FOXO-like Gene Is Involved in the Regulation of 20E Pathway through mTOR in Eriocheir sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Crabs and Sample Preparation
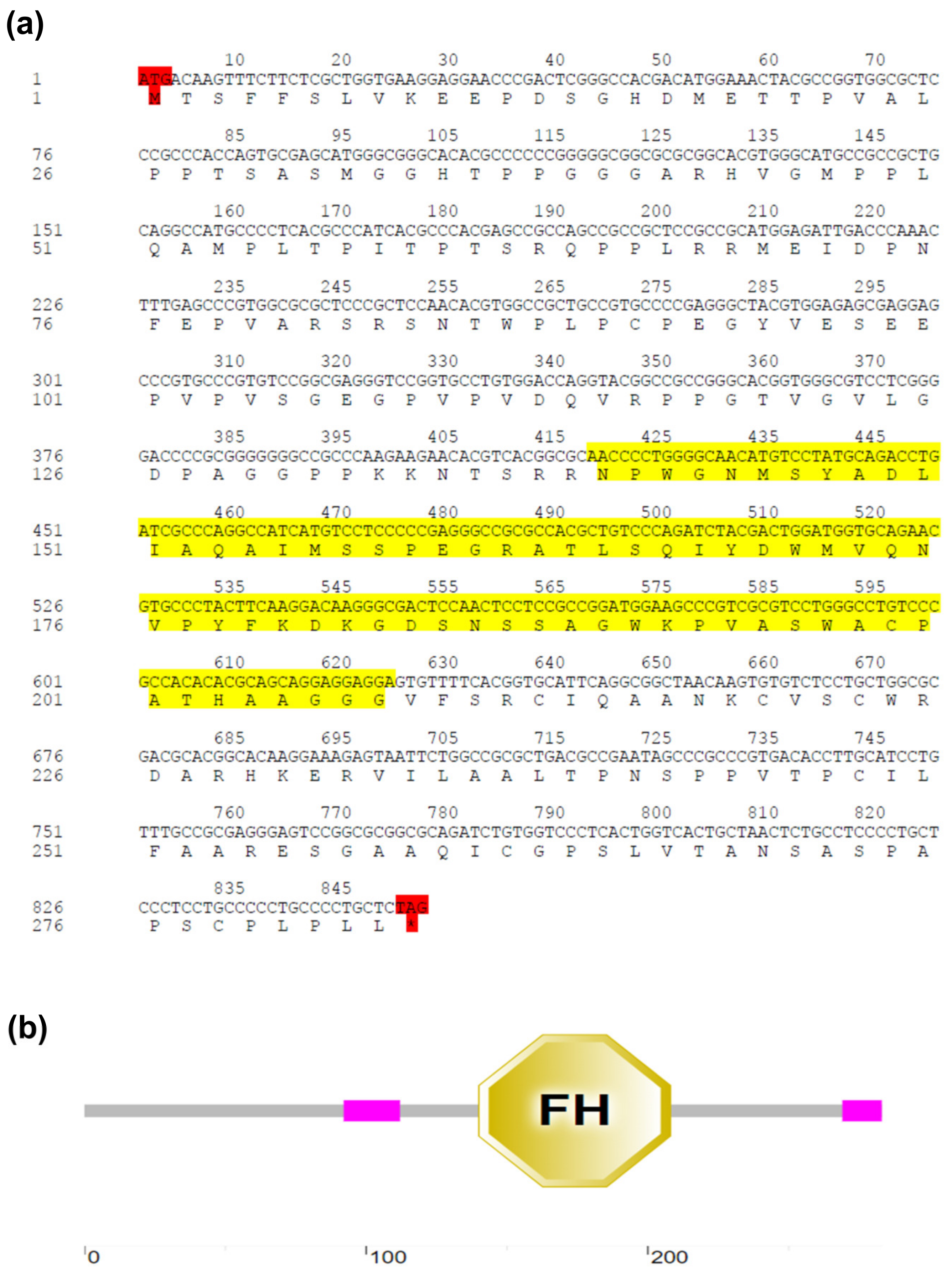
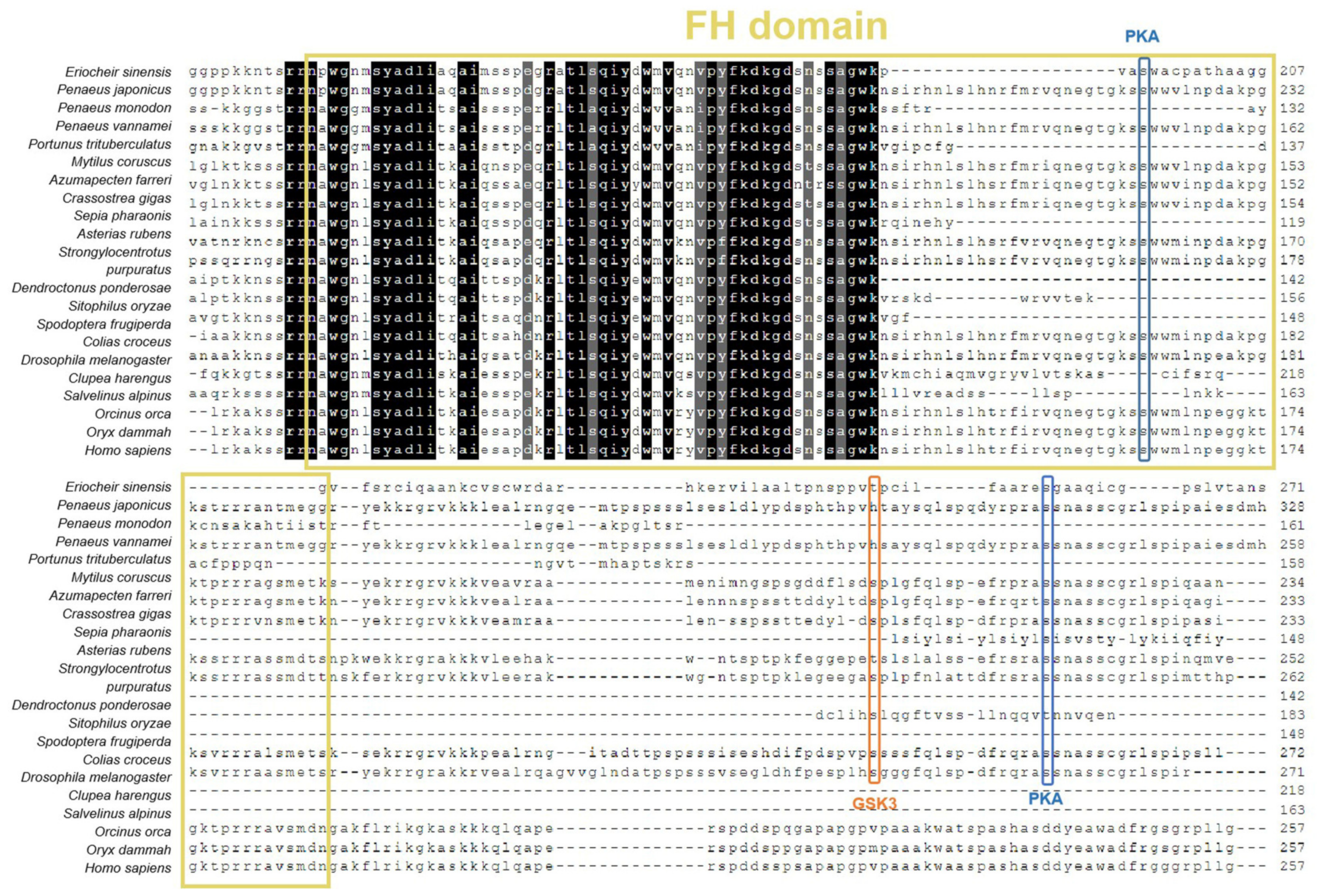
2.2. Identification and Sequence Analysis of EsFOXO-like
2.3. FOXO Inhibitor and Rapamycin Treatment
2.4. RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR Analysis
2.5. Detection of 20-Hydroxyecdysone (20E) Concentration
2.6. RNA Interference Assay
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
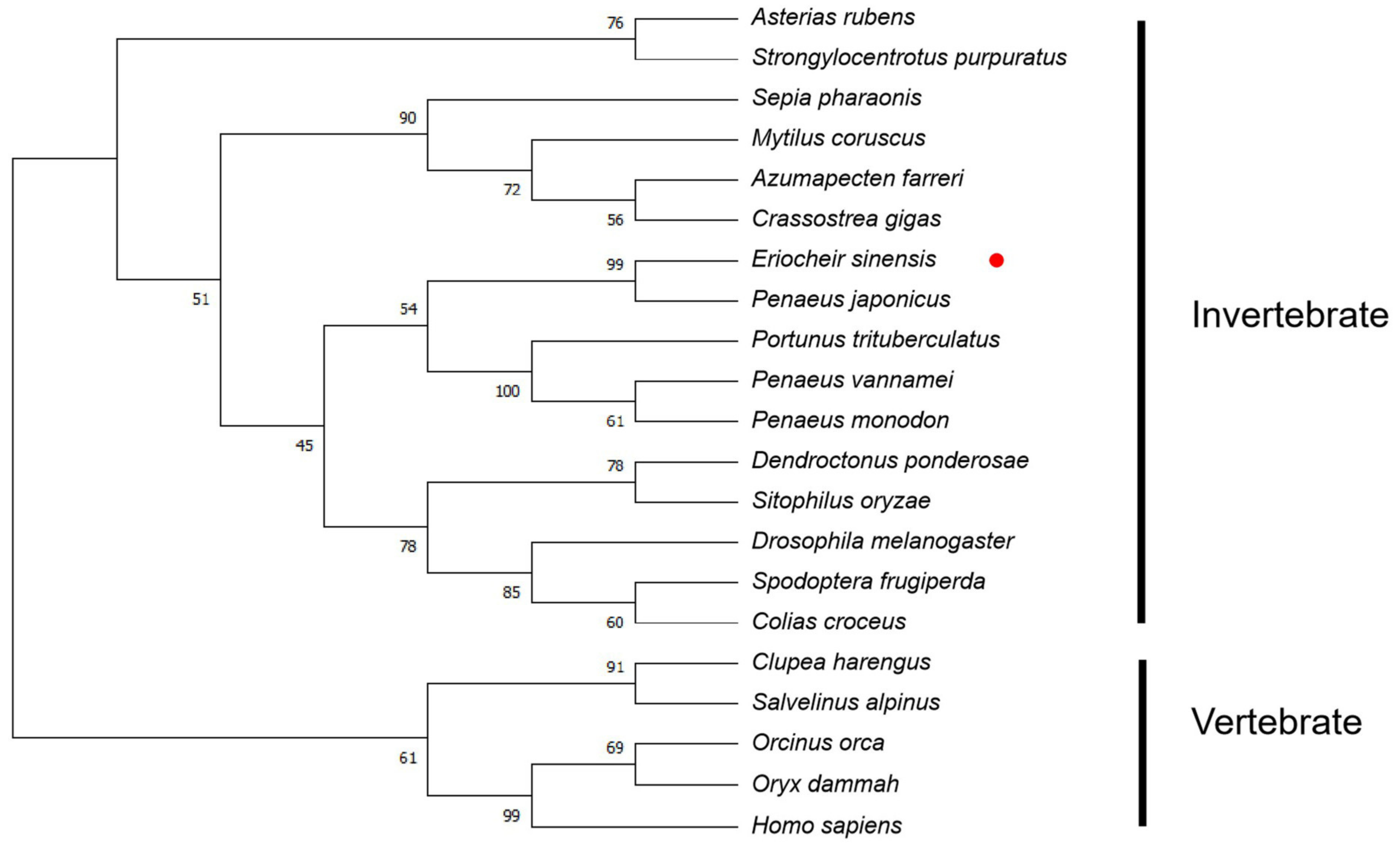
3.1. Sequence and Phylogenetic Analysis of EsFOXO-like Gene
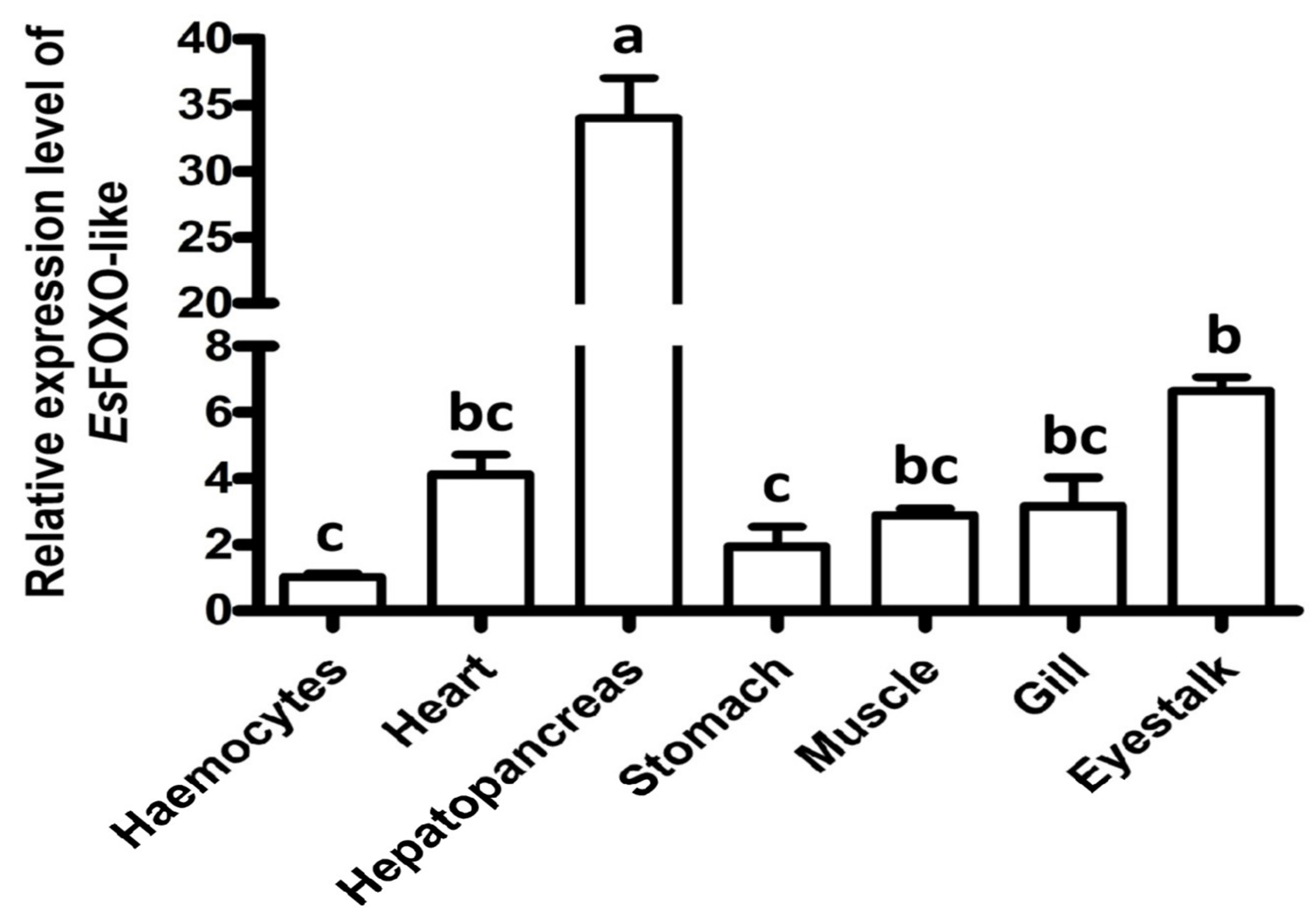
3.2. The Distribution of EsFOXO-like Gene in Different Tissues
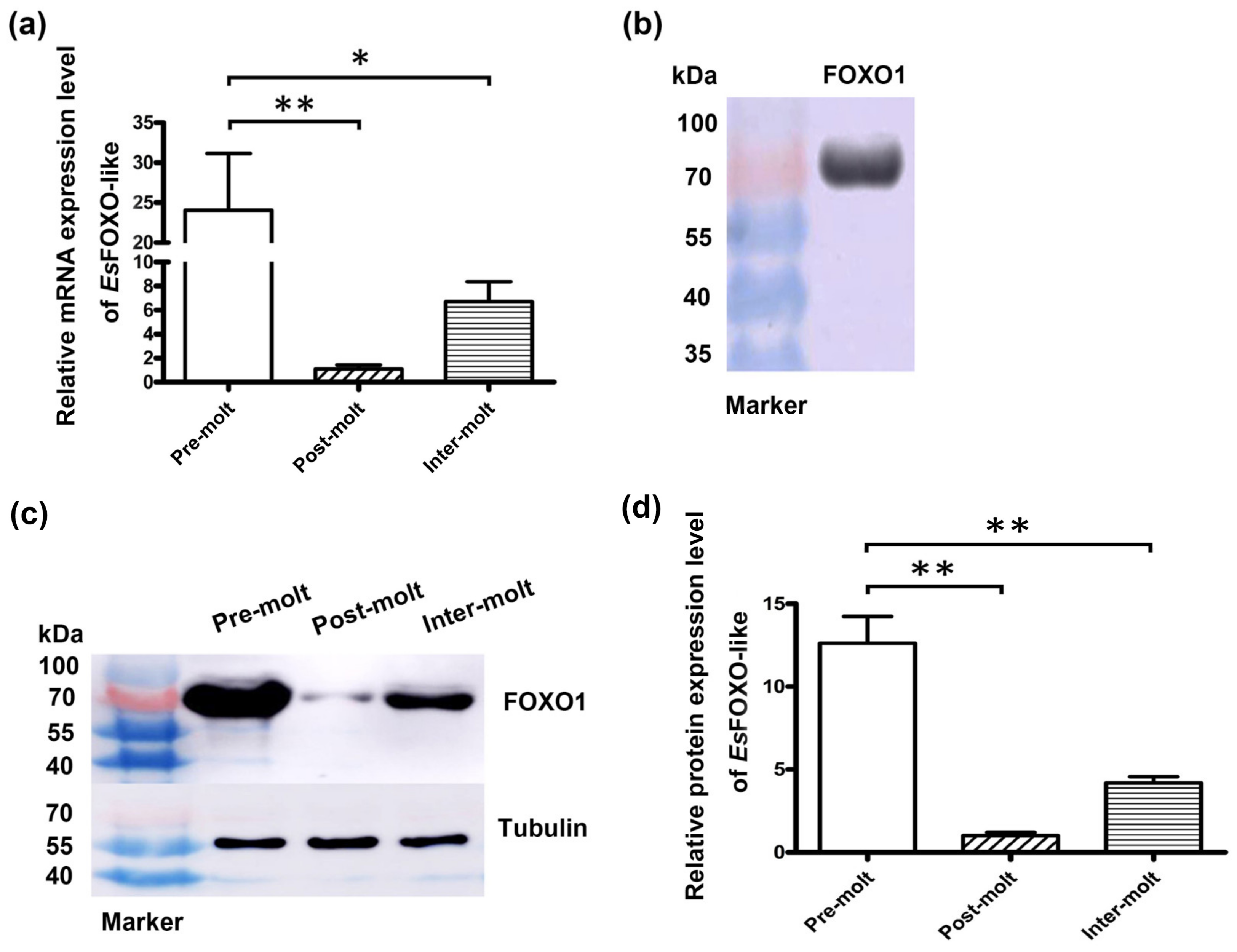
3.3. The EsFOXO-like mRNA and Protein Expression Characteristic in Hepatopancreas at Three Molting Stages
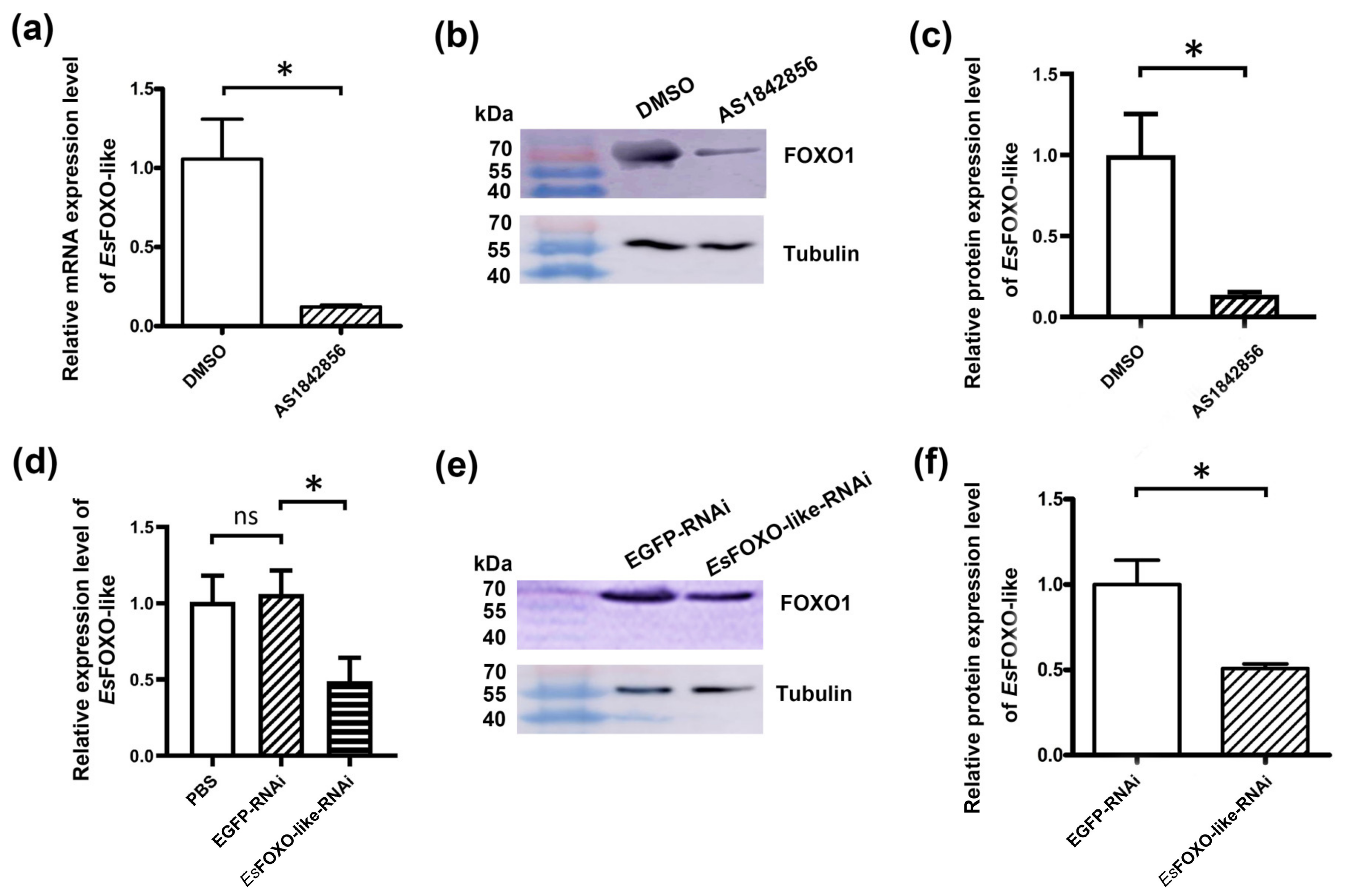
3.4. The EsFOXO-like mRNA and Protein Expression Levels after Inhibition of EsFOXO-like
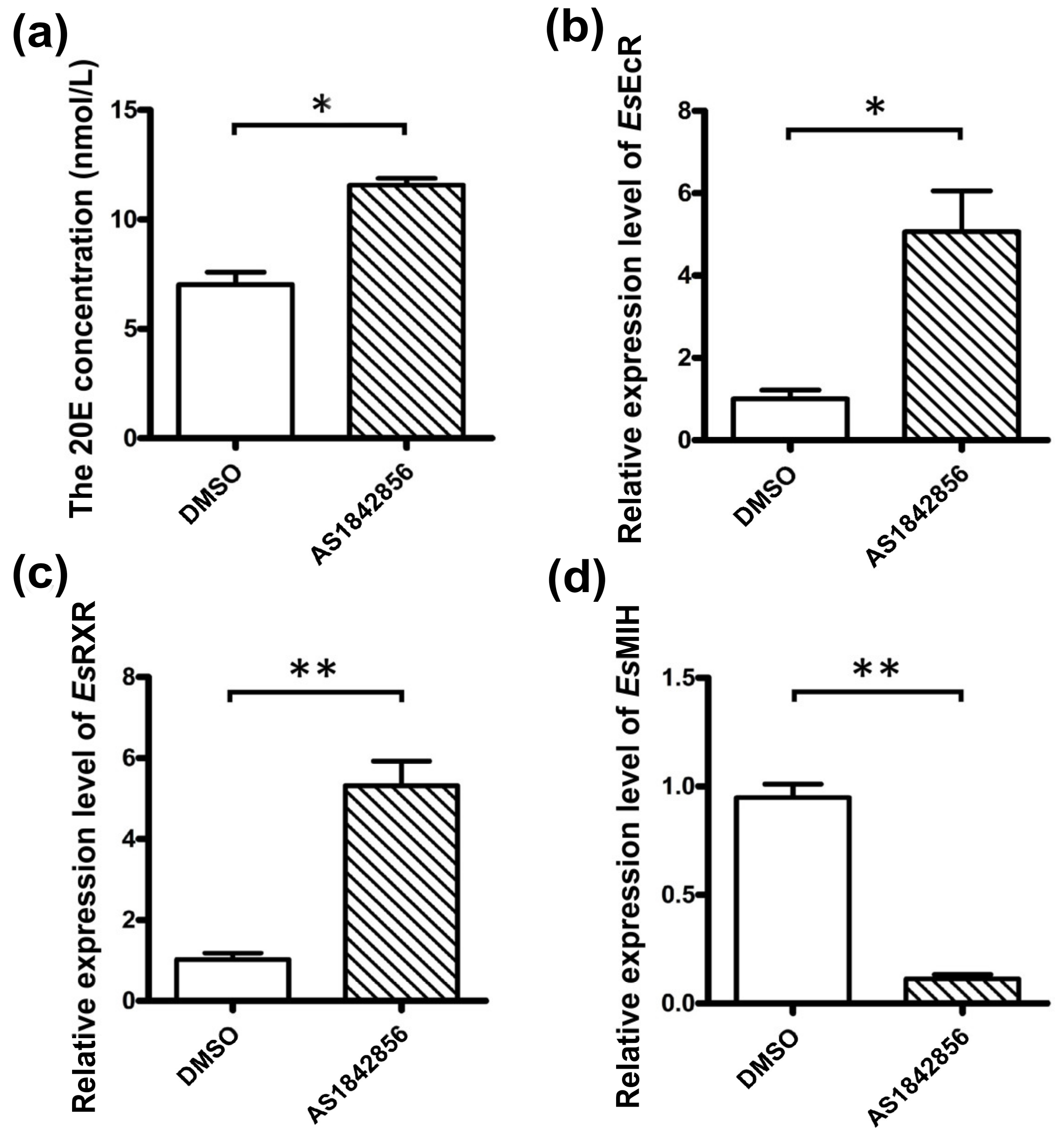
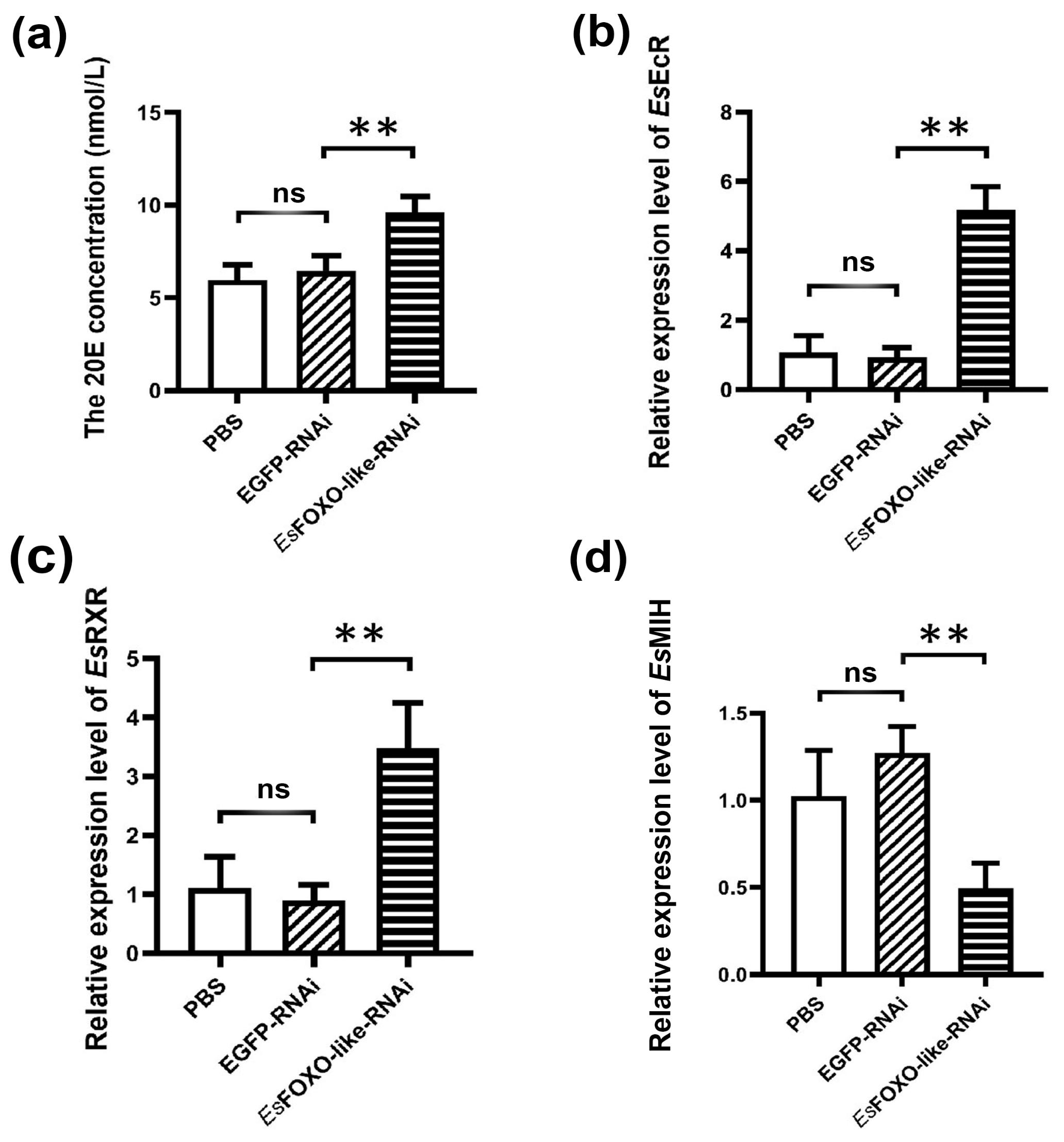
3.5. The 20E Concentration and Molting-Related Genes mRNA Expression Levels after EsFOXO-like Inhibition
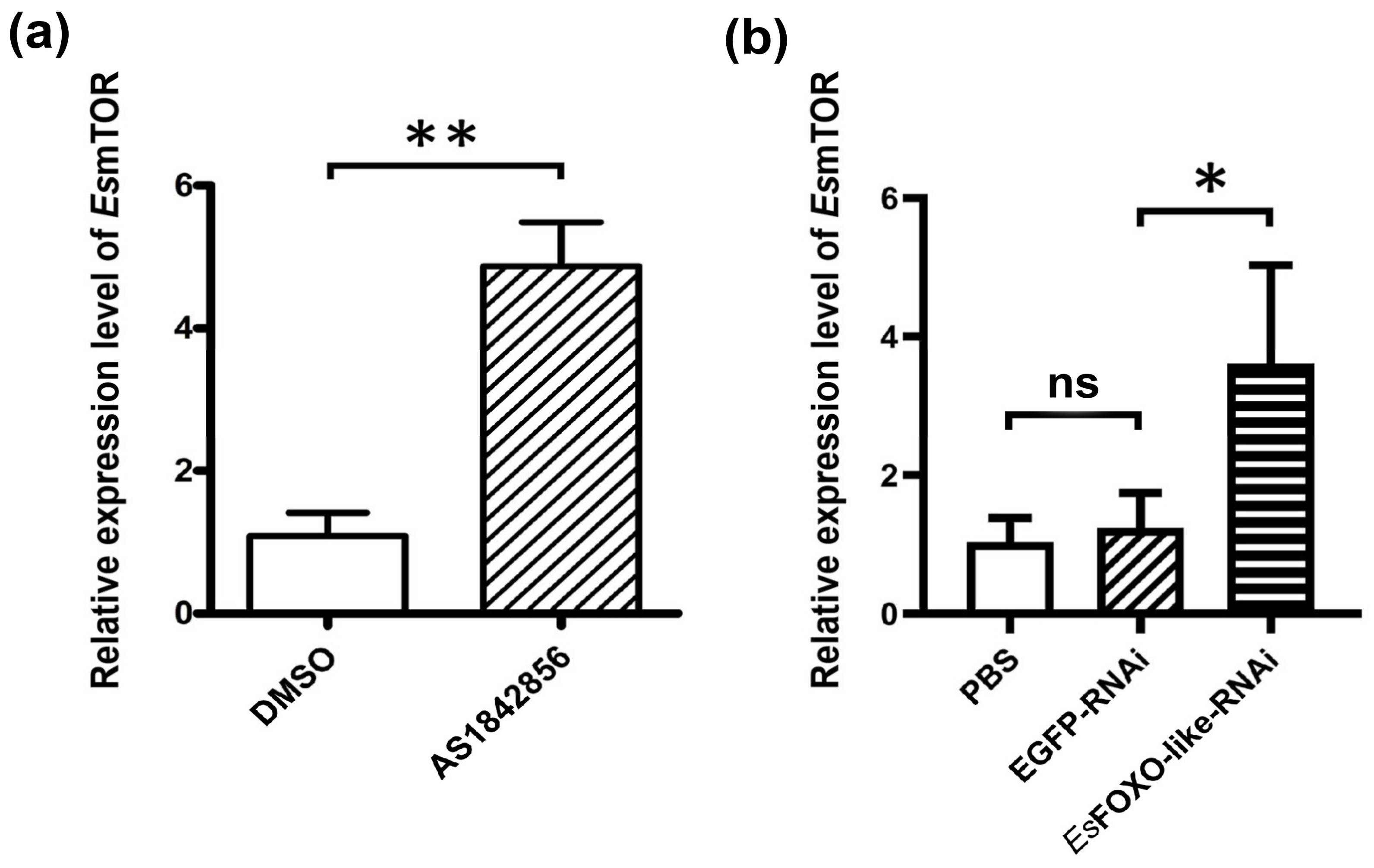
3.6. The mRNA Expression Level of EsmTOR after EsFOXO-like Inhibition
3.7. The EsmTOR mRNA Transcripts after AS1842856 and Rapamycin Injection
3.8. The 20E Concentration and EsEcR, EsRXR and EsMIH mRNA Expression Levels in AS1842856 + Rapamycin Group after Inhibiting mTOR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calissi, G.; Lam, E.W.; Link, W. Therapeutic strategies targeting FOXO transcription factors. Nat. Rev. Drug Discov. 2021, 20, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Jiramongkol, Y.; Lam, E.W. FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 2020, 39, 681–709. [Google Scholar] [CrossRef]
- Li, C.; Hong, P.P.; Yang, M.C.; Zhao, X.F.; Wang, J.X. FOXO regulates the expression of antimicrobial peptides and promotes phagocytosis of hemocytes in shrimp antibacterial immunity. PLoS Pathog. 2021, 17, e1009479. [Google Scholar] [CrossRef] [PubMed]
- Arden, K.C. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene 2008, 27, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Boura, E.; Silhan, J.; Herman, P.; Vecer, J.; Sulc, M.; Teisinger, J.; Obsilova, V.; Obsil, T. Both the N-terminal loop and wing W2 of the forkhead domain of transcription factor Foxo4 are important for DNA binding. J. Biol. Chem. 2007, 282, 8265–8275. [Google Scholar] [CrossRef]
- Clark, K.L.; Halay, E.D.; Lai, E.; Burley, S.K. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 1993, 364, 412–420. [Google Scholar] [CrossRef]
- Jacobs, F.M.; van der Heide, L.P.; Wijchers, P.J.; Burbach, J.P.; Hoekman, M.F.; Smidt, M.P. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J. Biol. Chem. 2003, 278, 35959–35967. [Google Scholar] [CrossRef]
- Arden, K.C.; Biggs, W.H. Regulation of the FoxO family of transcription factors by phosphatidylinositol-3 kinase-activated signaling. Arch. Biochem. Biophys. 2002, 403, 292–298. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Coffer, P.J. The extending network of FOXO transcriptional target genes. Antioxid. Redox Signal. 2011, 14, 579–592. [Google Scholar] [CrossRef]
