The Impacts of Dietary Curcumin on Innate Immune Responses and Antioxidant Status in Greater Amberjack (Seriola dumerili) under Ammonia Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish and Animal Husbandry
2.3. Acute Ammonia Challenge Experiment
2.4. Sample Collection and Chemical Analyses
2.5. Blood Biochemical Parameter Measurements
2.6. Tissues’ Enzyme Activity Measurements
2.7. Statistical Analysis
3. Results
3.1. Effect of Dietary Curcumin on Plasma ALP and ACP Activities of Greater Amberjack (Seriola dumerili)
3.2. Effect of Dietary Curcumin on Intestinal ALP and ACP Activities of Greater Amberjack (Seriola dumerili)
3.3. Effect of Dietary Curcumin on Hepatic Enzyme Activities of Greater Amberjack (Seriola dumerili)
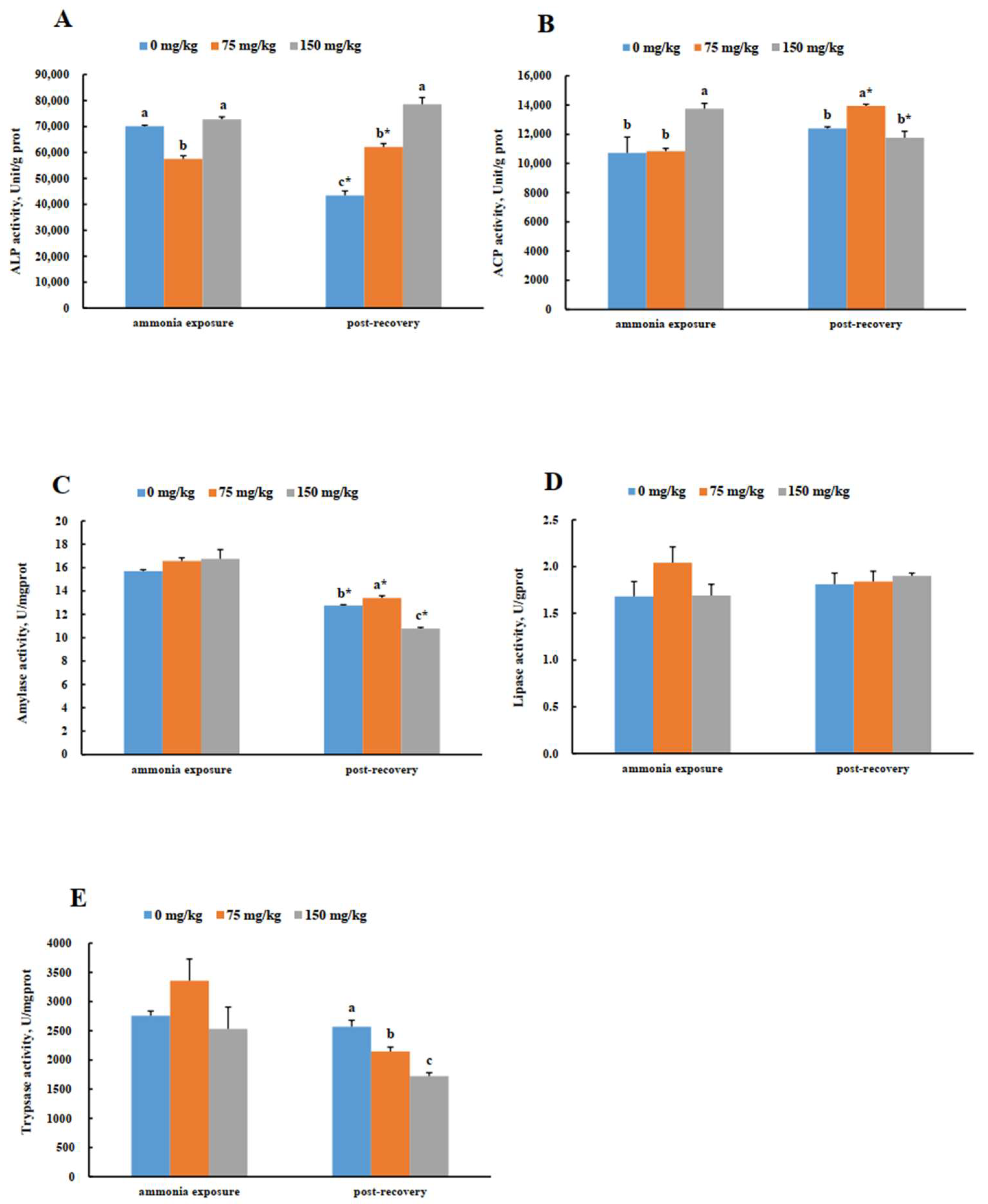
3.4. Intestinal Enzyme Activities in Greater Amberjack (S. dumerili) in Response to Acute Ammonia Exposure and Post-Recovery
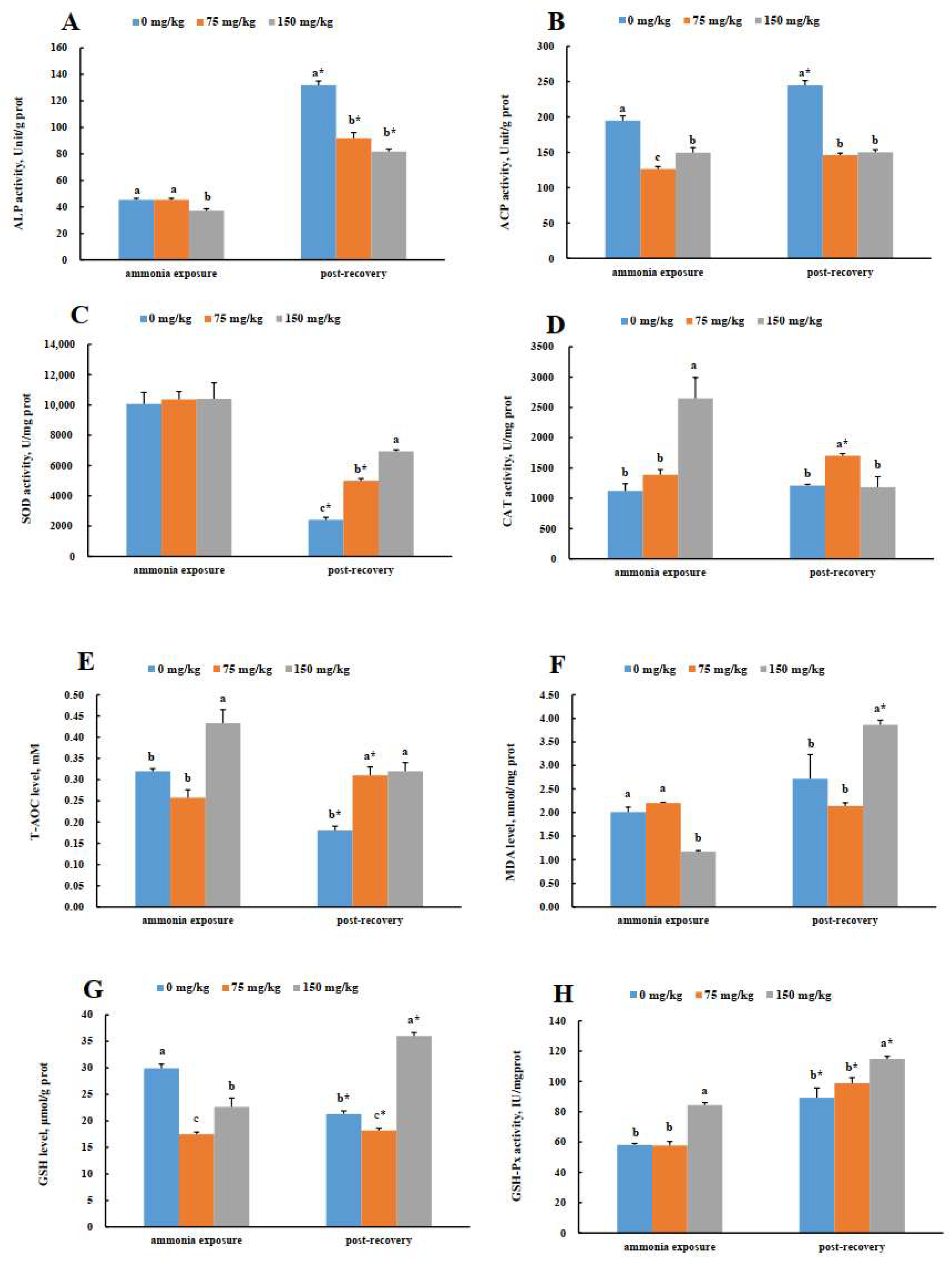
3.5. Hepatic Enzyme Activities in Greater Amberjack (S. dumerili) in Response to Acute Ammonia Exposure and Post-Recovery
3.6. Gill Enzyme Activities in Greater Amberjack (S. dumerili) in Response to Acute Ammonia Exposure and Post-Recovery
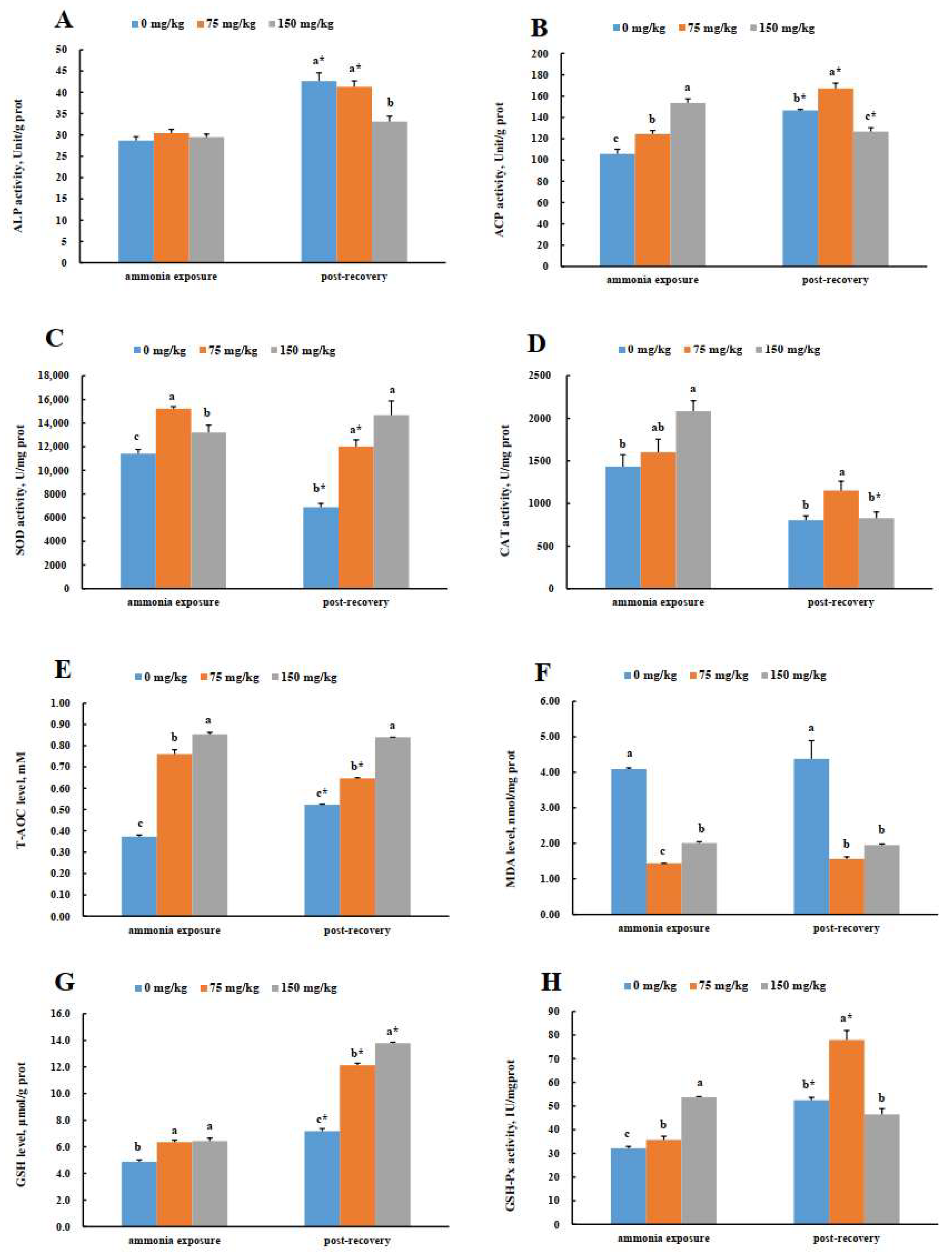
3.7. Spleen Enzyme Activities in Greater Amberjack (S. dumerili) in Response to Acute Ammonia Exposure and Post-Recovery
3.8. Head Kidney Enzyme Activities in Greater Amberjack (S. dumerili) in Response to Acute Ammonia Exposure and Post-Recovery
3.9. Brain Enzyme Activities in Greater Amberjack (S. dumerili) in Response to Acute Ammonia Exposure and Post-Recovery
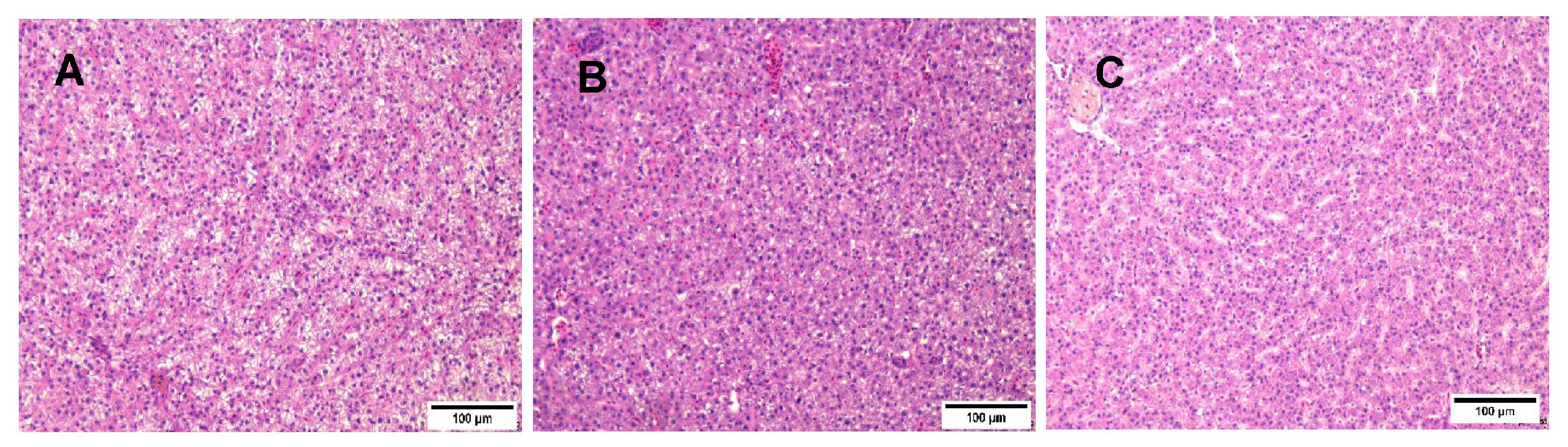
3.10. Effect of Dietary Curcumin on Hepatic Histological Changes of Greater Amberjack (Seriola dumerili)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, J.; Ge, X.; Liu, B.; Xie, J.; Cui, S.; Zhou, M.; Xia, S.; Chen, R. Effect of Dietary Vitamin C on Non-Specific Immunity and MRNA Expression of Three Heat Shock Proteins (HSPs) in Juvenile Megalobrama Amblycephala under PH Stress. Aquaculture 2014, 434, 325–333. