Abstract
Data acquired from stranded sea turtles can provide awareness of human activities that adversely affect sea turtle populations. We assessed strandings of five sea turtle species between 2017 and 2021. This study utilizes principal component analysis (PCA) and structural equation modeling (SEM) to reveal potential causes of sea turtle strandings linked to anthropogenic effects in Taiwan. Although our study did not observe a statistically significant impact of offshore wind turbines on sea turtle strandings, it did find evidence of a significant direct effect of coral colony density, heavy metals, and fishing disturbance on such strandings. For the conservation of endangered sea turtles, we recommend the incorporation of PCA and SEM in further contexts for validating anthropogenic impact assessments.
1. Introduction
Sea turtles can serve as sentinel indicators of the health of marine ecosystems [1,2]. Five of the seven internationally recognized marine turtle species are present in the coastal areas of Taiwan: the green turtle (Chelonia mydas; Endangered), hawksbill turtle (Eretmochelys imbricata; Critically endangered), olive ridley turtle (Lepidochelys olivacea; Vulnerable), leatherback turtle (Dermochelys coriacea; Vulnerable), and loggerhead turtle (Caretta caretta; Vulnerable) [2,3]. These species of sea turtles are all included in the Red List of Threatened Species maintained by the World Conservation Union (IUCN Red List), and are also listed under the Schedule of Protected Marine Species (Ocean Affairs council, Executive Yuan) in Taiwan. Of these, the green turtle is the most prevalent in Taiwan [2,3]. Furthermore, coastal areas in Taiwan contain important feeding and nesting sites for green turtles. However, as green turtles face numerous threats that are unrelated to wind farms (e.g., human activity, including fishery bycatch, illegal egg poaching, coastal development, marine debris, global environmental change, marine pollution, and anthropogenic-exacerbated disease including fibropapillomatosis (FP), etc.) [2,3,4,5,6,7,8,9,10,11,12], it can be difficult to distinguish between the effects of these threats and the impact of wind farms on the marine turtles.
The offshore wind energy industry has been growing since the 1990s, when the first offshore wind farms began operating [13]. Enthusiasm for offshore wind farms and other sources of sustainable energy is currently surging as the widespread development and adoption of sustainable energy are critical to mitigating climate change and its effects [13,14,15,16,17]. However, the precautionary principle dictates that offshore wind farms should not be deployed in close proximity to biodiversity hotspots, significant fish spawning areas, important migration routes, or sensitive habitats (including deep-sea corals, maerl beds, and crinoid assemblages), especially when these habitats occur within Marine Protected Areas [13].
Offshore wind turbines can have potential impacts on sea turtles in several ways. These include (1) vessel collisions with turtles, such as collisions involving the working boats of wind turbines [18]; (2) underwater sounds resulting from wind turbine operation, including pile driving [19,20]; and (3) local magnetic disturbances caused by cables, which can interfere with sea turtle migration [21,22]. Therefore, determining which effects result from offshore wind turbines, other human activities, and/or environmental factors can be difficult. Furthermore, collecting sufficient high-quality data on sea turtles over expansive ocean areas is also extremely challenging. For example, sea turtles generally spend more than 90% of their time submerged under the surface of the water [23,24,25,26,27,28]. This has limited our ability to decipher what problems sea turtles face underwater. Therefore, sea turtle stranding events could provide us with an opportunity to understand the potential negative impacts of anthropogenic activities (e.g., entanglement in fishing nets/lines/marine debris, ingestion of fish hooks, boat strikes, etc.) [4,8,10,22,26,29,30,31,32,33,34] and this could serve as a reference for sea turtle conservation management. In addition, recent advances in computer processing power and statistical methods, as well as the growing availability of large amounts of high-quality environmental data have greatly improved the ability to analyze the structure of ecological systems using mathematical methods. In recent years, the growing availability of publicly available environmental and species data has created opportunities for large-scale data analyses to investigate the impacts of specific anthropogenic disturbances on numerous species of conservation concern.
As a consequence of the aforementioned circumstances, data obtained from stranded sea turtles can provide insight into human activities that negatively impact sea turtle populations [2,8,9,26,27,29]. Such activities include vessel collisions or oil spills that damage feeding or swimming abilities [18,28,29]. This study quantitatively analyzed the impacts that numerous human disturbances and natural effects have on sea turtles, including the potential effects of offshore wind turbines. This study will contribute to our understanding of the potential causes for stranded marine turtles, thereby providing important information for application in sea turtle conservation efforts.
