Assessment of Risks Associated with Extreme Climate Events in Small-Scale Bivalve Fisheries: Conceptual Maps for Decision-Making Based on a Review of Recent Studies
Abstract
1. Introduction
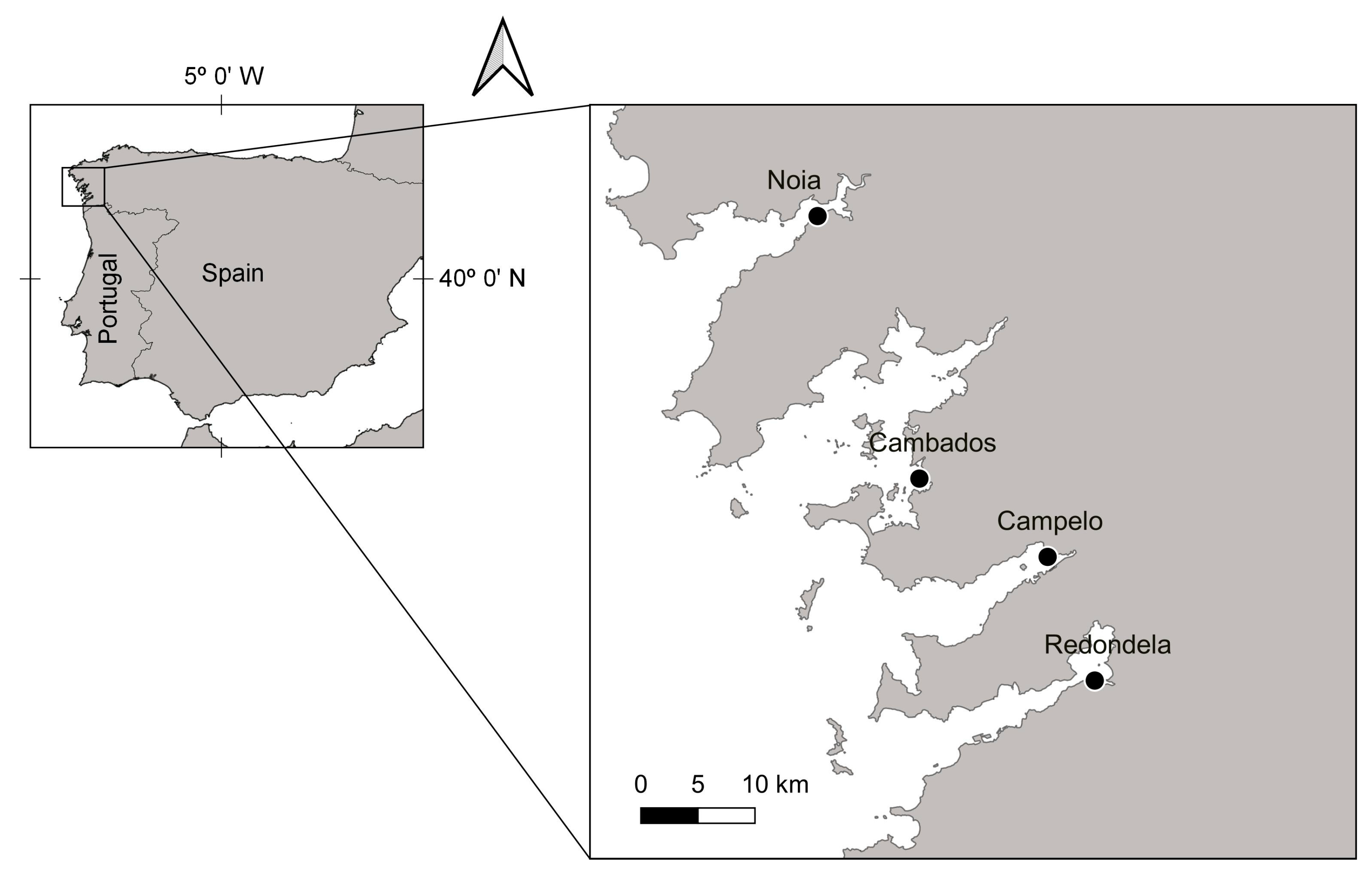
2. Materials and Methods
3. Results: Conceptual Maps for Fisheries Management
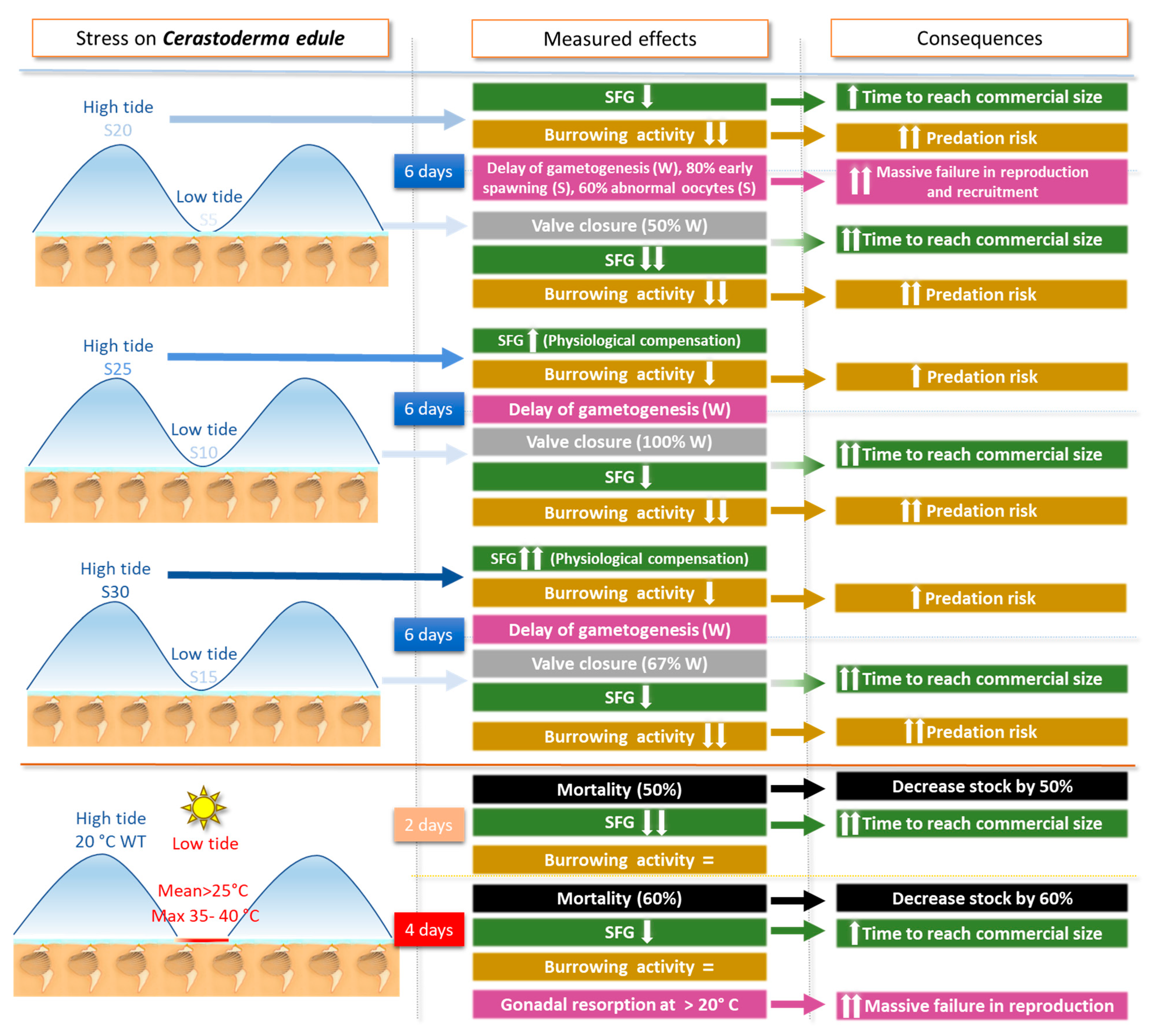
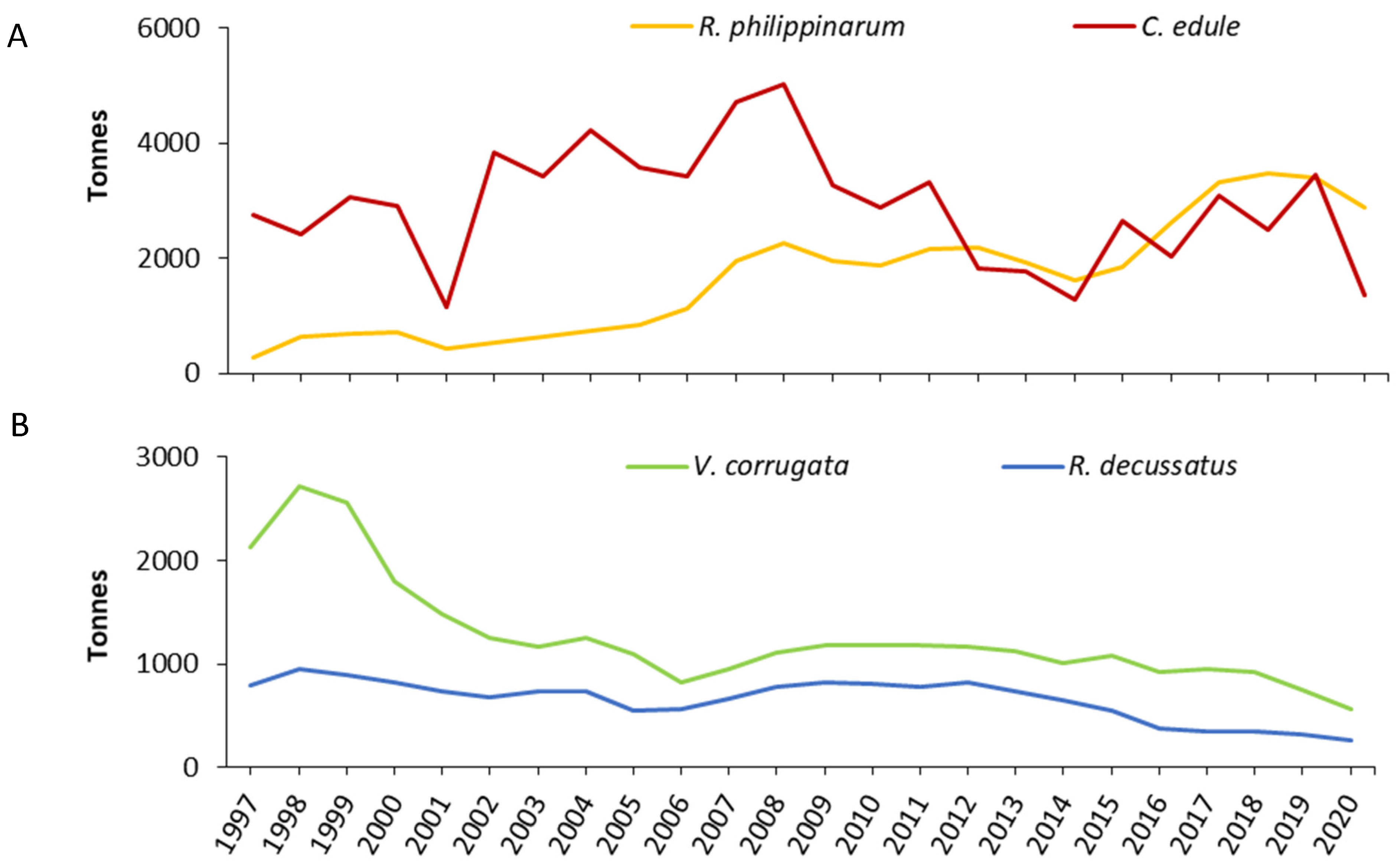
3.1. Results for Cerastoderma edule
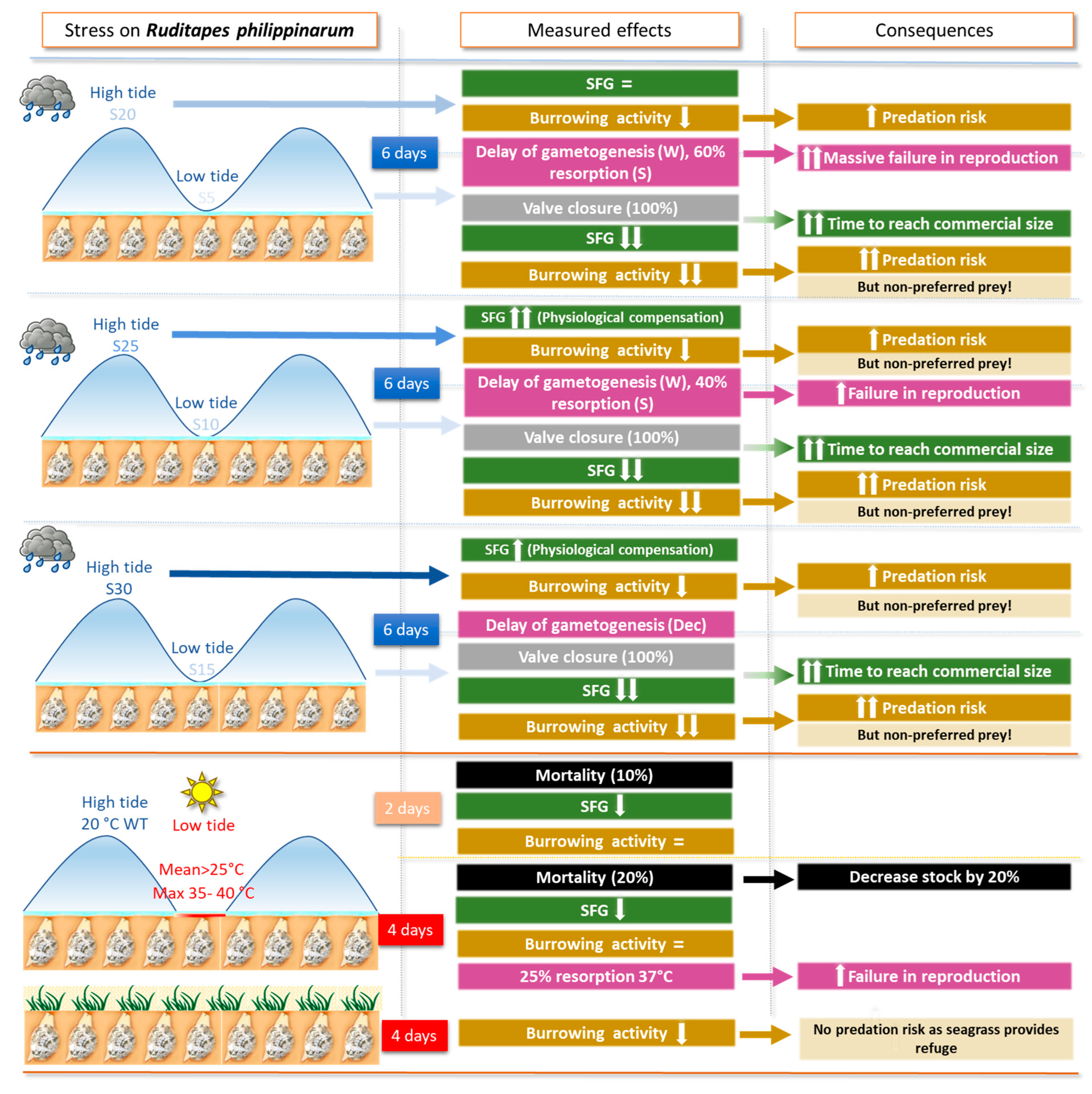
3.2. Results for Ruditapes philippinarum
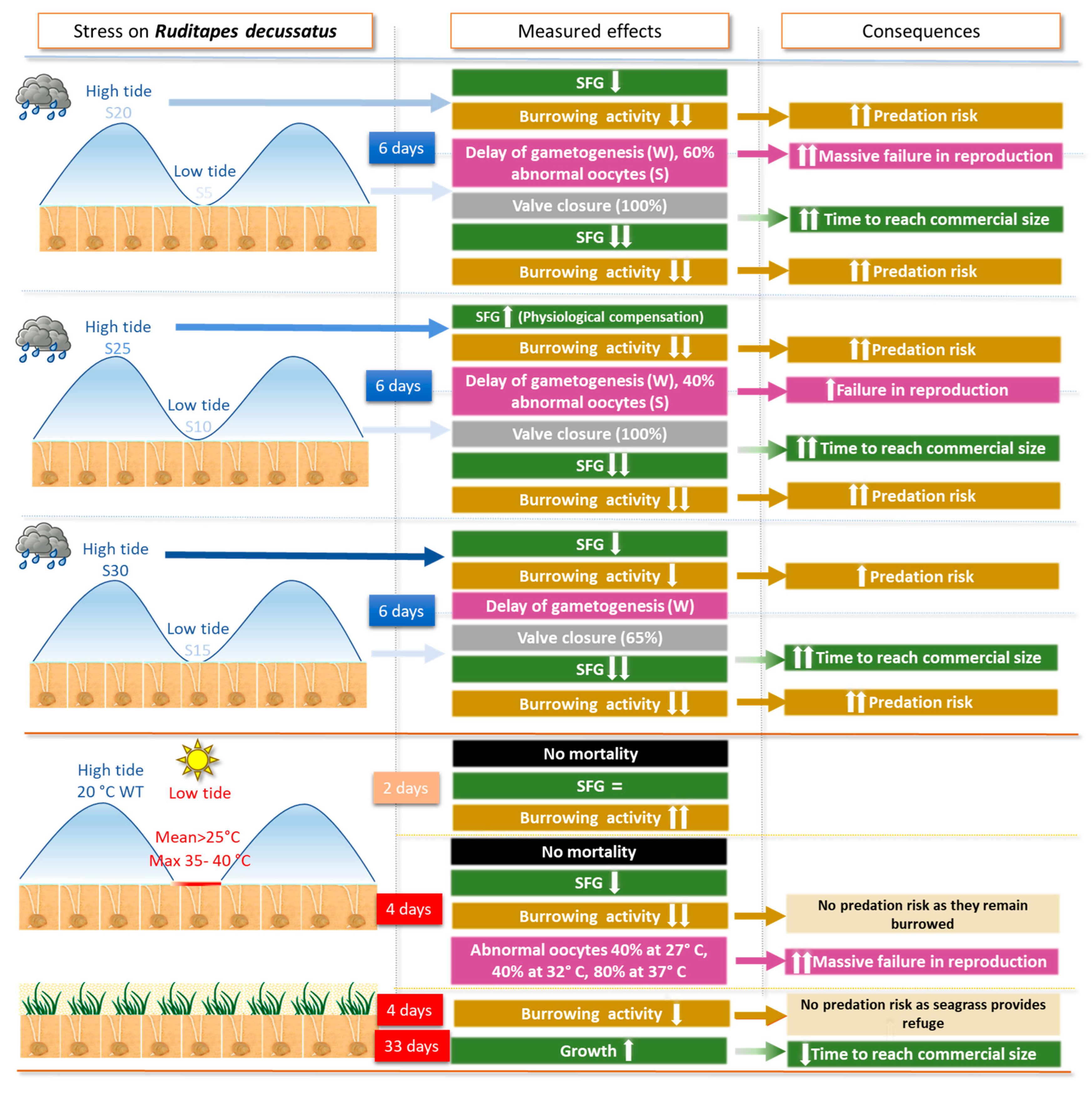
3.3. Results for Ruditapes decussatus
3.4. Results for Venerupis corrugata
4. Discussion: Consequences for Shellfisheries
5. Conclusions: Recommendations for Shellfishery Management
- Habitats occupied by shellfish beds should be characterized to determine the potential effects of climatic factors and anthropogenic stress. This can be done by:
- (i).
- Continuous automatic monitoring of physicochemical variables, such as water and sediment temperatures and salinity within the fishing beds. Although INTECMAR manages more than 50 oceanographic stations along the Galician coast (www.intecmar.gal/informacion/fito/estacions/, accessed on 1 May 2023), only five of the stations record data continuously, and sediment temperature is not recorded at any of the stations. This type of data could be used in local-scale modelling to create a warning system for each species, such as that automatically running for the Miño River [96]. In this system an alert is issued, including detailed hazards maps, which help decision makers to take precise and effective mitigation measures.
