The Association of Benthic Infauna with Fine-Grained Organic-Rich Sediments in a Shallow Subtropical Estuary
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Sediment Composition and Physical Parameters
3.2. Infaunal Density and Diversity Patterns
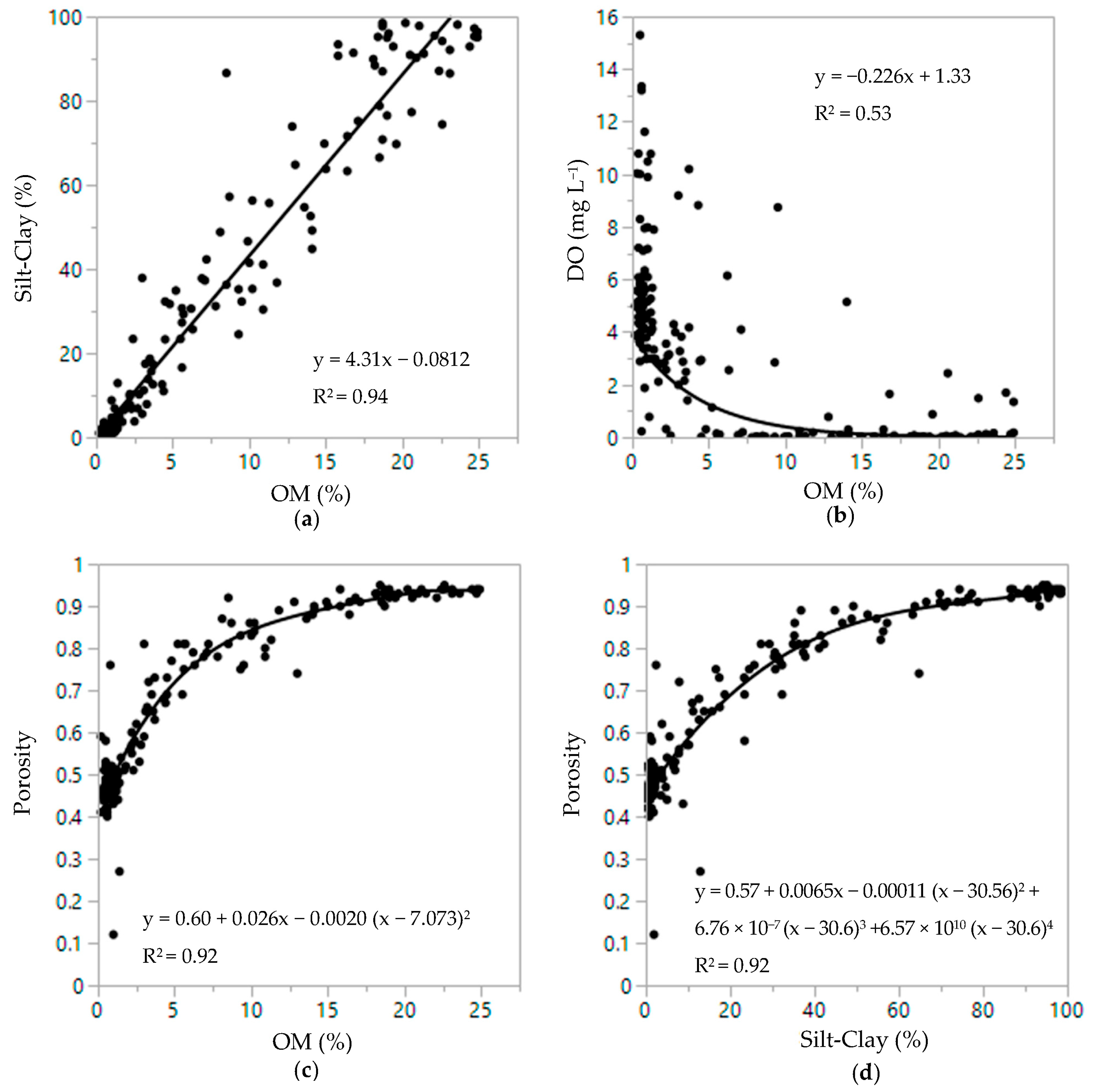
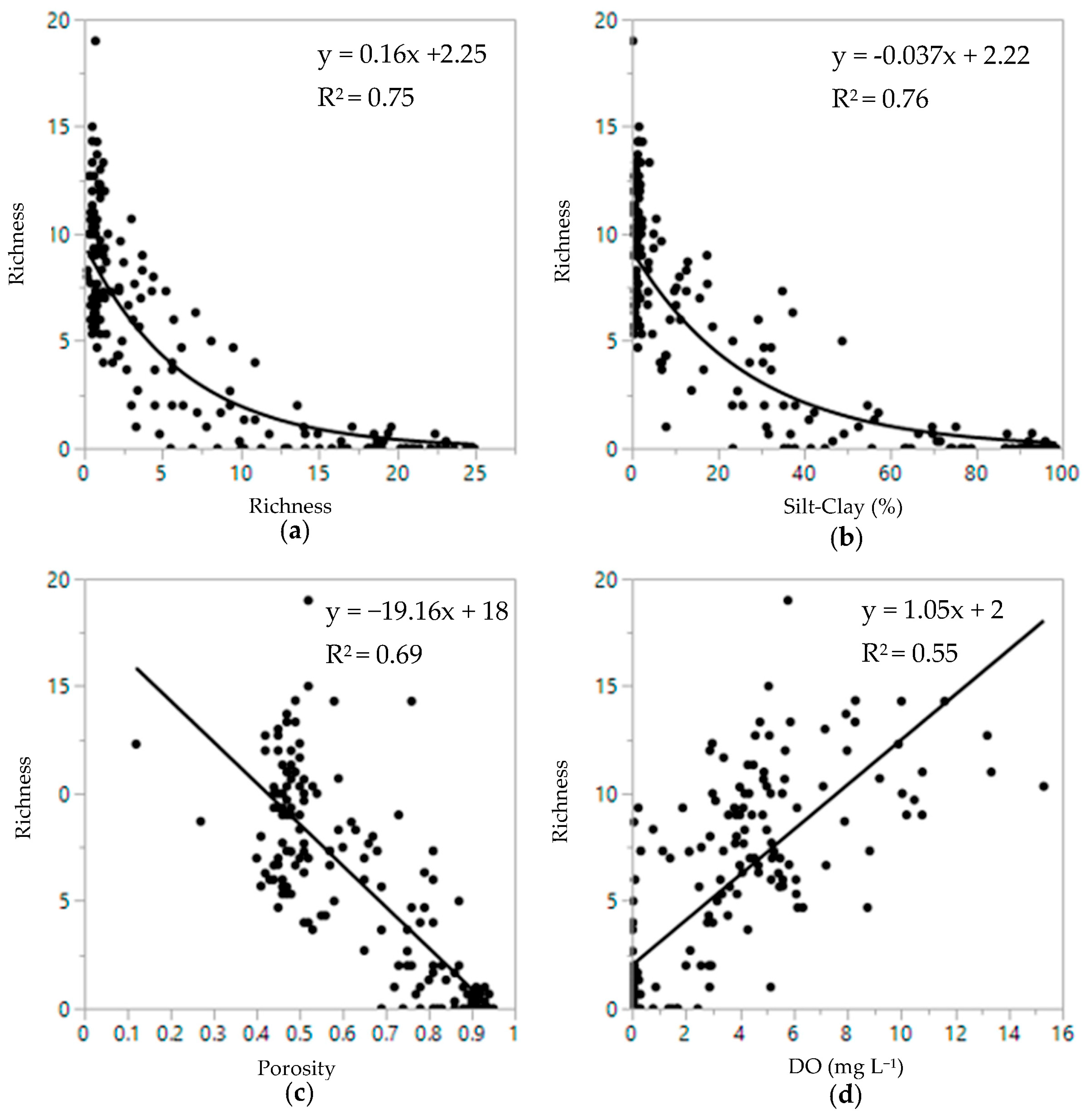
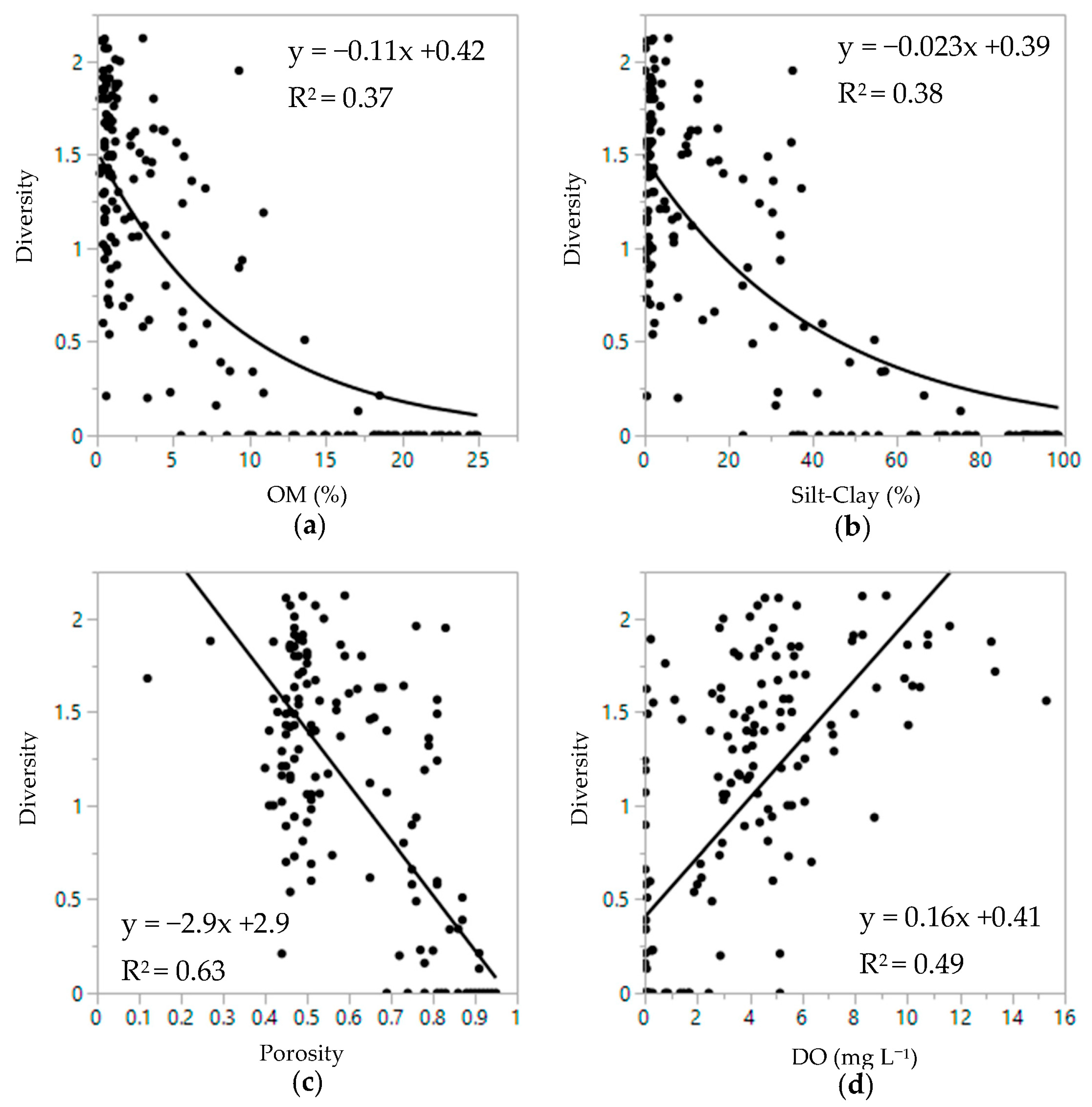
3.3. Sediment and Infauna Correlations
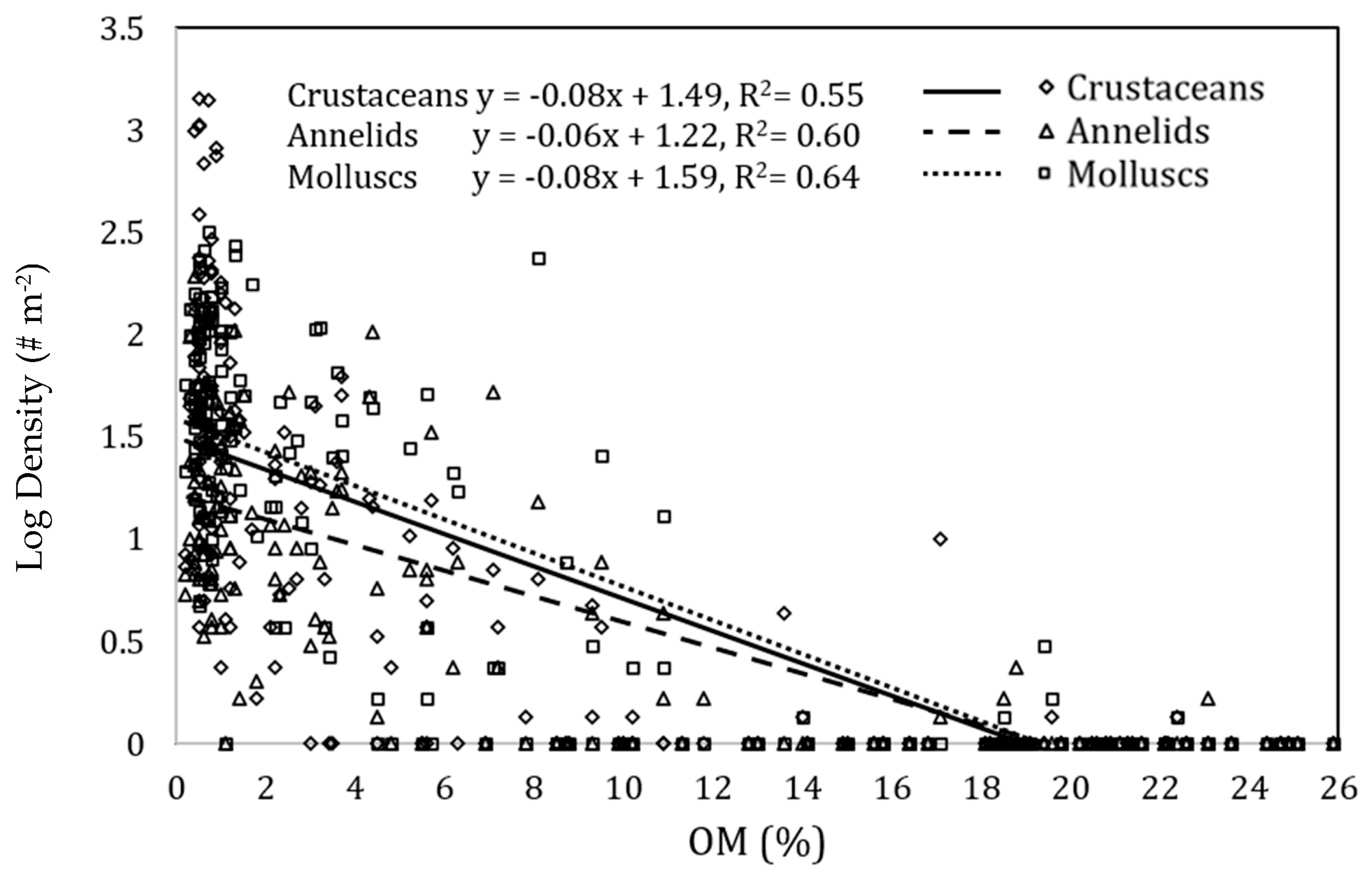
3.4. Taxonomic Sensitivity to OM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, P.M.; Wang, F. Assessing sediment contamination in estuaries. Environ. Toxicol. Chem. Int. J. 2001, 20, 3–22. [Google Scholar] [CrossRef]
- Harley, C.D.; Randall Hughes, A.; Hultgren, K.M.; Miner, B.G.; Sorte, C.J.; Thornber, C.S.; Rodriguez, L.F.; Tomanek, L.; Williams, S.L. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006, 9, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Sweat, L.H.; Alexander, H.; Phlips, E.J.; Johnson, K.B. Mesozooplankton community dynamics and grazing potential across algal bloom cycles in a subtropical estuary. Front. Mar. Sci. 2021, 8, 734270. [Google Scholar] [CrossRef]
