Porifera Associated with Deep-Water Stylasterids (Cnidaria, Hydrozoa): New Species and Records from the Ross Sea (Antarctica)
Abstract
1. Introduction
2. Materials and Methods
3. Results
Systematic Section
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cairns, S.D. Species Richness of Recent Scleractinia. Atoll Res. Bull. 1999, 459, 1–46. [Google Scholar] [CrossRef]
- Cairns, S.D. Global Diversity of the Stylasteridae (Cnidaria: Hydrozoa: Athecatae). PLoS ONE 2011, 6, e21670. [Google Scholar] [CrossRef]
- Bax, N.; Cairns, S. Chapter 5.7. Stylasteridae (Cnidaria: Hydrozoa). In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H.J., Raymond, B., Udekem d’Acoz, C., Van de Putte, A.P., Danis, B., David, B., Grant, S., Gutt, J., et al., Eds.; Scientific Committee on Antarctic Research: Cambridge, UK, 2014; pp. 107–112. [Google Scholar]
- Cairns, S.D. Antarctic and Subantarctic Stylasterina (Coelenterata: Hydrozoa). In Biology of the Antarctic Seas XIII; American Geophysical Union (AGU): Washington, DC, USA, 1983; pp. 61–164. ISBN 978-1-118-66672-2. [Google Scholar]
- Cairns, S.D. New Records of Stylasteridae (Hydrozoa: Hydroida) from the Galapagos and Cocos Islands. Proc. Biol. Soc. Wash. 1991, 104, 209–228. [Google Scholar]
- Heifetz, J. Coral in Alaska: Distribution, Abundance, and Species Associations. Hydrobiologia 2002, 471, 19–28. [Google Scholar] [CrossRef]
- Patton, W.K. Distribution and Ecology of Animals Associated with Branching Corals (Acropora spp.) from the Great Barrier Reef, Australia. Bull. Mar. Sci. 1994, 55, 193–211. [Google Scholar]
- Stella, J.S.; Pratchett, M.S.; Jones, P.A.H.G.P. Coral-Associated Invertebrates: Diversity, Ecological Importance and Vulnerability to Disturbance. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-0-429-10992-8. [Google Scholar]
- Hoeksema, B.W.; Van der Meij, S.E.T.; Fransen, C.H.J.M. The Mushroom Coral as a Habitat. J. Mar. Biol. Assoc. U. K. 2012, 92, 647–663. [Google Scholar] [CrossRef]
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A. Reefs of the Deep: The Biology and Geology of Cold-Water Coral Ecosystems. Science 2006, 312, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Häussermann, V.; Försterra, G. Extraordinary Abundance of Hydrocorals (Cnidaria, Hydrozoa, Stylasteridae) in Shallow Water of the Patagonian Fjord Region. Polar Biol. 2007, 30, 487–492. [Google Scholar] [CrossRef]
- Braga-Henriques, A.; Carreiro-Silva, M.; Porteiro, F.M.; de Matos, V.; Sampaio, Í.; Ocaña, O.; Ávila, S.P. The Association between a Deep-Sea Gastropod Pedicularia Sicula (Caenogastropoda: Pediculariidae) and Its Coral Host Errina dabneyi (Hydrozoa: Stylasteridae) in the Azores. ICES J. Mar. Sci. 2011, 68, 399–407. [Google Scholar] [CrossRef]
- Zibrowius, H. Associations of Hydrocorallia Stylasterina with Gall-Inhabiting Copepoda Siphonostomatoidea from the South-West Pacific. Part I. On the Stylasterine Hosts, Including Two New Species, Stylaster papuensis and Crypthelia cryptotrema. Bijdr. Tot Dierkd. 1981, 51, 268–281. [Google Scholar]
