Length–Weight Relationships of the Prized Sea Cucumber Holothuria lessoni from In Situ and Ex Situ Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Methods
2.2. Analytical Methods
- Body length in cm (L);
- Body basal area;
- Square root of the body length–width product;
- Recalculated length (Le), derived from the regression equations between L and SLW for both in situ and ex situ measurements:Le (in situ) = 0.288 + (1.551 × SLW (in situ))where W is the body width and L is the body length in cm.Le (ex situ) = 0.92 + (1.904 × SLW (ex situ))
- a is the coefficient;
- b is the exponent;
- x is the body length metric (L, SLW, BBA and Le);
- y is the body weight.
3. Results
4. Discussion
- For this species, and likely others, use in situ (underwater) length and width measurements to estimate the size of the sea cucumbers.
- Avoid using size–weight relationships from in situ data to estimate body weight from length and width measurements made ex situ, and vice versa.
- Collect data for size–weight relationships from a large sample of individuals (e.g., >100).
- For estimating body weight, use biometric indices such as SLW and Le rather than body length.
- Re-evaluate length–weight relationships within a fishery at regular intervals or if methodologies are adapted to ensure accuracy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Anderson, S.C.; Mills Flemming, J.; Watson, R.; Lotze, H.K. Rapid global expansion of invertebrate fisheries: Trends, drivers, and ecosystem effects. PLoS ONE 2011, 6, e14735. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.D.; Rothlisberg, P.C.; Munro, J.L. Restocking and stock enhancement of marine invertebrate fisheries. Adv. Mar. Biol. 2005, 49, xi-374. [Google Scholar] [PubMed]
- Pakoa, K.; Friedman, K.; Moore, B.; Tardy, E.; Bertram, I. Assessing Tropical Marine Invertebrates: A Manual for Pacific Island Resource Managers; Secretariat of the Pacific Community: Noumea, New Caledonia, 2014; p. 118. [Google Scholar]
- Caddy, J. Current usage of fisheries indicators and reference points, and their potential application to management of fisheries for marine invertebrates. Can. J. Fish. Aquat. Sci. 2004, 61, 1307–1324. [Google Scholar] [CrossRef]
- Léopold, M.; Cournet, N.; Andrefouët, S.; Moenteapo, Z.; Duvauchelle, C.; Raubani, J.; Ham, J.; Dumas, P. Comanaging small-scale sea cucumber fisheries in New Caledonia and Vanuatu using stock biomass estimates to set spatial catch quotas. Environ. Conserv. 2013, 40, 367–379. [Google Scholar] [CrossRef]