- (ii).
- Including other relevant variables, such as primary productivity in the models and maps [97]. With available information on food, coupled with environmental conditions, the critical conditions for each species could be predicted, thereby helping management decisions to be made on the basis of the environmental forecasts.
- (iii).
- Intensifying monitoring of shellfish stocks by fisher’s guilds (e.g., [98]) and standardizing and sharing field data to enhance the quality of the research. Although the fisheries authority has promoted surveys to solve specific situations that may place the fisheries at risk [29,35,84], available information about the catches of the Galician shellfisheries is not sufficient to verify the impact of climate change [24,99], since the shellfisheries are often influenced by management decisions driven by economic factors [82].
- (iv).
- Conducting experiments can be very useful for understanding species responses. Field experiments, such as those conducted by [28] in the inner parts of Ría de Arousa and Pontevedra, and those conducted by [21,22,102] in the Bay of Santander and by [70] in the Basque Country, are examples of what can be done to understand the dynamics in each area. The findings can also be useful for establishing baseline levels of natural mortality for each species to determine when exogenous factors should be considered a threat to the population or include them in an experimental design. Laboratory experiments, such as those summarized here, serve to accurately test the effects of main stressors on metrics, such as scope for growth, valve closure activity, burrowing activity, gametogenic cycle and molecular responses. Based on the results of the experiments presented, new designs can be developed, including other stress factors (e.g., pH, phytoplankton, see [103,104]), different duration or intensity of the stress events, or measuring different response variables (e.g., condition index, valve closure strength). The effects of synergistic or antagonistic interactions between environmental stressors should also be considered [28,105,106,107,108].
- Models including experimental data can be coupled with environmental data from different sources to generate precise predictions. Hindcasting models were developed to predict changes in the distribution of intertidal species related to temperature [109,110]. A recent study developed hindcasting and forecasting models by coupling hydrological models (based on precipitation), hydrodynamics (river outflow and shellfish bed salinity), and finally, by including bivalve mortality based on lethal physiological thresholds of the species [38]. This could also be done by including sublethal effects.