- Dong, X.; Zhai, Y.; Zhang, J.; Sun, Z.; Chen, J.; Chen, J.; Zhang, W. Fork head transcription factor is required for ovarian mature in the brown planthopper, Nilaparvata lugens (Stål). BMC Mol. Biol. 2011, 12, 53. [Google Scholar] [CrossRef]
- Zeng, B.; Huang, Y.; Xu, J.; Shiotsuki, T.; Bai, H.; Palli, S.R.; Huang, Y.; Tan, A. The FOXO transcription factor controls insect growth and development by regulating juvenile hormone degradation in the silkworm, Bombyx mori. J. Biol. Chem. 2017, 292, 11659–11669. [Google Scholar] [CrossRef]
- Giannakou, M.E.; Goss, M.; Jünger, M.A.; Hafen, E.; Leevers, S.J.; Partridge, L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 2004, 305, 361. [Google Scholar] [CrossRef]
- Wu, Y.B.; Yang, W.J.; Xie, Y.F.; Xu, K.K.; Tian, Y.; Yuan, G.R.; Wang, J.J. Molecular characterization and functional analysis of BdFoxO gene in the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Gene 2016, 578, 219–224. [Google Scholar] [CrossRef]
- Süren-Castillo, S.; Abrisqueta, M.; Maestro, J.L. FoxO inhibits juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2012, 42, 491–498. [Google Scholar] [CrossRef]
- Sim, C.; Denlinger, D.L. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA 2008, 105, 6777–6781. [Google Scholar] [CrossRef]
- Cai, M.J.; Zhao, W.L.; Jing, Y.P.; Song, Q.; Zhang, X.Q.; Wang, J.X.; Zhao, X.F. 20-Hydroxyecdysone activates Forkhead box O to promote proteolysis during Helicoverpa armigera molting. Development 2016, 143, 1005–1015. [Google Scholar] [CrossRef]
- Lin, X.; Yu, N.; Smagghe, G. FoxO mediates the timing of pupation through regulating ecdysteroid biosynthesis in the red flour beetle, Tribolium castaneum. Gen. Comp. Endocrinol. 2018, 258, 149–156. [Google Scholar] [CrossRef]
- Hossain, M.S.; Liu, Y.; Zhou, S.; Li, K.; Tian, L.; Li, S. 20-Hydroxyecdysone-induced transcriptional activity of FoxO upregulates brummer and acid lipase-1 and promotes lipolysis in Bombyx fat body. Insect Biochem. Mol. Biol. 2013, 43, 829–838. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, X.; Han, Z.; Liu, P.; Xu, H.; Huang, X.; Ren, Q. The forkhead box O transcription factor regulates lipase and anti-microbial peptide expressions to promote lipid catabolism and improve innate immunity in the Eriocheir sinensis with hepatopancreatic necrosis disease. Fish Shellfish Immunol. 2022, 124, 107–117. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Ma, W.M.; Yang, W.J.; Yang, J.S. The complete mitogenome of the lined shore crab Pachygrapsus crassipes Randall 1840 (Crustacea: Decapoda: Grapsidae). Mitochondrial DNA 2014, 25, 263–264. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Wei, J.; Sun, X.; Yuan, J.; Li, F.; Xiang, J. Whole Transcriptome Analysis Provides Insights into Molecular Mechanisms for Molting in Litopenaeus vannamei. PLoS ONE 2015, 10, e0144350. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, J.; Yue, W.; Chen, J.; Gaughan, S.; Lu, W.; Lu, G.; Wang, C. Transcriptomic variation of hepatopancreas reveals the energy metabolism and biological processes associated with molting in Chinese mitten crab, Eriocheir sinensis. Sci. Rep. 2015, 5, 14015. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.S.; Mykles, D.L. Regulation of crustacean molting: A review and our perspectives. Gen. Comp. Endocrinol. 2011, 172, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Henrich, V.C. Arthropod nuclear receptors and their role in molting. FEBS J. 2009, 276, 6128–6157. [Google Scholar] [CrossRef]
- Techa, S.; Chung, J.S. Ecdysone and retinoid-X receptors of the blue crab, Callinectes sapidus: Cloning and their expression patterns in eyestalks and Y-organs during the molt cycle. Gene 2013, 527, 139–153. [Google Scholar] [CrossRef]
- Techa, S.; Chung, J.S. Ecdysteroids regulate the levels of Molt-Inhibiting Hormone (MIH) expression in the blue crab, Callinectes sapidus. PLoS ONE 2015, 10, e0117278. [Google Scholar] [CrossRef]
- Mykles, D.L.; Chang, E.S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. Gen. Comp. Endocrinol. 2020, 294, 113493. [Google Scholar] [CrossRef]
- Shyamal, S.; Das, S.; Guruacharya, A.; Mykles, D.L.; Durica, D.S. Transcriptomic analysis of crustacean molting gland (Y-organ) regulation via the mTOR signaling pathway. Sci. Rep. 2018, 8, 7307. [Google Scholar] [CrossRef]
- Mykles, D.L. Signaling Pathways That Regulate the Crustacean Molting Gland. Front. Endocrinol. 2021, 12, 674711. [Google Scholar] [CrossRef]
- Gu, S.H.; Young, S.C.; Lin, J.L.; Lin, P.L. Involvement of PI3K/Akt signaling in PTTH-stimulated ecdysteroidogenesis by prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2011, 41, 197–202. [Google Scholar] [CrossRef]
- Gu, S.H.; Yeh, W.L.; Young, S.C.; Lin, P.L.; Li, S. TOR signaling is involved in PTTH-stimulated ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2012, 42, 296–303. [Google Scholar] [CrossRef]
- Lin, A.; Yao, J.; Zhuang, L.; Wang, D.; Han, J.; Lam, E.W.; Gan, B. The FoxO-BNIP3 axis exerts a unique regulation of mTORC1 and cell survival under energy stress. Oncogene 2014, 33, 3183–3194. [Google Scholar] [CrossRef]
- Teleman, A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2009, 425, 13–26. [Google Scholar] [CrossRef]
- Mirth, C.K.; Shingleton, A.W. Integrating body and organ size in Drosophila: Recent advances and outstanding problems. Front. Endocrinol. 2012, 3, 49. [Google Scholar] [CrossRef]
- Rewitz, K.F.; Yamanaka, N.; O’Connor, M.B. Developmental checkpoints and feedback circuits time insect maturation. Curr. Top. Dev. Biol. 2013, 103, 1–33. [Google Scholar]
- He, J.; Wu, X.; Li, J.Y.; Huang, Q.; Huang, Z.H.; Cheng, Y.X. Comparison of the culture performance and profitability of wild-caught and captive pond-reared Chinese mitten crab (Eriocheir sinensis) juveniles reared in grow-out ponds: Implications for seed selection and genetic selection programs. Aquaculture 2014, 434, 48–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Liu, Y.; Geng, X.; Wang, X.; Wang, Y.; Sun, J.; Yang, W. Molt-inhibiting hormone from Chinese mitten crab (Eriocheir sinensis): Cloning, tissue expression and effects of recombinant peptide on ecdysteroid secretion of YOs. Gen. Comp. Endocrinol. 2011, 173, 467–474. [Google Scholar] [CrossRef]