- Ma, X.; Jacoby, C.A.; Johnson, K.B. Grazing by the copepod Parvocalanus crassirostris on Picochlorum sp. at harmful bloom densities and the role of particle size. Front. Mar. Sci. 2021, 8, 664154. [Google Scholar] [CrossRef]
- Trefry, J.H.; Metz, S.; Trocine, R.P.; Iricanin, N.; Burnside, D.; Chen, N.; Webb, B. Design and Operation of a Muck Sediment Survey; Special Report SJ90-SP3; Saint Johns River Water Management District: Palatka, FL, USA, 1990. [Google Scholar]
- Hedges, J.I.; Keil, R.G.; Benner, R. What happens to terrestrial organic matter in the ocean? Org. Geochem. 1997, 27, 195–212. [Google Scholar] [CrossRef]
- Trefry, J.H.; Trocine, R.P.; Woodall, D.W. Composition and sources of suspended matter in the Indian River Lagoon, Florida. Fla. Sci. 2007, 70, 363–382. [Google Scholar]
- Gray, J.S.; Waldichuk, M.; Newton, A.J.; Berry, R.J.; Holden, A.V.; Pearson, T.H. Pollution-induced changes in populations [and discussion]. Philos. Trans. R. Soc. B Biol. Sci. 1979, 286, 545–561. [Google Scholar]