- Diversification of cultured species could diminish the risk of loss due to high mortality rates as well as overexploitation of and damage to habitats [111]. The global risk model developed by [112] shows that fisheries in Spain are at high risk due to a strong economic dependence, low diversification of cultured species and vulnerability to increased temperatures. These researchers globally identified the year 2060 as a tipping point for shellfish production. In light of the experimental results and characterization of the conditions in different shellfish beds, the most appropriate species for each habitat could be cultured, thus maintaining diversity.
- Protection for shellfish beds from contamination and human pressure (dams, industrial or agricultural effluents, artificial barriers, water depuration plants, etc.), including control of river outflows and management plans for catastrophic events, to reduce the impacts that commonly threaten shellfishery production in Galicia [113]. The creation of marine reserves and other protective figures is contemplated in the present legislation [114], although with a low degree of success to date [78].
- Monitoring the presence of invasive predator species on the shellfish beds [115], as such species could have unknown effects on more vulnerable species.
- Management plans, including conservation and maintenance of seagrasses patches within shellfish beds, to help sustainability and mitigation of climate change effects on harvested species.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orensanz, J.M.; Parma, A.; Jerez, G.; Barahona, N.; Montecinos, M.; Elias, I. What Are the Key Elements for the Sustainability of “S-Fisheries”? Insights from South America. Bull. Mar. Sci. 2005, 76, 527–556. [Google Scholar]
- Villasante, S.; Macho, G.; Silva, M.R.O.; Lopes, P.F.M.; Pita, P.; Simón, A.; Balsa, J.C.M.; Olabarria, C.; Vázquez, E.; Calvo, N. Resilience and Social Adaptation to Climate Change Impacts in Small-Scale Fisheries. Front. Mar. Sci. 2022, 9, 802762. [Google Scholar] [CrossRef]
- Villasante, S.; Tubío, A.; Gianelli, I.; Pita, P.; García-Allut, A. Ever Changing Times: Sustainability Transformations of Galician Small-Scale Fisheries. Front. Mar. Sci. 2021, 8, 712819. [Google Scholar] [CrossRef]
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global Production of Marine Bivalves. Trends and Challenges. In Goods and Services of Marine Bivalves; Smaal, A., Ferreira, J., Grant, J., Petersen, J., Strand, Ø., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Macho, G.; Woodin, S.A.; Wethey, D.S.; Vázquez, E. Impacts of Sublethal and Lethal High Temperatures on Clams Exploited in European Fisheries. J. Shellfish Res. 2016, 35, 405–419. [Google Scholar] [CrossRef]
- Volkenborn, N.; Woodin, S.A.; Wethey, D.S.; Polerecky, L. Bioirrigation. In Encyclopedia of Ocean Sciences; Elsevier: Amsterdam, The Netherlands, 2019; pp. 663–670. ISBN 978-0-12-813082-7. [Google Scholar]
- Chennu, A.; Volkenborn, N.; Beer, D.; Wethey, D.; Woodin, S.; Polerecky, L. Effects of Bioadvection by Arenicola Marina on Microphytobenthos in Permeable Sediments. PLoS ONE 2015, 10, e0134236. [Google Scholar] [CrossRef] [PubMed]
- McLeod, R.J.; Wing, S. Influence of an Altered Salinity Regime on the Population Structure of Two Infaunal Bivalve Species. Est. Coast. Shelf Sci. 2008, 78, 529–540. [Google Scholar] [CrossRef]
- FAO. FishStatJ: Universal Software for Fishery Statistical Time Series; FAO, Statistics and Information Service: Rome, Italy, 2012. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- Van Gils, J.A.; Piersma, T.; Dekinga, A.; Spaans, B.; Kraan, C. Shellfish Dredging Pushes a Flexible Avian Top Predator out of a Marine Protected Area. PLoS Biol. 2006, 4, e376. [Google Scholar] [CrossRef]
- Beukema, J.J.; Dekker, R. Decline of Recruitment Success in Cockles and Other Bivalves in the Wadden Sea: Possible Role of Climate Change, Predation on Postlarvae and Fisheries. Mar. Ecol. Progr. Ser. 2005, 287, 149–167. [Google Scholar] [CrossRef]
- Piñeiro-Antelo, M.; Santos, X.M. Shellfishing on Foot and the Road to Defeminization in Galicia (Spain). Marit. Stud. 2021, 20, 341–354. [Google Scholar] [CrossRef]
- Molares, J.; Freire, J. Development and Perspectives for Community-Based Management of the Goose Barnacle (Pollicipes Pollicipes) Fisheries in Galicia (NW Spain). Fish Res. 2003, 65, 485–492. [Google Scholar] [CrossRef]
- Pita, P.; Fernández-Márquez, D.; Antelo, M.; Macho, G.; Villasante, S. Social-Ecological Changes in Data-Poor S-Fisheries: A Hidden Shellfisheries Crisis in Galicia (NW Spain). Mar. Policy 2019, 101, 208–224. [Google Scholar] [CrossRef]
- Pita, P.; García-Allut, A.; Villasante, S. The role of marine stakeholders in the co-production of scientific knowledge: Lessons from Galicia (NW Spain). In Marine Species Interfering with Human Activities–Scientific vs Stakeholders’ Perceptions; CIESM Workshop Monograph n° 50; Briand, F., Ed.; CIESM Publisher: Paris, Monaco, 2018; p. 218. [Google Scholar]
- Frangoudes, K.; Marugán-Pintos, B.; Pascual-Fernández, J.J. From Open Access to Co-Governance and Conservation: The Case of Women Shellfish Collectors in Galicia (Spain). Mar. Policy 2008, 32, 223–232. [Google Scholar] [CrossRef]
- García-Flórez, L.; Morales, J.; Gaspar, M.B.; Castilla, D.; Mugerza, E. A Novel and Simple Approach to Define Artisanal Fisheries in Europe. Mar. Policy 2014, 44, 152–159. [Google Scholar] [CrossRef]
- Drummond, L.; Mulcahy, M.; Culloty, S. The Reproductive Biology of the Manila Clam, Ruditapes Philippinarum, from the North-West of Ireland. Aquaculture 2006, 254, 326–340. [Google Scholar] [CrossRef]
- Bidegain, G.; Juanes, J.A. Does Expansion of the Introduced Manila Clam Ruditapes Philippinarum Cause Competitive Displacement of the European Native Clam Ruditapes Decussatus? J. Exp. Mar. Biol. Ecol. 2013, 445, 44–52. [Google Scholar] [CrossRef]
- Bidegain, G.; Bárcena, J.F.; García, A.; Juanes, J.A. Predicting Coexistence and Predominance Patterns between the Introduced Manila Clam (Ruditapes philippinarum) and the European Native Clam (Ruditapes decussatus). Est. Coast. Shelf Sci. 2015, 152, 162–172. [Google Scholar] [CrossRef]
