Stromatolites and Their “Kin” as Living Microbialites in Contemporary Settings Linked to a Long Fossil Record
Abstract
1. Introduction and Perspective
2. Background Geography and Geological Oceanography
3. Definitions and Review Methods
3.1. Growth of Stromatolites and Kin
3.2. Types of Microbialites
3.3. Macroscopic Form of Stromatolites and Kin
4. Results
4.1. Review of Contemporary Regions with Stromatolites and Kin
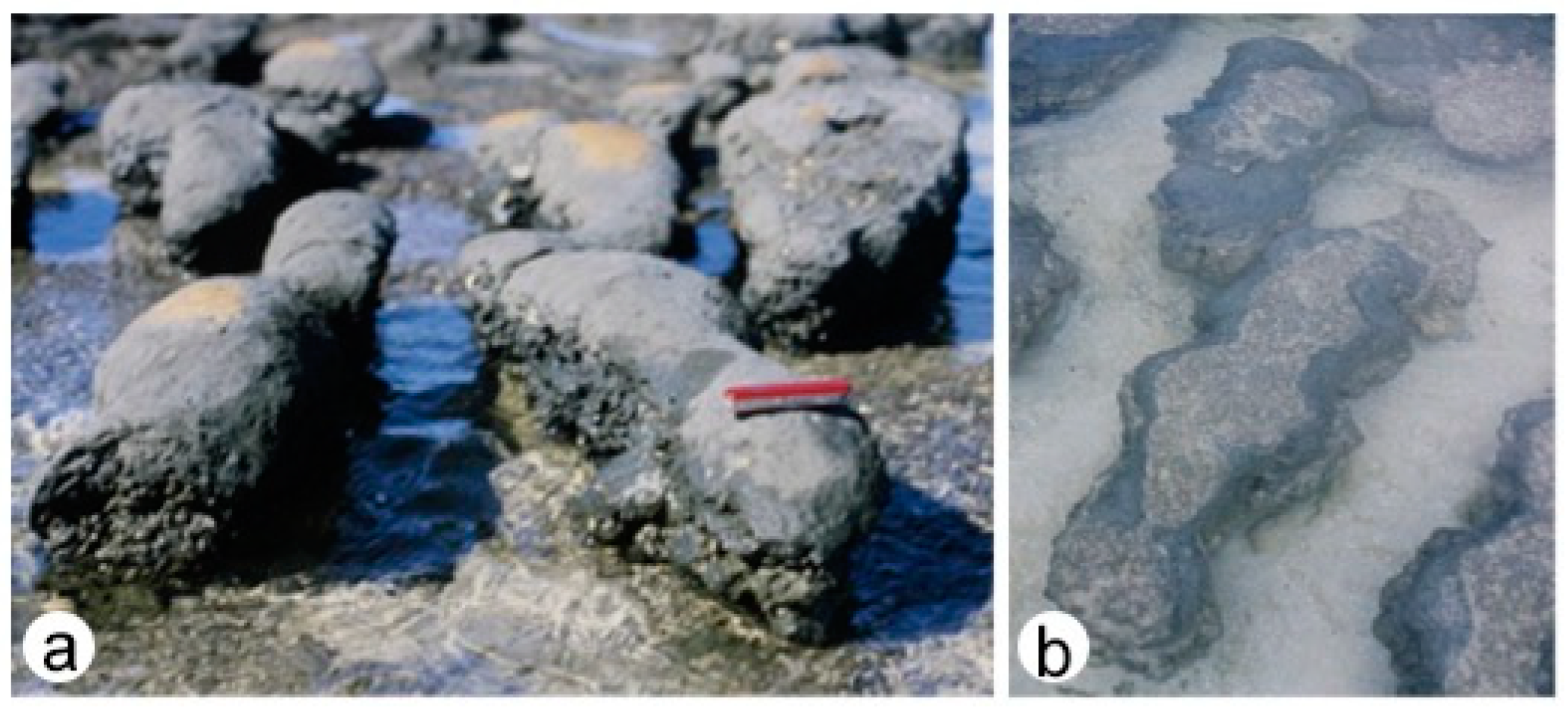
4.1.1. Hamelin Pool, Shark Bay, Western Australia
4.1.2. Exuma Cays in the Bahamas of the Western Atlantic Ocean
4.1.3. Lake Tanganyika in the East African Rift System
4.1.4. Saline Lakes of Northwestern Argentina
4.1.5. Hypersaline Pools from Islands in Mexico’s Gulf of California
4.2. Stromatolites from the Archean–Phanerozoic Eons
4.2.1. Cambrian Stromatolites from Lester Park in New York State
4.2.2. Distribution of Cryptozoon
5. Discussion of Modern Cryptozoon Research
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heard, A.W.; Bekker, A.; Kovalick, A.; Evans, H.; Ireland, T.; Dauphas, N. Oxygen production and rapid iron oxidation in stromatolites immediately preceding the Great Oxidation Event. Earth Planet. Sci. Lett. 2022, 282, 117416. [Google Scholar] [CrossRef]
- Grey, K.; Awramik, S.M. Handbook for the Study and Description of Microbialites; Geological Survey of Western Australia, Bulletin No. 147; Geological Survey, Western Australia: Perth, WA, Australia, 2020; 278p.
- Landing, E.; Geyer, G.; Brasier, M.D.; Bowring, S.A. Cambrian Evolutionary Radiation: Context, correlations, and chronostratigraphy—Overcoming deficiencies of the first appearance datum (FAD) concept. Earth-Sci. Rev. 2013, 123, 133–177. [Google Scholar] [CrossRef]
- Margulis, L.; Schwartz, K.V. Five Kingdoms—An Illustrated Guide to the Phyla of Life on Earth, 2nd ed.; W.H. Freeman & Co.: New York, NY, USA, 1982; 375p. [Google Scholar]
- Margulis, L.; Sagan, D. Microcosmos—Four Billion Years of Microbial Evolution; Allen & Unwin: Boston, MA, USA, 1987; 301p. [Google Scholar]
- Allwood, A.C.; Walter, M.R.; Kamber, B.S.; Marshall, C.P.; Burch, I.W. Stromatolite reef from the Early Archaean era of Australia. Nature 2006, 441, 04764. [Google Scholar] [CrossRef] [PubMed]
- Kalkowsky, E. Oölith und Stromatolith im norddeutchen Bundsandstein. Z. Deutshen Geol. Ges. 1908, 60, 68–125. [Google Scholar]
- Walcott, C.D. Cambrian geology and paleontology. III. No. 2.—Pre-Cambrian Algonkian algal flora. Smithson. Misc. Collect. 1914, 65, 79–156. [Google Scholar]
- Wieland, G.R. Further notes on Ozarkian seaweeds and oolites. Bull. Am. Mus. Nat. Hist. 1914, 33, 237–260. [Google Scholar]
- Barghoorn, E.S.; Stanley, A.T. Microorganisms from the Gunflint Chert. Science 1965, 147, 563–577. [Google Scholar] [CrossRef]
