Assessing the Interrelationship Between Microplastics and Polychlorinated Biphenyls Contamination in Chinese Mangrove Sediment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Area
2.3. Sample Collection
2.4. Sample Pretreatment and Extraction
2.4.1. Sample Pretreatment
2.4.2. Sample Extraction
2.5. Instrument Analysis
2.5.1. Microscopic Inspection and Raman Analysis
2.5.2. Instrumental Analysis of PCBs
2.6. Quality Assurance (QA) and Quality Control (QC)
2.7. Statistical Analysis
3. Results
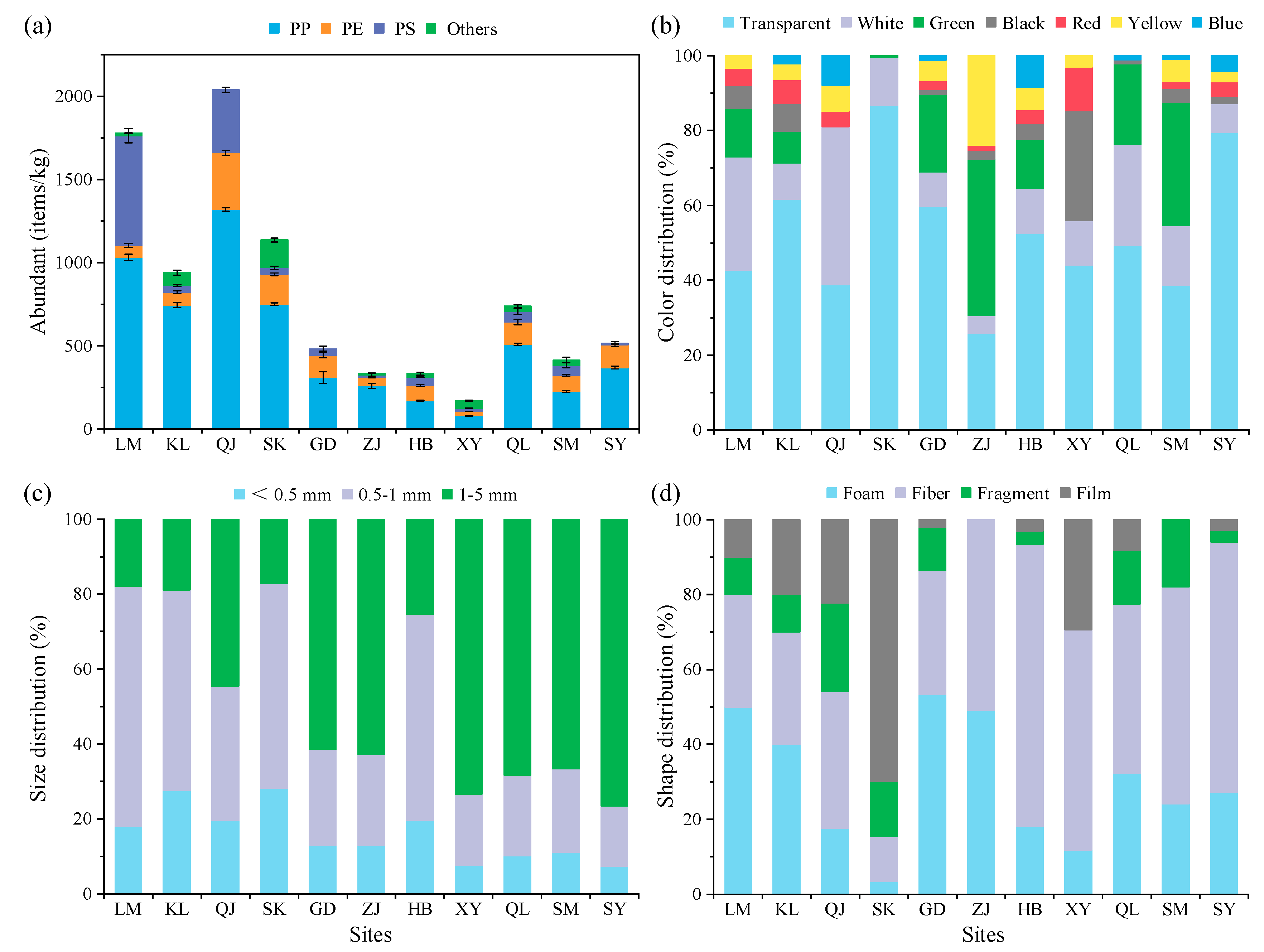
3.1. Abundance and Features of MPs from Different Mangrove Sediment Regions
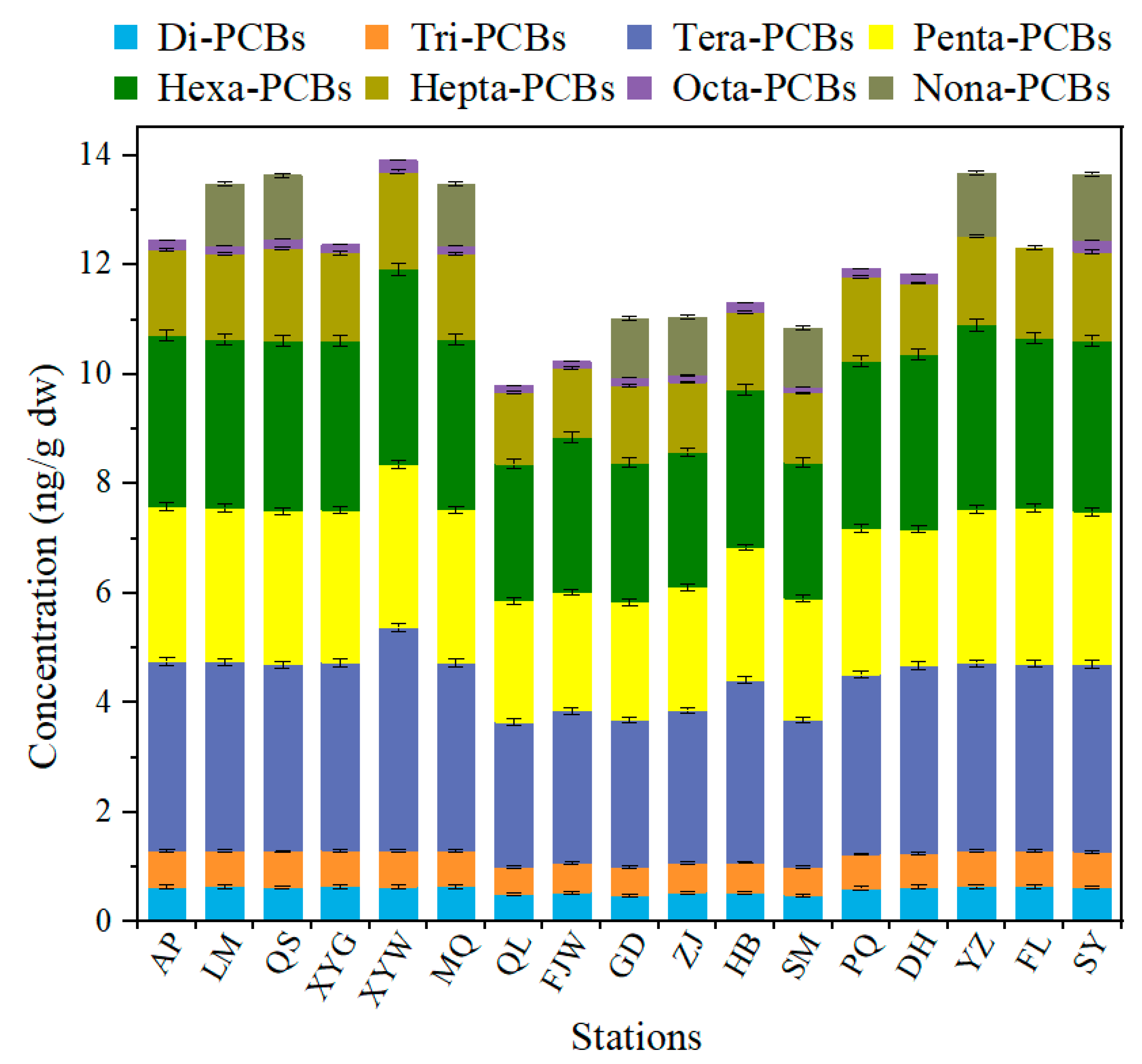
3.2. Characterization of PCBs Contamination in Different Mangrove Sediment Areas
3.3. Characteristics of Microplastics-Loaded PCBs in Different Mangrove Sediment Regions
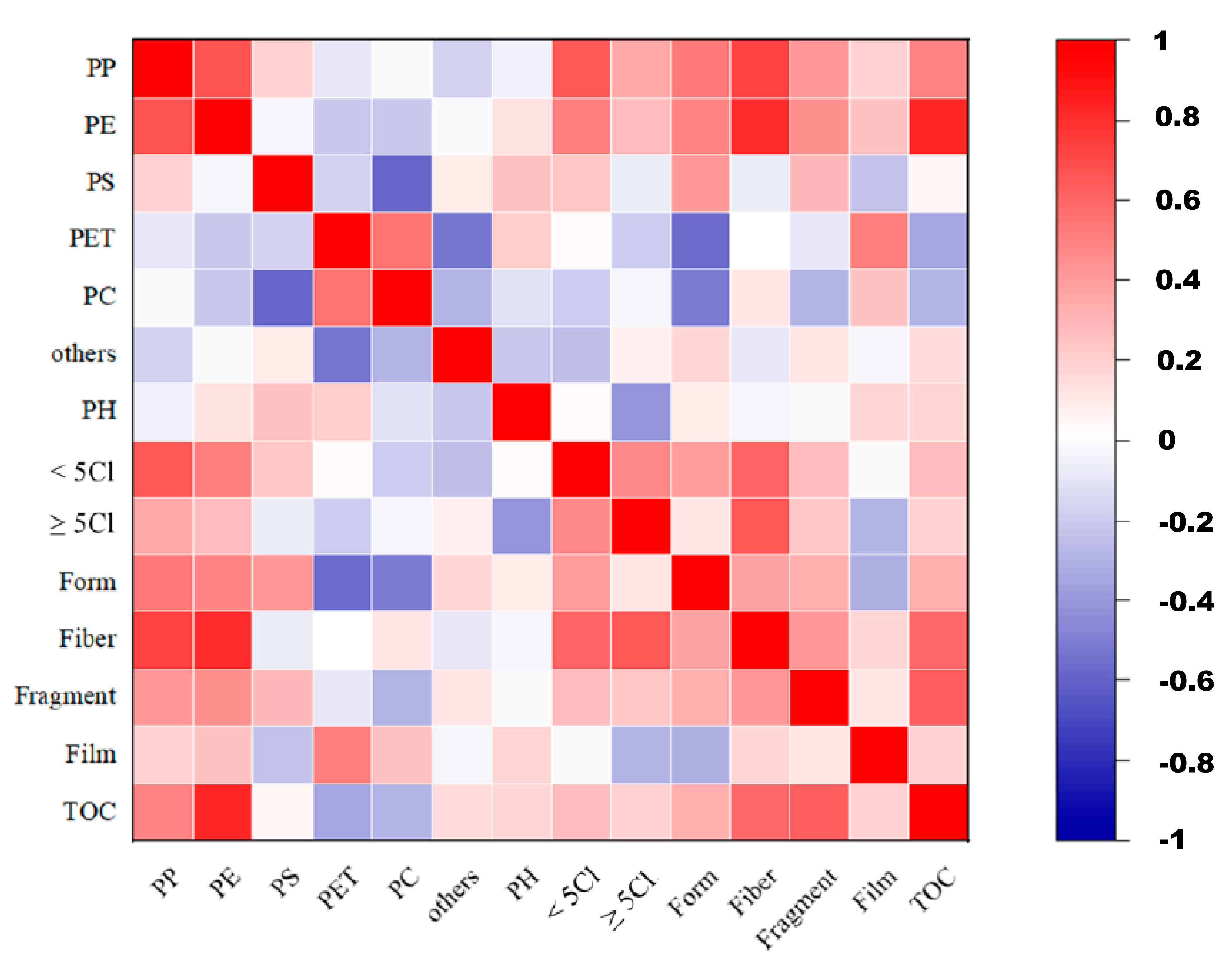
3.4. Analysis of Factors Affecting Microplastics-Loaded PCBs in Different Mangrove Sediment Regions
4. Discussion
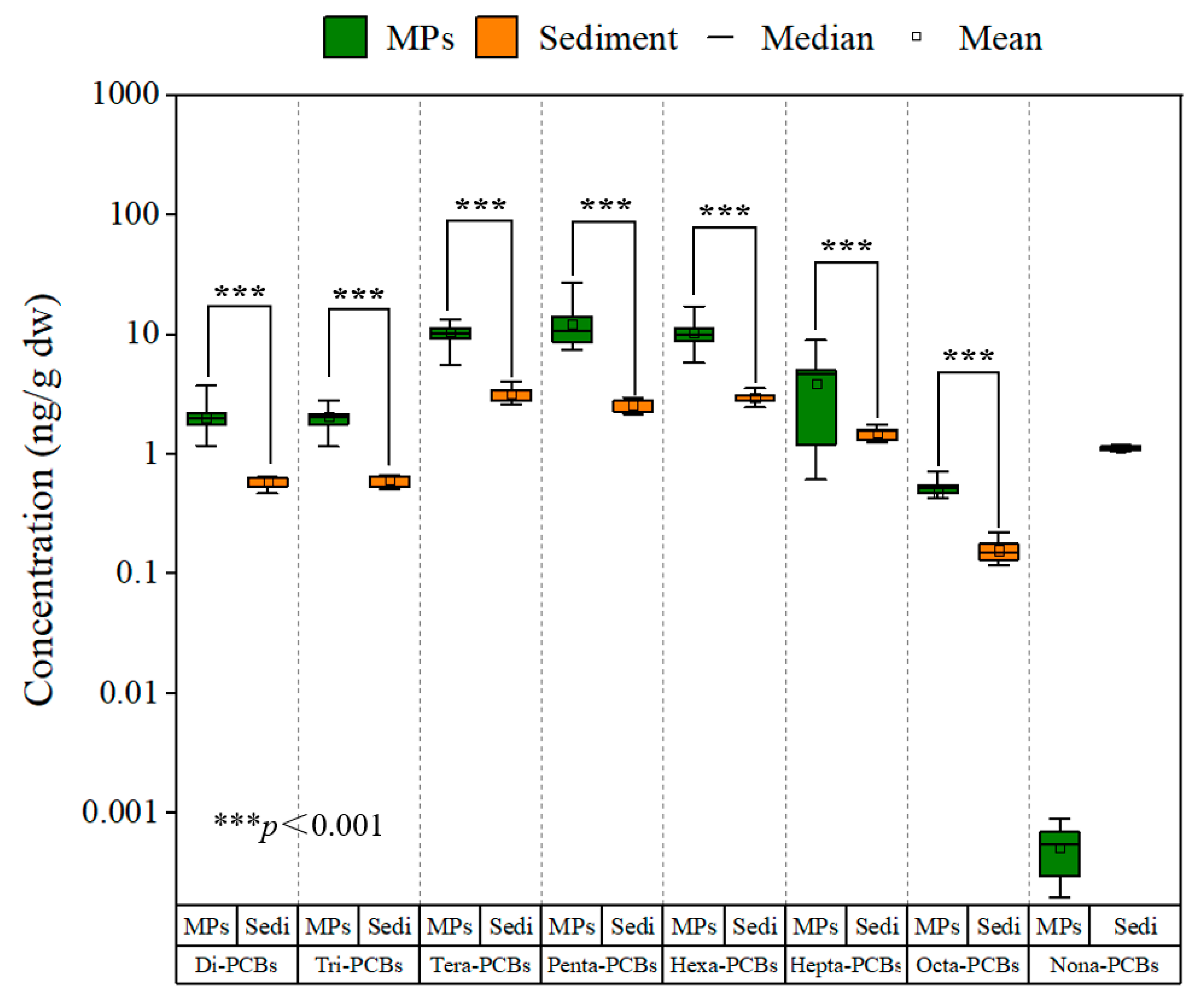
4.1. The Level of MPs-Bound PCBs and Microplastic Contamination
4.2. Sources of PCBs Laden on MPs and Sediment of Mangrove Ecosystem
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellasi, A.; Binda, G.; Pozzi, A.; Galafassi, S.; Volta, P.; Bettinetti, R. Microplastic Contamination in Freshwater Environments: A Review, Focusing on Interactions with Sediments and Benthic Organisms. Environments 2020, 7, 30. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Koutnik, V.S.; Leonard, J.; Alkidim, S.; DePrima, F.J.; Ravi, S.; Hoek, E.M.V.; Mohanty, S.K. Distribution of microplastics in soil and freshwater environments: Global analysis and framework for transport modeling. Environ. Pollut. 2021, 274, 116552. [Google Scholar] [CrossRef] [PubMed]
- Azaaouaj, S.; Nachite, D.; Anfuso, G.; Er-Ramy, N. Abundance and distribution of microplastics on sandy beaches of the eastern Moroccan Mediterranean coast. Mar. Pollut. Bull. 2024, 200, 116144. [Google Scholar] [CrossRef]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef]