- Juanes, J.A.; Bidegain, G.; Echavarri-Erasun, B.; Puente, A.; García, A.; García, A.; Bárcena, J.F.; Álvarez, C.; García-Castillo, G. Differential Distribution Pattern of Native Ruditapes decussatus and Introduced Ruditapes phillippinarum Clam Populations in the Bay of Santander (Gulf of Biscay): Considerations for Fisheries Management. Ocean Coast. Manag. 2012, 69, 316–326. [Google Scholar] [CrossRef]
- Sobral, P.; Widdows, J. Effects of Elevated Temperatures on the Scope for Growth and Resistance to Air Exposure of the Clam Ruditapes decussatus (L.) from southern Portugal. Sci. Mar. 1997, 61, 163–171. [Google Scholar] [CrossRef]
- Molares, J.; Parada, J.M.; Navarro-Pérez, E.; Fernández, A. Variabilidad interanual de las ventas de los principales recursos marisqueros de Galicia y su relación con las condiciones ambientales. Rev. Galega Rec. Mar. 2008, 2, 1–42. [Google Scholar]
- Parada, J.M.; Molares, J.; Otero, X. Multispecies Mortality Patterns of Commercial Bivalves in Relation to Estuarine Salinity Fluctuation. Est. Coasts 2012, 35, 132–142. [Google Scholar] [CrossRef]
- Parada, J.M.; Molares, J. Natural Mortality of the Cockle Cerastoderma edule (L.) from the Ria of Arousa (NW Spain) Intertidal Zone. Rev. Biol. Mar. Oceanogr. 2008, 43, 501–511. [Google Scholar] [CrossRef]
- Verdelhos, T.; Marques, J.C.; Anastácio, P. The Impact of Estuarine Salinity Changes on the Bivalves Scrobicularia plana and Cerastoderma edule, Illustrated by Behavioral and Mortality Responses on a Laboratory Assay. Ecol. Ind. 2015, 52, 96–104. [Google Scholar] [CrossRef]
- Aranguren, R.; Gomez-León, J.; Balseiro, P.; Costa, M.M.; Novoa, B.; Figueras, A. Abnormal Mortalities of the Carpet Shell Clam Ruditapes decussatus (Linnaeus 1756) in Natural Bed Populations: A Practical Approach. Aquacult. Res. 2014, 45, 1303–1310. [Google Scholar] [CrossRef]
- Parada, J.M.; Molares, J.; Sánchez-Mata, A.; Martínez, G.; Darriba, C.; Mariño, J. Plan de actuación para la recuperación del banco “Lombos do Ulla”: Campañas marisqueras desde 2002 a 2005. Rev. Galega Rec. Mar. 2006, 1, 1–37. [Google Scholar]
- Parada, J.M.; Molares, J.; Sánchez-Mata, A.; Martínez, G.; Darriba, C.; Mariño, J. Temperatura y salinidad en el banco marisquero de “Lombos do Ulla” (Ría de Arousa. Galicia) entre 2002 y 2006: Datos diarios. Rev. Galega Rec. Mar. Datos. 2007, 1, 226. [Google Scholar]
- IPCC. Summary for Policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Geneva, Switzerland, 2019; pp. 131–202. [Google Scholar]
- Viceto, C.; Cardoso Pereira, S.; Rocha, A. Climate Change Projections of Extreme Temperaturas for the Iberian Peninsula. Atmosphere 2019, 10, 229. [Google Scholar] [CrossRef]
- Cardoso-Pereira, S.; Marta-Almeida, M.; Carvalho, A.C.; Rocha, A. Extreme Precipitation Events under Climate Change in the Iberian Peninsula. Int. J. Clim. 2020, 40, 1255–1278. [Google Scholar] [CrossRef]
- Lorenzo, M.N.; Alvarez, I. Climate Change Patterns in Precipitation over Spain Using CORDEX Projections for 2021–2050. Sci. Total Environ. 2020, 723, 138024. [Google Scholar] [CrossRef]
- Villalba, A.; Iglesias, D.; Ramilo, A.; Darriba, S.; Parada, J.M.; No, E.; Abollo, E.; Molares, J.; Carballal, M.J. Cockle Cerastoderma edule Fishery Collapse in the Ría de Arousa (Galicia, Nw Spain) Associated with the Protistan Parasite Marteilia Cochilli. Dis. Aquat. Organ 2014, 109, 55–80. [Google Scholar] [CrossRef]
- Parada, J.M.; Miranda, M.; Martínez, G.; Carreira, P.; Molares, J. Temperatura y salinidad en los bancos marisqueros de Placeres, Sarrido, Bohído y Lombos do Ulla entre 2006 y 2010. Rías de Pontevedra y Arousa. Galicia. Rev. Galega Rec. Mar. Datos 2011, 2, 1–201. [Google Scholar]
- Castro-Olivares, A.; Des, M.; Olabarria, C.; deCastro, M.; Vázquez, E.; Sousa, M.C.; Gómez-Gesteira, M. Does Global Warming Threaten Small-Scale Bivalve Fisheries in NW Spain? Mar. Environ. Res. 2022, 180, 105707. [Google Scholar] [CrossRef] [PubMed]
- Des, M.; Fernández-Nóvoa, D.; Gómez-Gesteira, J.L.; Sousa, M.C.; Gómez-Gesteira, M. Modeling Salinity Drop in Estuarine Areas under Extreme Precipitation Events within a Context of Climate Change: Effect on Bivalve Mortality in Galician Rías Baixas. Sci. Total Environ. 2021, 790, 148147. [Google Scholar] [CrossRef]
- Elliott, M.; Whitfield, A. Challenging paradigms in estuarine ecology and management. Est. Coast. Shelf Sci. 2011, 94, 306–314. [Google Scholar] [CrossRef]
- Akberali, H.B. Behaviour of Scrobicularia plana (Da Costa) in water of various salinites. J. Exp. Mar. Biol. Ecol. 1978, 33, 237–249. [Google Scholar] [CrossRef]
- Kim, W.; Huh, H.; Huh, S.H. Effects of Salinity on Endogenous Rhythm of the Manila Clam, Ruditapes Philippinarum (Bivalvia: Veneridae). Mar. Biol. 2001, 138, 157–162. [Google Scholar] [CrossRef]
- Gosling, E. Marine Bivalve Molluscs; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9780470674949. [Google Scholar] [CrossRef]
- Haider, F.; Sokolov, E.P.; Sokolova, I.M. Effects of Mechanical Disturbance and Salinity Stress on Bioenergetics and Burrowing Behavior of the Soft Shell Clam Mya arenaria. J. Exp. Biol. 2018, 221, 172643. [Google Scholar] [CrossRef]
- Shumway, S.E. The Effect of Fluctuating Salinity on the Tissue Water Content of Eight Species of Bivalve Molluscs. J. Comp. Physiol. B 1977, 116, 269–285. [Google Scholar] [CrossRef]
- Shumway, S.E. Effect of Salinity Fluctuation on the Osmotic Pressure and Na+, Ca2+ and Mg2+ Ion Concentrations in the Hemolymph of Bivalve Molluscs. Mar. Biol. 1977, 41, 153–177. [Google Scholar] [CrossRef]
- Navarro, J.M.; Gonzalez, C.M. Physiological Responses of the Chilean Scallop Argopecten Purpuratus to Decreasing Salinities. Aquaculture 1998, 167, 315–327. [Google Scholar] [CrossRef]
- Urrutia, M.B.; Ibarrola, I.; Iglesias, J.I.P.; Navarro, E. Energetics of Growth and Reproduction in a High-Tidal Population of the Clam Ruditapes Decussatus from Urdaibai Estuary (Basque Country, N. Spain). J. Sea Res. 1999, 42, 35–48. [Google Scholar] [CrossRef]