- Schopf, J.W. Cradle of Life—The Discovery of Earth’s Earliest Fossils; Princeton University Press: Princeton, NJ, USA, 1999; 367p. [Google Scholar]
- You, X.L.; Sun, S.; Zhu, J.Q. Significance of fossilized microbes from the Cambrian stromatolites in the Tarim Basin, Northwest China. Sci. China Earth Sci. 2014, 57, 2901–2913. [Google Scholar] [CrossRef]
- Chang, Y.; Bai, W.; Qi, Y.; Sun, F.; Wang, M. Microfossil assemblage and its sedimentary environment in Cambrian stromatolites, western He’nan. Adv. Earth Sci. 2014, 29, 456–463, (In Chinese with English Summary). [Google Scholar]
- Klappa, C.F. Lichen stromatolites: Criterion for subaerial exposure and a mechanism for formation of lamellar calcretes (caliche). J. Sediment. Petrol. 1979, 49, 387–410. [Google Scholar]
- Landing, E. Tropical seas and volcanic fire in eastern New York. Nat. Hist. 2022, 130, 36–41. [Google Scholar]
- Riding, R. Microbialites, stromatolites, and thrombolites. In Encyclopedia of Geobiology; Reiter, J., Thiel, V., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 635–654. [Google Scholar]
- Landing, E.; Westrop, S.R. Upper Lower Cambrian depositional sequence in Avalonian New Brunswick. Can. J. Earth Sci. 1996, 33, 404–417. [Google Scholar] [CrossRef]
- Landing, E.; Westrop, S.R.; Geyer, G. Trans-Avalonian green–black boundary (early Middle Cambrian): Transform fault-driven epeirogeny and onset of 26 m.y. of shallow-marine, black mudstone in Avalonia (Rhode Island–Belgium) and Baltica. Can. J. Earth Sci. 2023, 60, 133–171. [Google Scholar] [CrossRef]
- Vanuxem, L. Geology of New York. Part III; Survey of the Third Geological District of New York: Albany, NY, USA, 1842; 306p. [Google Scholar]
- Aitken, J.D. Classification and environmental significance of cryptalgal limestones and dolomites with illustrations from the Cambrian and Ordovician of southwest Alberta. J. Sediment. Petrol. 1967, 37, 1163–1178. [Google Scholar] [CrossRef]
- Bernhard, J.M.; Fisher, L.A.; Murphy, Q.; Sen, L.; Yeh, H.D.; Louyakis, A.; Gomaa, F.; Reilly, M.; Batta-Lona, P.G.; Bucklin, A.; et al. Transition from stromatolite to thrombolite fabric: Potential role for reticulopodial protists in lake microbialites of a Proterozoic ecosystem analog. Front. Microbiol. 2023, 14, 1210781. [Google Scholar] [CrossRef]
- Dupraz, C.; Pattisina, R.; Verrecchia, E.P. Translation of energy into morphology: Simulation of stromatolite morphospace using a stochastic model. Sediment. Geol. 2006, 185, 185–203. [Google Scholar] [CrossRef]
- Logan, B.W. Cryptozoon and associated stromatolites from the Recent, Shark Bay, Western Australia. J. Geol. 1961, 69, 517–533. [Google Scholar] [CrossRef]
- Hall, J. Cryptozoön, n.g., Cryptozoön proliferum. n.sp. In Thirty-Sixth Annual Report of the Trustees of the State Museum of Natural History to the Legislature 1883. Transmitted January 10, 1883. New York State Senate Paper 53; Pierson, H.R., Ed.; (Two covers, same material, inner printed January 10, 1883, and outer January 10, 1884), unnumbered end page and Pl. 6 with caption; Weed, Parsons & Company: Albany, NY, USA, 1884. [Google Scholar] [CrossRef]