- Shi, R.; Liu, W.; Lian, Y.; Zeb, A.; Wang, Q. Type-dependent effects of microplastics on tomato (Lycopersicon esculentum L.): Focus on root exudates and metabolic reprogramming. Sci. Total Environ. 2023, 859, 160025. [Google Scholar] [CrossRef]
- Teng, L.; Zhu, Y.; Li, H.; Song, X.; Shi, L. The phytotoxicity of microplastics to the photosynthetic performance and transcriptome profiling of Nicotiana tabacum seedlings. Ecotoxicol. Environ. Saf. 2022, 231, 113155. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Lu, L.; Eom, J.; Ru, S.; Li, Y.; Wang, J. Trophic transfer of micro- and nanoplastics and toxicity induced by long-term exposure of nanoplastics along the rotifer (Brachionus plicatilis)-marine medaka (Oryzias melastigma) food chain. Environ. Pollut. 2024, 346, 123599. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, L.; Chai, M.; Wu, H.; Zhu, X. The distribution, characteristics and ecological risks of microplastics in the mangroves of Southern China. Sci. Total Environ. 2020, 708, 135025. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Chen, L.; Wang, X. Ecological interception effect of mangroves on microplastics. J. Hazard. Mater. 2022, 423, 127231. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qi, W.; Cao, X.; Hu, J.; Li, Y.; Peng, J.; Hu, C.; Qu, J. Microplastic residues in wetland ecosystems: Do they truly threaten the plant-microbe-soil system? Environ. Int. 2021, 156, 106708. [Google Scholar] [CrossRef]
- Song, X.; Zhuang, W.; Cui, H.; Liu, M.; Gao, T.; Li, A.; Gao, Z. Interactions of microplastics with organic, inorganic and bio-pollutants and the ecotoxicological effects on terrestrial and aquatic organisms. Sci. Total Environ. 2022, 838, 156068. [Google Scholar] [CrossRef]
- Su, X.; Liu, M.; Yuan, J.; Huang, X.; Lu, Z.; Xu, J.; He, Y. Potential risk of co-occurrence of microplastics and chlorinated persistent organic pollutants to coastal wetlands: Evidence from a case study. Environ. Pollut. 2023, 320, 121087. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Al-Odaini, N.A.; Song, Y.K.; Hong, S.H. Qualitative Analysis of Additives in Plastic Marine Debris and Its New Products. Arch. Environ. Contam. Toxicol. 2015, 69, 352–366. [Google Scholar] [CrossRef]