- Goud, J.; Hoeksema, B. Pedicularia Vanderlandi Spec. Nov., a Symbiotic Snail (Caenogastropoda: Ovulidae) on the Hydrocoral Distichopora Vervoorti Cairns & Hoeksema, 1998 (Hydrozoa: Stylasteridae), from Bali, Indonesia. Zool. Verh. 2001, 334, 77–97. [Google Scholar]
- Pica, D.; Bertolino, M.; Calcinai, B.; Puce, S.; Bavestrello, G. Boring and Cryptic Sponges in Stylasterids (Cnidaria: Hydrozoa). Ital. J. Zool. 2012, 79, 266–272. [Google Scholar] [CrossRef]
- Downey, R.V.; Griffiths, H.J.; Linse, K.; Janussen, D. Diversity and Distribution Patterns in High Southern Latitude Sponges. PLoS ONE 2012, 7, e41672. [Google Scholar] [CrossRef] [PubMed]
- Brey, T.; Klages, M.; Dahm, C.; Gorny, M.; Gutt, J.; Hain, S.; Stiller, M.; Arntz, W.E.; Wägele, J.W.; Zimmermann, A. Antarctic Benthic Diversity. Nature 1994, 368, 297. [Google Scholar] [CrossRef]
- Gray, J.S. Antarctic Marine Benthic Biodiversity in a World-Wide Latitudinal Context. Polar Biol. 2001, 24, 633–641. [Google Scholar] [CrossRef]
- Starmans, A.; Gutt, J. Mega-Epibenthic Diversity: A Polar Comparison. Mar. Ecol. Prog. Ser. 2002, 225, 45–52. [Google Scholar] [CrossRef]
- Janussen, D.; Tendal, O.S. Diversity and Distribution of Porifera in the Bathyal and Abyssal Weddell Sea and Adjacent Areas. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1864–1875. [Google Scholar] [CrossRef]
- Bavestrello, G.; Calcinai, B.; Cerrano, C.; Sarà, M. Alectona Species From North-Western Pacific (Demospongiae: Clionidae). J. Mar. Biol. Assoc. U. K. 1998, 78, 59–73. [Google Scholar] [CrossRef]
- Wisshak, M.; Correa, M.L.; Zibrowius, H.; Jakobsen, J.; Freiwald, A. Skeletal Reorganisation Affects Geochemical Signals, Exemplified in the Stylasterid Hydrocoral Errina dabneyi (Azores Archipelago). Mar. Ecol. Prog. Ser. 2009, 397, 197–208. [Google Scholar] [CrossRef]
- Costa, G.; Bavestrello, G.; Canese, S.; Canessa, M.; Mazzoli, C.; Montagna, P.; Puce, S.; Schiaparelli, S.; Bertolino, M. Sponges Associated with Stylasterid Thanatocoenosis (Cnidaria, Hydrozoa) from the Deep Ross Sea (Southern Ocean). Polar Biol. 2022, 45, 703–718. [Google Scholar] [CrossRef]
- Boothroyd, A.; Adams, V.; Alexander, K.; Hill, N. Priority Areas for Marine Protection in the Amundsen and Bellingshausen Seas, Antarctica. Mar. Policy 2024, 167, 106232. [Google Scholar] [CrossRef]
- Nissen, C.; Lovenduski, N.S.; Brooks, C.M.; Hoppema, M.; Timmermann, R.; Hauck, J. Severe 21st-Century Ocean Acidification in Antarctic Marine Protected Areas. Nat. Commun. 2024, 15, 259. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Mazzella, V.; Rispo, F.; Efremova, J.; Calcinai, B. DNA Barcoding Procedures for Taxonomical and Phylogenetic Studies in Marine Animals: Porifera as a Case Study. In Marine Genomics: Methods and Protocols; Verde, C., Giordano, D., Eds.; Springer: New York, NY, USA, 2022; pp. 195–223. ISBN 978-1-07-162313-8. [Google Scholar]
- Stosch, H.V. Pleurax, Seine Synthese Und Seine Verwendung Zur Einbettung Und Darstellung Det Zellwande von Diatomeen, Peridineen Und Anderen Algen, Sowie Für Eine Neue Methode Zur Electivfarbung von Dinoflagellaten-Penzern. Arch. Für Protistenkd. 1974, 116, 132–141. [Google Scholar]
- Rasband, W.S. ImageJ 2011. Available online: https://imagej.net/ij/ (accessed on 7 December 2024).