- Playford, P.E.; Cockbain, A.E. Modern algal stromatolites at Hamelin Pool, a hypersaline basin in Shark Bay, Western Australia. In Developments in Sedimentology, 20, Stromatolites; Walter, M.R., Ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA, 1976; pp. 389–411. [Google Scholar]
- Burne, R.V.; Moore, L.S. Microatoll microbialites of Lake Clifton, Western Australia: Morphological analogues of Cryptozoon proliferum, the first formally named stromatolite. Facies 1993, 29, 149–178. [Google Scholar] [CrossRef]
- Suosaari, E.P.; Reid, R.P.; Playford, P.E.; Foster, J.S.; Stolz, J.F.; Casaburi, G.; Hagan, P.D.; Chirayath, V.; Macintyre, I.G.; Planavsky, N.J.; et al. New multi-scale perspectives on the stromatolites of Shark Bay, Western Australia. Sci. Rep. 2016, 1, 20557. [Google Scholar] [CrossRef]
- Dravis, J.J. Hardened subtidal stromatolites Bahamas. Science 1983, 219, 385–386. [Google Scholar] [CrossRef]
- Dill, R.F.; Shinn, E.A.; Jones, A.T.; Kelly, K.; Steinen, R.P. Giant subtidal stromatolites forming in normal salinity waters. Nature 1986, 324, 57–58. [Google Scholar] [CrossRef]
- Reid, R.P.; Macintyre, I.G.; Browne, K.M.; Steneck, R.S.; Miller, T. Modern marine stromatolites in the Exuma Cays, Bahamas: Uncommonly common. Facies 1995, 33, 1–17. [Google Scholar] [CrossRef]
- Cohen, A.S.; Talbot, M.R.; Awramik, S.M.; Dettman, D.L.; Abell, P. Lake level and paleoenvironmental history of Lake Tanganyika, Africa, as inferred from late Holocene and modern stromatolites. Geol. Soc. Am. Bull. 1997, 109, 444–460. [Google Scholar] [CrossRef]
- West, K.; Cohen, A. Morphology and behavior of crabs and gastropods from Lake Tanganyika, Africa: Implications for lacustrine predator-prey coevolution. Evolution 1991, 45, 589–607. [Google Scholar]
- Argentinian Stromatolites. Available online: https://www.cnn.com/2023/12/16/americas/stromatolites-argentina-discovery-lagoons-ecosystem-scn/index.html (accessed on 14 November 2024).
- CU Boulder Today. Available online: https://www.colorado.edu/today/2023/12/06/deep-within-inhospitable-desert-window-first-life-earth (accessed on 14 November 2024).
- Horodyski, R.J.; Vonder Haar, S.P. Recent calcareous stromatolites from Laguna Mormona (Baja California) Mexico. J. Sed. Petrol. 1975, 45, 894–906. [Google Scholar]
- Johnson, M.E.; Ledesma-Vázquez, J.; Backus, D.H.; González, M.R. Lagoon microbialites on Isla Angel de la Guarda and associated peninsular shores, Gulf of California (Mexico). Sediment. Geol. 2012, 263–264, 76–84. [Google Scholar] [CrossRef]
- Bowen, T. On Desert Shores—Archaeology & History of the Western Midriff Islands in the Gulf of California; University of Utah Press: Salt Lake City, UT, USA, 2022; 313p. [Google Scholar]