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, S.; Ge, X.; Xia, S.; Zhu, J.; Miao, L.; Lin, Y.; Liang, H.; Pan, W.; Su, Y.; et al. Acute Effects of Ammonia Exposure on the Plasma and Haematological Parameters and Histological Structure of the Juvenile Blunt Snout Bream, Megalobrama Amblycephala, and Post-Exposure Recovery. Aquac. Res. 2018, 49, 1008–1019. [Google Scholar] [CrossRef]
- Wajsbrot, N.; Gasith, A.; Diamant, A.; Popper, D.M. Chronic Toxicity of Ammonia to Juvenile Gilthead Seabream Sparus Aurata and Related Histopathological Effects. J. Fish Biol. 1993, 42, 321–328. [Google Scholar] [CrossRef]
- Li, M.; Yu, N.; Qin, J.G.; Li, E.; Du, Z.; Chen, L. Effects of Ammonia Stress, Dietary Linseed Oil and Edwardsiella Ictaluri Challenge on Juvenile Darkbarbel Catfish Pelteobagrus Vachelli. Fish Shellfish. Immunol 2014, 38, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Benli, A.C.K.; Köksal, G.; Ozkul, A. Sublethal Ammonia Exposure of Nile Tilapia (Oreochromis Niloticus L.): Effects on Gill, Liver and Kidney Histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Safayhi, H.; Mack, T.; Sabieraj, J. Mechanism of Antiinflammatory Actions of Curcumine and Boswellic Acids. J. Ethnopharmacol. 1993, 38, 113–119. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and Liver Disease: From Chemistry to Medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef]
- Sharma, O.P. Antioxidant Activity of Curcumin and Related Compounds. Biochem. Pharmacol. 1976, 25, 1811–1812. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.O.; Elnesr, S.S.; Dhama, K. Curcumin and Its Different Forms: A Review on Fish Nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Al-Sagheer, A.A.