- Mai, L.; You, S.-N.; He, H.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Riverine Microplastic Pollution in the Pearl River Delta, China: Are Modeled Estimates Accurate? Environ. Sci. Technol. 2019, 53, 11810–11817. [Google Scholar] [CrossRef]
- Rohais, S.; Armitage, J.J.; Romero-Sarmiento, M.-F.; Pierson, J.-L.; Teles, V.; Bauer, D.; Cassar, C.; Sebag, D.; Klopffer, M.-H.; Pelerin, M. A source-to-sink perspective of an anthropogenic marker: A first assessment of microplastics concentration, pathways, and accumulation across the environment. Earth Sci. Rev. 2024, 254, 104822. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, M.; Zhao, Z.; Wang, Y.; Li, T.; Wei, Y.; Li, R.; Yang, F. Insight into the role of niche concept in deciphering the ecological drivers of MPs-associated bacterial communities in mangrove forest. Water Res. 2024, 249, 120995. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Asensio-Montesinos, F.; Reyes Almeida, G.; Solano Llanos, N.; Sánchez Moreno, H.; Orozco-Sánchez, C.J.; Villate Daza, D.A.; Iglesias-Navas, M.A.; Anfuso, G. Litter Content of Colombian Beaches and Mangrove Forests: Results from the Caribbean and Pacific Coasts. J. Mar. Sci. Eng. 2023, 11, 250. [Google Scholar] [CrossRef]
- De, K.; Sautya, S.; Dora, G.U.; Gaikwad, S.; Katke, D.; Salvi, A. Mangroves in the “Plasticene”: High exposure of coastal mangroves to anthropogenic litter pollution along the Central-West coast of India. Sci. Total Environ. 2023, 858, 160071. [Google Scholar] [CrossRef] [PubMed]
- Farzana, S.; Cheung, S.G.; Zhou, H.C.; Tam, N.F.Y. Growth and antioxidative response of two mangrove plants to interaction between aquaculture effluent and BDE-99. Sci. Total Environ. 2019, 662, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zeng, Y.; Kang, Z.; Cai, M.; Chen, K.; Zhao, Q.; Lin, J.; Liu, R.; Xu, G. Enrichment and ecological risks of microplastics in mangroves of southern Hainan Island, China. Sci. Total Environ. 2023, 889, 164160. [Google Scholar] [CrossRef]
- Yu, L.; Li, R.; Zhang, Z.; Wu, H.; Chai, M.; Zhu, X.; Guo, W. Distribution, characteristics, and human exposure to microplastics in mangroves within the Guangdong-Hong Kong-Macao Greater Bay Area. Mar. Pollut. Bull. 2022, 175, 113395. [Google Scholar] [CrossRef]