2. Materials and Methods
2.1. Data Collection
Sea turtle stranding records: This study collated stranded sea turtle data (data years: from 1 January 2017 to 30 September 2021) from the Marine Animal Rescue Network (MARN) of the Ocean Conservation Administration in Taiwan. Our dataset included data from each county and city where MARN operates. MARN records data from both dead and living sea turtles that were found (1) washed ashore, (2) floating in coastal waters, or (3) as fishery bycatch.
Marine environmental data: Marine environmental data (including salinity, water temperature, dissolved oxygen, and dissolved oxygen saturation as well as concentrations of chlorophyll a, ammonium, nitrate-nitrogen, phosphate, nitrite-nitrogen, silicate, copper, lead, and zinc) were obtained from the Ocean Conservation Administration website (https://iocean.oca.gov.tw/OCA_OceanConservation/PUBLIC/Marine_WaterQuality.aspx) (accessed on 10 March 2022).
The number of fishermen: We determined the number of people employed in the fishery industry using data from the Fishery Yearbook 2020, published by the Fisheries Agency of Taiwan (https://www.fa.gov.tw/view.php?theme=FS_AR&subtheme=&id=20) (accessed on 10 March 2022).
Coral coverage area: We determined the coral coverage area, using data obtained from the Report on the Results of the 2019 Coral Reef Ecosystem Survey Project [35] (https://www.oca.gov.tw/ch/home.jsp?id=394&parentpath=0,299&mcustomize=research_list.jsp) (accessed on 10 March 2022).
Additionally, we determined the number of offshore wind turbines under construction and operation in coastal areas of Taiwan (i.e., monthly turbine count) with which to estimate the impact of wind turbines on marine turtles in each county and city.
2.2. Study Area
Investigation areas were delineated around monitoring stations. These areas were typically large scale and included both coastal cities and counties in Taiwan. Specifically, these areas included New Taipei City (including Keelung City; A1), Taoyuan City (A2), Hsinchu County (A3), Miaoli County (A4), Taichung City (A5), Changhua County (A6), Yunlin County (A7), Chiayi County (A8), Tainan City (A9), Kaohsiung City (A10), Pingtung County [including North Pingtung (A11), Hengchun Peninsula (A12) and Liuchiu Island (A13)], Taitung County (A14), Hualien County (A15), and Yilan County (A16) (Figure 1). Therefore, datasets included data that fell within 16 different investigation areas. Data on stranded sea turtles were aggregated separately for the 16 investigation areas according to season [spring (S1): March to May, summer (S2): June to August, autumn (S3): September to November, winter (S4): December to February] and then matched with water quality, coral, and fisheries data. In total, there were 134 data (including salinity, water temperature, dissolved oxygen, and dissolved oxygen saturation as well as concentrations of chlorophyll a, ammonium, nitrate-nitrogen, phosphate, nitrite-nitrogen, silicate, copper, lead, and zinc) entries available for analysis.
Figure 1.
Study area located in Taiwan.
2.3. Data Analysis
We first employed principal component analysis (PCA) to conduct exploratory research on water quality data. Principal component axes identified by this analysis were considered latent variables [36,37] that influenced oceanic environmental changes. The meaning of each principal component (PC) axis was determined by examination of the factor loadings of the variables as well as the temporal and spatial changes of each PC score [38], which were assessed using two-way ANOVA and multiple comparisons (using Duncan’s Method). Once the primary environmental factors (latent variables) were recognized, we incorporated the data pertaining to the number of stranded sea turtles, the number of fishery employees, coral coverage, and offshore wind turbines to construct SEM for the conceptual model of ecological system changes in coastal area waters. In so doing, we used SEM, which employs both factor analysis and path analysis and can analyze complex relationships between organisms and their environment [36,39]. In other words, SEM can analyze the intricate networks of causal relationships in ecosystems [40,41,42]. In building our models, we used data on stranded sea turtles from the Marine Animal Rescue Network (MARN) of the Ocean Conservation Administration in Taiwan (Figure 1). Lastly, the LISREL8 program was used to perform structural equation modeling (SEM) and thereby verify conceptual models [36]. The detailed assumptions and the concept of SEM are elaborated by [36,42].
3. Results
During the study period, we recorded 810 sea turtle strandings: 692 green turtles (C. mydas) (85.4%) (juvenile = 567; sub-adult = 70; adult = 55), 48 hawksbill turtles (E. imbricata) (5.9%), 37 olive ridley turtles (L. olivacea) (4.5%), 30 loggerhead turtles (C. caretta) (3.7%), and 3 leatherback turtles (D. coriacea) (0.3%) (Table 1). For green turtles, juveniles (Curved carapace length; CCL < 67 cm) [2,9] were the most abundant size class (Table 2). The most prevalent oceanic-stage juveniles (CCL < 30 cm) [32,43] identified in our study were hawksbill turtles (12.5%; n = 6), followed by loggerhead turtles (6.6%; n = 2), olive ridley turtles (2.7%; n = 1), and green turtles (0.7%; n = 5) (Table 1). We found 90 turtles alive and 720 dead. The results of marine environmental data were shown in Table 3.