- Petes, L.E.; Menge, B.A.; Murphy, G.D. Environmental Stress Decreases Survival, Growth, and Reproduction in New Zealand Mussels. J. Exp. Mar. Biol. Ecol. 2007, 351, 83–91. [Google Scholar] [CrossRef]
- Carregosa, V.; Figueira, E.; Gil, A.M.; Pereira, S.; Pinto, J.; Soares, A.M.V.M.; Freitas, R. Tolerance of Venerupis Philippinarum to Salinity: Osmotic and Metabolic Aspects. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 171, 36–43. [Google Scholar] [CrossRef]
- Domínguez, R.; Vázquez, E.; Woodin, S.A.; Wethey, D.S.; Peteiro, L.G.; Macho, G.; Olabarria, C. Sublethal Responses of Four Commercially Important Bivalves to Low Salinity. Ecol. Indic. 2020, 111, 106031. [Google Scholar] [CrossRef]
- Domínguez, R.; Olabarria, C.; Woodin, S.A.; Wethey, D.S.; Peteiro, L.G.; Macho, G.; Vázquez, E. Contrasting Responsiveness of Four Ecologically and Economically Important Bivalves to Simulated Heat Waves. Mar. Environ. Res. 2021, 164, 105229. [Google Scholar] [CrossRef]
- Domínguez, R.; Vázquez, E.; Smallegange, I.M.; Woodin, S.A.; Wethey, D.S.; Peteiro, L.G.; Olabarria, C. Predation Risk Increases in Estuarine Bivalves Stressed by Low Salinity. Mar. Biol. 2021, 168, 132. [Google Scholar] [CrossRef]
- Vázquez, E.; Woodin, S.A.; Wethey, D.S.; LG, P.; Olabarria, C. Reproduction Under Stress: Acute Effect of Low Salinities and Heat Waves on Reproductive Cycle of Four Ecologically and Commercially Important Bivalves. Front. Mar. Sci. 2021, 8, 685282. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Somero, G.N. Evidence for Protein Damage at Environmental Temperatures: Seasonal Changes in Levels of Ubiquitin Conjugates and Hsp70 in the Intertidal Mussel Mytilus Trossulus. J. Exp. Biol. 1995, 198, 1509–1518. [Google Scholar] [CrossRef]
- Blanco, S.; Morán, P.; Diz, A.P.; Olabarria, C.; Vázquez, E. Effects of Short-Term Hyposalinity Stress on Four Commercially Important Bivalves: A Proteomic Perspective. Environ. Res. 2022, 215 Pt 3, 114371. [Google Scholar] [CrossRef]
- Kordas, R.L.; Harley, C.D.G.; O’Connor, M.I. Community Ecology in a Warming World: The Influence of Temperature on Interspecific Interactions in Marine Systems. J. Exp. Mar. Biol. Ecol. 2011, 400, 218–226. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Rosenblatt, A.E. The Temperature Dependence of Predation Stress and Prey Nutritional Stoichiometry. Front. Ecol. Evol. 2017, 5, 73. [Google Scholar] [CrossRef]
- Waggitt, J.J.; Torres, R.; Fraser, S. Foraging seabirds respond to an intermittent meteorological event in a coastal environment. Mar. Ornithol. 2020, 48, 123–131. [Google Scholar]
- Thiel, M. Epibenthic Predation in Marine Soft-Bottoms: Being Small and How to Get Away with It. In Interactions and Adaptation Strategies of Marine Organisms; Naumov, A.D., Hummel, H., Sukhotin, A.A., Ryland, J.S., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 11–19. ISBN 978-90-481-4988-9. [Google Scholar]
- Curtis, D.L.; Sauchyn, L.; Keddy, L.; Therriault, T.W.; Pearce, C.M. Prey Preferences and Relative Predation Rates of Adult European Green Crabs (Carcinus maenas) Feeding on Various Bivalve Species in British Columbia, Canada. Can. Tech. Rep. Fish. Aquat. Sci. 2012, 3014, 20. [Google Scholar]
- Gosselin, L.A.; Qian, P.Y. Juvenile Mortality in Benthic Marine Invertebrates. Mar. Ecol. Progr. Ser. 1997, 146, 265–282. [Google Scholar] [CrossRef]
- Romano, C.; Sarà, G.; Salvo, G.; Bishop, J.; Mazzola, A.; Widdows, J. Effect of the Presence of the Shore Crab, Carcinus maenas, on Burrowing Behaviour and Clearance Rate of the Common Cockle, Cerastoderma edule. Mar. Biol. 2011, 158, 2685–2694. [Google Scholar] [CrossRef]
- Finelli, C.M.; Pentcheff, N.D.; Zimmer, R.; Wethey, D.S. Physical Constraints on Ecological Processes: A Field Test of Odor-Mediated Foraging. Ecology 2000, 81, 784–797. [Google Scholar] [CrossRef]
- Seitz, R.D. Gradient Effects on Structuring of Soft-Bottom Benthic Infauna: Macoma Balthica and Predation, Recruitment, and Food Availability. J. Exp. Mar. Biol. Ecol. 2011, 409, 114–122. [Google Scholar] [CrossRef]
- Román, S.; Vázquez, E.; Román, M.; Viejo, R.M.; Woodin, S.A.; Wethey, D.S.; Troncoso, J.S.; Olabarria, C. Effects of Warming on Biological Interactions between Clams and the Seagrass Zostera noltei: A Case Study Using Open Top Chambers. Est. Coast. Shelf Sci. 2022, 276, 108027. [Google Scholar] [CrossRef]
- Román, M.; Gilbert, F.; Viejo, R.M.; Román, S.; Troncoso, J.S.; Vázquez, E.; Olabarria, C. Are Clam-Seagrass Interactions Affected by Heatwaves during Emersion? Mar. Environ. Res. 2023, 186, 105906. [Google Scholar] [CrossRef]
- Shaffrill, H.; Samaha, A.; D’Silva, J. Climate Change: Social Adaptation Strategies for Fishermen. Mar. Policy 2017, 81, 256–261. [Google Scholar] [CrossRef]
- Miller, D.D.; Ota, Y.; Sumaila, U.R.; Cisneros-Montemayor, A.M.; Cheung, W.W.L. Adaptation Strategies to Climate Change in Marine Systems. Glob. Chang. Biol. 2018, 24, e1–e14. [Google Scholar] [CrossRef]
- Bald, J.; Sinquin, A.; Borja, A.; Caill-Milly, N.; Duclercq, B.; Dang, C.; Montaudouin, X. A System Dynamics Model for the Management of the Manila Clam, Ruditapes philippinarum (Adams and Reeve, 1850) in the Bay of Arcachon (France). Ecol. Modell. 2009, 220, 2828–2837. [Google Scholar] [CrossRef]
- Borja, A.; Bald, J. Modelling the Management of Clam (Ruditapes decussatus) Exploitation in the Basque Country (Northern Spain). Period. Biol. 2000, 102, 395–406. [Google Scholar]
- Harvey, C.J.; Reum, J.C.P.; Poe, M.R.; Williams, G.D.; Kim, S.J. Using Conceptual Models and Qualitative Network Models to Advance Integrative Assessments of Marine Ecosystems. Coast. Manag. 2016, 44, 486–503. [Google Scholar] [CrossRef]
- Reum, J.C.P.; Mcdonald, P.S.; Ferriss, B.E.; Farrell, D.; Harvey, C.J.; Levin, P.S. Qualitative Network Models in Support of Ecosystem Approaches to Bivalve Aquaculture. Ices J. Mar. Sci. 2015, 72, 2278–2288. [Google Scholar] [CrossRef]