- Chen, H.; Gu, X.; Zeng, Q.; Mao, Z.; Liang, X.; Martyniuk, C.J. Carbamazepine disrupts molting hormone signaling and inhibits molting and growth of Eriocheir sinensis at environmentally relevant concentrations. Aquat. Toxicol. 2019, 208, 138–145. [Google Scholar] [CrossRef]
- Li, C.; Huang, L.; Zhang, Y.; Guo, X.; Cao, N.; Yao, C.; Duan, L.; Li, X.; Pang, S. Effects of triazole plant growth regulators on molting mechanism in Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2022, 131, 646–653. [Google Scholar] [CrossRef]
- Söderhäll, K.; Smith, V.J. Separation of the haemocyte populations of Carcinus maenas and other marine decapods, and prophenoloxidase distribution. Dev. Comp. Immunol. 1983, 7, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yi, Q.; Lian, X.; Xu, S.; Yang, C.; Sun, J.; Wang, L.; Song, L. The involvement of ecdysone and ecdysone receptor in regulating the expression of antimicrobial peptides in Chinese mitten crab, Eriocheir sinensis. Dev. Comp. Immunol. 2020, 111, 103757. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, C.; Xu, Q.; Qu, C.; Sun, J.; Huang, S.; Kong, N.; Lv, X.; Liu, Z.; Wang, L.; et al. Beclin-1 is involved in the regulation of antimicrobial peptides expression in Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2019, 89, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Liu, H.; Wang, H.S.; Cao, M.T.; Meng, X.J.; Xiang, Y.L.; Zhang, Y.Q.; Shu, F.; Zhang, Q.G.; Shan, H.; et al. Histone deacetylase inhibitors promote epithelial-mesenchymal transition in Hepatocellular Carcinoma via AMPK-FOXO1-ULK1 signaling axis-mediated autophagy. Theranostics 2020, 10, 10245–10261. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fan, S.; Wang, D.; Huyan, T.; Chen, J.; Chen, J.; Su, J.; Li, X.; Wang, Z.; Xie, S.; et al. FOXO1 inhibition potentiates endothelial angiogenic functions in diabetes via suppression of ROCK1/Drp1-mediated mitochondrial fission. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2481–2494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Qin, Y.; Song, Y.; Zhao, K.; Pan, W.; Nan, X.; Wang, Y.; Wang, Q.; Li, W. A novel Ig domain-containing C-type lectin triggers the intestine-hemocyte axis to regulate antibacterial immunity in crab. J. Immunol. 2022, 208, 2343–2362. [Google Scholar] [CrossRef]
- Bulut-Karslioglu, A.; Biechele, S.; Jin, H.; Macrae, T.A.; Hejna, M.; Gertsenstein, M.; Song, J.S.; Ramalho-Santos, M. Inhibition of mTOR induces a paused pluripotent state. Nature 2016, 540, 119–123. [Google Scholar] [CrossRef]
- Abuhagr, A.M.; MacLea, K.S.; Mudron, M.R.; Chang, S.A.; Chang, E.S.; Mykles, D.L. Roles of mechanistic target of rapamycin and transforming growth factor-β signaling in the molting gland (Y-organ) of the blackback land crab, Gecarcinus lateralis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 198, 15–21. [Google Scholar] [CrossRef]