- Mendes, D.S.; Silva, D.N.N.; Santiago, L.E.P.; Gomes, V.J.C.; Beasley, C.R.; Fernandes, M.E.B. Comprehensive risk assessment of microplastics in tidal channel sediments in amazonian mangroves (northern Brazil). J. Environ. Manag. 2024, 366, 121826. [Google Scholar] [CrossRef]
- Qian, X.; Liang, B.; Fu, W.; Liu, X.; Cui, B. Polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the intertidal zone of Bohai Bay, Northeast China: Spatial distribution, composition, sources and ecological risk assessment. Mar. Pollut. Bull. 2016, 112, 349–358. [Google Scholar] [CrossRef]
- Ranjbar Jafarabadi, A.; Riyahi Bakhtiari, A.; Moghimi, H.; Gorokhova, E. Assessment of parent and alkyl -PAHs in surface sediments of Iranian mangroves on the northern coast of the Persian Gulf: Spatial accumulation distribution, influence factors, and ecotoxicological risks. Chemosphere 2024, 358, 142176. [Google Scholar] [CrossRef]
- Prajapati, A.; Narayan Vaidya, A.; Kumar, A.R. Microplastic properties and their interaction with hydrophobic organic contaminants: A review. Environ. Sci. Pollut. Res. 2022, 29, 49490–49512. [Google Scholar] [CrossRef]
- Tan, B.; Li, Y.; Xie, H.; Dai, Z.; Zhou, C.; Qian, Z.-J.; Hong, P.; Liang, Y.; Ren, L.; Sun, S.; et al. Microplastics accumulation in mangroves increasing the resistance of its colonization Vibrio and Shewanella. Chemosphere 2022, 295, 133861. [Google Scholar] [CrossRef]
- Olisah, C.; Malloum, A.; Adegoke, K.A.; Ighalo, J.O.; Conradie, J.; Ohoro, C.R.; Amaku, J.F.; Oyedotun, K.O.; Maxakato, N.W.; Akpomie, K.G.; et al. Scientometric trends and knowledge maps of global polychlorinated naphthalenes research over the past four decades. Environ. Pollut. 2024, 357, 124407. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, Y.; Shi, L.; Song, J.; Jiang, Y. Organochlorine pesticides and polychlorinated biphenyls in sediments of the Lanzhou reach of Yellow River (China): Spatial distribution, sources and risk assessment. Mar. Pollut. Bull. 2024, 208, 116962. [Google Scholar] [CrossRef]
