Abstract
This study investigates methods for reducing air pollution in the shipping sector, particularly in port areas. The study examines the use of fuel cells as an alternative to diesel generators. Environmental pollution at ports remains a critical issue, so using fuel cells as an alternative to conventional energy systems warrants further research. This study compares commercial fuel cell types that can be used on a case study very large crude carrier (VLCC) vessel specifically, although the technology is applicable to other vessels and requirements. Seven different fuel cell types were ranked based on five criteria to accomplish this. The proton-exchange membrane cell type was found to be the most suitable fuel cell type for the case study vessel. Based on the input fuel, ammonia-based hydrogen storage has been identified as the most promising option, along with using an ammonia reforming unit to produce pure hydrogen. Furthermore, this study provides an integrated fuel cell module and highlights the economic, environmental, and maintenance aspects of implementing the proton-exchange membrane fuel cell module for this case study. It also calculates the required space as a crucial constraint of implementing fuel cell technology at sea.
Keywords:
shipping emission; alternative fuels; fuel cell; hydrogen; port; diesel power generator; ammonia; PEMFC 1. Introduction
Ref. [1] Global warming and air pollution are significant concerns, given that worldwide maritime shipping accounts for more than 80% of all trade. By 2050, the UK’s maritime sector envisions a zero-carbon shipping industry [2]. The International Maritime Organization (IMO) has set a short-term goal of reducing CO2 emissions by 40% by 2030. Developing a carbon-neutral propulsion system is not within the scope of current technology, and further research and development are required. The infrastructure for supplying zero-carbon fuels is not yet ready for a full-scale transition to zero-carbon marine propulsion systems [3]. It is, therefore, the case that novel approaches are needed to address the level of greenhouse gas (GHG) emissions in the marine industry.
A total of 40% of the world’s population resides within 100 km of ports and coastal areas, according to the United Nations [4]; in port, ships spend significant time loading and unloading while operating their electrical generators. In addition, pollution at ports is a concern, as ports are typically congested and populated areas. Switching to alternative energy sources for power generation is a potential option for the marine industry. Among the potential alternatives, fuel cells represent a promising option. Marine fuel cell systems can produce electricity at 60% efficiency with lower heating values (LHV) [5].
A significant focus of the existing research is on propulsion systems. The studies range from feasibility studies of various diesel-fuelled fuel cell systems to commercialised hydrogen-fuelled, air-independent submarine propulsion systems. Furthermore, several demonstrator systems have been developed and tested on board ships. Studying them is beneficial because, in most cases, a ship’s primary propulsion and power units are powered by diesel fuel. Whether fuel cell systems are likely to be used more widely in maritime environments depends on their ability to meet the demands of onboard power generation.
Contemporary studies have considered the life cycle analysis and greenhouse gas emissions of the technology on VLCCs [6] with particular comparison to conventional fuels; similarly, other studies have analysed the fuel consumption in comparison to fuel cell and fuel cell hybrid systems compared to conventional systems [7]. It is the case that the majority of available studies have focused upon the emissions, cost, and safety or a combination of the three [8].
The study conducted in this paper aimed to consider the technology from a broader range of criteria than strictly emissions to present a more comprehensive view on its viability as an alternative to conventional systems.
The proposal in this study relates to the utilisation of fuel cell technologies and their accompanying systems. Adoption of fuel cells as a replacement to diesel fuel generators is significant in the efforts towards achieving stated environmental targets, and the analysis conducted in this study hopes to address the question of the technology’s viability by proposing the most viable fuel cell system for a specified case study vessel, of which the analysis could be applied further to other vessels.
2. Decision-Making Approach
Criteria were selected for comparing fuel cells for onboard use, and fuel cell types were evaluated and ranked according to these criteria using multi-criteria decision analysis (MCDA). The scoring method uses a weighting system (scale of 1 to 3, with 3 indicating the highest importance) based on the importance of the criteria conducted during the integration and operation of the fuel cell system on the vessel under study [9], as well as a ranking system illustrated in Table 1, Table 2 and Table 3, ranking the qualitative criteria on a scale 1 to 5, with 5 indicating the highest rank. The final comparison tables provide scores for each of the fuel cell options based on evaluation points and weights assigned to each criterion. Seven alternative fuel cell technologies (A1 to A7) and five criteria (C1 to C5) are considered. These criteria have been selected as they are considered vital for determining whether a fuel cell technology will be suitable for the application. To ensure all criteria are compared on the same basis, the linear normalisation method is applied to both beneficial criteria (the criteria for which the higher value is desired) and non-beneficial criteria (the criteria for which the lower value is desired).

Table 1.
Various alternative fuel cell technologies in this study.

Table 2.
The attributes of a fuel cell discussed in this study.

Table 3.
Evaluation scale for assessments of different criteria.
The fuel cell types considered are proton exchange membrane fuel cells (PEMFC), high-temperature proton exchange membrane fuel cells (HTPEM), molten-carbonate fuel cells (MCFC), solid oxide fuel cells (SOFC), alkaline fuel cells (AFC), phosphoric acid fuel cells (PAFC), and direct-methanol fuel cells (DMFC).
Assuming that the ranking point for each criterion is :
3. Comparison Criteria for Fuel Cells
3.1. Efficiency
The electrical efficiency of fuel cells ranges from 50 to 65% [10]. Compared to diesel engines, this higher efficiency is primarily due to direct chemical conversion rather than thermal and mechanical conversion. Fuel cells with high temperatures, such as solid oxide SOFC and MCFC, can be more efficient when combined with cogeneration units [11]. The hot air produced by the fuel cell, in the form of exhaust gas, can generate electricity via gas or steam turbine systems. This would result in a total system efficiency of 70–80% [12]. Based on a review paper on fuel cell systems for maritime applications [8], Table 4 compares the efficiency and ranking of proposed fuel cell technologies. In this case, efficiency refers to the totality of stand-alone electric efficiency. In the final comparison matrix, this parameter has been weighted as 3 to reflect its expected importance.

Table 4.
An analysis of the relative efficiency of proposed fuel cell technologies.
3.2. Emissions
Regarding emissions, alternative fuel cells have varying impacts depending on the types of fuel they use. Fuel cells that use hydrogen emit only water as a by-product. However, after processing, fuel cells such as solid oxide fuel cells, phosphoric acid fuel cells, or molten-carbonate fuel cells can also process carbon-containing fuels such as diesel or liquefied natural gas (LNG). As a fuel, hydrocarbons provide flexibility and may be more cost-effective than hydrogen. It is essential to note that, in addition to water and heat, substantial amounts of carbon dioxide (CO2) and low levels of nitrogen oxides (NOX) are emitted as pollutants when utilising hydrocarbons [13]. To reflect that all fuel cell types can run on hydrogen, the criterion is given the weighting of 2 to reflect that emission levels are mainly affected by fuel choice. According to the US Department of Energy report [14], Table 5 compares the emissions of each fuel cell technology on an equivalent ranking scale.