- Boury-Esnault, N.; Van Beveren, M. Les Démosponges Du Plateau Continental de Kerguellen-Heard. Com. Natl. Français Des Rech. Antarct. 1982, 52, 1–175. [Google Scholar]
- Hansen, G.A. Spongiadae. The Norwegian North-Atlantic Expedition 1876–1878. Zoology 1885, 13, 1–26. [Google Scholar]
- Lundbeck, W. Porifera. (Part II.) Desmacidonidae. Dan. Ingolf-Exped. 1905, 6, 1–219. [Google Scholar]
- Tompkins, G.; Baker, E.; Anstey, L.; Walkusz, W.; Siferd, T.; Kenchington, E. Sponges from the 2010-2014 Paamiut Multispecies Trawl Surveys, Eastern Arctic and Subarctic: Class Demospongiae, Subclass Heteroscleromorpha, Order Poecilosclerida, Family Coelosphaeridae, Genera Forcepia and Lissodendoryx; Canadian Technical Report of Fisheries and Aquatic Sciences; Dartmouth: Hanover, NH, USA, 2017; p. 129. [Google Scholar]
- Hentschel, E. Monaxone Kieselschwämme Und Hornschwämme Der Deutschen Südpolar-Expedition 1901–1903. Dtsch. Südpolar-Exped. 1914, 15, 35–141. [Google Scholar]
- Göcke, C.; Janussen, D. Demospongiae of ANT XXIV/2 (SYSTCO I) Expedition—Antarctic Eastern Weddell Sea. Zootaxa 2013, 3692, 28–101. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Hajdu, E. Family Esperiopsidae Hentschel, 1923. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Willenz, P., Eds.; Springer: Boston, MA, USA, 2002; pp. 656–664. ISBN 978-1-4615-0747-5. [Google Scholar]
- Carter, H.J. XXIX.—Descriptions and Figures of Deep-Sea Sponges and Their Spicules from the Atlantic Ocean, Dredged up on Board H.M.S. ‘Porcupine,’ Chiefly in 1869; with Figures and Descriptions of Some Remarkable Spicules from the Agulhas Shoal and Colon, Panama. Ann. Mag. Nat. Hist. 1874, 14, 207–221. [Google Scholar] [CrossRef]
- de Voogd, N.; Alvarez, B.; Boury-Esnault, N.; Cárdenas, P.; Díaz, M.-C.; Dohrmann, M.; Downey, R.; Goodwin, C.; Hajdu, E.; Hooper, J.; et al. World Porifera Database. Available online: https://www.marinespecies.org/porifera (accessed on 23 January 2024).
- Kirkpatrick, R. Porifera (Sponges). II. Tetraxonida, Dendy. National Antarctic Expedition, 1901–1904 Natural History. Zoology 1908, 4, 1–56. [Google Scholar]
- Schmidt, O. Grundzüge Einer Spongien-Fauna Des Atlantischen Gebietes. Wilhelm Engelmann Leipz. 1870, iii–iv, 1–88. [Google Scholar]
- Topsent, E. Révision Des Mycales de l’Europe Occidentale. Ann. L’institut Océanographique 1924, 1, 77–118. [Google Scholar]