- Van Wynsberge, S.; Andrefouët, S.; Gilbert, A.; Stein, A.; Remoissenet, G. Best management strategies for sustainable giant clam fishery in French Polynesia Islands: Answers from a spatial modeling approach. PLoS ONE 2013, 8, e64641. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.; Georget, S.; Guillemot, N.; Ton, C.; Léopold, M.; Purcell, S.; Van Wynsberge, S.; Andréfouët, S. État des Lieux des Stocks d’Holothuries Commerciales en Nouvelle Calédonie (2021–2022). Rapport ADECAL Technopole—Projet PROTÉGÉ; Secretariat of the Pacific Community: Noumea, New Caledonia, 2022; p. 178. [Google Scholar]
- Gilbert, A.; Baletaud, F.; Seguin, F.; Ravail, B.; Purcell, S. Etudes des Stocks d’Holothuries Commerciales du Lagon de l’Atoll d’APATAKI—Archipel des Tuamotu—et Recommandations de Gestion (2023). Rapport DRM, Projet PROTEGE; Secretariat of the Pacific Community: Noumea, New Caledonia, 2023; p. 54. [Google Scholar]
- Feary, D.; Hamilton, R.; Matawai, M.; Molai, C.; Karo, M.; Almany, G. Assessing Sandfish Population Stocks within the South Coast of Manus, and a Summary Report of Sandfish Connectivity Field Research May 19–June 27, 2014. Final Report; The Nature Conservancy: Arlington, VA, USA, 2015; pp. 5–51. [Google Scholar]
- Helidoniotis, F. Stock Assessment of White Teatfish (Holothuria fuscogilva) in Queensland, Australia; Department of Agriculture and Fisheries: Brisbane, QLD, Australia, 2021; p. 29. [Google Scholar]
- Helidoniotis, F. Stock Assessment of Black Teatfish (Holothuria whitmaei) in Queensland, Australia; Department of Agriculture and Fisheries: Brisbane, QLD, Australia, 2021; p. 25. [Google Scholar]
- González-Wangüemert, M.; Valente, S.; Henriques, F.; Domínguez-Godino, J.A.; Serrão, E.A. Setting preliminary biometric baselines for new target sea cucumbers species of the NE Atlantic and Mediterranean fisheries. Fish. Res. 2016, 179, 57–66. [Google Scholar] [CrossRef]
- Portz, D.E.; Woodley, C.M.; Cech, J.J. Stress-associated impacts of short-term holding on fishes. Rev. Fish Biol. Fish. 2006, 16, 125–170. [Google Scholar] [CrossRef]