- Baumgartner, R.J.; Van Kranendonk, M.J.; Wacey, D.; Fiorentini, M.L.; Saunders, M.; Caruso, S.; Pages, A.; Homann, M.; Guagliardo, P. Nano-porous pyrite and OM in 3.5-billion-year-old stromatolites record primordial life. Geology 2019, 47, 1039–1043. [Google Scholar] [CrossRef]
- Hickman-Lewis, K.; Cavalazzi, B.; Giannoukos, K.; D’Amico, L.; Vrbaski, S.; Saccomano, G.; Dreossi, D.; Tromba, G.; Foucher, F.; Brownscombe, W.; et al. Advanced two- and three-dimensional insights into Earth’s oldest stromatolites (ca. 3.5 Ga): Prospects for life on Mars. Geology 2022, 51, 33–38. [Google Scholar] [CrossRef]
- Beukes, N.J. Facies relations, depositional environments and diagenesis in a major early Proterozoic stromatolitic carbonate platform to basinal sequence, Campbellrand Subgroup, Transvaal Supergroup, Southern Africa. Sediment. Geol. 1987, 54, 1–46. [Google Scholar] [CrossRef]
- Sumner, D.Y.; Grotzinger, J.P. Implications for Neoarchaean ocean chemistry from primary carbonate mineralogy of the Campbellrand-Malmani platform, South Africa. Sedimentology 2004, 51, 1273–1299. [Google Scholar] [CrossRef]
- Awramik, S.M.; Sprinkle, J. Proterozoic stromatolites: The first marine evolutionary biota. Hist. Biol. 1999, 13, 241–253. [Google Scholar] [CrossRef]
- Kah, L.C.; Riding, R. Mesoproterozoic carbon dioxide levels inferred from calcified cyanobacteria. Geology 2007, 35, 799–802. [Google Scholar] [CrossRef]
- Aitken, J.D.; Narbonne, G.M. Two occurrences of Precambrian thrombolites from the Mackenzie Mountains, northwestern Canada. Palaios 1989, 4, 384–388. [Google Scholar] [CrossRef]
- Riding, R. Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic-Cambrian changes in atmospheric composition. Geobiology 2006, 4, 299–316. [Google Scholar] [CrossRef]
- Grotzinger, J.P. Geochemical model for Proterozoic stromatolite decline. Am. J. Sci. 1990, 290-A, 80–103. [Google Scholar]
- Chumakov, N.M.; Semikhatov, M.A. Riphean and Vendian of the USSR. Precambrian Res. 1981, 15, 229–253. [Google Scholar] [CrossRef]
- Sami, T.T.; James, N.P. Synsedimentary cements as Paleoproterozoic platform building blocks, Pethei Group, northwestern Canada. J. Sediment. Res. 1996, 66, 209–222. [Google Scholar]
- Riding, R. Abiogenic, microbial and hybrid authigenic carbonate crusts: Components of Precambrian stromatolites. Geol. Croat. 2008, 61, 73–103. [Google Scholar] [CrossRef]
- Walter, M.R. Stromatolites and the biostratigraphy of the Australian Precambrian and Cambrian. Spec. Pap. Palaeontol. 1972, 11, 190. [Google Scholar]
- Landing, E. Time-specific black mudstones and global hyperwarming on the Cambrian-Ordovician slope and shelf of the Laurentia palaeocontinent. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 367–368, 256–272. [Google Scholar] [CrossRef]