Table 5.
Emissions comparison of each fuel cell technology on an equivalent scale.
3.3. Safety
For SOFCs and MCFCs, the exhaust gas temperature is higher than for other fuel cells. To prevent leaks during exhaust discharge from the fuel cell, pipes must have a double insulation layer and be well-insulated [8]. There is a significant risk of harm in cases of a leak in the electrolyte from the cell unit; the storage tank and delivery pipes for hydrogen using fuel cell units must be insulated as an additional precaution. Given hydrogen’s physical characteristics, which are volatile and highly explosive, the transfer between the storage tank and the anode side of the fuel cell must adhere to the two-barrier principle.
Due to their safe operation, low-temperature fuel cells such as PEMFCs offer an advantage in terms of safety, but their need for hydrogen or units for hydrogen production creates additional regulations and standards [12]. In traditional MCFCs and SOFCs, hydrogen is only present within the cell and balance of the plant (BOP), eliminating the need for separate tanks and distribution systems for the gas. Per a study conducted by the European Maritime Safety Agency (EMSA) on the use of fuel cells in shipping [11], the safety score comparisons for the alternatives have been summarised in Table 6. To reflect the importance of safety in new technologies, the parameter has a weighting of 3.

Table 6.
A comparison of the safety scores of the alternatives on an equivalent scale from different safety perspectives.
3.4. Cost
Fuel cells are more expensive than diesel engines; however, many companies see the opportunity to reduce the cost of fuel cells [11]. It was estimated in a US Department of Energy report in 2017 that direct hydrogen PEM fuel cell stacks would cost almost US$45/kW at a volume of 100,000 units per year and US$53/kW at a larger volume of 500,000 units per year [15], but recent releases from fuel cell manufacturers have indicated that with the advancement of the technology and manufacturing improvements, fuel cell prices are targeted to drop by 70–80% as progress continues towards the 2030 deadline for electric and diesel road vehicles [16]. Furthermore when considering the comparison of fuel cells to diesel engines, we must consider that cost continues to be a large barrier in 2023, as indicated in some recently published review articles and industry publications [17].
Fuel cell costs are divided into capital expenditures (CapEx) and operational expenditures (OpEx). Hydrogen, LNG, and diesel oil are the most common fuels used in fuel cells. Diesel oil is one of the most prominent fuels in shipping, and its storage conditions are well-tested and costed compared to alternatives. LNG and hydrogen are the most significant cost drivers for all storage vessels. According to the Department of Energy (DOE) fuel cell technologies market report [18], capital expenses for LNG tanks can range between 5 and 20 million US dollars depending on the tank capacity. For hydrogen, the situation is similar. Hydrogen is processed and transported using the three main procedures of compression, liquefaction, and hydrogenation of carrier substances, which are more expensive than conventional fuel storage systems. While hydrogen storage costs are higher than those of the storage of conventional fuels, diesel oil and LNG must be processed to obtain hydrogen for fuel cell systems. This increases the complexity and cost of the system’s operating costs.
Among fuel cell types, the effect of maintenance costs is far lower than that of fuel price. As a result, it is necessary to consider the prices of fuel types. There is a weighting of ‘2’ assigned to this criterion because budget is always an essential factor, but in order to move toward decarbonisation, the cost factor should be given a lower priority than others in favour of accelerating this transformation. A detailed comparison of the cost of fuel cell technologies is provided in Table 7, with the technologies ranked according to data presented in the Fuel Cell Technologies market report 2019/20 [18] as well as the DOE Hydrogen and Fuel Cells cost analysis paper in 2016 [19]. We have taken consideration of the predicted trends going forward which indicate the potential for major cost reductions as we approach 2030 [16], as well as the current progress on fuel cells as of 2023 [17].

Table 7.
A comparison of the relative costs of the alternatives on an equivalent scale.
3.5. Size
Depending on the power density or specific power of fuel cells, we can compare the size of the fuel cells. Specific power and power density are shown.
A maximum value for both is targeted, meaning the fuel cell has relatively less mass and a smaller volume with maximised power output. Currently it is indicated that PEMFCs have the highest power density compared to PAFCs and SOFCs, which have the lowest. Fuel processing and hydrogen-obtaining systems for high-temperature working fuel cells are also considered when discussing the size of fuel cells. When using diesel oil or LNG as fuel, MCFC or SOFC require an additional fuel conditioning system, which increases both volume and mass, thereby reducing power density. Based on a report published in 2019 [14], and considering the same power output of the fuel cell modules, Table 8 compares the sizes of fuel cell technologies and their rankings. The size parameter is determined by balancing the energy density of the core cell with the amount of space required by all ancillary components of the installation. This parameter has been weighted at ‘3’, indicating that it is desirable to adopt compact systems.

Table 8.
A comparison of the sizes of the fuel cell technologies.
4. Decision on Fuel Cell Technology
Several criteria have been defined, scored, and explained within the context of shipping, and their importance has been acknowledged. Following applying the MCDA technique and combining the normalised ranking points for each criterion considering their weighting points (WP), the criteria are ranked as indicated in Table 9. PEMFCs, AFCs, HTPEMs, and SOFCs rank among the top four fuel cell types. The PEMFC technology and the HTPEM technology have many similarities, but they also differ in many essential aspects, including installation complexity, fuel options, tolerance for fuel impurities, and total efficiency. DNV GL reviewed the potential of each of these technologies in 2016 [11], and except for AFCs, the other three options were found to be the most promising fuel cell technologies for maritime applications. The study highlights the drawback of AFC as the high degree of sensitivity to impurities, which requires high-purity hydrogen and oxygen supplies, adding complexity and cost to commercial marine applications. PEMFC has proved to be the most suitable alternative fuel cell technology to replace the auxiliary power generator among all the alternative fuel cell technologies examined in this chapter.

Table 9.
The final comparison matrix between fuel cells technologies considering the weighting points for each criterion.
4.1. Decision on Input Fuel
PEM fuel cells are an established technology that has been successfully applied to marine and other high-energy applications. As a result of their technological characteristics, PEM fuel cells present a specific regulatory challenge because they can only utilise hydrogen as a primary fuel. This section reviews and compares onboard hydrogen storage methods for ships. A discussion of the obstacles that prevent using these methods is presented. Generally, bunkering infrastructure, global hydrogen fuel availability, and safety issues for reliable operations appear to be the biggest challenges for the marine industry.
The required mass of hydrogen that would need to be stored for the case study vessel, and the voyage in question, is 57.577 kg; this is assuming a PEMFC efficiency of 56%, a hydrogen energy density of 33.3 kWh/kg and an energy demand of 1,073,684 kWh.
4.1.1. Compressed Hydrogen Storage
Compressed hydrogen storage is the most well-established method among the technologies available. Hydrogen is stored under extremely high pressures (350–700 bar), resulting in densities of 23.3 kg/m3 and 39.3 kg/m3, respectively [20]. Compressed hydrogen storage comprises two main components: storage tanks and compressors. Table 10 presents the four tank types’ materials, typical pressures, and approximate costs [19].