- Dinn, C.; Leys, S.P. Field Guide to Sponges of the Eastern Canadian Arctic; Department of Biological Sciences, University of Alberta: Edmonton, AB, USA, 2018. [Google Scholar]
- Koltun, V.M. Sponges of the Antarctic. 1 Tetraxonida and Cornacuspongida. In Biological Reports of the Soviet Antarctic Expedition (1955–1958); Pavlovskii, E.P., Andriyashev, A.P., Ushakov, P.V., Eds.; Akademya Nauk SSSR: Moscow, Russia, 1964. [Google Scholar]
- Erpenbeck, D.; Van Soest, R.W.M. Family Halichondriidae Gray, 1867. In Systema Porifera. A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Eds.; Springer: New York, NY, USA, 2002; pp. 787–816. ISBN 0-306-47260-0. [Google Scholar]
- Sollas, W.J. Report on the Tetractinellida Collected by H.M.S. Challenger, during the Years 1873–1876. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the Years 1873–1876. Zoology 1888, 25, 1–458. [Google Scholar]
- Topsent, E. Spongiaires de l’Expédition Antarctique Nationale Ecossaise. Earth Environ. Sci. Trans. R. Soc. Edinb. 1913, 49, 579–643. [Google Scholar] [CrossRef]
- Post, A.L.; O’Brien, P.E.; Beaman, R.J.; Riddle, M.J.; Santis, L.D. Physical Controls on Deep Water Coral Communities on the George V Land Slope, East Antarctica. Antarct. Sci. 2010, 22, 371–378. [Google Scholar] [CrossRef]
- J. Miller, K.; Mundy, C.N.; Lindsay Chadderton, W. Ecological and Genetic Evidence of the Vulnerability of Shallow-Water Populations of the Stylasterid Hydrocoral Errina Novaezelandiae in New Zealand’s Fiords. Aquat. Conserv. Mar. Freshw. Ecosyst. 2004, 14, 75–94. [Google Scholar] [CrossRef]
- Waller, R.G.; Robinson, L.F. Southern Ocean Corals: Cabo de Hornos. Coral Reefs 2012, 31, 205. [Google Scholar] [CrossRef]
- Kaiser, S.; Brandão, S.N.; Brix, S.; Barnes, D.K.A.; Bowden, D.A.; Ingels, J.; Leese, F.; Schiaparelli, S.; Arango, C.P.; Badhe, R.; et al. Patterns, Processes and Vulnerability of Southern Ocean Benthos: A Decadal Leap in Knowledge and Understanding. Mar. Biol. 2013, 160, 2295–2317. [Google Scholar] [CrossRef]
- Cerrano, C.; Bertolino, M.; Valisano, L.; Bavestrello, G.; Calcinai, B. Epibiotic Demosponges on the Antarctic Scallop Adamussium Colbecki (Smith, 1902) and the Cidaroid Urchins Ctenocidaris perrieri Koehler, 1912 in the Nearshore Habitats of the Victoria Land, Ross Sea, Antarctica. Polar Biol. 2009, 32, 1067–1076. [Google Scholar] [CrossRef]