- Zhao, C.; Peng, C.; Wang, P.; Yan, L.; Fan, S.; Qiu, L. Identification of a Shrimp E3 Ubiquitin Ligase TRIM50-like involved in restricting White Spot Syndrome virus proliferation by its mediated autophagy and ubiquitination. Front. Immunol. 2021, 12, 682562. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Yue, W.; Huang, S.; Chen, J.; Chen, Y.; Wang, C. Structure and function of the alternatively spliced isoforms of the ecdysone receptor gene in the Chinese mitten crab, Eriocheir sinensis. Sci. Rep. 2017, 7, 12993. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, S.; Zhang, M.; Gong, Y.; Ma, H.; Tran, N.T.; Zhang, Y.; Li, S. SpRab11a-regulated exosomes inhibit bacterial infection through the activation of antilipopolysaccharide factors in crustaceans. J. Immunol. 2022, 209, 710–722. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Z.; Li, J.; Ma, Y.; Tu, Y.; Zeng, X.; Wang, Q.; Jiang, Y.; Huang, S.; Yi, Q. The involvement of hypoxia inducible factor-1α on the proportion of three types of haemocytes in Chinese mitten crab under hypoxia stress. Dev. Comp. Immunol. 2023, 140, 104598. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Su, S.; Tang, Y.; Xu, G.; Li, J.; Yu, F.; Li, H.; Song, C.; Liang, M.; et al. Comparative transcriptome analysis on the regulatory mechanism of thoracic ganglia in Eriocheir sinensis at post-molt and inter-molt Stages. Life 2022, 12, 1181. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Coffer, P.J. FOXO-binding partners: It takes two to tango. Oncogene 2008, 27, 2289–2299. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Huang, G.; Zhu, P.; Liu, J.; Ye, B.; Du, Y.; Fan, Z. FoxO1-mediated autophagy is required for NK cell development and innate immunity. Nat. Commun. 2016, 7, 11023. [Google Scholar] [CrossRef]
- Iyer, S.; Ambrogini, E.; Bartell, S.M.; Han, L.; Roberson, P.K.; de Cabo, R.; Jilka, R.L.; Weinstein, R.S.; O’Brien, C.A.; Manolagas, S.C.; et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Investig. 2013, 123, 3409–3419. [Google Scholar] [CrossRef]
- Barbieri, M.; Bonafè, M.; Franceschi, C.; Paolisso, G. Insulin/IGF-I-signaling pathway: An evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1064–E1071. [Google Scholar] [CrossRef]
- Park, D.; Hahm, J.H.; Park, S.; Ha, G.; Chang, G.E.; Jeong, H.; Kim, H.; Kim, S.; Cheong, E.; Paik, Y.K. A conserved neuronal DAF-16/FoxO plays an important role in conveying pheromone signals to elicit repulsion behavior in Caenorhabditis elegans. Sci. Rep. 2017, 7, 7260. [Google Scholar] [CrossRef]
- Obsil, T.; Obsilova, V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 2008, 27, 2263–2275. [Google Scholar] [CrossRef]
- Mazet, F.; Yu, J.K.; Liberles, D.A.; Holland, L.Z.; Shimeld, S.M. Phylogenetic relationships of the Fox (Forkhead) gene family in the Bilateria. Gene 2003, 316, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Marshall, C.B.; Yamamoto, K.; Li, G.Y.; Plevin, M.J.; You, H.; Mak, T.W.; Ikura, M. Biochemical and structural characterization of an intramolecular interaction in FOXO3a and its binding with p53. J. Mol. Biol. 2008, 384, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, A.; Li, S.; Wang, G.; Ye, H. Hepatopancreas immune response during molt cycle in the mud crab, Scylla paramamosain. Sci. Rep. 2020, 10, 13102. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ni, H.; Zhang, X.; Sun, Q.; Wu, X.; He, J. Comparative transcriptomics reveals the immune dynamics during the molting cycle of swimming crab Portunus trituberculatus. Front. Immunol. 2022, 13, 1037739. [Google Scholar] [CrossRef]