Table 10.
A comparison of different compressed hydrogen tank types based on their materials, typical pressures, and approximate costs.
Considering the power demand of the case study, its energy density, and its density factor at 700 bars, as shown in the following, we require 1370 m3 of space to accommodate the fuel consumption requirements during this voyage, which is not feasible. Currently, no storage technology can accommodate such a large volume of fuel. Even if the fuel could be stored in a series of tanks, based on Table 10, it would cost approximately US$40 million for storage alone. Some of the calculated amounts from the next chapter were used in the calculation of the following.
The total volume of compressed hydrogen is determined by its density factor at 700 bar (42 kg/m3), meaning the stored volume for 57.577 kg of hydrogen in these conditions would be 1370 m3.
The cost incurred by the type IV storage method is 700 US$/kg, and therefore the predicted cost of storage for this mass and subsequent volume would be US$40,303,900.
The calculations completed here indicate that despite compressed hydrogen being the most mature method of storing hydrogen, it is not the most suitable choice for the case study vessel.
4.1.2. Liquid Hydrogen Storage
Liquification is the second method of storing hydrogen. In order to store hydrogen in liquid form, it is necessary to reduce the temperature to −253 °C. Liquefaction has the advantage of being able to attain high hydrogen densities at atmospheric pressures (70 kg/m3). For 57.577 kg of hydrogen under these conditions, this would require 822 m3 of storage.
Heat transfer must be minimised to maintain the desired temperature for cryogenic liquid storage. For this reason, maximising the volume-to-surface ratio of the tank is imperative. This can be achieved using spherical tank shapes with the largest volume-to-surface ratios and insulation. Considering a volume of 822 m3, the boil-off rate would exceed the standard of 0.1% per day in the best-case scenario. In addition, cooling hydrogen to −253 °C reduced the overall efficiency of the module significantly. In light of the available technology, applying this storage method is neither technically feasible nor cost-effective.
4.1.3. Solid-State Hydrogen Storage
Metal hydrides are considered a promising method for storing hydrogen. Metal hydrides store hydrogen by chemically bonding it to the metal. Due to their unique properties, metal hydrides adsorb and release hydrogen at different temperatures and pressures, so each metal hydride has its specific characteristics. The main disadvantage of this method is its heavy weight, which makes it unsuitable for marine applications. Considering commercial models available, a storage tank that can hold 250 kg of metal hydrides would weigh as much as 35 tonnes [21].
Based on the total amount of hydrogen required in this case study, the total mass of storage in this method will be 8050 tonnes. This is not feasible for a tanker vessel to accommodate. It is almost equal to the entire bunkering capacity of the vessel.
4.1.4. Ammonia
Ammonia is a promising hydrogen carrier and is the main chemical in producing fertilisers, accounting for 80% of global consumption [22], so there is existing knowledge regarding its use and storage. There are more than 120 ports with infrastructure for bunkering ammonia, and it is already synthesised to a large extent. The project pipeline for renewable ammonia currently stands at 15 Mt/year by 2030 and 71 Mt/year by 2040. The gravimetric hydrogen storage capacity of ammonia at a 10-bar liquid state is 17.7% (wt) [5]. Due to its easy-to-handle properties and ability to be produced without carbon dioxide, ammonia-based hydrogen storage is an attractive option for ships [23]. A further benefit of ammonia is its potential as a transition fuel. With no carbon emissions, it can be burned directly in an internal combustion engine (ICE) or cracked to provide hydrogen for a PEMFC. As demonstrated in Figure 1 [24], based on analysis published on the US government’s website, ammonia storage is among the most economical methods of storing gas and provides the smallest footprint and capital expenditures. Aside from ammonia and metal alloy hydrides, all other hydrogen storage candidate compounds contain carbon.
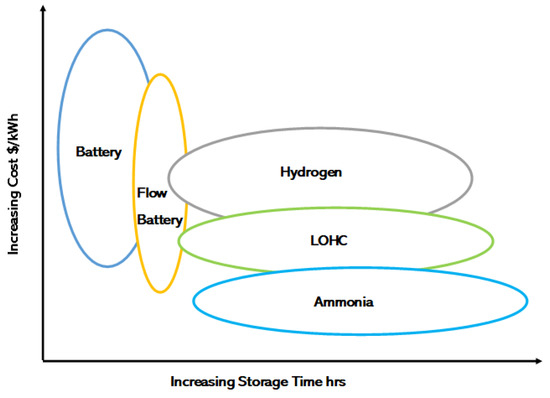
Figure 1.
A comparison of the cost, and storage time, of ammonia with other promising energy storage technologies. Adapted the from US Government [24].
Regarding energy density and cost, Table 11 compares ammonia pressurised storage with its strongest competitor, metal hybrid storage [5]. Due to its existing infrastructure, ease of storage, well-defined regulations, and excellent safety record, ammonia is a prime candidate for providing a secure supply of renewable hydrogen to stationary and mobile applications in the UK. While the production, handling, and supply of ammonia are well-established, efficient processes to separate hydrogen from ammonia have emerged more recently. Liquefied petroleum gas storage tanks are similar in design and function to ammonia tanks and have already been constructed for up to 130,000 m3. The storage solution does not have any geographical limitations and can be scaled up in a modular manner as needed.

Table 11.
An evaluation of the energy density and cost of ammonia pressurised storage compared to metal hybrid storage.
The PEM fuel cell can only be powered by hydrogen. Therefore, ammonia must be converted into hydrogen. It is anticipated that Air Products will make the largest-ever investment of US$500 million in the United States to build, own, and operate its largest-ever hydrogen production from ammonia [25]. According to a study published by the UK government [26], four dominant companies developed and evaluated a large-scale ammonia plant capable of generating fuel-cell grade purity hydrogen at large scales. With an overall efficiency of around 69%, their large-scale ammonia cracking unit can deliver high-purity hydrogen at 250 bars with a specific energy consumption of 12.65 MJ/Nm3. Figure 2 shows the breakdown of energy consumption for cracking ammonia units of kJ/mol of hydrogen produced [26]. Based on the estimated daily fuel consumption of the fuel cell module in the following chapter, this reforming unit would need to be sized to 1800 kg per day, since the estimated daily fuel consumption is approximately 1650 kg. In summary, this study concludes that ammonia as fuel and PEM fuel cells are the most promising options for the case study vessel.
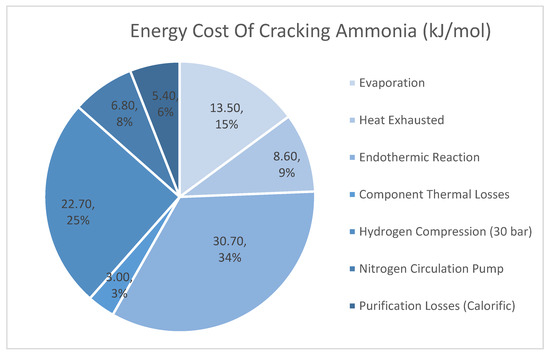
Figure 2.
A breakdown of the energy required to crack ammonia in units of kJ/mol of hydrogen generated. Adapted from [26].
5. Decision on Fuel Cell Type for the Case Study
In this chapter, the advantages and disadvantages of different fuels and fuel cells have been discussed. The objective of this chapter was to find the most suitable fuel cell type for the case-study vessel, considering the conditions of the maritime industry. Based on applied analysis, criteria were defined and explained from the shipping perspective, and PEM fuel cells were recognised as the most promising fuel cell technology for the case-study vessel. PEM fuel cells can only be fueled with hydrogen; after investigating various hydrogen storage options, ammonia was determined to represent the most appropriate storage solution due to the availability of decomposition technology. The next chapter will present a detailed discussion of how the PEMFC could be used to replace diesel generator modules by implementing an ammonia reforming unit.
5.1. Case Study
The vessel considered is a VLCC tanker with a gross tonnage of 165,784. Table 12 shows the specifications of the case study vessel. This vessel’s main engine is a B & W six-cylinder capable of producing 39,900 kW at a speed of 76.0 revolutions per minute. This is the maximum power the engine can produce while operating continuously within safe limits. The generators on the ship are Wartsila Engines. This engine provides a power range of 1200 kW in a 9 L cylinder configuration with a nominal speed of 900 revolutions per minute.