- Jenzri, M.; Gharred, C.; Bouraoui, Z.; Guerbej, H.; Jebali, J.; Gharred, T. Evisceration of Holothuria poli by mechanical, chemical and hypoxia stress methods and its bioremediation potentials for the pisciculture wastewater. Aquac. Res. 2022, 53, 3309–3317. [Google Scholar] [CrossRef]
- Rodríguez-Barreras, R.; Serrano-Torres, S.; Macías-Reyes, D. A study of two tagging methods in the Caribbean sea cucumber Holothuria mexicana. Mar. Biodivers. Rec. 2014, 7, e118. [Google Scholar] [CrossRef]
- Bonneel, M.; Hennebert, E.; Byrne, M.; Flammang, P. Chapter 36—Mutable collagenous tissues in sea cucumbers. In The World of Sea Cucumbers; Mercier, A., Hamel, J.-F., Suhrbier, A.D., Pearce, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 573–584. [Google Scholar]
- Skewes, T.; Dennis, D.; Burridge, C. Survey of Holothuria scabra (Sandfish) on Warrior Reef, Torres Strait. January, 2000; CSIRO Division of Marine Research: Brisbane, Australia, 2000; p. 28. [Google Scholar]
- Robinson, L.A.; Greenstreet, S.P.R.; Reiss, H.; Callaway, R.; Craeymeersch, J.; de Boois, I.; Degraer, S.; Ehrich, S.; Fraser, H.M.; Goffin, A. Length–weight relationships of 216 North Sea benthic invertebrates and fish. J. Mar. Biol. Assoc. UK 2010, 90, 95–104. [Google Scholar] [CrossRef]
- Azevedo, E.; Silva, F.; Brito, A.; Simões, T.; Pombo, A.; Marques, T.; Rocha, C.; Sousa, J.; Venâncio, E.; Félix, P. Allometric relationships to assess ontogenetic adaptative changes in three NE Atlantic commercial sea cucumbers (Echinodermata, Holothuroidea). Aquat. Ecol. 2021, 55, 711–720. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Moura, P.; Pereira, F.; Pereira, A.M.; Gaspar, M.B. Morphometric relationships and relative growth of 20 uncommon bivalve species from the Algarve coast (Southern Portugal). J. Mar. Biol. Assoc. UK 2018, 98, 463–474. [Google Scholar] [CrossRef]
- Saleh, A.; Hasan, M.M.; Raadsma, H.W.; Khatkar, M.S.; Jerry, D.R.; Rahimi Azghadi, M. Prawn morphometrics and weight estimation from images using deep learning for landmark localization. Aquac. Eng. 2024, 106, 102391. [Google Scholar] [CrossRef]
- Choo, P.S. Population Status, Fisheries and Trade of Sea Cucumbers in Asia. In Sea Cucumbers. A Global Review of Fisheries and Trade. FAO Fisheries and Aquaculture Technical Paper 516; Toral-Granda, V., Lovatelli, A., Vasconcellos, M., Eds.; FAO: Rome, Italy, 2008; pp. 81–118. [Google Scholar]