- Landing, E.; Keppie, J.D.; Keppie, F.D.; Geyer, G.; Westrop, S.R. Greater Avalonia—Latest Ediacaran–Ordovician “peribaltic” terrane bounded by continental margin prisms (“Ganderia,” Harlech Dome, Meguma): Review, tectonic implications, and paleogeography. Earth-Sci. Rev. 2022, 255, 103863. [Google Scholar] [CrossRef]
- Kiessling, W. Secular variations in the Phanerozoic reef ecosystem. In Phanerozoic Reef Patterns; Kiessling, W., Flügel, E., Golonka, J., Eds.; Society of Economic Paleontologists and Mineralogists—SEPM Special Publication; SEPM: Tulsa, OK, USA, 2002; Volume 72, pp. 625–690. [Google Scholar]
- Pratt, B.R. Stromatolite decline—A reconsideration. Geology 1982, 10, 512–515. [Google Scholar] [CrossRef]
- Fischer, A.G. Fossils, early life, and atmospheric history. Proc. Natl. Acad. Sci. USA 1965, 53, 1205–1215. [Google Scholar] [CrossRef]
- Landing, E.; Antcliffe, J.B.; Geyer, G.; Kouchinsky, A.; Bowser, S.S.; Andreas, A. Early evolution of colonial animals (Ediacaran Evolutionary Revolution-Cambrian Evolutionary Radiation-Great Ordovician Diversification Interval). Earth-Sci. Rev. 2018, 178, 105–135. [Google Scholar] [CrossRef]
- Foster, G.; Royer, D.; Lunt, D. Future climate forcing potentially without precedent in the last 420 million years. Nat. Commun. 2017, 8, 14845. [Google Scholar] [CrossRef]
- Goldring, W. Algal barrier reefs in the Lower Ozarkian of New York with a chapter on the importance of coralline algae as reef builders through the ages. N. Y. State Mus. Bull. 1938, 315, 5–75. [Google Scholar] [CrossRef]
- Friedman, G.M. Hoyt Limestone of Late Cambrian, eastern New York State: Spectacular domed stromatolites as Lester Park and Petrified Sea Gardens. Northeast. Geol. Environ. Sci. 2000, 22, 336–349. [Google Scholar]
- Lindemann, R.H. The historic significance of a petrified cabbage patch at Greenfield, New York. Northeast. Geol. Environ. Sci. 2006, 28, 101–109. [Google Scholar]
- Friedman, G.M. Late Cambrian cabbage-head stromatolites from Saratoga Springs, New York, USA. Carbonates Evaporites 2012, 15, 37–44. [Google Scholar] [CrossRef]
- Lee, J.-H. Stromatolite. In Encyclopedia of Geology, 2nd ed.; Alderton, D., Elias, S.A., Eds.; Elsevier: Rotterdam, The Netherlands, 2019; pp. 75–388. [Google Scholar]
- Lee, J.-H.; Riding, R. The ‘classic stromatolite’ Cryptozoon is a keratose sponge-microbial consortium. Geobiology 2021, 9, 189–198. [Google Scholar] [CrossRef]
- Lee, J.-H.; Riding, R. Keratolite–stromatolite consortia mimic domical and branched columnar stromatolites. Palaeogeography, Palaeoclimatology. Palaeoecology 2021, 571, 110288. [Google Scholar] [CrossRef]
- Kershaw, S.; Li, Q.; Li, Y. Phanerozoic carbonate facies conundrum—Sponges or clotted fabric? Evidence form Early Silurian reefs. Stratigr. Rec. 2021, 19, 3–10. [Google Scholar]