Table 12.
The case study vessel’s relevant particulars.
5.2. The Voyage Scenario
Determining the exact amount of auxiliary power demand for a real voyage of the case study vessel is necessary. This is to determine whether fuel cell modules can replace auxiliary power generators. For this purpose, the authors refer to fuel consumption data collected during a real journey for the vessel. The voyage scenario is chosen from the latest voyage of this vessel. The voyage lasted 35 days and covered 6380 nautical miles from the Persian Gulf to China. The generators of this ship consumed 204 metric tons of low-sulphur heavy fuel oil during this period. Today, there are more than 120 ports with existing infrastructure for ammonia bunkering. Therefore, a bunkering facility is assumed to be available for the ship at the destination port. A Wartsila 9 L20 engine operating according to standard conditions consumes 190 g of fuel per kilowatt-hour as per a Wartsila manual, the total energy demand for the whole journey would be 1,073,684 kWh.
5.3. Storage Tank Sizing
5.3.1. Ammonia
A significant advantage of ammonia is that it can be stored in liquid form at 25 °C and 10 bar pressure. This is done in standard steel tanks already used for liquified petroleum gas. It is important to note that although no unique material is required for ammonia storage tanks, ammonia has a high coefficient of thermal expansion. Ammonia has a volumetric density of 636 kg/m3 at saturation pressure and 0 °C, but a reduced density of 609 kg/m3 at 20 °C [23]. As a result, tank walls must be insulated to avoid material stress and potential ruptures. The ammonia cracking plant mentioned in the previous chapter was implemented to decompose ammonia and the production of hydrogen. With an overall efficiency of approximately 69%, this scaled-up cracking plant can deliver 1800 kg/day of high-purity hydrogen at 250 bars [26]. This reactor was designed to provide fuel to a fuel cell over a wide dynamic range and with a fast response time. Reflecting the proposed reactor’s efficiency, the total mass of ammonia required was calculated to be 301,562 kg. This calculation assumes an energy density of 5.16 kWh/kg, a subsequent required mass of ammonia for the voyage requirements of 208,078 kg, and a reformer efficiency of 69%.
A 301,562 kg mass of ammonia would occupy a volume of 442 m3, assuming an energy density of 682.6 kg/m3 at 25 °C and 10 bars.
A total of 682.6 kg/m3 of liquified ammonia can be stored in one large-scale full containment storage tank, typically a stainless steel atmospheric cryogenic bimetal tank.
According to the case study ship’s manual, heavy fuel oil is stored on board in four tanks designated for heavy fuel oil and low-sulphur heavy fuel oil. The vessel can bunker 9762 m3, excluding eight service and settling tanks and diesel oil tanks capable of bunkering 600 m3. The remaining 99.5% bunkering capacity (9711 m3) could accommodate the main engine and boiler fuel consumption during this voyage scenario. In addition, the ship has 15 cargo oil tanks with a combined capacity of 341,528.60 m3 at 98% loading. There are also two slop tanks covered in tar epoxy with a capacity of 9800.80 m3. As a result, this amount of ammonia represents about 0.01% of the total cargo capacity of the vessel.
5.3.2. Hydrogen
There is a need to store the hydrogen generated from the ammonia cracking plant in a tank. Since the daily hydrogen production of the ammonia reforming unit is 1800 kg, two liquid hydrogen storage tanks are considered. One is for fuel cell consumption, and one is a spare. If necessary, both tanks can be supplied with liquified hydrogen directly from a port bunkering facility. Assuming a density of 71 kg/m3 for liquified hydrogen, the space required for each of these tanks would be 25.3 m3, for a total volume of 50.6 m3.
5.4. Fuel Cell Sizing
Based on the calculation mentioned above, the total energy demand of the vessel during the 35 days of the voyage scenario was 1,073,684 kWh. In addition, based on the recorded output power of the diesel generators in the engine logbook, the average load supplied by the diesel generator module on this vessel is estimated to be 950 kW. To meet the total power demand of the vessel, there are three diesel generator modules, each with maximum outputs of 1200 kw. In normal operation, however, only one or two are used based on the vessel’s running hours and maintenance schedule. Considering all the assumptions made, a 2600 kW fuel cell module would be sufficient to meet this vessel’s power demands. This is three times as much power as the vessel typically consumes. The Ballard PEM fuel cell, a well-established solution in the marine industry, is a possible alternative to the Wartsila 9 L20 diesel generator module.
Consequently, 13 modules of 200 kW Ballard fuel cells would be required to achieve the desired power output. According to the manufacturer’s datasheet, each of the six modules on a single skid occupies less than 5.5 m3. Therefore, all 13 modules together would occupy approximately 12 m3. This is less than half of the area occupied by the current diesel generators (27 m3). Table 13 provides detailed dimensions and other specifications for one module of the PEM fuel cell [27].