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Dietary Curcumin Supplement Influence on Growth, Immunity, Antioxidant Status, and Resistance to Aeromonas Hydrophila in Oreochromis Niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Yonar, M.E. Chlorpyrifos-Induced Biochemical Changes in Cyprinus Carpio: Ameliorative Effect of Curcumin. Ecotoxicol. Environ. Saf. 2018, 151, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Effects of Ammonia,Nitrite and Curcumin on Growth Metabolism and Disease-Resistant Genes in Japanese Sea Bass, Lateolabrax Japonicus. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2020. [Google Scholar]
- Zhao, L.; Tang, G.; Xiong, C.; Han, S.; Yang, C.; He, K.; Liu, Q.; Luo, J.; Luo, W.; Wang, Y.; et al. Chronic Chlorpyrifos Exposure Induces Oxidative Stress, Apoptosis and Immune Dysfunction in Largemouth Bass (Micropterus Salmoides). Environ. Pollut. 2021, 282, 117010. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Yazlak, H.; Tuzcu, M.; Sahin, N. Lycopene Improves Activation of Antioxidant System and Nrf2/HO-1 Pathway of Muscle in Rainbow Trout (Oncorhynchus Mykiss) with Different Stocking Densities. Aquaculture 2014, 430, 133–138. [Google Scholar] [CrossRef]
- Zhang, Y. Effects of Curcumin on Growth and Liver-Protection in Common Carp, Cyprinus Carpio. Pak. J. Zool. 2020, 53, 1211–1220. [Google Scholar] [CrossRef]
- El-abd, H.; Abd El-latif, A.; Shaheen, A. Effect of Curcumin on Growth Performance and Antioxidant Stress Status of Nile Tilapia (Oreochromis Niloticus). Iran. J. Fish. Sci. 2021, 20, 1234–1246. [Google Scholar] [CrossRef]
- Hamasaki, K.; Tsuruoka, K.; Teruya, K.; Hashimoto, H.; Hamada, K.; Hotta, T.; Mushiake, K. Feeding Habits of Hatchery-Reared Larvae of Greater Amberjack Seriola Dumerili. Aquaculture 2009, 288, 216–225. [Google Scholar] [CrossRef]
- Takakuwa, F.; Fukada, H.; Hosokawa, H.; Masumoto, T. Optimum Digestible Protein and Energy Levels and Ratio for Greater Amberjack Seriola Dumerili (Risso) Fingerling. Aquac. Res. 2006, 37, 1532–1539. [Google Scholar] [CrossRef]
- Bureau of Fisheries, Ministry of Agriculture and Rural Affairs; National Fisheries Technology Extension Center; China Society of Fisheries. 2022 China Fisheries Yearbook; China Agriculture Press: Beijing, China, 2022. [Google Scholar]