- Bruckner, A.W.; Johnson, K.A.; Field, J. Conservation strategies for sea cucumbers: Can a CITES Appendix II listing promote sustainable international trade? SPC Beche-de-Mer Inf. Bull. 2003, 18, 24–33. [Google Scholar]
- Ramírez-González, J.; Moity, N.; Andrade-Vera, S.; Reyes, H. Overexploitation and more than a decade of failed management leads to no recovery of the Galápagos sea cucumber fishery. Front. Mar. Sci. 2020, 7, 920. [Google Scholar] [CrossRef]
- Purcell, S.W.; Polidoro, B.A.; Hamel, J.-F.; Gamboa, R.U.; Mercier, A. The cost of being valuable: Predictors of extinction risk in marine invertebrates exploited as luxury seafood. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133296. [Google Scholar] [CrossRef]
- Wolfe, K.; Mercier, A.; Hamel, J.-F. Chapter 17—Biology and Ecology of Wild Juvenile Sea Cucumbers: What do We Know? In The World of Sea Cucumbers; Mercier, A., Hamel, J.-F., Suhrbier, A.D., Pearce, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 257–283. [Google Scholar]
- Lorenzen, K.; Leber, K.M.; Loneragan, N.R.; Schloesser, R.W.; Taylor, M.D. Developing and integrating enhancement strategies to improve and restore fisheries. Bull. Mar. Sci. 2021, 97, 475–487. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Logan, M.; Purcell, S. Analysis of optimal habitat for captive release of the sea cucumber Holothuria scabra. Mar. Ecol. Prog. Ser. 2018, 588, 85–100. [Google Scholar] [CrossRef]
- Osathanunkul, M.; Suwannapoom, C. Sustainable fisheries management through reliable restocking and stock enhancement evaluation with environmental DNA. Sci. Rep. 2023, 13, 11297. [Google Scholar] [CrossRef]
- Haddi, I.; Benzha, F.; Maanan, M.; Siddique, S.; Rhinane, H.; Charouki, N.; Zidane, H. Population index and growth estimation based on SLW index of the sea cucumber (Holothuria arguinensis Koehler & Vaney, 1906) in the Moroccan waters of the Atlantic coast. Thalassas Int. J. Mar. Sci. 2022, 38, 535–551. [Google Scholar] [CrossRef]
- Hannah, L.; Duprey, N.; Blackburn, J.; Hand, C.M.; Pearce, C.M. Growth rate of the California sea cucumber Parastichopus californicus: Measurement accuracy and relationships between size and weight metrics. N. Am. J. Fish. Manag. 2012, 32, 167–176. [Google Scholar] [CrossRef]
- Mercier, A.; Battaglene, S.C.; Hamel, J.-F. Periodic movement, recruitment and size-related distribution of the sea cucumber Holothuria scabra in Solomon Islands. Hydrobiologia 2000, 440, 81–100. [Google Scholar] [CrossRef]