- Neuweiler, F.; Kershaw, S.; Boulva, F.; Matysik, M.; Sendino, C.; McMenamin, M.; Munnecke, A. Keratose sponges in ancient carbonates—A problem of interpretation. Sedimentology 2023, 70, 927–969. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication. National University of Ireland, Galway. Available online: https://algaebase.org (accessed on 1 October 2024).
- World Register of Marine Species. Available online: https://www.marinespecies.org/ (accessed on 19 November 2024).
- Steele, J.H. A description of the oolite formation lately described in the County of Saratoga and in the State of New York. Am. J. Sci. 1825, 9, 16. [Google Scholar]
- Flick, A.C. Suggestions of a state policy relating to historic and scientific reservations. Twenty-third Report of the Director of the Division of Science and the State Museum. N. Y. State Mus. Bull. 1929, 284, 68–71. [Google Scholar]
- Cushing, H.P.; Ruedemann, R. Geology of Saratoga Springs and vicinity. N. Y. State Mus. Bull. 1914, 169, 177. [Google Scholar]
- Landing, E. Ediacaran–Ordovician of east Laurentia―geologic setting and controls on deposition along the New York Promontory. In Ediacaran–Ordovician of East Laurentia―S. W. Ford Memorial Volume. New York State Museum Bulletin 510; Landing, E., Ed.; The NewYork State Education Department: Albany, NY, USA, 2007; pp. 5–24. [Google Scholar]
- Landing, E.; Westrop, S.R.; Kröger, B.; English, A.M. Left behind—Extinction and a relict trilobite fauna in the Cambrian–Ordovician boundary succession, (northeast Laurentian platform, New York). Geol. Mag. 2010, 148, 529–557. [Google Scholar] [CrossRef]
- Friedman, G.M.; Sanders, J.E. Time-temperature-burial significance of Devonian anthracite implies former great (~6.5 km) depth of burial of Catskill Mountains, New York. Geology 1982, 10, 93–96. [Google Scholar] [CrossRef]
- Landing, E.; Webster, M.; Bowser, S.S. Terminal Ediacaran–Late Ordovician of the NE Laurentia palaeocontinent: Rift-drift–onset of Taconic orogeny, sea-level change, and “Hawke Bay” onlap (not offlap). In Supercontinents, Orogenesis, and Magmatism; Nance, R.D., Strachan, R.A., Quesada, Q., Lin, S., Eds.; Geological Society of London, Special Publication; Geological Society Publications: London, UK, 2023; Volume 542. [Google Scholar] [CrossRef]
- Hall, J. Natural History of New York. Geology of New-York. Part 4—Comprising the Geology of the Fourth Geological District; Carroll and Cook: Albany, NY, USA, 1843; 683p. [Google Scholar]
- Mather, W.W. Geology of the First Geologic District of New York; Carroll and Cook: Albany, NY, USA, 1843; 832p. [Google Scholar]
- Hall, J. Palaeontology of New York. 1. Organic Remains of the New York System; C. van Benthuysen: Albany, NY, USA, 1847; 338p. [Google Scholar]