Table 1.
Sea turtle strandings recorded from 1 January 2017 to 30 September 2021 in Taiwan.

Table 2.
The life stage of green turtles in this study.

Table 3.
Ranges and mean values (±SD) of the hydrological and metal variables included in the study (N = 134).
Table 4 presents the results of a two-factor analysis of variance conducted using data from 401 stranded sea turtles across 16 county districts (paired with water quality data). Results indicated that the number of stranded sea turtles in spring (March to May) was significantly higher than in autumn (September to November), winter (December to February), and summer (June to August). Furthermore, New Taipei City, the Hengchun Peninsula, Yilan-Hualien, and Liuchiu Island had significantly more stranded turtle events than did Taichung-Changhua, and Yunlin-Chiayi.

Table 4.
Results of two-way ANOVA for the influence of station and seasons and the interaction between these two factors upon the number of stranded sea turtles.
Regarding the geographical distribution of the number of fishermen (Table 5), Kaohsiung City (48,540) and New Taipei City (including Keelung City) (41,909) had the highest “total number of professional fishermen”, while Hsinchu County (1902) and Taoyuan City (623) had the lowest total number of professional fishermen. North Pingtung (5112) and Kaohsiung City (15,221) had the highest “number of coastal professional fishermen”, while Changhua County (453) and Taichung City (0) had the lowest numbers of coastal professional fishermen.

Table 5.
The geographical distribution of the number of fishermen included in this study.
The largest extent of coral coverage was found in the Hengchun Peninsula (44.69%), followed by Taitung (38.21%), New Taipei City (30.55%), Hualien (26.88%), Yilan (23.02%), and Liuchiu Island (15.70%) (Table 6).

Table 6.
The geographical distribution of the coral cover used in this study.
Results of principal component analysis (PCA) on water quality data from 16 coastal districts in Taiwan between 2017 and 2021 revealed that the first (PC1) to fourth (PC4) principal components explained 68.42% of the overall water quality variation (Table 7). Among these components, the loadings of salinity, ammonium, nitrate-nitrogen, nitrite-nitrogen, phosphate, and silicate on the first component axis (PC1) were relatively high, with salinity having a negative value and the other nutrients having positive values. These results were indicative of the effects of nutrient input from freshwater rivers into the sea. Therefore, we named this component axis “river-derived nutrients”. Variance analysis of the component scores through this axis showed that river-derived nutrients were significantly higher in northern Pingtung and significantly lower in the Hengchun Peninsula, Yilan, Hualien, and Taitung (Table 8).

Table 7.
The loadings of the principal components (PC) 1–4 for abiotic variables assessed in this study area (N = 134).