- Noman, M.A.; Adyel, T.M.; Macreadie, P.I.; Trevathan-Tackett, S.M. Prioritising plastic pollution research in blue carbon ecosystems: A scientometric overview. Sci. Total Environ. 2024, 914, 169868. [Google Scholar] [CrossRef] [PubMed]
- Zanardi-Lamardo, E.; Mitra, S.; Vieira-Campos, A.A.; Cabral, C.B.; Yogui, G.T.; Sarkar, S.K.; Biswas, J.K.; Godhantaraman, N. Distribution and sources of organic contaminants in surface sediments of Hooghly river estuary and Sundarban mangrove, eastern coast of India. Mar. Pollut. Bull. 2019, 146, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Xu, M.; Zou, X.; Wang, C. The changing Changjiang River estuarine-coastal ocean continuum in the anthropocene. Catena 2024, 238, 107904. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, L.; Li, R.; Wang, Y.; Guo, J.; Yu, K.; Wang, S. Underestimated Microplastic Pollution Derived from Fishery Activities and “Hidden” in Deep Sediment. Environ. Sci. Technol. 2020, 54, 2210–2217. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Xue, B.; Wang, Y. Abundance and characteristics of microplastics in the mangrove sediment of the semi-enclosed Maowei Sea of the south China sea: New implications for location, rhizosphere, and sediment compositions. Environ. Pollut. 2019, 244, 685–692. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, F.; Li, J.; Wang, Z.; Sun, C.; Wang, Z.; Fu, L.; Ding, N.X.; He, C. Microplastics in the Coral Reef Systems from Xisha Islands of South China Sea. Environ. Sci. Technol. 2019, 53, 8036–8046. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef]
- Zhang, D.; Dang, H.; Li, Z.; Zhang, C. Redox characteristics of humins and their coupling with potential PCB dechlorinators in southern Yellow Sea sediments. Environ. Pollut. 2019, 252, 296–304. [Google Scholar] [CrossRef]
- Zhang, Z.; Pei, N.; Sun, Y.; Li, J.; Li, X.; Yu, S.; Xu, X.; Hu, Y.; Mai, B. Halogenated organic pollutants in sediments and organisms from mangrove wetlands of the Jiulong River Estuary, South China. Environ. Res. 2019, 171, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Q.; Li, Y.; Tan, S.; Kang, Z.; Yu, X.; Lan, W.; Cai, L.; Wang, J.; Shi, H. Microplastic pollution in the Maowei Sea, a typical mariculture bay of China. Sci. Total Environ. 