Table 13.
A table of specifications of Ballard’s 200 kW FcWave fuel cell power module for marine applications.
The stack’s final dimensions, weight, and power output will be 25.7 m3, 11,375 kg, and 2600 kW, respectively. Assuming that the fuel cell module is operated at full load, the daily fuel consumption would be 1644 kg, assuming a daily power demand of 1,073,684 kWh over 35 days, and a peak fuel efficiency of 56%.
5.5. Battery Unit Sizing
Battery banks are commonly combined with fuel cells to take advantage of their superior energy density and ability to respond to transient conditions. Using the case study vessel and the calculated energy demand during the voyage, the maximum load on the vessel will not exceed 2000 kW. In addition, the daily electricity demand will not exceed 30 MWh. Based on the mentioned data, this case study vessel considers a lithium titanate battery SCiB module with a capacity of 1 MWh and a power output of 2000 kW. With a vessel’s daily power demand of 30 MW, the SCiB module can support the vessel’s power requirements for approximately 1.5 h without relying on the fuel cell module [28]. According to the datasheet for this module, it has a volume of 9.15 m3 and dimensions of 4.89 m × 0.90 m × 2.09 m. This battery unit’s total mass, based on its energy density of 140 W/kg or 0.14 kWh/kg, is 7142 kg.
5.6. System Integration
According to the current design, three diesel generator engines are designed to run continuously on heavy fuel oil. There are several systems associated with the main generator:
- The fuel system consists of heavy fuel oil service and settling tanks, two supply pumps, two circulating pumps, a deaeration tank, a filtration unit, a viscometer, and a fuel purification system;
- The compressed air system includes compressors, air dryers, air bottles, and an air starting system;
- The cooling water system consists of low-temperature units and high-temperature units;
- The Lubricating oil system, including pumps and filters, drain tank, and distribution system.
Removing and replacing all these systems with a PEMFC module will increase engine room space. This will allow for the replacement of the fuel cell module’s subsystems. Furthermore, noise, thermal waste, and air pollution will be reduced by removing the generator. The following sections discuss the ammonia reforming unit and the integrated fuel cell system as alternatives to the generator module.
5.6.1. Ammonia Reforming Unit
A scaled-up advanced membrane reactor with an integrated purification membrane utilising a commercial catalyst for pure hydrogen generation from ammonia is proposed for this case study, which has significantly lower capital and operating costs than existing solutions. The decomposition unit produced from the convention unit was a key innovation of this design developed by four dominant companies, including Siemens, STFC, Equity, and Energy [26]. As illustrated in Figure 3, the convection section is divided into four compartments or banks, each with a different function. This configuration recovers most of the heat in flue gases leaving the firebox. Thus, the design achieved 93.1% thermal efficiency at an 84.6% ammonia conversion rate. Table 14 summarises each stream’s composition and operating conditions in the integrated design flowsheet.
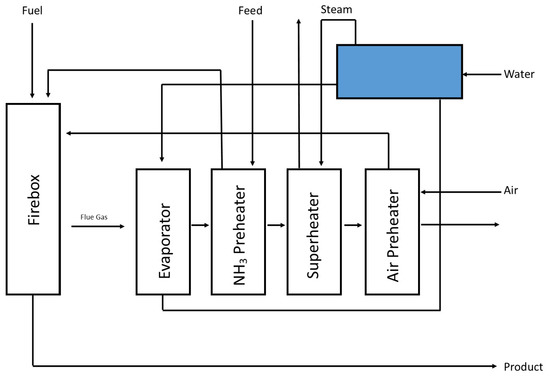
Figure 3.
An illustration of different compartments in the final integrated design flowsheet for the proposed ammonia reforming plant adapted from [26].

Table 14.
Overview of the composition and operating conditions of each stream in the integrated design flowsheet of the ammonia reforming plant.
The reformer plant produces a forming gas containing 69% hydrogen, 23% nitrogen, 8% NH3, and 18 kg/s of high-pressure steam. Ammonia is recovered from the forming gas by an ammonia recovery unit and returned to the reforming unit as uncracked ammonia. The electricity generated by the high-pressure steam is used to partially cover the work of gas compression in the following steps. To achieve the required hydrogen purity, a liquefaction cycle is employed to purify the syngas.
As a result of the process, the hydrogen product reaches a purity of 99.97%, which makes it suitable for use in fuel cells. Figure 4 illustrates the complete model of a large-scale ammonia cracking plant.
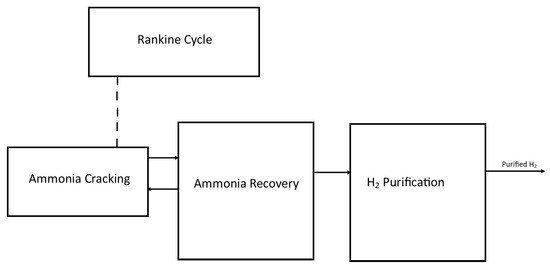
Figure 4.
A block diagram of the original design for a large-scale ammonia cracking plant adapted from [26].
5.6.2. Fuel Cell Module
The PEMFC module subsystem consists of a stack, a hydrogen delivery unit, an air delivery unit, and a cooling unit [29]. According to the proposed design, 240 bar of hydrogen is compressed at the purification section of the reforming plant. It is cooled by nitrogen in a heat exchanger and stored in the liquified form in the insulated storage tank. The hydrogen is converted to gasified hydrogen and is stored in a daily hydrogen bottle as required.
Components should be employed between the fuel inlet and outlet of the stack in order to facilitate hydrogen recirculation [28]. Since this case study is a maritime application, degradation of the polymer membrane in the fuel cell may occur due to the cathode being exposed to sea air. As a result, sodium chloride vapour may need to be removed from the inlet air through an appropriate pretreatment process. A cooling module consisting of a water tank, a water pump, and a heat exchanger removes the heat generated by the stack. Heat is transferred from the heat exchanger to the seawater outboard. The cooling water temperature at the stack inlet is controlled by adjusting the seawater flow. In contrast, the temperature at the stack outlet is controlled by adjusting the flow of coolant water [30].
5.6.3. Power System
The essential components of a marine fuel cell power system include fuel storage, a fuel cell module and control unit, a DC-DC converter, battery banks and chargers, DC-AC inverters, and DC-AC loads.
Battery Banks
Fuel cells are commonly combined with battery banks to take advantage of fuel cell systems’ superior energy density and the batteries’ transient response capabilities. The use of battery banks allows the load change to be balanced, and the fuel cells can be operated more steadily [31]. The battery banks are automatically connected to the power distribution network when the external load increases during regular operation. As a result, the fuel cell module will be able to gradually increase its power output to the required power level within a short period after the load is increased. If the external load decreases, the excess power generated by the fuel cell module can be used to charge the battery banks.
Battery Management System
Battery chargers are designed to charge batteries using either the fuel cell module or an external source of electricity. In accordance with the state of charge, the power output of the fuel cell module is automatically adjusted. To ensure the safety of the battery banks, a battery management system monitors the voltage, temperature, and state of charge of the batteries.
DC-DC Converter
Typical fuel cells produce a DC voltage between 0.5 and 1.0 volts at a rated load, which decreases with a higher current. To achieve a much greater voltage, individual cells can be stacked together, the maximum limit of which is usually determined by the manufacturing process. For the fuel cells to produce the desired power, a higher current is obtained by increasing the surface area of the cells or combining them in parallel. Fuel cell modules are modified with DC-DC converters to raise their voltage to meet industrial applications’ demands [30].
DC-AC Inverter
DC buses distribute power from fuel cell modules’ DC-DC converters to DC loads. A DC-AC inverter is installed to convert the DC power from the fuel cell module to the currents and voltage levels required to power AC loads, such as auxiliary pumps and blowers of the fuel cell module, and onboard auxiliary equipment. AC buses also distribute power from fuel cell modules’ DC-AC converters to AC loads. An illustration of the process flow diagram (PFD) of the fuel cell module that includes all the associated machinery and systems, including the power management system, can be seen in Figure 5.
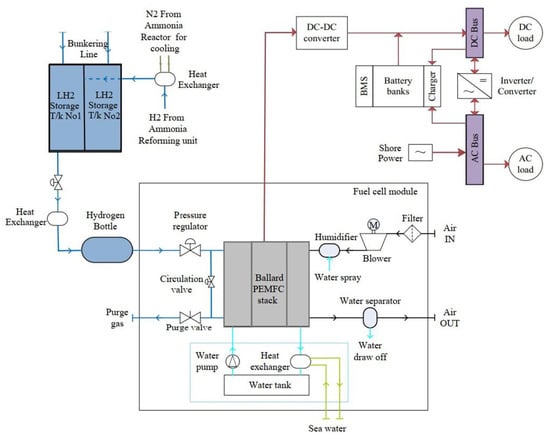
Figure 5.
An illustration of the designed process flow diagram (PFD) of the integrated PEMFC fuel cell module that includes all the associated machinery and systems, including the power management system. A battery management system is referred to as a BMS.
5.7. Emission Reduction
Since ships are subject to environmental regulations; the potential to reduce local emissions during operation is an imperative factor in the decision to use fuel cell systems. A PEM fuel cell system emits no nitrogen oxides, particulates, volatile organic compounds, or carbon dioxide, and is also more efficient electrically, resulting in lower CO2 emissions. The purpose of this section is to calculate how much emissions have been saved because of the proposed changes made to the auxiliary power unit. Each year, the United Kingdom government releases a conversion factors workbook to assist UK-based organisations and operations in reporting greenhouse gas emissions and carbon dioxide emissions. According to the latest published dataset on the UK government website on 27 July 2022 [32], the factors included in Table 15 pertain to marine fuel oil.