- Hyland, J.; Balthis, L.; Karakassis, I.; Magni, P.; Petrov, A.; Shine, J.; Warwick, R. Organic carbon content of sediments as an indicator of stress in the marine benthos. Mar. Ecol. Prog. Ser. 2005, 295, 91–103. [Google Scholar] [CrossRef]
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Ann. Rev. 1978, 16, 229–311. [Google Scholar]
- Viaroli, P.; Bartoli, M.; Giordani, G.; Naldi, M.; Orfanidis, S.; Zaldivar, J.M. Community shifts, alternative stable states, biogeochemical controls and feedbacks in eutrophic coastal lagoons: A brief overview. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, S105–S117. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 1995, 33, 3. [Google Scholar]
- Gibson, R.N.; Barnes, M.; Atkison, R.J.A. Functional group ecology in softsediment marine benthos: The role of bioturbation. Oceanogr. Mar. Biol. Annu. Rev. 2001, 39, 233–267. [Google Scholar]
- Aller, R.C. Bioturbation and remineralization of sedimentary organic matter: Effects of redox oscillation. Chem. Geol. 1994, 114, 331–345. [Google Scholar] [CrossRef]
- Rhoads, D.C. Organism-sediment relations on the muddy sea floor. Oceanogr. Mar. Biol. Ann. Rev. 1974, 12, 263–300. [Google Scholar]
- Cox, A.; Hope, D.; Angelica Zamora-Duran, M.; Johnson, K.B. Environmental factors influencing benthic polychaete distributions in a subtropical lagoon. Mar. Technol. Soc. J. 2018, 52, 58–74. [Google Scholar] [CrossRef]
- Mallick, N.; Johnson, K.B.; Jacoby, C.A. The effect of environmental dredging of muck on an assemblage of benthic amphipods. J. Mar. Sci. Eng. 2023, 11, 444. [Google Scholar] [CrossRef]
- Magni, P.; De Falco, G.; Como, S.; Casu, D.; Floris, A.; Petrov, A.N.; Perilli, A. Distribution and ecological relevance of fine sediments in organic-enriched lagoons: The case study of the Cabras lagoon (Sardinia, Italy). Mar. Pollut. Bull. 2008, 56, 549–564. [Google Scholar] [CrossRef]
- Gray, J.S. The Ecology of Marine Sediments; Cambridge University Press: Cambridge, UK, 1981; Volume 2. [Google Scholar]
- Dybas, C.L. Florida’s Indian River Lagoon: An estuary in transition. BioScience 2002, 52, 554–559. [Google Scholar] [CrossRef]
- Rhoads, D.C.; Germano, J.D. Interpreting long-term changes in benthic community structure: A new protocol. Hydrobiologia 1986, 142, 291–308. [Google Scholar] [CrossRef]
- Burd, B.; Macdonald, R.; Boyd, J. Punctuated recovery of sediments and benthic infauna: A 19-year study of tailings deposition in a British Columbia fjord. Mar. Environ. Res. 2000, 49, 145–175. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, A.; Holme, N.A. Macrofauna techniques. In Methods for the Study of Marine Benthos; Wiley: Hoboken, NJ, USA, 1984; pp. 140–216. [Google Scholar]
- Ruso, Y.D.P.; De la Ossa Carretero, J.A.; Casalduero, F.G.; Lizaso, J.S. Spatial and temporal changes in infaunal communities inhabiting soft-bottoms affected by brine discharge. Mar. Environ. Res. 2007, 64, 492–503. [Google Scholar] [CrossRef] [PubMed]
- WoRMS Editorial Board. World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 30 March 2016).