- Poot-Salazar, A.; Hernández-Flores, Á.; Ardisson, P.-L. Use of the SLW index to calculate growth function in the sea cucumber Isostichopus badionotus. Sci. Rep. 2014, 4, 5151. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Ayub, Z. To estimate growth function by the use of SLW index in the sea cucumber Holothuria arenicola (Holothuroidea: Echinodermata) of Pakistan (Northern Arabian Sea). Thalassas Int. J. Mar. Sci. 2019, 35, 123–132. [Google Scholar] [CrossRef]
- Danni, L.H.; Bethoney, N.D.; Kevin, D.E.S.; Mark, L.; Montana, F.M.; Michael, J.W.S. Standard methods for the collection of morphometric data for the commercially fished sea cucumber Cucumaria frondosa in Eastern Canada. J. Shellfish Res. 2020, 39, 481–489. [Google Scholar] [CrossRef]
- Skewes, T.; Smith, L.; Dennis, D.; Rawlinson, N.; Donovan, A.; Ellis, N. Conversion Ratios for Commercial Beche-de-Mer Species in Torres Strait; Final Report; Australian Fisheries Management Authority, Torres Strait Research Program: Canberra, Australia, 2004; p. 20. [Google Scholar]
- Fan, X.; Wu, K.; Tian, X.; Benjakul, S.; Li, Y.; Sang, X.; Zhao, Q.; Zhang, J. Endogenous proteases in sea cucumber (Apostichopus japonicas): Deterioration and prevention during handling, processing, and preservation. Foods 2024, 13, 2153. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Zhao, J.; Li, S.; Xing, J.; Ai, C.; Yu, C.; Yang, S.; Yang, J. Oxygenated storage alleviates autolysis of the sea cucumber Apostichopus japonicus during transport. Aquac. Int. 2023, 31, 2779–2798. [Google Scholar] [CrossRef]
- Hou, S.; Jin, Z.; Jiang, W.; Chi, L.; Xia, B.; Chen, J. Physiological and immunological responses of sea cucumber Apostichopus japonicus during desiccation and subsequent resubmersion. PeerJ 2019, 7, e7427. [Google Scholar] [CrossRef]
- Prescott, J.; Zhou, S.; Prasetyo, A.P. Soft bodies make estimation hard: Correlations among body dimensions and weights of multiple species of sea cucumbers. Mar. Freshwater Res. 2015, 66, 857–865. [Google Scholar] [CrossRef]
- Massin, C.; Uthicke, S.; Purcell, S.; Rowe, F.; Samyn, Y.; Rowe, F. Taxonomy of the heavily exploited Indo-Pacific sandfish complex (Echinodermata: Holothuriidae). Zool. J. Linn. Soc. 2009, 155, 40–59. [Google Scholar] [CrossRef]
- Purcell, S.W.; Shea, S.K.H.; Gray, B.C.T. Decadal changes in value of dried sea cucumbers (bêche-de-mer) in Hong Kong markets. Mar. Policy 2024, 171, 106450. [Google Scholar] [CrossRef]
