands by Stimulation of Native Microorganisms Induced Mineralization. Materials 2022, 16, 251. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Chen, H.Y.; Meng, Q.S.; Wang, R. Microscopic characterization of intra-pore structures of calcareous sands. Rock Soil Mech. 2014, 35, 1831–1836. [Google Scholar]
- Liu, H.L.; Ma, G.L.; Xiao, Y.; Ding, X.M.; Fang, X.W. In situ experimental research on calcareous foundation stabilization using MICP technique on the reclaimed coral reef islands. Chin. Ground Improv. 2019, 1, 26–31. [Google Scholar]
- Gao, X.Y. Study on the Technology of Solidifying Coral Sand with Struvite Induced by Microorganisms in High Salt Environment. Master’s Thesis, Huaqiao University, Quanzhou, China, 2020. [Google Scholar]
- Liu, J.; Li, G.; Li, X.A. Geotechnical Engineering Properties of Soils Solidified by Microbially Induced CaCO3 Precipitation (MICP). Adv. Civ. Eng. 2021, 2021, 6683930. [Google Scholar] [CrossRef]
- Peng, J.; Tian, Y.M.; Yang, J.G. Experiments of coral sand reinforcement using MICP in seawater environment. Adv. Sci. Technol. Water Resour. 2019, 39, 58–62. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Amini, F.; Li, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotech. 2020, 15, 17–27. [Google Scholar] [CrossRef]
- Tang, C.S.; Pan, X.H.; Lv, C.; Dong, Z.C.; Liu, B.; Wang, D.L.; Li, H.; Cheng, Y.J.; Shi, B. Bio-geoengineering Technology and the Applications. Geol. J. China Univ. 2021, 27, 625–654. [Google Scholar] [CrossRef]
- Ding, R.; Wang, Z.; Zhao, X.; Cao, P.; Chen, X.; Chen, W. Experimental Study on Coastal Sediment Reinforcement by Induced Carbonate Precipitation by Different Enzyme Sources. Water 2023, 15, 1525. [Google Scholar] [CrossRef]
- Bu, C.; Lu, X.; Zhu, D.; Liu, L.; Sun, Y.; Wu, Q.; Zhang, W.; Wei, Q. Soil improvement by microbially induced calcite precipitation (MICP): A review about mineralization mechanism, factors, and soil properties. Arab. J. Geosci. 2022, 15, 863. [Google Scholar] [CrossRef]
- Park, G.; Kim, Y.; Lee, H.H.; Lee, O.; Park, J.; Kim, Y.; Lee, K.M.; Heo, M.; Son, H. Characterization and applicability of novel alkali–tolerant carbonatogenic bacteria as environment-friendly bioconsolidants for management of concrete structures and soil erosion. J. Environ. Manag. 2022, 321, 115929. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, H.; Stuedlein, A.W.; Evans, T.M.; Chu, J.; Cheng, L.; Jiang, N.; Lin, H.; Liu, H.; Aboel-Naga, H.M. Restraint of particle breakage by biotreatment method. J. Geotech. Geoenviron. 2020, 146, 4020123. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Stuedlein, A.W.; Chu, J.; Matthew Evans, T.; Van Paassen, L.A. Crystal growth of MICP through microfluidic chip tests. J. Geotech. Geoenviron. 2022, 148, 6022002. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Konstantinou, C. Strength Behavior of Temperature-Dependent MICP-Treated Soil. J. Geotech. Geoenviron. 2023, 149, 4023116. [Google Scholar] [CrossRef]
- Safavizadeh, S.; Montoya, B.M.; Gabr, M.A.; Knappe, D.R. Factors Affecting the Kinetics of Urea Hydrolysis via Sporoscarcina pasteurii; American Society of Civil Engineers: Reston, VA, USA, 2019; pp. 105–114. [Google Scholar]
- Al-Salloum, Y.; Abbas, H.; Sheikh, Q.I.; Hadi, S.; Alsayed, S.; Almusallam, T. Effect of some biotic factors on microbially-induced calcite precipitation in cement mortar. Saudi J. Biol. Sci. 2017, 24, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tang, C.; Pan, X.; Xu, J.; Zhang, X. Suppressing Drought-Induced Soil Desiccation Cracking Using MICP: Field Demonstration and Insights. J. Geotech. Geoenviron. 2024, 150, 4024006. [Google Scholar] [CrossRef]
- Mujah, D.; Cheng, L.; Shahin, M.A. Microstructural and geomechanical study on biocemented sand for optimization of MICP process. J. Mater. Civil. Eng. 2019, 31, 4019025. [Google Scholar] [CrossRef]
- Ghasemi, P.; Zamani, A.; Montoya, B. The Effect of Chemical Concentration on the Strength and Erodibility of MICP Treated Sands; American Society of Civil Engineers: Reston, VA, USA, 2019; pp. 241–249. [Google Scholar]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors affecting improvement of engineering properties of MICP-treated soil catalyzed by bacteria and urease. J. Mater. Civil. Eng. 2014, 26, 4014094. [Google Scholar] [CrossRef]
- Zheng, J.J.; Wu, C.C.; Song, Y.; Cui, M.J. Study of the strength test and strength dispersion of MICP-treated calcareous sand. J. Harbin Eng. Univ. 2020, 41, 250–256. [Google Scholar] [CrossRef]