Table 15.
Emission conversion factors for marine fuel oil.
The case-study vessel was fed with marine fuel oil (low-sulphur heavy fuel oil) as the auxiliary power fuel. Based on the conversion factor and based on metric tonnes of fuel consumption, the following emissions would be produced, assuming 204 tonnes of fuel oil is utilised: 635,253 kg of CO2; 259 kg of CH4; 9024 kg of N2O.
Therefore, because the substitute auxiliary power generator is a PEM fuel cell fueled solely by hydrogen, there will be no emissions other than water.
As part of the next stage of this system, water and nitrogen will be captured, and nitrogen will be used as a coolant to liquefy pressurised hydrogen. The vessel will be supplied with more than five tonnes of fresh water by replacing the fuel cell module associated with the ammonia reforming plant. This could result in a reduction in fuel consumption for the generation of freshwater onboard and, therefore, a reduction in air pollution. On the other hand, according to the ammonia reforming plant, the only by-products of this system are 67% N2, 31% H2O, and 2% O2 [26]. In this scenario, 100% of the abovementioned pollutants will be prevented.
5.8. Cost of Integration
Several factors contribute to the performance of the PEM system, including heat recovery and fuel processing. For PEMFCs to be a viable option for generating power at sea, the cost of purchasing and operating the fuel cell system, which comprises all the components, must be comparable to that of an internal combustion engine. According to the United States government, the stack accounts for no more than 31% of the system cost [14]. This contributes less to the overall cost of most system production volumes. Regarding balance of plant (BOP) costs, power electronics represent the most significant contributor for all systems [33], as illustrated in Figure 6. Table 16 shows the total costs for the 250 Kw size PEMFC at two representative production volumes per year based on data from the US Department of Energy (DOE) [14].
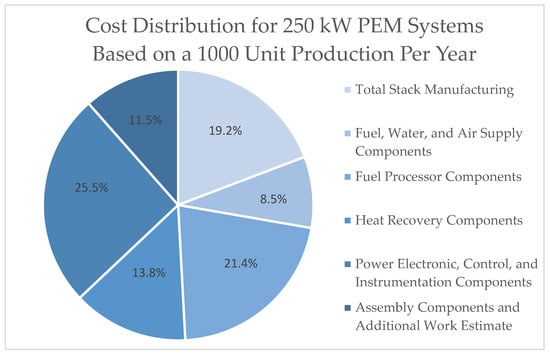
Figure 6.
A pie chart illustrating the cost distribution for 250 kW PEM systems based on a 1000-unit production each year to the nearest 0.1%. Adapted from [14].