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Dean Jr, W.E. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. J. Sediment. Res. 1974, 44, 242–248. [Google Scholar]
- Birchenough, S.N.; Frid, C.L. Macrobenthic succession following the cessation of sewage sludge disposal. J. Sea Res. 2009, 62, 258–267. [Google Scholar] [CrossRef]
- Lawless, A.S.; Seitz, R.D. Effects of shoreline stabilization and environmental variables on benthic infaunal communities in the Lynnhaven River System of Chesapeake Bay. J. Exp. Mar. Biol. Ecol. 2014, 457, 41–50. [Google Scholar] [CrossRef]
- Robertson, B.P.; Gardner, J.P.; Savage, C. Macrobenthic–mud relations strengthen the foundation for benthic index development: A case study from shallow, temperate New Zealand estuaries. Ecol. Indic. 2015, 58, 161–174. [Google Scholar]
- Milliman, J.D. Organic matter content in US Atlantic continental slope sediments: Decoupling the grain-size factor. Deep Sea Res. Part II Top. Stud. Oceanogr. 1994, 41, 797–808. [Google Scholar]
- Hedges, J.I.; Keil, R.G. Sedimentary organic matter preservation: An assessment and speculative synthesis. Mar. Chem. 1995, 49, 81–115. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F.; Rosenberg, R.; François-Carcaillet, F.; Norling, K.; Mauclaire, L. Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment. Aquat. Microb. Ecol. 2004, 36, 271–284. [Google Scholar] [CrossRef]
- Giblin, A.E.; Hopkinson, C.S.; Tucker, J. Benthic metabolism and nutrient cycling in Boston Harbor, Massachusetts. Estuaries 1997, 20, 346–364. [Google Scholar] [CrossRef]
- Gray, J.S.; Mirza, F.B. A possible method for the detection of pollution-induced disturbance on marine benthic communities. Mar. Pollut. Bull. 1979, 10, 142–146. [Google Scholar] [CrossRef]
- Puente, A.; Diaz, R.J. Response of benthos to ocean outfall discharges: Does a general pattern exist? Mar. Pollut. Bull. 2015, 101, 174–181. [Google Scholar] [CrossRef]
- Luther, G.W.; Church, T.M.; Powell, D. Sulfur speciation and sulfide oxidation in the water column of the Black Sea. Deep Sea Research Part A. Oceanogr. Res. Pap. 1991, 38, S1121–S1137. [Google Scholar]
- Leong, L.S.; Tanner, P.A. Comparison of methods for determination of organic carbon in marine sediment. Mar. Pollut. Bull. 1999, 38, 875–879. [Google Scholar] [CrossRef]
- Kodama, K.; Lee, J.H.; Oyama, M.; Shiraishi, H.; Horiguchi, T. Disturbance of benthic macrofauna in relation to hypoxia and organic enrichment in a eutrophic coastal bay. Mar. Environ. Res. 2012, 76, 80–89. [Google Scholar] [CrossRef]
- Warwick, R. A new method for detecting pollution effects on marine macrobenthic communities. Mar. Biol. 1986, 92, 557–562. [Google Scholar] [CrossRef]
- Gray, J.S.; Pearson, T.H. Objective Selection of Sensitive Species Indicative of Pollution-Induced Change in Benthic Communities. I. Comparative Methodology. Mar. Ecol. Prog. Ser. 1982, 9, 111–119. [Google Scholar] [CrossRef]
- Maurer, D.; Robertson, G.; Gerlinger, T. San Pedro Shelf California: Testing the Pearson-Rosenberg Model (PRM). Mar. Environ. Res. 1993, 35, 303–321. [Google Scholar] [CrossRef]
- Heip, C. Eutrophication and zoobenthos dynamics. Ophelia 1995, 41, 113–136. [Google Scholar] [CrossRef]
- Gray, J.S.; Wu, R.S.S.; Or, Y.Y. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 2002, 238, 249–279. [Google Scholar] [CrossRef]
- Tsutsumi, H. Population dynamics of Capitella capitata (Polychaeta; Capitellidae) in an organically polluted cove. Mar. Ecol. Prog. Ser. 1987, 36, 139–149. [Google Scholar] [CrossRef]
- Grizzle, R.E. Pollution indicator species of macrobenthos in a coastal lagoon. Mar. Ecol. Prog. Ser. 1984, 18, 191–200. [Google Scholar] [CrossRef]