- Liu, H.L.; Xiao, P.; Xiao, Y.; Wang, J.P.; Chen, Y.M.; Chu, J. Dynamic behaviors of MICP-treated calcareous sand in cyclic tests. Chin. J. Geotech. Eng. 2018, 40, 38–45. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yu, J.; Han, L.; Cai, Y.Y.; Tu, B.X.; Zhou, J.F. Experimental study on water resistance of tabia surface with microbially induced carbonate precipitation. Chin. J. Rock. Mech. Eng. 2019, 38, 1718–1728. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Wang, L.; Ye, Z.; Shen, C.; Zhou, W. Interface Shear Behavior between MICP-Treated Calcareous Sand and Steel. J. Mater. Civil. Eng. 2021, 33, 04020455. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Stuedlein, A.W.; Evans, T.M.; Xiao, Y. Strength, stiffness, and microstructure characteristics of biocemented calcareous sand. Can. Geotech. J. 2019, 56, 1502–1513. [Google Scholar] [CrossRef]
- Montoya, B.M.; Dejong, J.T.; Boulanger, R.W. Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation. Géotechnique 2013, 63, 302–312. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, H.; Zhang, Y.; Jiang, X.; Li, C.; Chu, J.; Xiao, Y. Dynamic strength of temperature-controlled MICP-treated calcareous sand. Chin. J. Geotech. Eng. 2021, 43, 511–519. [Google Scholar]
- Yu, Z.X. Microbial Solidification Technology of Coral Sand in High Salt Environment in South Island Reef. Master’s Thesis, Huaqiao University, Quanzhou, China, 2019. [Google Scholar]
- Dong, B.; Liu, S.; Yu, J.; Xiao, Y.; Cai, Y.; Tu, B. Evaluation of the effect of natural seawater solidifying calcareous sand based on MICP. Rock Soil Mech. 2021, 42, 1104–1114. [Google Scholar]
- Ding, X.C.; Chen, Y.M.; Zhang, X.L. Experimental study on microbial reinforced calcareous sand using ring shear apparatus. J. Zhejiang Univ. (Eng. Sci.) 2020, 54, 1690–1696. [Google Scholar] [CrossRef]
- Peng, J.; Cao, T.; He, J.; Dai, D.; Tian, Y. Improvement of Coral Sand With MICP Using Various Calcium Sources in Sea Water Environment. Front. Phys. 2022, 10, 825409. [Google Scholar] [CrossRef]
- Ou, Y.X.; Fang, W.X.; Zhang, N.; Li, J. Influence of Solution Salinity on Microbial Biocementation of Coral Sand. J. Logist. Eng. Univ. 2016, 32, 78–82. [Google Scholar]
- Xiao, Y.; Deng, H.; Li, J.; Cheng, L.; Zhu, W. Study on domestication of Sporosarcina pasteurii and cementation effect of calcareous sand in seawater environment. Rock Soil Mech. 2022, 43, 5. [Google Scholar]
- Li, Y.L.; Guo, Z.; Xu, Q.; Li, Y.J. Experimental research on wet-dry cycle of MICP cemented calcareous sand in seawater environment. J. Zhejiang Univ. (Eng. Sci.) 2022, 56, 1740–1749. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yu, W.Y.; Qi, C.N.; Zhao, X.Y. Reaction mechanism and influencing factors of MICP in seawater environment. J. Civ. Environ. Eng. 2022, 44, 128–135. [Google Scholar]
- Bao, Z.; Wang, X.; Wang, Q.; Zou, L.; Peng, L.; Li, L.; Tu, W.; Li, Q. A novel method of domestication combined with ARTP to improve the reduction ability of Bacillus velezensis to Cr(VI). J. Environ. Chem. Eng. 2023, 11, 109091. [Google Scholar] [CrossRef]
- Feng, L.; Wu, G.; Zhang, Z.; Tian, Z.; Li, B.; Cheng, J.; Yang, G. Improving denitrification performance of biofilm technology with salt-tolerant denitrifying bacteria agent for treating high-strength nitrate and sulfate wastewater from lab-scale to pilot-scale. Bioresour. Technol. 2023, 387, 129696. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Wan, Z.H.; Dai, G.L.; Gong, W.M.; Gao, L.C. Experimental study on micro-erosion mechanism of cement stabilized calcareous sand under seawater environment. Rock Soil Mech. 2021, 42, 1871–1882. [Google Scholar] [CrossRef]
- Teng, X.Y.; Wang, Z.Y.; Jia, Y.G.; Chen, W.J. Effect of ion concentration on MICP reaction in natural seawater environment. J. Civ. Environ. Eng. 2023. Available online: http://qks.cqu.edu.cn/cqdxxbcn/article/abstract/tm-202210069 (accessed on 21 March 2024).
- Sun, X.H.; Miao, L.C.; Tong, T.Z.; Wang, C.C. Comparison between microbiologically-induced calcium carbonate precipitation and magnesium carbonate precipitation. Chin. J. Geotech. Eng 2018, 40, 1309–1315. [Google Scholar] [CrossRef]
- Long, X.; Ma, Y.; Qi, L. Biogenic and synthetic high magnesium calcite—A review. J. Struct. Biol. 2014, 185, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Tang, C.; Zhang, J.; Pan, X.; Liu, H. Effects of calcium sources and magnesium ions on the mechanical behavior of MICP-treated calcareous sand: Experimental evidence and precipitated crystal insights. Acta Geotech. 2023, 18, 2703–2717. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, L.; Xu, S.; Zhao, X.; Cao, P.; Wang, J. Strength mechanism and electrochemical characterization of cement-bonded calcareous sand in different water environments. Constr. Build. Mater. 2023, 389, 131751. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).