- Hosaka, T.; Biggs, W.H.; Tieu, D.; Boyer, A.D.; Varki, N.M.; Cavenee, W.K.; Arden, K.C. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. USA 2004, 101, 2975–2980. [Google Scholar] [CrossRef]
- Lin, L.; Hron, J.D.; Peng, S.L. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 2004, 21, 203–213. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, Q.; Peng, Y.; Zhang, Q.J.; Castrillon, D.H.; DePinho, R.A.; Liu, Z.P. FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology 2009, 137, 1403–1414. [Google Scholar] [CrossRef]
- Salih, D.A.M.; Rashid, A.J.; Colas, D.; de la Torre-Ubieta, L.; Zhu, R.P.; Morgan, A.A.; Santo, E.E.; Ucar, D.; Devarajan, K.; Cole, C.J.; et al. FoxO6 regulates memory consolidation and synaptic function. Genes Dev. 2012, 26, 2780–2801. [Google Scholar] [CrossRef]
- Panganiban, G.; Sebring, A.; Nagy, L.; Carroll, S. The development of crustacean limbs and the evolution of arthropods. Science 1995, 270, 1363–1366. [Google Scholar] [CrossRef]
- Hopkins, P.M.; Chung, A.C.-K.; Durica, D.S. Limb Regeneration in the Fiddler Crab, Uca pugilator: Hormonal and Growth Factor Control. Am. Zool. 2001, 41, 389–398. [Google Scholar] [CrossRef]
- Morris, S.; Postel, U.; Mrinalini; Turner, L.M.; Palmer, J.; Webster, S.G. The adaptive significance of crustacean hyperglycaemic hormone (CHH) in daily and seasonal migratory activities of the Christmas Island red crab Gecarcoidea natalis. J. Exp. Biol. 2010, 213 Pt 17, 3062–3073. [Google Scholar] [CrossRef]
- Jung, H.; Lyons, R.E.; Hurwood, D.A.; Mather, P.B. Genes and growth performance in crustacean species: A review of relevant genomic studies in crustaceans and other taxa. Rev. Aquacult. 2013, 5, 77–110. [Google Scholar] [CrossRef]
- Li, K.; Jia, Q.Q.; Li, S. Juvenile hormone signaling—A mini review. Insect. Sci. 2019, 26, 600–606. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, N.; Jiang, H.; Meng, X.; Ge, H.; Yang, X.; Xu, X.; Qian, K.; Park, Y.; Zheng, Y.; et al. 20-hydroxyecdysone regulates expression of methioninesulfoxide reductases through transcription factor FOXO in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2021, 131, 103546. [Google Scholar] [CrossRef]
- Yu, F.; Wei, R.; Yang, J.; Liu, J.; Yang, K.; Wang, H.; Mu, Y.; Hong, T. FoxO1 inhibition promotes differentiation of human embryonic stem cells into insulin producing cells. Exp. Cell Res. 2018, 362, 227–234. [Google Scholar] [CrossRef]
- Zou, P.; Liu, L.; Zheng, L.; Liu, L.; Stoneman, R.E.; Cho, A.; Emery, A.; Gilbert, E.R.; Cheng, Z. Targeting FoxO1 with AS1842856 suppresses adipogenesis. Cell Cycle 2014, 13, 3759–3767. [Google Scholar] [CrossRef]
- Durica, D.S.; Arthur, C.-K.C.; Hopkins, P.M. Characterization of EcR and RXR gene homologs and receptor expression during the molt cycle in the crab, Uca pugilator. Am. Zool. 1999, 39, 758–773. [Google Scholar] [CrossRef]