Table 16.
A summary of the total cost of PEMFC production at two representative production volumes per year for 250 kW size, based on data from the United States Department of Energy (DOE) [14].
With the US DOE estimation and considering the lowest annual production volume of 1000 units, which is US$2040 per KWnet, as well as the total power we require in this case study, the integrated PEMFC module cost would be US$4,366,783.
It has been estimated by the International Energy Agency [34] that the ammonia production costs for CO2-free ammonia ranged from £0.11–£0.31 per kg worldwide over the last five years. Whilse plant size/capacity/age (efficiency) and other fixed/capex/local costs play a role, the cost driver is the natural gas cost, which can result in price swings as steep as that of the natural gas market. According to the International Energy Agency and considering the current capex costs of £0.73/kg for ammonia, as well as a renewable electricity cost of £24.40/MWhe, a value of £0.44/kg can be estimated for carbon-free ammonia production. Assuming the case study vessel is fueled with carbon-free ammonia derived from renewable hydrogen, and based on the mass of ammonia consumed in the voyage scenario, the total cost of fuel consumption would be £132,687.
It is estimated that the capital cost of the ammonia cracking plant, based on the techno-economic assessment applied in the Ammonia to Green Hydrogen project, will be £4.3 m, which includes 76% direct costs and 24% indirect costs. The total capital investment (TCI) consists of the following included in Figure 7 [26]:
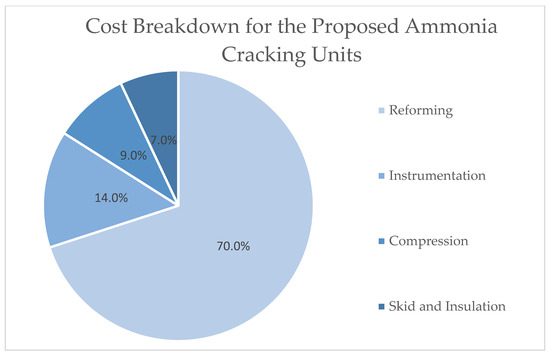
Figure 7.
An illustration of the cost breakdown for the proposed ammonia cracking units in a pie chart adapted from [26].
- Costs associated with purchased equipment—all costs related to the purchase of equipment for the control system (see Figure 6);
- Direct installation costs: the costs associated with labour and materials for the installation of the equipment;
- Indirect installation costs: the cost of preparing the site and building structures, as well as certain other costs;
- The cost of working capital and off-site facilities.
5.9. Maintenance
Fuel cells have no moving parts, making them potentially much more reliable than combustion engines (and significantly quieter) [35]. Fuel cells, in particular, require minimal maintenance (usually once every one to three years). A PEM fuel cell requires less maintenance than other fuel cell types since it operates at lower temperatures and has a simple structure [36]. However, several possible fault modes may arise with PEM fuel cells, including membrane dehydration, fuel/gas starvation, physical defects of the membrane, and catalyst poisoning [35]. This could be due to the fuel not being adequately humidified, the electrode channels not being adequately flooded with liquid water, a local hotspot, an incorrect pressure difference, or other factors. The fragility of fuel cell components and their proneness to further damage when operating under fault conditions make condition monitoring particularly critical. It has been demonstrated that novel sensor technologies, including nanosensors, are helpful for the condition monitoring of PEM fuel cell systems. PEM fuel cell condition monitoring can be applied in a variety of ways in order to prevent such failures [35]. The condition-based approach to maintenance should be developed based on a thorough analysis of the potential failure modes. Multiple condition measurements are often necessary to characterise cell failure modes since a problem is often diagnosed using two parameters. For example, a voltage drop in a particular cell with increased hydrogen pressure indicates a ruptured membrane. Choosing suitable sensors is critical to successfully implementing fuel cell condition monitoring technology. Sensors allow detection of faults, but the differences between conventional systems and fuel cell systems means that in order for the vessel to be capable of dealing with potential faults at sea, there would need to be specific processes in place and crew knowledge of fuel cell systems.
6. Conclusions
A comprehensive change will be necessary to meet the IMO’s goal of a 50% reduction in emissions from shipping by 2050. This article considers fuel cell technology a promising option for decarbonising power generation at sea and port. Fuel cell systems can be used to generate electricity onboard from a variety of logistics fuels with minimal hazardous emissions. This work used a case study vessel to study the application to an auxiliary power generator unit, and a review of different fuel cell technologies was presented. The criteria for selecting a fuel cell technology were defined and explained from the shipping perspective. Based on applied analysis, PEM fuel cells were considered the most promising option for the case study vessel. Besides having a high power-to-weight ratio (100–1000 W/kg), this mature technology also has a low operating temperature, allowing for flexible operation and less specific material requirements. The PEMFC is also an ideal fuel cell for power generation at sea due to its safety and flexibility regarding load changes.
Among the hydrogen carrier options available, ammonia in liquid form was considered the most promising fuel for the case study. As a carbon-free and readily dispatchable hydrogen carrier, ammonia makes it possible to store and distribute large quantities of renewable energy at a reasonable cost. Owing to its existing infrastructure, ease of storage, well-defined regulations, and excellent safety history, it is a candidate to provide a secure supply of renewable hydrogen for the presented fuel cell module. In this case study, a state-of-the-art design of an ammonia reforming plant was chosen because fuels other than hydrogen must be converted into hydrogen before they can be injected into PEMFCs. This scaled-up cracking plant can produce 1800 kg/day of high-purity hydrogen at 250 bars with an overall efficiency of 69%. The reactor can operate over a wide dynamic range and respond very quickly to supply hydrogen to the PEM fuel cell.
In addition to proposing the ammonia reforming system, a commercial PEM fuel cell was deployed for integration. All associated equipment and systems, such as a power management system, were defined for the case study. The emissions that could be avoided using the proposal were calculated, and this calculation showed that the reduction in emissions could be considerable. Calculations have shown that using PEM fuel cell technology for a real voyage scenario from the Persian Gulf to China can save up to 635,259 kg of CO2, 3.92 kg of CH4, and 9.024 kg of N2O.
In terms of economics, a techno-economic evaluation was carried out based on updated market data and integrated module capital costs. The capital cost for both the fuel cell module and the ammonia cracking plant is estimated at £4.3 million each, totalling £8.6 million combined. In comparison with diesel engines, fuel cell systems are relatively expensive. However, significant cost reductions have been demonstrated, and novel concepts have shown potential for further reductions. Ultimately, the reductions in fuel consumption, emissions, noise, and vibrations are considered by the authors to be sufficient to justify the higher capital costs.
As a final part of the study, a comparison was made between the maintenance of diesel generators and fuel cell modules. A condition monitoring strategy was presented as a vital tool for maintaining fuel cells.
Author Contributions
Formal analysis, H.S. and D.H.; investigation, H.S. and D.H.; visualisation, D.H.; project administration, M.A. and E.B.-D.; supervision, M.A. and E.B.-D.; writing—original draft, D.H.; writing—review and editing, D.H., M.A. and E.B.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
List of Symbols and Abbreviations
| VLCC | Very large crude carrier |
| CO2 | Carbon dioxide |
| GHG | Greenhouse gas |
| IMO | International Maritime Organization |
| UK | United Kingdom |
| LHV | Lower heating values |
| MCDA | Multi-criteria decision analysis |
| PEMFC | Proton exchange membrane fuel cell |
| HTPEM | High-temperature proton exchange membrane |
| MCFC | Molten-carbonate fuel cell |
| SOFC | Solid oxide fuel cell |
| AFC | Alkaline fuel cell |
| PAFC | Phosphoric acid fuel cell |
| DMFC | Direct-methanol fuel cell |
| LNG | Liquified natural gas |
| NOX | Nitrogen oxides |
| US | United States |
| BOP | Balance of plant |
| EMSA | European Maritime Safety Agency |