- Gutt, J.; Schickan, T. Epibiotic Relationships in the Antarctic Benthos. Antarct. Sci. 1998, 10, 398–405. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Aguilar, C.; Dorado, D.; Manrique, N. Phenotypic Plasticity and Morphological Integration in a Marine Modular Invertebrate. BMC Evol. Biol. 2007, 7, 122. [Google Scholar] [CrossRef]
- Guardiola, M.; Frotscher, J.; Uriz, M.-J. High Genetic Diversity, Phenotypic Plasticity, and Invasive Potential of a Recently Introduced Calcareous Sponge, Fast Spreading across the Atlanto-Mediterranean Basin. Mar. Biol. 2016, 163, 123. [Google Scholar] [CrossRef]
- Wolfe, K.; Kenyon, T.M.; Mumby, P.J. The Biology and Ecology of Coral Rubble and Implications for the Future of Coral Reefs. Coral Reefs 2021, 40, 1769–1806. [Google Scholar] [CrossRef]
- Kenyon, T.M.; Doropoulos, C.; Wolfe, K.; Webb, G.E.; Dove, S.; Harris, D.; Mumby, P.J. Coral Rubble Dynamics in the Anthropocene and Implications for Reef Recovery. Limnol. Oceanogr. 2023, 68, 110–147. [Google Scholar] [CrossRef]
- Tunnicliffe, V. Breakage and Propagation of the Stony Coral Acropora cervicornis. Proc. Natl. Acad. Sci. USA 1981, 78, 2427–2431. [Google Scholar] [CrossRef]
- Pica, D.; Calcinai, B.; Anderson, M. Excavating sponges boring into the precious red coral from Cape Verde Archipelago. Biol. Mar. Mediterr. 2011, 18, 278–279. [Google Scholar]
- Weinstein, D.K.; Maher, R.L.; Correa, A.M.S. Bioerosion. In Mesophotic Coral Ecosystems; Loya, Y., Puglise, K.A., Bridge, T.C.L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 829–847. ISBN 978-3-319-92735-0. [Google Scholar]
- Pulido Mantas, T.; Bavestrello, G.; Bertolino, M.; Cerrano, C.; Pica, D.; Roveta, C.; Calcinai, B. A 3D Innovative Approach Supporting the Description of Boring Sponges of the Precious Red Coral Corallium rubrum. J. Mar. Sci. Eng. 2022, 10, 868. [Google Scholar] [CrossRef]
- Meyer, N.; Wisshak, M.; Freiwald, A. Bioerosion Ichnodiversity in Barnacles from the Ross Sea, Antarctica. Polar Biol 2021, 44, 667–682. [Google Scholar] [CrossRef]
- Brandt, A.; De Broyer, C.; Gooday, A.; Hilbig, B.; Thomson, M. Introduction to ANDEEP (ANtarctic Benthic DEEP-Sea Biodiversity: Colonization History and Recent Community Patterns)—A Tribute to Howard L. Sanders. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 1457–1465. [Google Scholar] [CrossRef]



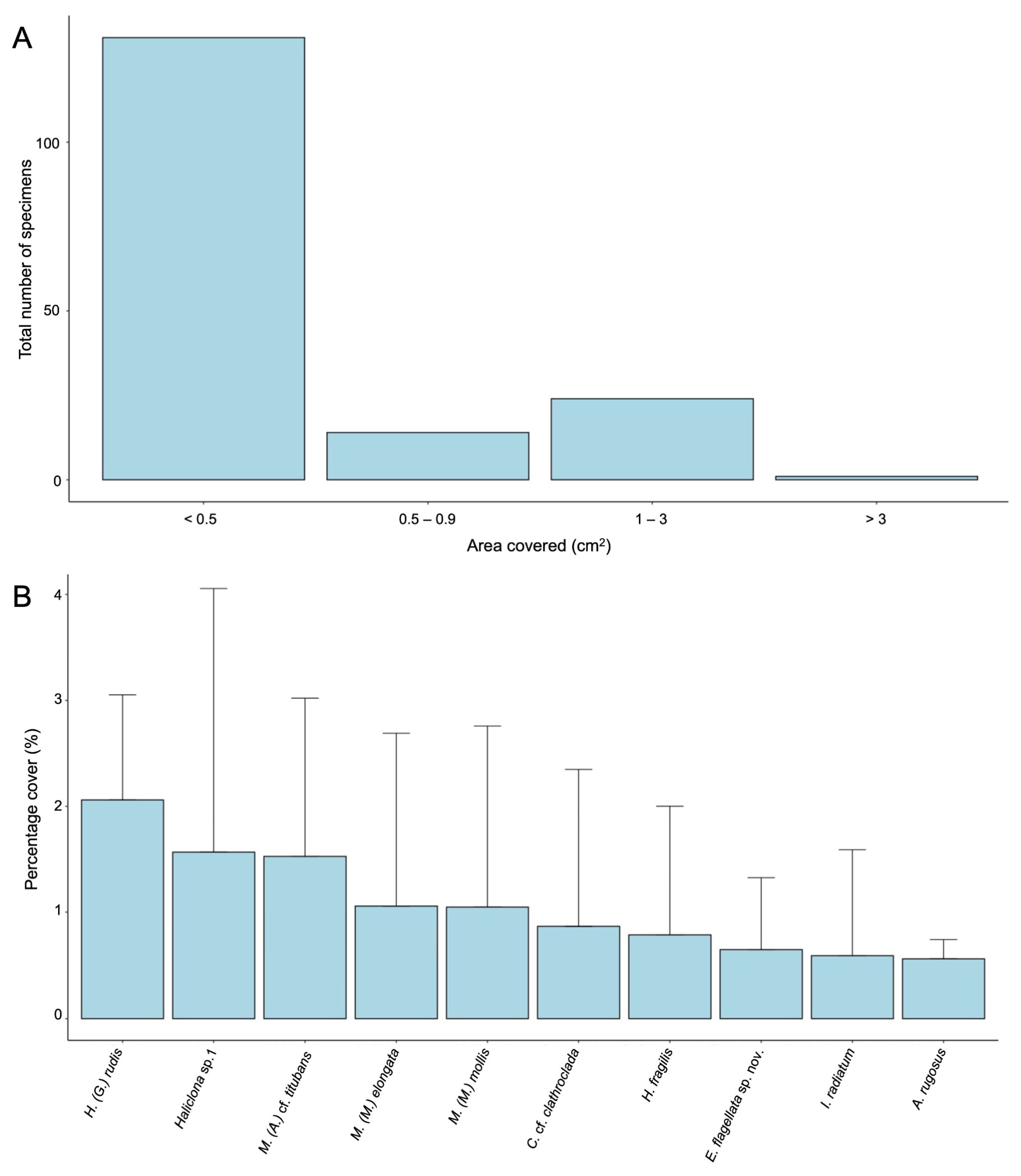








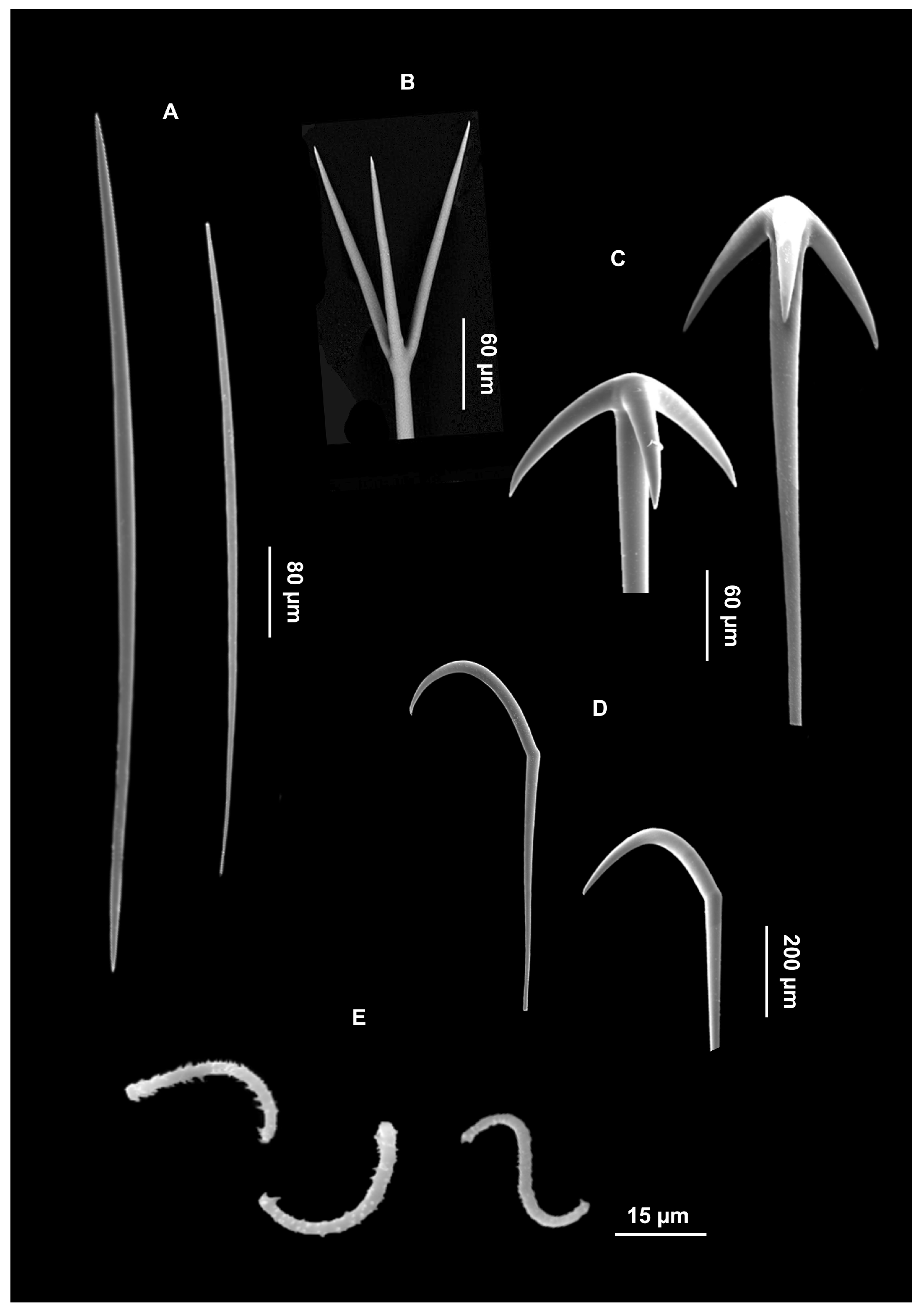

| Station | Station Code | Latitude | Longitude |
|---|---|---|---|
| Cape Hallett Canyon | CHC | −71.98111 | 172.19383 |