- Lim, H.S.; Diaz, R.J.; Hong, J.S.; Schaffner, L.C. Hypoxia and benthic community recovery in Korean coastal waters. Mar. Pollut. Bull. 2006, 52, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Baustian, M.M.; Rabalais, N.N. Seasonal composition of benthic macroinfauna exposed to hypoxia in the northern Gulf of Mexico. Estuaries Coasts 2009, 32, 975–983. [Google Scholar] [CrossRef]
- Smith, N.P. Tidal and nontidal flushing of Florida’s Indian River Lagoon. Estuaries 1993, 16, 739–746. [Google Scholar] [CrossRef]
- Clark, K.B. Rheophilic/oligotrophic lagoonal communities: Through the eyes of slugs (Mollusca: Opisthobranchia). Bull. Mar. Sci. 1995, 57, 242–251. [Google Scholar]
- De Freese, D.E. Threats to biological diversity in marine and estuarine ecosystems of Florida. Coast. Manag. 1991, 19, 73–101. [Google Scholar] [CrossRef]
- Tortonese, E. Distribution and ecology of endemic elements in the Mediterranean fauna (fishes and echinoderms). In Mediterranean Marine Ecosystems; Springer: New York, NY, USA, 1985; pp. 57–83. [Google Scholar]
- Danovaro, R.; Della Croce, N.; Eleftheriou, A.; Fabiano, M.; Papadopoulou, N.; Smith, C.; Tselepides, A. Meiofauna of the deep Eastern Mediterranean Sea: Distribution and abundance in relation to bacterial biomass, organic matter composition and other environmental factors. Prog. Oceanogr. 1995, 36, 329–341. [Google Scholar] [CrossRef]
- Whomersley, P.; Huxham, M.; Bolam, S.; Schratzberger, M.; Augley, J.; Ridland, D. Response of intertidal macrofauna to multiple disturbance types and intensities–an experimental approach. Mar. Environ. Res. 2010, 69, 297–308. [Google Scholar] [CrossRef]
- Kondoh, M. Unifying the relationships of species richness to productivity and disturbance. Proc. R. Soc. Lond. B Biol. Sci. 2001, 268, 269–271. [Google Scholar] [CrossRef]
- Connell, J.H. The consequences of variation in initial settlement vs. post-settlement mortality in rocky intertidal communities. J. Exp. Mar. Biol. Ecol. 1985, 93, 11–45. [Google Scholar] [CrossRef]
- Arnold, W.S.; Hitchcock, G.L.; Frischer, M.E.; Wanninkhof, R.; Sheng, Y.P. Dispersal of an introduced larval cohort in a coastal lagoon. Limnol. Oceanogr. 2005, 50, 587–597. [Google Scholar] [CrossRef]
- Johnson, D.R. Wind forced surface currents at the entrance to Chesapeake Bay: Their effect on blue crab larval dispersion and post-larval recruitment. Bull. Mar. Sci. 1995, 57, 726–738. [Google Scholar]
- Dauer, D.M. Biological criteria, environmental health and estuarine macrobenthic community structure. Mar. Pollut. Bull. 1993, 26, 249–257. [Google Scholar] [CrossRef]
- Levinton, J. Stability and trophic structure in deposit-feeding and suspension-feeding communities. Am. Nat. 1972, 106, 472–486. [Google Scholar] [CrossRef]
- Bayne, B.L. Phenotypic flexibility and physiological tradeoffs in the feeding and growth of marine bivalve molluscs. Integr. Comp. Biol. 2004, 44, 425–432. [Google Scholar] [CrossRef]
- Miller, D.C.; Poucher, S.L.; Coiro, L.; Rego, S.; Munns, W. Effects of Hypoxia on Growth and Survival of Crustaceans and Fishes of Long Island Sound; New York Sea Grant Inst.: Stony Brook, NY, USA, 1995; Volume 92. [Google Scholar]
- Bayne, B.L. (Ed.) Marine Mussels: Their Ecology and Physiology; Cambridge University Press: Cambridge, UK, 1976; Volume 10. [Google Scholar]
- Gäde, G.; Ellington, W.R. The anaerobic molluscan heart: Adaptation to environmental anoxia. Comparison with energy metabolism in vertebrate hearts. Comp. Biochem. Physiol. Part A Physiol. 1983, 76, 615–620. [Google Scholar] [CrossRef]
- Nepote, E.; Bianchi, C.N.; Morri, C.; Ferrari, M.; Montefalcone, M. Impact of a harbour construction on the benthic community of two shallow marine caves. Mar. Pollut. Bull. 2017, 114, 35–45. [Google Scholar] [CrossRef]