| DOE | Department of Energy |
| CapEx | Capital expenditures |
| OpEx | Operational expenditures |
| RP | Ranking point |
| WP | Weighting point |
| DNV GL | Det Norske Veritas and Germanischer Lloyd (Now commonly referred to solely as DNV) |
| ICE | Internal combustion engine |
| B & W | Burmeister & Wain |
| LOA | Length overall |
| LBP | Length between perpendiculars |
| FWA | Fresh water allowance |
| TPC | Tonnes per centimetre |
| G.R.T. | Gross registered tonnage |
| DWT | Deadweight Tonnage |
| NRT | Net Register Tonnage |
| DISPL | Displacement |
| FWD | Forward |
| SDWT | Summer deadweight tonnage |
| SDW | Summer deadweight |
| ISO | International Organization for Standardization |
| PEM | Proton exchange membrane |
| APP. | Approximately |
| SCiB | A Rechargeable Battery using lithium titanium oxide for the anode, trademarked by Toshiba |
| STFC | UK’s Science & Technology Facilities Council |
| NH3 | Ammonia |
| H2 | Hydrogen |
| DC | Direct current |
| AC | Alternating current |
| PFD | Process flow diagram |
| CH4 | Methane |
| O2 | Oxygen |
| H2O | Water |
| TCI | Total capital investment |
References
- Lindstad, E.; Lagemann, B.; Rialland, A.; Gamlem, G.M.; Valland, A. Reduction of maritime GHG emissions and the potential role of E-fuels. Transp. Res. Part D Transp. Environ. 2021, 101, 103075. [Google Scholar] [CrossRef]
- UK to Go Further and Faster to Tackle Climate Change. UK GOV: United Kingdom Government Website. 2019. Available online: https://www.gov.uk/government/news/uk-to-go-further-and-faster-to-tackle-climate-change (accessed on 25 July 2023).
- Welaya, Y.M.; El Gohary, M.M.; Ammar, N.R. A comparison between fuel cells and other alternatives for marine electric power generation. Int. J. Nav. Archit. Ocean Eng. 2011, 3, 141–149. [Google Scholar] [CrossRef]
- Nations, T.U. Factsheet: People and Oceans. In Proceedings of the Ocean Conference, New York, NY, USA, 5–9 June 2017. [Google Scholar]
- Inal, O.B.; Dere, C.; Zincir, B.; Deniz, C. Hybrid propulsion and alternative fuels education in the course of decarbonised shipping. Aust. J. Marit. Ocean Aff. 2022, 14, 97–113. [Google Scholar] [CrossRef]
- Huang, J.; Fan, H.; Xu, X.; Liu, Z. Life Cycle Greenhouse Gas Emission Assessment for Using Alternative Marine Fuels: A Very Large Crude Carrier (VLCC) Case Study. J. Mar. Sci. Eng. 2022, 10, 1969. [Google Scholar] [CrossRef]
- Roh, G.; Kim, H.; Jeon, H.; Yoon, K. Fuel Consumption and CO2 Emission Reductions of Ships Powered by a Fuel-Cell-Based Hybrid Power Source. J. Mar. Sci. Eng. 2019, 7, 230. [Google Scholar] [CrossRef]
- van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P. A review of fuel cell systems for maritime applications. J. Power Source 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Yatsalo, B.; Radaev, A.; Martínez, L. Presumption of model adequacy or is every fuzzification of an mCDA method justified? Inf. Sci. 2022, 587, 371–392. [Google Scholar] [CrossRef]
- Kandidayeni, M.; Soleymani, M.; Macias, A.; Trovão, J.P.; Boulon, L. Online power and efficiency estimation of a fuel cell system for adaptive energy management designs. Energy Convers. Manag. 2022, 255, 115324. [Google Scholar] [CrossRef]
- Tronstad, T.; Åstrand, H.H.; Haugom, G.-P.; Langfeldt, L. Study on the Use of Fuel Cells in Shipping; EMSA European Maritime Safety Agency: Lisbon, Portugal, 2017. [Google Scholar]
- de-Troya, J.J.; Alvarez, C.; Fernández-Garrido, C.; Carral, L. Analysing the possibilities of using fuel cells in ships. Int. J. Hydrogen Energy 2016, 41, 2853–2866. [Google Scholar] [CrossRef]
- Mohammed, H.; Al-Othman, A.; Nancarrow, P.; Tawalbeh, M.; Assad, M.E.H. Direct hydrocarbon fuel cells: A promising technology for improving energy efficiency. Energy 2019, 172, 207–219. [Google Scholar] [CrossRef]
- Energy, U.S. Office of Energy, Efficiency and Renewable Energy. Fuel Cells. Available online: https://www.energy.gov/eere/fuelcells/fuel-cells (accessed on 22 April 2023).
- Papageorgopoulos, D.C. Advancements and Prospects of DOE Fuel Cell Research and Development Activities; Electrochemical Society Meeting Abstracts 231; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2017; p. 1614. [Google Scholar]
- Pocard, N. Fuel Cell Price to Drop 70–80% as Production Volume Scales. In Zero Emission Fuel Cells; Ballard, Ed.; Ballard: Burnaby, BC, Canada, 2022. [Google Scholar]
- Aminudin, M.A.; Kamarudin, S.K.; Lim, B.H.; Majilan, E.H.; Masdar, M.S.; Shaari, N. An overview: Current progress on hydrogen fuel cell vehicles. Int. J. Hydrogen Energy 2023, 48, 4371–4388. [Google Scholar] [CrossRef]
- Dolan, C.; Gangi, J.; Homann, Q.; Fink, V.; Kopasz, J. 2019 Fuel Cell Technologies Market Report; Argonne National Lab. (ANL): Argonne, IL, USA, 2020. [Google Scholar]
- James, B.D.; Houchins, C.; Huya-Kouadio, J.M.; DeSantis, D.A. Hydrogen Storage System Cost Analysis; Strategic Analysis Inc.: Arlington, VA, USA, 2016. [Google Scholar]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Hydrogen, G. Green Energy Storage HY2MEGA. Available online: https://www.gknhydrogen.com/wp-content/uploads/2022/07/GKN_HY2MEGA_ProductSheet.pdf (accessed on 24 April 2023).
- Hansson, J.; Brynolf, S.; Fridell, E.; Lehtveer, M. The potential role of ammonia as marine fuel—Based on energy systems modeling and multi-criteria decision analysis. Sustainability 2020, 12, 3265. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Szymanski, S. Green Ammonia and H2@Scale: An Industry Perspective. Available online: https://www.energy.gov/sites/prod/files/2017/05/f34/fcto_may_2017_h2_scale_wkshp_szymanski.pdf (accessed on 26 April 2023).
- Products, A. Air Products to Make Largest-Ever U.S. Investment of $500 Million to Build, Own and Operate Its Largest-Ever Hydrogen SMR, a Nitrogen ASU and Utilities Facilities, and Wins Long-Term Contract to Supply Gulf Coast Ammonia’s New World-Scale Texas Production Plant. Available online: https://www.airproducts.com/company/news-center/2020/01/0108-air-products-to-build-its-largest-smr-to-supply-gulf-coast-ammonia (accessed on 1 August 2022).
- Makhloufi, C.; Kezibri, N. Large-scale decomposition of green ammonia for pure hydrogen production. Int. J. Hydrogen Energy 2021, 46, 34777–34787. [Google Scholar] [CrossRef]
- Ballard Marine Modules—Fuel Cell Power Products. Available online: https://www.ballard.com/fuel-cell-solutions/fuel-cell-power-products/marine-modules (accessed on 2 March 2023).
- Toshiba. Toshiba to Supply Lithium-Titanate Battery for 2 MW Energy Storage System Project in UK Led by the University of Sheffield; Global Toshiba: Tokyo, Japan, 2014; p. 1. [Google Scholar]
- Li, Q.; Yang, H.; Han, Y.; Li, M.; Chen, W. A state machine strategy based on droop control for an energy management system of PEMFC-battery-supercapacitor hybrid tramway. Int. J. Hydrogen Energy 2016, 41, 16148–16159. [Google Scholar] [CrossRef]
- Choi, C.H.; Yu, S.; Han, I.-S.; Kho, B.-K.; Kang, D.-G.; Lee, H.Y.; Seo, M.-S.; Kong, J.-W.; Kim, G.; Ahn, J.-W. Development and demonstration of PEM fuel-cell-battery hybrid system for propulsion of tourist boat. Int. J. Hydrogen Energy 2016, 41, 3591–3599. [Google Scholar] [CrossRef]
- Borgogna, G.; Speranza, E.; Lamberti, T.; Traverso, A.N.; Magistri, L.; Gadducci, E.; Massardo, A.F.; Olivieri, P. Design and Development of a Laboratory for the Study of PEMFC System for Marine Applications; E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2019; p. 02020. [Google Scholar]
- Government, U. Greenhouse gas reporting: Conversion factors 2022. Department for Energy Security and Net Zero, D. f. B. Energy & Industrial Strategy, Ed. Research and Analysis: 2022. Government Document. Available online: https://www.gov.uk/government/publications/greenhouse-gas-reporting-conversion-factors-2022 (accessed on 24 April 2022).
- Horvath, S.; Fasihi, M.; Breyer, C. Techno-economic analysis of a decarbonized shipping sector: Technology suggestions for a fleet in 2030 and 2040. Energy Convers. Manag. 2018, 164, 230–241. [Google Scholar] [CrossRef]
- Agency, I.E. Ammonia—The CO2-Free Fuel of the Future? IEA: Paris, France, 2020. [Google Scholar]
- Bahrebar, S.; Zhou, D.; Rastayesh, S.; Wang, H.; Blaabjerg, F. Reliability assessment of power conditioner considering maintenance in a PEM fuel cell system. Microelectron. Reliab. 2018, 88, 1177–1182. [Google Scholar] [CrossRef]
- Hua, Z.; Zheng, Z.; Pahon, E.; Péra, M.-C.; Gao, F. A review on lifetime prediction of proton exchange membrane fuel cells system. J. Power Source 2022, 529, 231256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).