| −71.98752 | 172.1767 | ||
| Iselin Bank | IB | −72.26866 | −176.60469 |
| −72.26288 | −176.59273 | ||
| Hallett Ridge | HR | −72.3839 | 176.1017 |
| −72.38978 | 176.10349 | ||
| Unknown station 1 | US1 | −71.64022 | 172.15508 |
| Unknown station 2 | US2 | −72.49782 | 174.99664 |
| Unknown station 3 | US3 | −72.49855 | 174.99802 |
| Unknown station 4 | US4 | −72.49892 | 175.00003 |
| Unknown station 5 | US5 | −72.4995 | 174.99637 |
| Unknown station 6 | US6 | −72.49971 | 174.96788 |
| Unknown station 7 | US7 | −72.494803 | 174.94212 |
| Unknown station 8 | US8 | −72.4956 | 174.94267 |
| Unknown station 9 | US9 | −72.487563 | 174.94933 |
| Class | Sponge Species | Growth Habit | Sponge Size (Min–Max) | Locality | Number of Specimens |
|---|---|---|---|---|---|
| Demospongiae Sollas, 1885 | Biemna chilensis Thiele, 1905 | ME | 0.027–1.407 cm2 | IB | 2 |
| Haliclona cf. (Flagellia) flagellifera (Ridley & Dendy, 1886) | ME | 0.482 cm2 | IB | 1 | |
| Haliclona (Gellius) rudis (Topsent, 1901) | Ec | 0.447–1.079 cm2 | IB | 3 | |
| CHC | |||||
| Haliclona cf. virens (Topsent, 1908) | Ec | 0.085–0.596 cm2 | IB | 5 | |
| Haliclona sp. 1 | Ec | 0.350–0.665 cm2 | IB | 3 | |
| HR | |||||
| Haliclona sp. 2 | Ec | 0.115 cm2 | IB | 1 | |
| Iophon abnormalis Ridley & Dendy, 1886 ^ | Ec | 0.061–0.234 cm2 | IB | 2 | |
| Iophon radiatum Topsent, 1901 | Ec | 0.006–1.692 cm2 | IB | 47 | |
| CHC | |||||
| HR | |||||
| Iophon unicorne Topsent, 1907 | Ec | 0.017–3.611 cm2 | IB | 2 | |
| CHC | |||||
| Asbestopluma (Asbestopluma) sinuosa Costa & Bertolino, 2022 | ME | 0.01 cm2 | IB | 1 | |
| Inflatella belli (Kirkpatrick, 1907) | ME | 0.034 cm2 | IB | 1 | |
| Lissodendoryx (Lissodendoryx) stylosa sp. nov. ° | Ec | 0.027–0.132 cm2 | IB | 3 | |
| Lissodendoryx (Lissodendoryx) styloderma Hentschel, 1914 ^ | Ec | 0.045–0.564 cm2 | IB | 2 | |
| CHC | |||||
| Lissondendoryx (Ectyodoryx) nobilis (Ridley & Dendy, 1886) | Ec | 0.105 cm2 | IB | 1 | |
| Lissondendoryx sp. | Ec | 1.483 cm2 | IB | 1 | |
| Crella (Crella) tubifex (Hentschel, 1914) ^ | Ec | 0.084–0.103 cm2 | IB | 2 | |
| Amphilectus rugosus (Thiele, 1905) ^ | Ec | 0.037–0.178 cm2 | IB | 3 | |
| Esperiopsis flagellata sp. nov. ° | Ec | 0.037–0.464 cm2 | IB | 4 | |
| Isodictya cf. spinigera (Kirkpatrick, 1907) | Ec | 0.003 cm2 | IB | 2 | |
| Clathria (Clathria) pauper Brøndsted, 1927 | Ec | 0.078 cm2 | IB | 1 | |
| Clathria (Microciona) sp. 1 | Ec | 0.193 cm2 | HR | 1 | |