- Jover, M.; García-Gómez, A.; Tomás, A.; De la Gándara, F.; Pérez, L. Growth of Mediterranean Yellowtail (Seriola Dumerilii) Fed Extruded Diets Containing Different Levels of Protein and Lipid. Aquaculture 1999, 179, 25–33. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Z.; Lin, H.; Ma, Z.; Wang, J.; Wang, Y.; Yu, W. Rhizoma Curcumae Longae Ameliorates High Dietary Carbohydrate-Induced Hepatic Oxidative Stress, Inflammation in Golden Pompano Trachinotus Ovatus. Fish Shellfish. Immunol. 2022, 130, 31–42. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Zhou, S.; Peng, X.; Ma, Z. Effects of Acute Ammonia Nitrogen Stress on Antioxidant Enzymes Activity and Digestive Enzymes Activity in Larval Lates Calcarifer. Journal of Southern Agriculture. J. South. Agric. 2018, 49, 2087–2095. [Google Scholar]
- Rajput, Y.S.; Srivastava, R.K. Distribution of Acid and Alkaline Phosphatase in Buffalo Spermatozoa. Arch. Exp. Vet. 1980, 34, 673–675. [Google Scholar]
- Chi, C.; Yun, S.; Giri, S.S.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Park, S.C. Effect of the Algicide Thiazolidinedione 49 on Immune Responses of Bay Scallop Argopecten Irradians. Molecules 2019, 24, E3579. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Metcalf, W.W. A New Activity for an Old Enzyme: Escherichia Coli Bacterial Alkaline Phosphatase Is a Phosphite-Dependent Hydrogenase. Proc. Natl. Acad. Sci. USA 2004, 101, 7919–7924. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Harrington, D.; Sutcliffe, I.C. Characterization of Acid Phosphatase Activities in the Equine Pathogen Streptococcus Equi. Syst. Appl. Microbiol. 2000, 23, 325–329. [Google Scholar] [CrossRef]
- Lackie, A.M. Invertebrate Immunity. Parasitology 1980, 80, 393–412. [Google Scholar] [CrossRef]