- Pearson, T.H. Marine pollution effects of pulp and paper industry wastes. Helgoländer Meeresunters. 1980, 33, 340–365. [Google Scholar] [CrossRef]





| Species | Complete Absence Window | High Density (# m−2 ± SE) | High Density | High-Density Sample Month |
|---|---|---|---|---|
| Leptochelia dubia | Oct., Nov., Dec. (2015), and August (2016) | 53,000 ± 14,000 | TCL | June 2016 |
| Peratocytheridea Setipunctata | Almost always present in non-muck | 12,000 ± 3900 | TCL | July 2016 |
| Parastarte triquetra | Always present in non-muck | 3900 ± 860 | CCL | January 2016 |
| Mulinia lateralis | Almost always present in non-muck | 7400 ± 1700 | CCL | April 2016 |
| Ammonia parkinsoniana | October (2015) | 10,000 ± 6400 | TC | May 2015 |
| Nereis succinea | Always present in non-muck | 2500 ± 520 | CCL | August 2016 |
| Unidentified Tanaid | Almost always present in non-muck | 1100 ± 650 | TCL | July 2016 |
| Oxyurostylis smith | October (2015) and August (2016) | 2600 ± 710 | CCL | January 2016 |
| Acteocina canaliculata | Almost always present in non-muck | 2000 ± 390 | TCL | April 2016 |
| Unidentified Gammarid Amphipod A | Nov. (2015), Feb., Mar., and April (2016) | 1600 ± 480 | CCL | July 2016 |
| Unidentified Polychaete A | Oct. (2015) and June (2016) | 1800 ± 810 | TC | December 2015 |
| Unidentified Gammarid Amphipod B | Nov. (2015), Jan., Feb., Mar., and April (2016) | 1600 ± 480 | CCL | July 2016 |
| Unidentified Polychaete B | Almost always present in non-muck | 590 ± 270 | CCL | May 2015 |
| Paradiopatra hispanica | Almost always present in non-muck | 610 ± 220 | TCL | April 2016 |
| Japonactaeon Punctostriatus | May, June, July (2015) | 990 ± 270 | CCL | January 2016 |
| Glycera A | Almost always present in non-muck | 500 ± 250 | CCL | January 2016 |
| Pectinaria Gouldii | Almost always present in non-muck | 740 ± 380 | TC | May 2016 |
| Diopatra cuprea | Almost always present in non-muck | 180 ± 80 | CCL | May 2016 |
| Hemipholis elongate | July (2016) | 330 ± 310 | CCL | April 2016 |
| Eusirus cuspidatus | Jan., Feb., Jul., and August (2016) | 300 ± 150 | CCL | May 2016 |
| Capitella capitata | Nov. (2015), Apr., and August (2016) | 410 ± 220 | TCL | August 2015 |
| Haminoaea succinea | July (2016) | 190 ± 120 | TCL | December 2015 |
| Unidentified Polychaete C | Nov., Dec. (2015), Apr., and August (2016) | 400 ± 240 | CCL | June 2015 |
| Hargeria rapax | Nov., Dec. (2015), Mar., and August (2016) | 270 ± 270 | TCL | July 2015 |
| Amygdalum papyrium | Dec. (2015), Feb., Mar., Apr., and June (2016) | 150 ± 100 | TC | July 2015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hope, D.; Cox, A.; Zamora-Duran, A.; Johnson, K.B. The Association of Benthic Infauna with Fine-Grained Organic-Rich Sediments in a Shallow Subtropical Estuary. J. Mar. Sci. Eng. 2024, 12, 2184. https://doi.org/10.3390/jmse12122184
Hope D, Cox A, Zamora-Duran A, Johnson KB. The Association of Benthic Infauna with Fine-Grained Organic-Rich Sediments in a Shallow Subtropical Estuary. Journal of Marine Science and Engineering. 2024; 12(12):2184. https://doi.org/10.3390/jmse12122184
Chicago/Turabian StyleHope, Daniel, Anthony Cox, Angelica Zamora-Duran, and Kevin B. Johnson. 2024. "The Association of Benthic Infauna with Fine-Grained Organic-Rich Sediments in a Shallow Subtropical Estuary" Journal of Marine Science and Engineering 12, no. 12: 2184. https://doi.org/10.3390/jmse12122184
APA StyleHope, D., Cox, A., Zamora-Duran, A., & Johnson, K. B. (2024). The Association of Benthic Infauna with Fine-Grained Organic-Rich Sediments in a Shallow Subtropical Estuary. Journal of Marine Science and Engineering, 12(12), 2184. https://doi.org/10.3390/jmse12122184