2019, 658, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lao, Q.; Chen, F.; Zhou, X.; Chen, C.; Zhu, Q. Assessing the sources and dynamics of organic matter in a high human impact bay in the northern Beibu Gulf: Insights from stable isotopes and optical properties. Front. Mar. Sci. 2022, 9, 1043278. [Google Scholar] [CrossRef]
- Mai, L.; He, H.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Plastics Are an Insignificant Carrier of Riverine Organic Pollutants to the Coastal Oceans. Environ. Sci. Technol. 2020, 54, 15852–15860. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, Y.-W.; Chen, G.-Z. The effect of mangrove reforestation on the accumulation of PCBs in sediment from different habitats in Guangdong, China. Mar. Pollut. Bull. 2012, 64, 1614–1619. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Wang, Y.; Hu, S. Distribution, sources, and ecological risk assessment of polychlorinated biphenyls (PCBs) in the estuary of Dagu River, China. Mar. Pollut. Bull. 2023, 194, 115340. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Wen, L.; Li, Z.; Peng, M.; Wu, H.; Xie, L. Linking human activity to spatial accumulation of microplastics along mangrove coasts. Sci. Total Environ. 2022, 825, 154014. [Google Scholar] [CrossRef]
- Xin, X.; Chen, B.; Yang, M.; Gao, S.; Wang, H.; Gu, W.; Li, X.; Zhang, B. A critical review on the interaction of polymer particles and co-existing contaminants: Adsorption mechanism, exposure factors, effects on plankton species. J. Hazard. Mater. 2023, 445, 130463. [Google Scholar] [CrossRef]
- Delle Site, A. Factors Affecting Sorption of Organic Compounds in Natural Sorbent/Water Systems and Sorption Coefficients for Selected Pollutants. A Review. J. Phys. Chem. Ref. Data 2001, 30, 187–439. [Google Scholar] [CrossRef]
- Rai, P.K.; Sonne, C.; Brown, R.J.C.; Younis, S.A.; Kim, K.-H. Adsorption of environmental contaminants on micro- and nano-scale plastic polymers and the influence of weathering processes on their adsorptive attributes. J. Hazard. Mater. 2022, 427, 127903. [Google Scholar] [CrossRef]