- Zhou, C.; Ge, X.; Lin, H.; Niu, J. Effect of Dietary Carbohydrate on Non-Specific Immune Response, Hepatic Antioxidative Abilities and Disease Resistance of Juvenile Golden Pompano (Trachinotus Ovatus). Fish Shellfish Immunol. 2014, 41, 183–190. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Carvan, M.J.; Di Giulio, R.T. Oxidative Stress Responses in Aquatic and Marine Fishes. In Studies on Experimental Toxicology and Pharmacology; Roberts, S.M., Kehrer, J.P., Klotz, L.-O., Eds.; Oxidative Stress in Applied Basic Research and Clinical Practice; Springer International Publishing: Cham, Switzerland, 2015; pp. 481–493. ISBN 978-3-319-19096-9. [Google Scholar]
- Jia, R.; Cao, L.; Xu, P.; Jeney, G.; Yin, G. In Vitro and in Vivo Hepatoprotective and Antioxidant Effects of Astragalus Polysaccharides against Carbon Tetrachloride-Induced Hepatocyte Damage in Common Carp (Cyprinus Carpio). Fish Physiol. Biochem. 2012, 38, 871–881. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Khani Oushani, A.; Najafi Enferadi, M.H. Effects of Haematococcus Pluvialis Supplementation on Antioxidant System and Metabolism in Rainbow Trout (Oncorhynchus Mykiss). Fish Physiol. Biochem. 2012, 38, 413–419. [Google Scholar] [CrossRef]
- Guglielmo, A.; Sabra, A.; Elbery, M.; Cerveira, M.M.; Ghenov, F.; Sunasee, R.; Ckless, K. A Mechanistic Insight into Curcumin Modulation of the IL-1β Secretion and NLRP3 S-Glutathionylation Induced by Needle-like Cationic Cellulose Nanocrystals in Myeloid Cells. Chem. -Biol. Interact. 2017, 274, 1–12. [Google Scholar] [CrossRef]
- Mostafavi, Z.S.; Shekarabi, S.P.H.; Mehrgan, M.S.; Islami, H.R. Amelioration of Growth Performance, Physio-Metabolic Responses, and Antioxidant Defense System in Rainbow Trout, Oncorhynchus Mykiss, Using Dietary Dandelion, Taraxacum Officinale, Flower Extract. Aquaculture 2022, 546, 737296. [Google Scholar] [CrossRef]
- Jędrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of Antioxidant Activity of Phenolic Fractions from the Leaves and Petals of Dandelion in Human Plasma Treated with H2O2 and H2O2/Fe. Chem. -Biol. Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Hoseinifar, S.H.; Nejadmoghadam, S.; Jafar, A. Transciptomic Study of Mucosal Immune, Antioxidant and Growth Related Genes and Non-Specific Immune Response of Common Carp (Cyprinus Carpio) Fed Dietary Ferula (Ferula Assafoetida). Fish Shellfish. Immunol. 2016, 55, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Shekarabi, S.P.H.; Mehrgan, M.S.; Ramezani, F.; Dawood, M.A.O.; Van Doan, H.; Moonmanee, T.; Hamid, N.K.A.; Kari, Z.A. Effect of Dietary Barberry Fruit (Berberis Vulgaris) Extract on Immune Function, Antioxidant Capacity, Antibacterial Activity, and Stress-Related Gene Expression of Siberian Sturgeon (Acipenser Baerii). Aquac. Rep. 2022, 23, 101041. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Xu, B.; Sagada, G.; Chen, K.; Xiao, J.; Zhang, J.; Shao, Q. Effects of Berberine Supplementation in High Starch Diet on Growth Performance, Antioxidative Status, Immune Parameters and Ammonia Stress Response of Fingerling Black Sea Bream (Acanthopagrus Schlegelii). Aquaculture 2020, 527, 735473. [Google Scholar] [CrossRef]
- Esteban, M.A.; Cordero, H.; Martínez-Tomé, M.; Jiménez-Monreal, A.M.; Bakhrouf, A.; Mahdhi, A. Effect of Dietary Supplementation of Probiotics and Palm Fruits Extracts on the Antioxidant Enzyme Gene Expression in the Mucosae of Gilthead Seabream (Sparus Aurata L.). Fish Shellfish. Immunol. 2014, 39, 532–540. [Google Scholar] [CrossRef]
- Craig, P.M.; Wood, C.M.; McClelland, G.B. Oxidative Stress Response and Gene Expression with Acute Copper Exposure in Zebrafish (Danio Rerio). Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2007, 293, R1882–R1892. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Pérez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and Liver Disease. BioFactors 2013, 39, 88–100. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chen, J.-C. Effect of Ammonia on the Immune Response of White Shrimp Litopenaeus Vannamei and Its Susceptibility to Vibrio Alginolyticus. Fish Shellfish Immunol. 