- Hall, J. Report of the Director. Albany December 23, 1882, p. 1–17. In Thirty-Sixth Annual Report of the Trustees of the State Museum of Natural History to the Legislature. Transmitted January 10, 1883: New York State Senate Paper, 53; Pierson, H.R., Ed.; Weed, Parsons & Company: Albany, NY, USA, 1884; printed 10 January 1884. [Google Scholar]
- Monty, C. Spongiostromate vs. porostromate stromatolites and oncolites. In Phanerozoic Stromatolites. Case Histories 1–4; Monty, C., Ed.; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Smith, G.L.; Clark, D.L. Conodonts of the Lower Ordovician Prairie du Chien Group of Wisconsin and Minnesota. Micropaleontology 1996, 42, 363–373. [Google Scholar] [CrossRef]
- Winchell, N.H. VII New species of fossils. In The Fourteenth Annual Report for the Year 1885. Geological and Natural Historical Survey of Minnesota; Winchell, N.H., Ed.; Minnesota Geological Survey; Pioneer Press Company: St. Paul, MN, USA, 1886; pp. 313–318. [Google Scholar]
- Chaney, L.W., Jr. Cryptozoon minnesotense in the Shakopee Limestone at Northfield, Minnesota. Minn. Acad. Nat. Sci. Bull. 1889, 3, 280–284. [Google Scholar]
- Stauffer, C.R. Cryptozoons of the Shakopee Dolomite. J. Paleontol. 1945, 19, 376–379. [Google Scholar]
- Brainerd, E.; Seely, H.M. The Calciferous Formation in the Champlain valley. Geol. Soc. Am. Bull. 1890, 1, 501–516. [Google Scholar] [CrossRef]
- Bassler, R.S. Systematic paleontology of the Cambrian and Ordovician deposits of Maryland. In The Cambrian and Ordovician deposits of Maryland; Bassler, R.S., Ed.; Maryland Geological Survey Report; The Johns Hopkins Press: Baltimore, MD, USA, 1919; pp. 184–373. [Google Scholar]
- Walcott, C.D. Cambrian geology and paleontology II No. 9. New York Potsdam–Hoyt fauna. Smithson. Misc. Collect. 1912, 57, 251–304. [Google Scholar]
- Rothpletz, A. Über die systematische Deutung und die stratigraphishe Stellung der ältesten Versteinerungen Europas und Nordamerika mit besonderer Berücksichtigung der Cryptozoon und Oolithe. Teil II: Über Cryptozoon, Eozoon und Artikokania. Abh. Könglich Bayer. Akad. Wiss. Matimatische-Phys. Kl. 1916, 28, 91. [Google Scholar]
- Dawson, J.W. Notes on Cryptozoon and other ancient fossils. Can. Rec. Sci. 1896, 7, 203–209. [Google Scholar]
- Seely, H.M. Beekmantown and Chazy formations in the Champlain Valley. Contribution to their geology and paleontology. In Fifth Report of the Vermont State Geologist; Vermont Department of Environmental Conservation: Montpelier, VT, USA, 1906; pp. 115–145. [Google Scholar]
- Fenton, C.L.; Fenton, M.A. Algae and algal beds in the Belt Series of Glacier National Park. J. Geol. 1931, 39, 670–688. [Google Scholar] [CrossRef]
- Rezak, R. Stromatolites of the Belt Series in Glacier National Park, Montana. U. S. Geol. Surv. Prof. Pap. 1957, 294, 127–150. [Google Scholar]
- Wilks, M.E.; Nisbet, E.G. Archaean stromatolites from the Steep Rock Group, northwestern Ontario, Canada. Can. J. Earth Sci. 1985, 22, 792–797. [Google Scholar] [CrossRef]