- Purcell, S.W.; Lovatelli, A.; González-Wangüemert, M.; Solís-Marín, F.A.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World. Second Edition, FAO Species Catalogue for Fishery Purposes; FAO: Rome, Italy, 2023; p. 256. [Google Scholar]
- Azari, B.G.D.; Ivy, W.G. Aquaculture potential of the tropical sea cucumbers Holothuria scabra and H. lessoni in the Indo-Pacific region. SPC Beche-de-Mer Inf. Bull. 2010, 30, 29–32. [Google Scholar]
- Uthicke, S.; Purcell, S.; Blockmans, B. Natural hybridization does not dissolve species boundaries in commercially important sea cucumbers. Biol. J. Linn. Soc. 2005, 85, 261–270. [Google Scholar] [CrossRef]
- Guénette, S.; Chuenpagdee, R.; Jones, R. Marine Protected Areas with an Emphasis on Local Communities and Indigenous Peoples: A Review; Fisheries Centre Research Reports; Fisheries Centre, University of British Columbia: Vancouver, BC, Canada, 2000; p. 55. [Google Scholar]
- Payri, C.E.; Allain, V.; Aucan, J.; David, C.; David, V.; Dutheil, C.; Loubersac, L.; Menkes, C.; Pelletier, B.; Pestana, G. Chapter 27—New Caledonia. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: London, UK, 2019; pp. 593–618. [Google Scholar]
- Conand, C. Reproductive biology of the holothurians from the major communities of the New Caledonian lagoon. Mar. Biol. 1993, 116, 439–450. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Conand, C. Croissance et mortalité de quelques holothuries de Nouvelle-Calédonie. Vie Mar. 1989, 10, 160–176. [Google Scholar]
- Purcell, S.; Agudo, N.; Gossuin, H. Status and Management of the Sea Cucumber Fishery of La Grande Terre, New Caledonia; WorldFish Center Studies and Review Nº 1901; The WorldFish Center: Penang, Malaysia, 2009; p. 136. [Google Scholar]
- Yamana, Y.; Hamano, T. New size measurement for the Japanese sea cucumber Apostichopus japonicus (Stichopodidae) estimated from the body length and body breadth. Fish. Sci. 2006, 72, 585–589. [Google Scholar] [CrossRef]
- Froese, R. Estimating somatic growth of fishes from maximum age or maturity. Acta Ichthyol. Piscat. 2022, 52, 125–133. [Google Scholar] [CrossRef]
- Li, D.; Hao, Y.; Duan, Y. Nonintrusive methods for biomass estimation in aquaculture with emphasis on fish: A review. Rev. Aquac. 2020, 12, 1390–1411. [Google Scholar] [CrossRef]