2004, 16, 321–334. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, S.; Cao, D.; Bin, L.U.; Chang, Q.; Liu, C.; Yan, J. Effects of Acute Ammonia Nitrogen Stress on Histopathology of Gill and Liver and Enzyme Activities of Juvenile Verasper Variegatus. Prog. Fish. Sci. 2017, 38, 59–70. [Google Scholar]
- Liu, H.Z.; Zheng, F.R.; Sun, X.Q.; Tang, X.X.; Dong, S.L. Effect of Exposure to Ammonia Nitrogen Stress on Immune Enzyme of Holothurian Apostichopus Japonicus. Mar. Sci. 2012, 36, 47–52. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.-C. The Virulence of Enterococcus to Freshwater Prawn Macrobrachium Rosenbergii and Its Immune Resistance under Ammonia Stress. Fish Shellfish. Immunol. 2002, 12, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Pervin, S.; MG, S.J.; Md, M.R.A.; Sana, N.K.; Rahman, H.; Shaha, R.K. Effects of Some Environmental Variables on Urease in Germinating Chickpea (Cicer Arietinum L.) Seed. J. Stress Physiol. Biochem. 2013, 9, 345–356. [Google Scholar]



| Ingredient | (% of Dry Matter) |
|---|---|
| Fish meal | 60 |
| Corn gluten meal | 8 |
| Soybean meal | 10 |
| Corn starch | 8 |
| Microcrystalline cellulose | 2 |
| Fish oil | 7 |
| Lecithin | 1 |
| Vitamin mixture 1 | 0.5 |
| Mineral mixture 2 | 0.5 |
| Choline chloride | 0.5 |
| Betaine | 0.5 |
| Carboxyl-methyl cellulose | 2 |
| Total | 100 |
| Proximate composition | |
| Dry matter | 87.9 |
| Crude protein | 49.7 |
| Crude lipid | 12.7 |
| Crude ash | 10.7 |
| Gross energy (kJ/g) | 18.5 |
| Dietary Curcumin (mg/kg) | 0 | 75 | 150 |
|---|---|---|---|
| ALP (king’s unit/100 mL) | 2.3 ± 0.15 | 2.17 ± 0.04 | 2.10 ± 0.10 |
| ACP (king’s unit/100 mL) | 1.45 ± 0.02 b | 2.67 ± 0.20 a | 2.45 ± 0.09 a |
| Dietary Curcumin (mg/kg) | 0 | 75 | 150 |
|---|---|---|---|
| ALP (king’s unit/gprot) | 28,222 ± 2008 b | 41,407 ± 2055 a | 42,254 ± 3654 a |
| ACP (king’s unit/gprot) | 8710 ± 183 b | 11,693 ± 419 a | 11,330 ± 562 a |
| Dietary Curcumin (mg/kg) | 0 | 75 | 150 |