- Forget, N.L.; Duplisea, D.E.; Sardenne, F.; McKindsey, C.W. Using Qualitative Network Models to Assess the Influence of Mussel Culture on Ecosystem Dynamics. Ecol. Model. 2020, 430, 109070. [Google Scholar] [CrossRef]
- Peteiro, L.G.; Woodin, S.A.; Wethey, D.S.; Costas-Costas, D.; Martínez-Casal, A.; Olabarria, C.; Vázquez, E. Responses to salinity stress in bivalves: Evidence of ontogenetic changes in energetic physiology on Cerastoderma edule. Sci. Rep. 2018, 8, 8329. [Google Scholar] [CrossRef]
- Woodin, S.A.; Wethey, D.S.; Olabarria, C.; Vázquez, E.; Domínguez, R.; Macho, G.; Peteiro, L. Behavioral Responses of Three Venerid Bivalves to Fluctuating Salinity Stress. J. Exp. Mar. Biol. Ecol. 2020, 522, 151256. [Google Scholar] [CrossRef]
- Widdows, J.; Donkin, P.; Staff, F.J.; Matthiessen, P.; Law, R.J.; Allen, Y.T.; Thain, J.E.; Allchin, C.R.; Jones, B.R. Measurement of Stress Effects (Scope for Growth) and Contaminant Levels in Mussels (Mytilus Edulis) Collected from the Irish Sea. Mar. Environ. Res. 2002, 53, 327–356. [Google Scholar] [CrossRef]
- Dethier, M.N.; Dobkowski, K.; Noreen, A.; Yun, M.; Moosmiller, A. Vulnerability of Juvenile Clams to Predation by Shore Crabs. Aquaculture 2019, 506, 350–354. [Google Scholar] [CrossRef]
- Anta, J.; Peña, E.; Puertas, J. Análisis Hidrodinámico Del Arrastre Del Bereberecho Cerastoderma edule de Los Lombos Del Ulla. Rev. Real Acad. Galega Cienc. 2007, XXVI, 53–90. [Google Scholar]
- Chícharo, L.; Chícharo, M.A. Effects of Environmental Conditions on Planktonic Abundances, Benthic Recruitment and Growth Rates of the Bivalve Mollusc Ruditapes Decussatus in a Portuguese Coastal Lagoon. Fish Res. 2001, 53, 235–250. [Google Scholar] [CrossRef]
- Sobral, P.; Widdows, J. Effects of Increasing Current Velocity, Turbidity and Particle-Size Selection on the Feeding Activity and Scope for Growth of Ruditapes Decussatus from Ria Formosa, Southern Portugal. J. Exp. Mar. Biol. Ecol. 2000, 245, 111–125. [Google Scholar] [CrossRef]
- De Coo, A. Revisión a Dez Anos de Marisqueo. In X Foro dos Recursos Mariños e da Acuicultura das Rías Galegas e I Foro Iberoamericano dos Recursos Mariños e da Acuicultura: Normativa europea na calidade das augas para o marisqueo en Galicia; Rey-Méndez, M., Fernández Casal, J., Guerra, A., Lodeiros, C., Eds.; Universidade de Santiago de Compostela: Santiago, Spain, 2008; pp. 41–128. ISBN 978-84-608-0755-1. [Google Scholar]
- De Coo, A. Marisqueo de Bivalvos e Crise Económica En Galicia. In XI Foro dos Recursos Mariños e da Acuicultura das Rías Galegas; Rey-Méndez, M., Fernández Casal, J., Guerra, A., Lodeiros, C., Eds.; Universidade de Santiago de Compostela: Santiago, Spain, 2009; pp. 507–520. ISBN 978-84-608-0886-2. [Google Scholar]
- Malham, S.K.; Hutchinson, T.H.; Longshaw, M. A Review of the Biology of European Cockles (Cerastoderma spp.). J. Mar. Biol. Assoc. UK 2012, 92, 1563–1577. [Google Scholar] [CrossRef]
- Villalba, A.; Casas, S.M.; López, C.; Carballal, M.J. Study of Perkinsosis in the Carpet Shell Clam Tapes Decussatus in Galicia (NW Spain). II. Temporal Pattern of Disease Dynamics and Association with Clam Mortality. Dis. Aquat. Organ. 2005, 65, 257–267. [Google Scholar] [CrossRef]
- Cruz, R.; Lago, A.; Lage, A.; Rial, M.E.; Diaz-Fierros, F.; Salsón, S. Evolución recente do clima de Galicia. Tendencias observadas nas variables meteorolóxicas. In Evidencias e Impactos do Cambio Climático en Galicia; Xunta de Galicia Ed.: Santiago de Compostela, Spain, 2009. [Google Scholar]
- Sáez De Cámara, E.; Gangoiti, G.; Alonso, L.; Iza, J. Daily Precipitation in Northern Iberia: Understanding the Recent Changes after the Circulation Variability in the North Atlantic Sector. J. Geophys Res. Atmos. 2015, 120, 9981. [Google Scholar] [CrossRef]
- Bartolomeu, S.; Carvalho, M.J.; Marta-Almeida, M.; Melo-Gonçalves, P.; Rocha, A. Recent Trends of Extreme Precipitation Indices in the Iberian Peninsula Using Observations and WRF Model Results. Phys. Chem. Earth 2016, 94, 10–21. [Google Scholar] [CrossRef]
- Santos, F.; Álvarez, I. Upwelling along the Western Coast of the Iberian Peninsula: Dependence of Trends on Fitting Strategy. Clim. Res. 2011, 48, 213–218. [Google Scholar] [CrossRef]
- Pérez, F.F.; Padín, X.A.; Pazos, Y.; Gilcoto, M.; Cabanas, M.; Pardo, P.C.; Doval, M.D.; Farina-Busto, L. Plankton response to weakening of the Iberian coastal upwelling. Glob. Chang. Biol. 2010, 16, 1258–1267. [Google Scholar] [CrossRef]
- Dumbauld, B.R.; Ruesink, J.L.; Rumrill, S.S. The Ecological Role of Bivalve Shellfish Aquaculture in the Estuarine Environment: A Review with Application to Oyster and Clam Culture in West Coast (USA) Estuaries. Aquaculture 2009, 290, 196–223. [Google Scholar] [CrossRef]
- Glude, J.B. The Effects of Temperature and Predators on the Abundance of the Soft- Shell Clam, Mya arenaria, in New England. Trans. Am. Fish Soc. 1955, 84, 13–26. [Google Scholar] [CrossRef]
- Moulton, J.M.; Gustafson, A.H. Green Crabs and the Redistribution of Quahogs. Science 1956, 123, 992. [Google Scholar] [CrossRef] [PubMed]
- Hanich, Q.; Wabnitz, C.C.C.; Ota, Y.; Amos, M.; Donato-Hunt, C.; Hunt, A. Small-Scale Fisheries under Climate Change in the Pacific Islands Region. Mar. Policy 2018, 88, 279–284. [Google Scholar] [CrossRef]
- Defeo, O.; Castrejón, M.; Ortega, L.; Kuhn, A.M.; Gutiérrez, N.L.; Castilla, J.C. Impacts of Climate Variability on Latin American Small-Scale Fisheries. Ecol. Soc. 2013, 18, 30. [Google Scholar] [CrossRef]
- Barange, M.; Merino, G.; Blanchard, J. Impacts of Climate Change on Marine Ecosystem Production in Societies Dependent on Fisheries. Nat. Clim. Change 2014, 4, 211–216. [Google Scholar] [CrossRef]
- Fernández-Nóvoa, D.; García-Feal, O.; González-Cao, J.; Gonzalo, C.; Rodríguez-Suárez, J.A.; Portal, C.R.; Gómez-Gesteira, M. MIDAS: A New Integrated Flood Early Warning System for the Miño River. Water 2020, 12, 2319. [Google Scholar] [CrossRef]
- Ojea, J.; Pazos, A.J.; Martínez, D.; Novoa, S.; Sánchez, J.L.; Abad, M. Seasonal Variation in Weight and Biochemical Composition of the Tissues of Ruditapes Decussatus in Relation to the Gametogenic Cycle. Aquaculture 2004, 238, 451–468. [Google Scholar] [CrossRef]