| Clathria (Clathria) toxipraedita Topsent, 1913 | Ec | 0.086–0.125 cm2 | IB | 2 | |
| HR | |||||
| Artemisina plumosa Hentschel, 1914 ^ | ME | 0.031–0.188 cm2 | IB | 5 | |
| HR | |||||
| Mycale (Anomomycale) cf. titubans (Schmidt, 1870) | Ec | 0.07–1.615 cm2 | IB | 5 | |
| CHC | |||||
| Myxilla (Myxilla) elongata Topsent, 1916 | Ec | 0.005–0.440 cm2 | IB | 3 | |
| CHC | |||||
| Myxilla (Myxilla) mollis Ridley & Dendy, 1886 | Ec | 0.179–0.757 cm2 | IB | 7 | |
| CHC | |||||
| HR | |||||
| Tedania (Tedaniopsis) oxeata Topsent, 1916 | Ec | 1.463 cm2 | IB | 1 | |
| Tedania (Tedaniopsis) tantula (Kirkpatrick, 1907) | Ec | 0.004–0.192 cm2 | IB | 2 | |
| Polymastia invaginata Kirkpatrick, 1907 | Ec | 0.039–0.279 cm2 | IB | 6 | |
| Hymeniacidon fragilis (Koltun, 1964) ^ comb. nov. | ME/Ec | 0.01–1.794 cm2 | IB | 20 | |
| CHC | |||||
| Pseudosuberites cf. sulcatus (Thiele, 1905) | Ec | 0.072 cm2 | IB | 1 | |
| Halichondria (Halichondria) sp. | Ec | 0.212 cm2 | HR | 1 | |
| Halichondria (Halichondria) prostrata Thiele, 1905 | ME | 0.400 cm2 | CHC | 1 | |
| Poecillastra antarctica Koltun, 1964 ^ comb. nov. | ME | 0.015–0.315 cm2 | IB | 3 | |
| Tetilla coronida Sollas, 1888 * | ME | 0.300 cm2 | US | 1 | |
| Hexactinellida Schmidt, 1870 | Clathrochone cf. clathroclada (Lévi & Lévi, 1982) | ME | 0.008–3.563 cm2 | IB | 21 |
| HR | |||||
| Acanthascus (Rhabdocalyptus) australis (Topsent, 1901) | ME | 0.109–0.234 cm2 | IB | 2 | |
| Tot species: 37 | Tot specimens: 169 | ||||
| Locality | Styles | Dermal Spicules | Isochelae | Sigma I | Sigma II | Author |
|---|---|---|---|---|---|---|
| North Atlantic | 420–680 × 16–25 | strongyles with transition to tylotes: 220–400 × 3.5–7 | 40–58 × 5–12 | 42–55 × 2 | 17–23 × 1 | [31] |
| Eastern Arctic and Subarctic | 448.4–690.3 × 9.3–25.5 | tylotes: 208.6–359.9 × 3.7–8.5 | 27.8–68.9 × 5–13.2 | 31.4–60.3 × 1.5–3.9 | 15–23.2 × 1.9–3 | [32] |
| Kerguelen Archipelago | 557–787 × 19–26 | styles: 262–314 × 6.4 | 45–58 × 10–13 | 51–64 × 2 | 19–26 × 1 | [29] |
| Antarctic (Ross Sea) | 580–880 × 20–30 | styles II: 290–425 × 10–15 | 25–42.5 × 15 | 25–40 × 5 | – | Present work |
| Locality | Mycalostyle I | Mycalostyle II | Anomochelae | Sigma | Author |
|---|---|---|---|---|---|
| Florida | – | – | 30 | 3–250 | [39] |
| West coast of Brittany | 875 × 27 | 430 × 8 | 21–35 | 50–130 × 2–5 | [40] |
| Denmark | 590–900 × 15–19 | 320–400 × 5–8 | 24–52 | 50–140 × 1.8–5.7 | [31] |