- Hammond, A.R.; Purcell, S.W. Length–weight and body condition relationships of the exploited sea cucumber Pearsonothuria graeffei. J. Mar. Sci. Eng. 2024, 12, 371. [Google Scholar] [CrossRef]
- Gray, B.C.T.; Byrne, M.; Clements, M.; Foo, S.A.; Purcell, S.W. Length-weight relationship for the dragonfish, Stichopus cf. monotuberculatus (Holothuroidea). Fish. Res. 2023, 268, 106851. [Google Scholar] [CrossRef]
- Murphy, N.E.; Plaganyi, E.; Edgar, S.; Salee, K.; Skewes, T. Stock Survey of Sea Cucumbers in East Torres Strait. In AFMA Project, 2019/0826; Final Report; CSIRO: Canberra, Australia, 2021; p. 138. [Google Scholar]
- Veronika, K.; Edirisinghe, U.; Kuganathan, S.; Athauda, S. Length-weight relationships of four different sea cucumber species in North-East coastal region of Sri Lanka. Trop. Agric. Res. 2018, 29, 212. [Google Scholar] [CrossRef]
- Ramili, Y.; Umasangaji, H.; Legohiwo, M. Length-weight relationship and condition factors of sea cucumber on Mare and Moti Islands conservation areas in North Maluku. J. Kelaut. Tropis 2024, 27, 150–160. [Google Scholar] [CrossRef]
- Azevedo e Silva, F.; Brito, A.C.; Simões, T.; Pombo, A.; Sousa, J.; Venâncio, E.; Félix, P.M. Estimating age and growth parameters for three commercial NE-Atlantic sea cucumbers, Holothuria mammata, H. forskali and H. arguinensis, in a marine protected area. Front. Mar. Sci. 2024, 11, 1295196. [Google Scholar] [CrossRef]
- Jones, R.E.; Petrell, R.J.; Pauly, D. Using modified length–weight relationships to assess the condition of fish. J. Aquac. Eng. 1999, 20, 261–276. [Google Scholar] [CrossRef]
- Jellyman, P.G.; Brooker, D.J.; Crow, S.K.; Bonnett, M.L.; Jellyman, D.J. Does all size fit one? An evaluation of length-weight relationships for New Zealand’s freshwater fish species. N. Z. J. Mar. Freshw. Res. 2013, 47, 450–468. [Google Scholar] [CrossRef]
- Navarro, P.G.; Garcia-Sanz, S.; Barrio, J.M.; Tuya, F. Feeding and movement patterns of the sea cucumber Holothuria sanctori. Mar. Biol. 2013, 160, 2957–2966. [Google Scholar] [CrossRef]
- Garcin, M.; Vendé-Leclerc, M.; Maurizot, P.; Le Cozannet, G.; Robineau, B.; Nicolae-Lerma, A. Lagoon islets as indicators of recent environmental changes in the South Pacific—The New Caledonian example. Cont. Shelf Res. 2016, 122, 120–140. [Google Scholar] [CrossRef]