- Riddiford, L.M.; Hiruma, K.; Zhou, X.; Nelson, C.A. Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem. Mol. Biol. 2003, 33, 1327–1338. [Google Scholar] [CrossRef]
- Koyama, T.; Rodrigues, M.A.; Athanasiadis, A.; Shingleton, A.W.; Mirth, C.K. Nutritional control of body size through FoxO-Ultraspiracle mediated ecdysone biosynthesis. eLife 2014, 3, e03091. [Google Scholar] [CrossRef]
- Mirth, C.K.; Tang, H.Y.; Makohon-Moore, S.C.; Salhadar, S.; Gokhale, R.H.; Warner, R.D.; Koyama, T.; Riddiford, L.M.; Shingleton, A.W. Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, 7018–7023. [Google Scholar] [CrossRef] [PubMed]
- Abuhagr, A.M.; Maclea, K.S.; Chang, E.S.; Mykles, D.L. Mechanistic target of rapamycin (mTOR) signaling genes in decapod crustaceans: Cloning and tissue expression of mTOR, Akt, Rheb, and p70 S6 kinase in the green crab, Carcinus maenas, and blackback land crab, Gecarcinus lateralis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 168, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Goldbraikh, D.; Neufeld, D.; Eid-Mutlak, Y.; Lasry, I.; Gilda, J.E.; Parnis, A.; Cohen, S. USP1 deubiquitinates Akt to inhibit PI3K-Akt-FoxO signaling in muscle during prolonged starvation. EMBO Rep. 2020, 21, e48791. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Gupta, A.; Stocker, H. FoxO restricts growth and differentiation of cells with elevated TORC1 activity under nutrient restriction. PLoS Genet. 2018, 14, e1007347. [Google Scholar] [CrossRef]
- Benjamin, D.; Colombi, M.; Moroni, C.; Hall, M.N. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011, 10, 868–880. [Google Scholar] [CrossRef]
- Kemirembe, K.; Liebmann, K.; Bootes, A.; Smith, W.A.; Suzuki, Y. Amino acids and TOR signaling promote prothoracic gland growth and the initiation of larval molts in the tobacco hornworm Manduca sexta. PLoS ONE 2012, 7, e44429. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Q.Y.; Fu, K.Y.; Guo, W.C.; Li, G.Q. RNA interference against the putative insulin receptor substrate gene chico affects metamorphosis in Leptinotarsa decemlineata. Insect. Biochem. Mol. Biol. 2018, 103, 1–11. [Google Scholar] [CrossRef]


| Primer Name | Sequences (5′–3′) | Tm (°C) | Size (bp) | Efficiency (%) |
|---|---|---|---|---|
| Cloning primers | ||||
| EsFOXO-F | ATGACAAGTTTCTTCTCGCT | 52.5 | 852 | |
| EsFOXO-R | CTAGAGCAGGGGCAGGGG | 62.3 | ||
| qRT-PCR primers | ||||
| EsFOXO-like-F | GGCTACGTGGAGAGCGAGGA | 60.6 | 142 | 99% |
| EsFOXO-like-R | CCTGGGCGATCAGGTCTGC | 60.0 | ||
| EsEcR-F | GAGAGAACAGAAAAAGGCACGA | 57.3 | 105 | 102% |
| EsEcR-R | ATGGCTGACATTGGACTAATGG | 58.8 | ||
| EsMIH-F | TGAAGACTGCGCCAACATCT | 56.2 | 90 | 103% |
| EsMIH-R | CGTGAGGTCGTCCTTCTGTG | 60.4 | ||
| EsRXR-F | AGGCTTCAGGTTCCACTCGC | 58.3 | 131 | 98% |
| EsRXR-R | GTGTACGCTGCCCTGGAGGA | 60.1 | ||
| EsmTOR-F | CTTGAGGAGTTCTACCCTGCGT | 60.5 | 115 | 101% |
| EsmTOR-R | GGACCACCTGGGCAAGGTAT | 56.9 | ||
| Esβ-actin-F | GCATCCACGAGACCACTTACA | 56.4 | 223 | 102% |
| Esβ-actin-R | CTCCTGCTTGCTGATCCACATC | 60.1 | ||
| RNAi primers | ||||