|---|---|---|---|
| ALP (king’s unit/gprot) | 16.62 ± 1.99 b | 55.71 ± 4.07 a | 52.25 ± 2.38 a |
| ACP (king’s unit/gprot) | 78.27 ± 4.48 b | 90.44 ± 6.99 b | 108.08 ± 1.91 a |
| SOD (U/mgprot) | 6598 ± 772 b | 8793 ± 768 a | 5765 ± 204 b |
| CAT (U/mgprot) | 172.89 ± 14.5 | 178.66 ± 4.88 | 160.87 ± 6.24 |
| T-AOC (mM) | 0.93 ± 0.02 ab | 0.9 ± 0.01 b | 0.98 ± 0.01 a |
| MDA (nmol/mgprot) | 1.25 ± 0.11 | 1.16 ± 0.08 | 1.28 ± 0.05 |
| GSH (μmol/gprot) | 21.41 ± 1.37 ab | 24.16 ± 1.05 a | 18.12 ± 0.51 b |
| GPX (IU/mgprot) | 9.19 ± 2.01 b | 20.84 ± 1.06 a | 11.29 ± 0.66 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Huang, Z.; Zhou, S.; Hu, J.; Yang, R.; Wang, J.; Wang, Y.; Yu, W.; Lin, H.; Ma, Z. The Impacts of Dietary Curcumin on Innate Immune Responses and Antioxidant Status in Greater Amberjack (Seriola dumerili) under Ammonia Stress. J. Mar. Sci. Eng. 2023, 11, 300. https://doi.org/10.3390/jmse11020300
Zhou C, Huang Z, Zhou S, Hu J, Yang R, Wang J, Wang Y, Yu W, Lin H, Ma Z. The Impacts of Dietary Curcumin on Innate Immune Responses and Antioxidant Status in Greater Amberjack (Seriola dumerili) under Ammonia Stress. Journal of Marine Science and Engineering. 2023; 11(2):300. https://doi.org/10.3390/jmse11020300
Chicago/Turabian StyleZhou, Chuanpeng, Zhong Huang, Shengjie Zhou, Jing Hu, Rui Yang, Jun Wang, Yun Wang, Wei Yu, Heizhao Lin, and Zhenhua Ma. 2023. "The Impacts of Dietary Curcumin on Innate Immune Responses and Antioxidant Status in Greater Amberjack (Seriola dumerili) under Ammonia Stress" Journal of Marine Science and Engineering 11, no. 2: 300. https://doi.org/10.3390/jmse11020300
APA StyleZhou, C., Huang, Z., Zhou, S., Hu, J., Yang, R., Wang, J., Wang, Y., Yu, W., Lin, H., & Ma, Z. (2023). The Impacts of Dietary Curcumin on Innate Immune Responses and Antioxidant Status in Greater Amberjack (Seriola dumerili) under Ammonia Stress. Journal of Marine Science and Engineering, 11(2), 300. https://doi.org/10.3390/jmse11020300