| Labrador Sea | 484–646 × 16–24 | 350–595 × 7.5–16 | 26–30 | 55–97 | [41] |
| Kerguelen Archipelago | 704–960 × 22.4–25.6 | 432.4–580.4 × 6.4–8.3 | 70.4–83.2 × 3.2–6.4 | Sigma I: 93.4–119 × 1.9–5.7 | [29] |
| Sigma II: 190.7–262.4 × 5.7–9.6 | |||||
| Antarctica (Ross Sea) | 600–940 × 20–30 | 380–480 × 10 | 27.5–50 | 65–142 × 2.5 | Present work |
| Species | Shape | Ectosomal Skeleton | Choanosomal Skeleton | Style I (μm) | Style II (μm) | Author |
|---|---|---|---|---|---|---|
| Plicatellopsis fragilis Koltun, 1964 | lamellar | tangential auxiliary styles | irregular reticulum of poorly developed longitudinal fiber bundles and isolated spicules | 550–800 × 20–30 | 250–380 × 6–11 | [42] |
| Hymeniacidon fragilis (Koltun, 1964) comb. nov. | massively encrusting, lamellar | tangential auxiliary styles | ascending bundles are connected by transversal bundles and free spicules, creating an irregular network; smaller styles are scattered between fibers | 640–754 × 40–50 | 193–377 × 10 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcinai, B.; Marrocco, T.; Roveta, C.; Puce, S.; Montagna, P.; Mazzoli, C.; Canese, S.; Vultaggio, C.; Bertolino, M. Porifera Associated with Deep-Water Stylasterids (Cnidaria, Hydrozoa): New Species and Records from the Ross Sea (Antarctica). J. Mar. Sci. Eng. 2024, 12, 2317. https://doi.org/10.3390/jmse12122317
Calcinai B, Marrocco T, Roveta C, Puce S, Montagna P, Mazzoli C, Canese S, Vultaggio C, Bertolino M. Porifera Associated with Deep-Water Stylasterids (Cnidaria, Hydrozoa): New Species and Records from the Ross Sea (Antarctica). Journal of Marine Science and Engineering. 2024; 12(12):2317. https://doi.org/10.3390/jmse12122317
Chicago/Turabian StyleCalcinai, Barbara, Teo Marrocco, Camilla Roveta, Stefania Puce, Paolo Montagna, Claudio Mazzoli, Simonepietro Canese, Carlo Vultaggio, and Marco Bertolino. 2024. "Porifera Associated with Deep-Water Stylasterids (Cnidaria, Hydrozoa): New Species and Records from the Ross Sea (Antarctica)" Journal of Marine Science and Engineering 12, no. 12: 2317. https://doi.org/10.3390/jmse12122317
APA StyleCalcinai, B., Marrocco, T., Roveta, C., Puce, S., Montagna, P., Mazzoli, C., Canese, S., Vultaggio, C., & Bertolino, M. (2024). Porifera Associated with Deep-Water Stylasterids (Cnidaria, Hydrozoa): New Species and Records from the Ross Sea (Antarctica). Journal of Marine Science and Engineering, 12(12), 2317. https://doi.org/10.3390/jmse12122317