- Hobday, A.J.; Tegner, M.J.; Haaker, P.L. Over-exploitation of a broadcast spawning marine invertebrate: Decline of the white abalone. Rev. Fish Biol. Fish. 2000, 10, 493–514. [Google Scholar] [CrossRef]

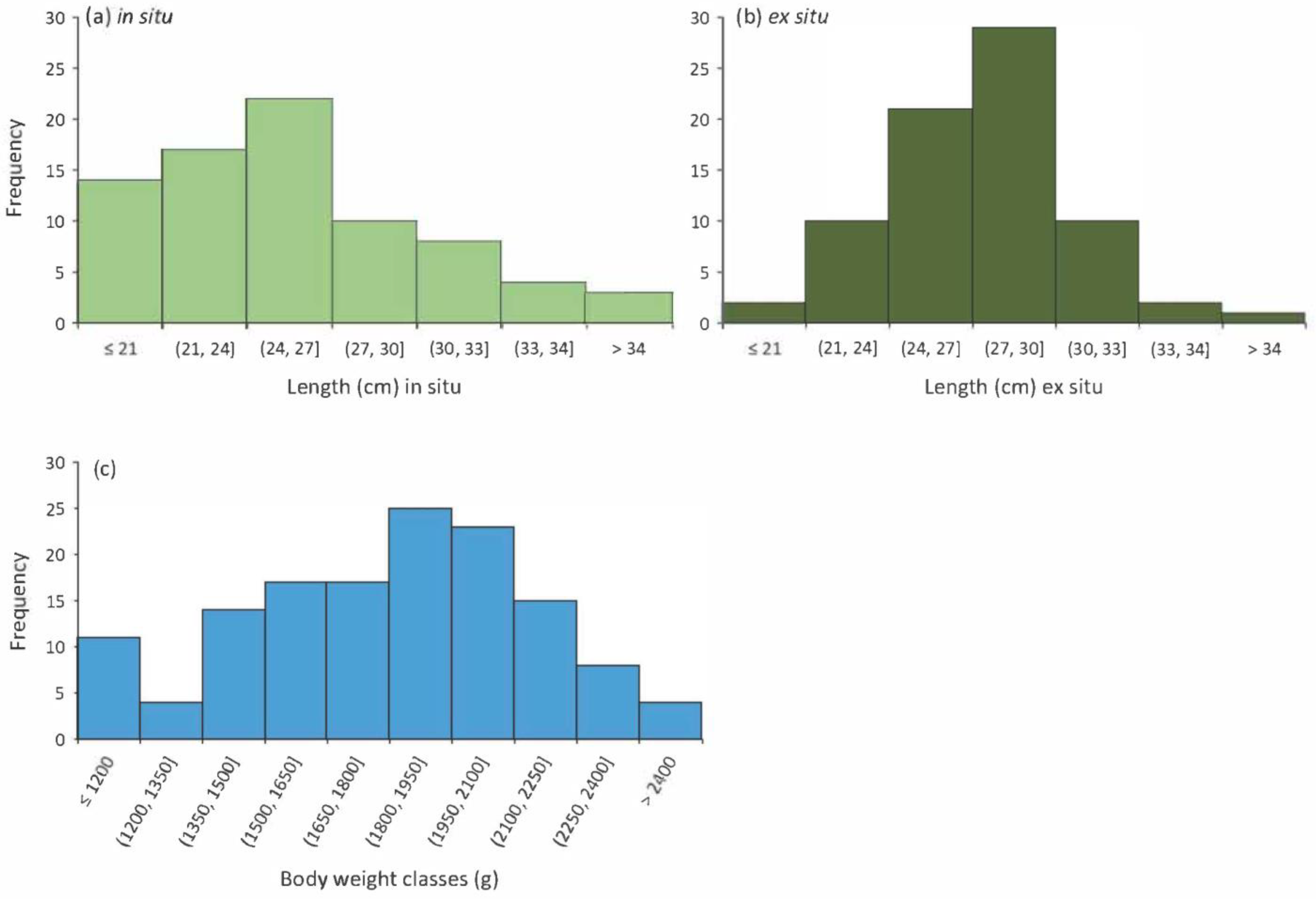
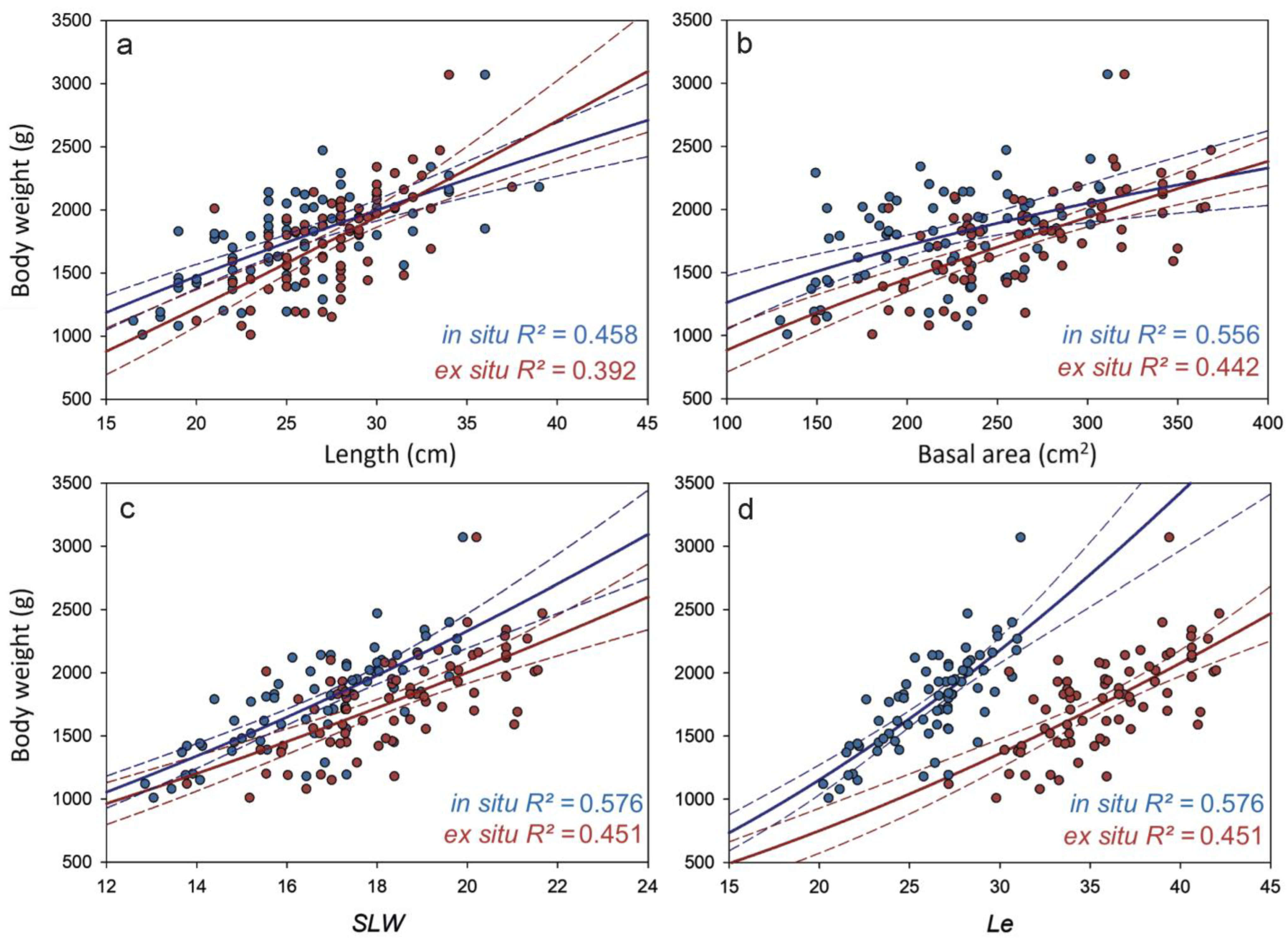

| In Situ | Ex Situ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relationship | R2 | a | b | RMSE | CV | R2 | a | b | RMSE | CV | p |
| Length–weight | 0.458 | 163.2 | 0.75 | 272 | 19 | 0.392 | 41.9 | 1.14 | 288 | 11.9 | <0.001 |
| Basal area–weight | 0.556 | 32.4 | 0.70 | 242 | 20.8 | 0.449 | 33.5 | 0.72 | 276 | 19.5 | <0.001 |
| SLW–weight | 0.576 | 22.3 | 1.55 | 242 | 10.6 | 0.451 | 28.2 | 1.49 | 276 | 9.9 | <0.001 |
| Le–weight | 0.576 | 13.2 | 1.57 | 242 | 10.5 | 0.451 | 14.2 | 1.53 | 276 | 9.6 | <0.001 |
| Study | Mean (±SD) | a | b | R2 | K (±SD) | Relationship | p |
|---|---|---|---|---|---|---|---|
| Conand (1989) [51] | 34.8 (5.4) | 2.48 | 1.78 | 0.51 | 3.51 (1.4) | Purcell et al. (2009) [52] | <0.001 |
| Purcell et al. (2009) [52] | 30.9 (2.6) | 2.93 | 1.80 | 0.59 | 4.94 (0.9) | Djenidi et al. | 0.0018 |
| Djenidi et al. | 27.6 (3.3) | 41.87 | 1.12 | 0.40 | 8.67 (2.7) | Conand (1989) [51] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djenidi, L.A.F.; Purcell, S.W.; Thornton, A.W.; Gossuin, H.; Gilbert, A. Length–Weight Relationships of the Prized Sea Cucumber Holothuria lessoni from In Situ and Ex Situ Measurements. J. Mar. Sci. Eng. 2024, 12, 2283. https://doi.org/10.3390/jmse12122283
Djenidi LAF, Purcell SW, Thornton AW, Gossuin H, Gilbert A. Length–Weight Relationships of the Prized Sea Cucumber Holothuria lessoni from In Situ and Ex Situ Measurements. Journal of Marine Science and Engineering. 2024; 12(12):2283. https://doi.org/10.3390/jmse12122283
Chicago/Turabian StyleDjenidi, Lea A. F., Steven W. Purcell, Aaron W. Thornton, Hugues Gossuin, and Antoine Gilbert. 2024. "Length–Weight Relationships of the Prized Sea Cucumber Holothuria lessoni from In Situ and Ex Situ Measurements" Journal of Marine Science and Engineering 12, no. 12: 2283. https://doi.org/10.3390/jmse12122283
APA StyleDjenidi, L. A. F., Purcell, S. W., Thornton, A. W., Gossuin, H., & Gilbert, A. (2024). Length–Weight Relationships of the Prized Sea Cucumber Holothuria lessoni from In Situ and Ex Situ Measurements. Journal of Marine Science and Engineering, 12(12), 2283. https://doi.org/10.3390/jmse12122283