- Xu, H.; Hu, Z.; Sun, Y.; Xu, J.; Huang, L.; Yao, W.; Yu, Z.; Xie, Y. Microplastics supply contaminants in food chain: Non-negligible threat to health safety. Environ. Geochem. Health 2024, 46, 276. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Xu, D.; Yang, X.; Hu, J. Microplastics and PAHs mixed contamination: An in-depth review on the sources, co-occurrence, and fate in marine ecosystems. Water Res. 2024, 257, 121622. [Google Scholar] [CrossRef] [PubMed]
- Souza ASd Torres, J.P.M.; Meire, R.O.; Neves, R.C.; Couri, M.S.; Serejo, C.S. Organochlorine pesticides (OCs) and polychlorinated biphenyls (PCBs) in sediments and crabs (Chasmagnathus granulata, Dana, 1851) from mangroves of Guanabara Bay, Rio de Janeiro State, Brazil. Chemosphere 2008, 73 (Suppl. 1), S186–S192. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Luo, X.-J.; Mai, B.-X.; Sheng, G.-Y.; Fu, J.-M.; Zeng, E.Y. Distribution and Mass Inventories of Polycyclic Aromatic Hydrocarbons and Organochlorine Pesticides in Sediments of the Pearl River Estuary and the Northern South China Sea. Environ. Sci. Technol. 2006, 40, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Alagan, M.; Chandra Kishore, S.; Perumal, S.; Manoj, D.; Raji, A.; Kumar, R.S.; Almansour, A.I.; Lee, Y.R. Narrative of hazardous chemicals in water: Its potential removal approach and health effects. Chemosphere 2023, 335, 139178. [Google Scholar] [CrossRef]
- Qamar, A.; Waheed, J.; Ghulam Mohyuddin, S.; Chen, Z.-B.; Kang, D.-J.; Li, Z.; Anthony, G.H.; Gooneratne, R.; Nie, F.-H.; Chen, J.-J. The Status of Polychlorinated Biphenyls (PCBs) Extract from Zhanjiang Mangrove Sediments and the Effects on Tissue Structure and Inflammatory Cytokines in Zebrafish Liver. Bull. Environ. Contam. Toxicol. 2022, 108, 890–900. [Google Scholar] [CrossRef]
- Zhu, M.; Yuan, Y.; Yin, H.; Guo, Z.; Wei, X.; Qi, X.; Liu, H.; Dang, Z. Environmental contamination and human exposure of polychlorinated biphenyls (PCBs) in China: A review. Sci. Total Environ. 2022, 805, 150270. [Google Scholar] [CrossRef]
- Šrédlová, K.; Cajthaml, T. Recent advances in PCB removal from historically contaminated environmental matrices. Chemosphere 2022, 287, 132096. [Google Scholar] [CrossRef]