- Fralick, P.; Riding, R. Steep Rock Lake: Sedimentology and geochemistry of an Archean carbonate platform. Earth-Sci. Rev. 2015, 151, 132–175. [Google Scholar] [CrossRef]
- Johnson, J.H. Lime-secreting algae from the Pennsylvanian and Permian of Kansas. Geol. Soc. Am. Bull. 1946, 57, 1087–1120. [Google Scholar] [CrossRef]
- Vahrenkamp, V.; Chandra, V.; Garuglieri, E.; Marasco, R.; Hachmann, K.; Khanna, P.; Daffonchio, D.; Petrovic, D. Discovery of modern living intertidal stromatolites on Sheybarah Island, Red Sea, Saudi Arabia. Geology 2024, 52, 347–351. [Google Scholar] [CrossRef]
- Shapiro, R.S. Stromatolites: A 3.5 billion year ichnologic record. In Trace Fossils: Concepts, Problems, Prospects; Ajello, M., Brotton, S.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 381–389. [Google Scholar]
- Neuweiler, F.; Mueller, M.; Walter, B.; Landing, E.; Beranoaguirre, A.; Sendino, C.; Amati, L.; Kershaw, S. Fossil record misconstrued: Sponge-like fabric reflect incipient carbonate metamorphism. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Turner, E.C. Possible poriferan body fossils in early Neoproterozoic microbial reefs. Nature 2021, 596, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358. [Google Scholar] [CrossRef]
- Hoffman, H.J. Stromatoid morphometrics. In Stromatolites. Developments in Sedimentology 20; Walter, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1976; pp. 45–54. [Google Scholar]
- Reid, P.R.; James, N.P.; Macintyre, I.G.; Dupraz, C.P.; Burne, R.V. Shark Bay stromatolites: Microfabrics and reinterpretation of origins. Facies 2013, 49, 299–324. [Google Scholar] [CrossRef]
- Landing, E.; Geyer, G.; Schmitz, M.D.; Wotte, T.; Kouchinsky, A. (Re)proposal of three Cambrian Subsystems and their geochronology. Episodes 2021, 44, 273–283. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landing, E.; Johnson, M.E. Stromatolites and Their “Kin” as Living Microbialites in Contemporary Settings Linked to a Long Fossil Record. J. Mar. Sci. Eng. 2024, 12, 2127. https://doi.org/10.3390/jmse12122127
Landing E, Johnson ME. Stromatolites and Their “Kin” as Living Microbialites in Contemporary Settings Linked to a Long Fossil Record. Journal of Marine Science and Engineering. 2024; 12(12):2127. https://doi.org/10.3390/jmse12122127
Chicago/Turabian StyleLanding, Ed, and Markes E. Johnson. 2024. "Stromatolites and Their “Kin” as Living Microbialites in Contemporary Settings Linked to a Long Fossil Record" Journal of Marine Science and Engineering 12, no. 12: 2127. https://doi.org/10.3390/jmse12122127
APA StyleLanding, E., & Johnson, M. E. (2024). Stromatolites and Their “Kin” as Living Microbialites in Contemporary Settings Linked to a Long Fossil Record. Journal of Marine Science and Engineering, 12(12), 2127. https://doi.org/10.3390/jmse12122127