- Mucientes-Sandoval, G.; Fernández Barreiro, J.A.; No Couto, E. Muestreos periódicos para el seguimiento y gestión de los recursos marinos en Cambados. In XI Foro Rec Mar Ac Rías Gal; Rey-Méndez, M., Fernández Casal, J.C., Guerra, A., Lodeiros, C., Eds.; Universidade de Santiago de Compostela: Santiago, Spain, 2009; pp. 521–524. [Google Scholar]
- Bode, A.; Álvarez-Salgado, X.A.; Ruiz-Villarreal, M.; Bañón, R.; Castro, C.G.; Molares Vila, J.; Otero, J.; Rosón, G.; Varela, M. Impacto do cambio climático nas condicions oceanograficas e nos recursos marinos. In Evidencias e Impactos do Cambio Climático en Galicia; Xunta de Galicia Ed.: Santiago de Compostela, Spain, 2009; pp. 619–636. [Google Scholar]
- Fernández, E.; Álvarez-Salgado, X.A.; Beiras, R.; Ovejero, A.; Méndez, G. Coexistence of Urban Uses and Shellfish Production in an Upwelling-Driven, Highly Productive Marine Environment: The Case of the Ría de Vigo (Galicia, Spain). Reg. Stud. Mar. Sci. 2016, 8, 362–370. [Google Scholar] [CrossRef]
- Altas, G.R. Estudo Integral das rías de Ribadeo, Foz, Viveiro, O Barqueiro e Ortigueira. Hidrografía, Dinámica, Sedimentoloxía, Ecotoxicoloxía, Microbioloxía e Bioloxía das zonas de Interese Marisqueiro; Xunta de Galicia Consellería do Medio Rural e do Mar. Ed.: Lugo, Spain, 2012. [Google Scholar]
- Bidegain, G.; Sestelo, M.; Roca-Pardiñas, J.; Juanes, J.A. Estimating a New Suitable Catch Size for Two Clam Species: Implications for Shellfishery Management. Ocean Coast. Manag. 2013, 71, 52–63. [Google Scholar] [CrossRef]
- Dickinson, G.H.; Matoo, O.B.; Tourek, R.T.; Sokolova, I.M.; Beniash, E. Environmental Salinity Modulates the Effects of Elevated CO2 Levels on Juvenile Hard-Shell Clams, Mercenaria Mercenaria. J. Exp. Biol. 2013, 216, 2607–2618. [Google Scholar] [CrossRef]
- Catalán, I.A.; Auch, D.; Kamermans, P.; Morales-Nin, B.; Angelopoulos, N.V.; Reglero, P.; Sandersfeld, T.; Peck, M.A. Critically Examining the Knowledge Base Required to Mechanistically Project Climate Impacts: A Case Study of Europe’s Fish and Shellfish. Fish Fish. 2019, 20, 501–517. [Google Scholar] [CrossRef]
- Kinne, O. Marine Ecology. A Comprehensive, Integrated Treatise on Life in Oceans and Coastal Waters. Environ. Factors Part 1971, 2, 1. [Google Scholar]
- Pörtner, H.-O. Oxygen- and Capacity-Limitation of Thermal Tolerance: A Matrix for Integrating Climate-Related Stressor Effects in Marine Ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Armstrong, E.J.; Stillman, J.H. Multiple Stressors in a Changing World: The Need for an Improved Perspective on Physiological Responses to the Dynamic Marine Environment. An. Rev. Mar. Sci. 2016, 8, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Van Colen, C.; Ong, E.Z.; Briffa, M.; Wethey, D.S.; Abatih, E.; Moens, T.; Woodin, S.A. Clam Feeding Plasticity Reduces Herbivore Vulnerability to Ocean Warming and Acidification. Nat. Clim. Chang. 2020, 10, 162–166. [Google Scholar] [CrossRef]
- Wethey, D.S.; Woodin, S.A. Ecological Hindcasting of Biogeographic Responses to Climate Change in the European Intertidal Zone. Hydrobiologia 2008, 606, 139–151. [Google Scholar] [CrossRef]
- Wethey, D.S.; Woodin, S.A.; Hilbish, T.J.; Jones, S.J.; Lima, F.P.; Brannock, P.M. Response of intertidal populations to climate: Effects of extreme events versus long term change. J. Exp. Mar. Biol. Ecol. 2011, 400, 132–144. [Google Scholar] [CrossRef]
- García, S.M.; Kolding, J.; Rice, J.; Rochet, M.; Zhou, S.; Arimoto, T.; Beyer, J.E.; Borges, L.; Bundy, A.; Dunn, D.; et al. Reconsidering the Consequences of Selective Fisheries. Science 2012, 335, 1045–1047. [Google Scholar] [CrossRef]
- Stewart-Sinclair, P.J.; Last, K.S.; Payne, B.L.; Wilding, T.A. A Global Assessment of the Vulnerability of Shellfish Aquaculture to Climate Change and Ocean Acidification. Ecol. Evol. 2020, 10, 3518–3534. [Google Scholar] [CrossRef]
- Micheli, F.; Saenz-Arroyo, A.; Greenley, A.; Vazquez, L.; Espinoza Montes, J.A.; Rossetto, M.; Leo, G.A. Evidence That Marine Reserves Enhance Resilience to Climatic Impacts. PLoS ONE 2012, 7, 40832. [Google Scholar] [CrossRef]
- «BOE» Núm. 15, de 17 de Enero de 2009, Páginas 5443 a 5506 (64 Págs.) Comunidad Autónoma de Galicia Referencia: BOE-A-2009-805. Available online: https://www.boe.es/eli/es-ga/l/2008/12/03/11 (accessed on 1 May 2023).
- Bañón, R.; Almón, B.; Trigo, J.E.; Pérez-Dieste, J. Especies marinas exóticas e inmigrantes en las costas de Galicia. In Especies Exóticas Invasoras: Cátedra Parques Nacionales; Junoy, J., Ed.; Universidad de Alcalá: Madrid, Spain, 2019; pp. 81–94. ISBN 978-84-17729-30-1. [Google Scholar]
- Folke, C. Resilience: The Emergence of a Perspective for Social–Ecological Systems Analyses. Glog. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, R.; Olabarria, C.; Vázquez, E. Assessment of Risks Associated with Extreme Climate Events in Small-Scale Bivalve Fisheries: Conceptual Maps for Decision-Making Based on a Review of Recent Studies. J. Mar. Sci. Eng. 2023, 11, 1216. https://doi.org/10.3390/jmse11061216
Domínguez R, Olabarria C, Vázquez E. Assessment of Risks Associated with Extreme Climate Events in Small-Scale Bivalve Fisheries: Conceptual Maps for Decision-Making Based on a Review of Recent Studies. Journal of Marine Science and Engineering. 2023; 11(6):1216. https://doi.org/10.3390/jmse11061216
Chicago/Turabian StyleDomínguez, Rula, Celia Olabarria, and Elsa Vázquez. 2023. "Assessment of Risks Associated with Extreme Climate Events in Small-Scale Bivalve Fisheries: Conceptual Maps for Decision-Making Based on a Review of Recent Studies" Journal of Marine Science and Engineering 11, no. 6: 1216. https://doi.org/10.3390/jmse11061216
APA StyleDomínguez, R., Olabarria, C., & Vázquez, E. (2023). Assessment of Risks Associated with Extreme Climate Events in Small-Scale Bivalve Fisheries: Conceptual Maps for Decision-Making Based on a Review of Recent Studies. Journal of Marine Science and Engineering, 11(6), 1216. https://doi.org/10.3390/jmse11061216