- Valizadeh, S.; Lee, S.S.; Baek, K.; Choi, Y.J.; Jeon, B.-H.; Rhee, G.H.; Andrew Lin, K.-Y.; Park, Y.-K. Bioremediation strategies with biochar for polychlorinated biphenyls (PCBs)-contaminated soils: A review. Environ. Res. 2021, 200, 111757. [Google Scholar] [CrossRef]
- Passatore, L.; Rossetti, S.; Juwarkar, A.A.; Massacci, A. Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): State of knowledge and research perspectives. J. Hazard. Mater. 2014, 278, 189–202. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M.N. Genetically engineered microorganisms for environmental remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef] [PubMed]
- Hatje, V.; Sarin, M.; Sander, S.G.; Omanović, D.; Ramachandran, P.; Völker, C.; Barra, R.O.; Tagliabue, A. Emergent interactive effects of climate change and contaminants in coastal and ocean ecosystems. Front. Mar. Sci. 2022, 9, 936109. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Deng, J.; Zhang, G.; Yi, G.; Peng, G.; Wei, Y.; Ren, L.; Wang, Y. Assessing the Interrelationship Between Microplastics and Polychlorinated Biphenyls Contamination in Chinese Mangrove Sediment. J. Mar. Sci. Eng. 2024, 12, 2216. https://doi.org/10.3390/jmse12122216
He J, Deng J, Zhang G, Yi G, Peng G, Wei Y, Ren L, Wang Y. Assessing the Interrelationship Between Microplastics and Polychlorinated Biphenyls Contamination in Chinese Mangrove Sediment. Journal of Marine Science and Engineering. 2024; 12(12):2216. https://doi.org/10.3390/jmse12122216
Chicago/Turabian StyleHe, Jianqiao, Jun Deng, Guochao Zhang, Guoqiang Yi, Gen Peng, Yihua Wei, Lu Ren, and Yinghui Wang. 2024. "Assessing the Interrelationship Between Microplastics and Polychlorinated Biphenyls Contamination in Chinese Mangrove Sediment" Journal of Marine Science and Engineering 12, no. 12: 2216. https://doi.org/10.3390/jmse12122216
APA StyleHe, J., Deng, J., Zhang, G., Yi, G., Peng, G., Wei, Y., Ren, L., & Wang, Y. (2024). Assessing the Interrelationship Between Microplastics and Polychlorinated Biphenyls Contamination in Chinese Mangrove Sediment. Journal of Marine Science and Engineering, 12(12), 2216. https://doi.org/10.3390/jmse12122216