| EGFP-RNAi-F | TAATACGACTCACTATAGGGCGACGTAAACGGCCACAAGT | |||
| EGFP-RNAi-R | TAATACGACTCACTATAGGGCTTGTACAGCTCGTCCATGC | |||
| EsFOXO-RNAi-F | TAATACGACTCACTATAGGGATGACAAGTTTCTTCTCGCTGGTGA | |||
| EsFOXO-RNAi-R | TAATACGACTCACTATAGGGATCAGGTCTGCATAGGACATGTTGC | |||
| Species | Gene Name | Accession Number | Query Cover % | Identity % |
|---|---|---|---|---|
| Azumapecten farreri | FOXO-like protein | QFR 39803.1 | 43 | 53.66 |
| Crassostrea gigas | forkhead box protein O | XM 011416057.3 | 43 | 60.16 |
| Mytilus coruscus | FOXO3 | CAC 5392548.1 | 43 | 57.03 |
| Sepia pharaonis | FOXO3 | CAE 1327826.1 | 39 | 60.53 |
| Asterias rubens | forkhead box protein O3-like | XP 033635399.1 | 43 | 54.48 |
| Strongylocentrotus purpuratus | forkhead transcription factor O | DQ 286746.2 | 43 | 49.30 |
| Dendroctonus ponderosae | forkhead box protein O isoform X3 | XP 048524044.1 | 52 | 51.23 |
| Sitophilus oryzae | forkhead box protein O isoform X2 | XP 030755757.1 | 50 | 59.17 |
| Drosophila melanogaster | forkhead box, sub-group O isoform C | NP 996204.1 | 42 | 55.37 |
| Spodoptera frugiperda | forkhead box protein O-like isoform X2 | XP 035439085.1 | 44 | 54.33 |
| Colias croceus | forkhead box protein O isoform X1 | XP 045494104.1 | 54 | 47.67 |
| Penaeus vannamei | forkhead box protein O-like isoform X2 | XP 027228067.1 | 40 | 75.00 |
| Penaeus monodon | forkhead box protein O4-like | XP 037792098.1 | 40 | 88.37 |
| Portunus trituberculatus | Forkhead box protein O | MPC 39137.1 | 42 | 88.55 |
| Penaeus japonicus | Fork box protein O | MW080526.1 | 67 | 83.51 |
| Clupea harengus | forkhead box protein O1-B-like | XP 031428678.1 | 30 | 83.33 |
| Salvelinus alpinus | forkhead box protein O1 | XP 023824765.1 | 45 | 85.45 |
| Orcinus orca | forkhead box protein O6 | XP 033277871.1 | 43 | 52.67 |
| Oryx dammah | forkhead box protein O6 | XP 040087454.1 | 43 | 52.67 |
| Homo sapiens | forkhead box protein O6 | NP 001278210.2 | 43 | 49.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, Y.; Yang, Z.; Wang, F.; Li, J.; Jiang, Y.; Yang, D.; Yi, Q.; Huang, S. FOXO-like Gene Is Involved in the Regulation of 20E Pathway through mTOR in Eriocheir sinensis. J. Mar. Sci. Eng. 2023, 11, 1225. https://doi.org/10.3390/jmse11061225
Li J, Ma Y, Yang Z, Wang F, Li J, Jiang Y, Yang D, Yi Q, Huang S. FOXO-like Gene Is Involved in the Regulation of 20E Pathway through mTOR in Eriocheir sinensis. Journal of Marine Science and Engineering. 2023; 11(6):1225. https://doi.org/10.3390/jmse11061225
Chicago/Turabian StyleLi, Jiaming, Yuhan Ma, Zhichao Yang, Fengchi Wang, Jialin Li, Yusheng Jiang, Dazuo Yang, Qilin Yi, and Shu Huang. 2023. "FOXO-like Gene Is Involved in the Regulation of 20E Pathway through mTOR in Eriocheir sinensis" Journal of Marine Science and Engineering 11, no. 6: 1225. https://doi.org/10.3390/jmse11061225
APA StyleLi, J., Ma, Y., Yang, Z., Wang, F., Li, J., Jiang, Y., Yang, D., Yi, Q., & Huang, S. (2023). FOXO-like Gene Is Involved in the Regulation of 20E Pathway through mTOR in Eriocheir sinensis. Journal of Marine Science and Engineering, 11(6), 1225. https://doi.org/10.3390/jmse11061225