Table 8.
Results from two-way analysis of variance (ANOVA) tests and all pairwise multiple comparisons (using Duncan’s Method) on principal components 1 (PC1) to 4 (PC4).
The second component axis (PC2) (Table 8) had larger loadings for dissolved oxygen, saturated dissolved oxygen, and chlorophyll a. All of these values were positive, and we posited that this was indicative of vigorous photosynthesis by phytoplankton, which is an oxygen-releasing process. We, therefore, named this axis “phytoplankton photosynthesis”. Results of variance analysis revealed that phytoplankton photosynthesis was significantly higher in northern Pingtung and significantly lower in Yilan, Hualien, Keelung, and the Hengchun Peninsula.
The third component axis (PC3) (Table 8) had larger loadings of copper, zinc, and lead, indicating that these three heavy metals were found together. Therefore, this component axis was named “heavy metals”.
The fourth component axis (PC4) (Table 8) had larger loadings of water temperature and salinity, with the former being negative and the latter being positive overall. In Taiwan, the low winter water temperature coincides with the dry season, which is characterized by reduced river flow and freshwater input to the sea. Therefore, this component axis was named “seasonal variation”.
In SEM, squares represent observed variables while circles represent latent variables estimated and identified by observed variables [38,42]. Path coefficients between environmental and biological latent variables range from −1 to 1, with the absolute value indicating the degree of influence of a given latent variable. Solid lines indicate significant effects while dotted lines indicate insignificant effects, with arrowheads indicating the direction of influence. Figure 2 presents the ecological model constructed in this study. Specifically, our model was constructed by combining environmental data, fishery statistics, water quality data, coral data, and sea turtle data. The latent variables used in this model, including “nutrients (−) from rivers”, “phytoplankton photosynthesis”, and “heavy metals”, were derived from the principal component analysis of water quality in the model. The latent variable “fishing disturbance” was estimated using two measured parameters: “the total number of all professional fishery personnel” and “the number of coastal fishery personnel”. The latent variables “coral colony density”, “stranded sea turtle quantity”, and “offshore wind turbine effects” were estimated using a single measured variable: “coral coverage area”, “the number of stranded turtles”, and “offshore wind turbine (unit) operating quantity”, respectively.
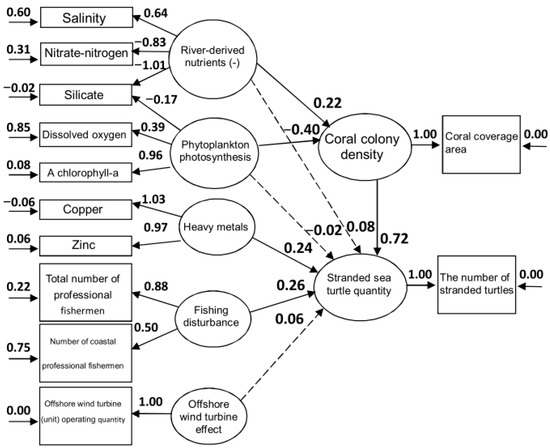
Figure 2.
Final structural equation modeling (SEM) with standardized coefficients. Solid arrows: significant impact; dashed arrows: insignificant impact.
In our model, “nutrients (−) from rivers” and “phytoplankton photosynthesis” had significant effects on “coral density”, with coefficients of 0.22 and −0.40, respectively. The former indicates that environments characterized by excessive nutrient input from rivers are not suitable for coral growth, while the latter indicates that areas characterized by vigorous phytoplankton photosynthesis are also unsuitable (because they are likewise nutrient-rich, eutrophic environments). With regard to the impact of “nutrients originating from rivers” and “photosynthesis of phytoplankton” on the “stranded sea turtle quantity”, the values of 0.08 and −0.02, respectively, indicate that the nutrients brought by rivers and the strength of photosynthesis in the water were not related to the stranding of sea turtles. Conversely, “heavy metals” and “fishing disturbance” had significant and positive effects (0.24 and 0.26, respectively) on the stranded sea turtle quantity. The impact of “offshore wind turbine effect” on stranded sea turtles (a key focus of this study) was not significant at 0.06. This strongly suggests that, to date, the installation of wind turbines in Miaoli and Changhua has not directly increased the number of sea turtle strandings.
4. Discussion
Knowledge of the interactions between offshore wind farms and sea turtles in foraging areas/migratory corridors in Taiwan remains very limited. This is the first study to employ SEM and data from multiple databases to conduct quantitative analysis and establish a model to elucidate the potential causes of sea turtle strandings in Taiwan. This study analyzed the coastal areas of major counties and cities in Taiwan using publicly available water quality data.
In the present study, the C. mydas was the most frequent species involved in stranding. As has been found in previous studies, the green turtle is the most common species of sea turtle in Taiwan [3,10,44,45]. Furthermore, green turtle strandings have been recorded more frequently than other species in all regions of Taiwan [34,45,46]. We also found that coastal areas of densely populated western regions were characterized by greater eutrophication and land-based pollution than coastal areas in the eastern, northern, and southern regions. The water quality monitoring station of Pingtung is adjacent to Donggang Creek, where the livestock industry is highly developed and eutrophication is most severe, while areas with fewer river-derived nutrients were associated with sparsely populated eastern counties and better river water quality (in most cases). Lower nutrient concentrations can also be explained by the dilution effect of the Pacific Ocean and the lack of large rivers in the Hengchun Peninsula. The high level of photosynthesis in northern Pingtung can be primarily explained by the severe eutrophication of Donggang Creek, while lower photosynthesis levels are found in marine areas that are strongly diluted by the eastern ocean and do not feature large rivers that input high amounts of land-based pollutants. The study conducted by Liu et al. (2015) [47] suggested that southwestern Taiwan is also a highly problematic region for coastal eutrophication. With regard to the possible effects of the increased eutrophication in these areas on the populations of sea turtles, previous studies also found that fibropapillomatosis (FP) is more prevalent in areas exposed to greater eutrophication [48,49,50]. FP is a tumor-forming disease that affects all sea turtle species and is most common in green turtles [5]. Reports on FP in green turtles in Asia are still very limited. However, the disease has recently been described in endangered green turtles in Taiwan [6,44,51].
SEM is a powerful multivariate statistical technique increasingly used in scientific research to evaluate and test multivariate causal relationships [42]. In addition, over the past 20 years ecologists have applied SEM to test multi-variable hypotheses. The complex networks of causal relationships in ecosystems can be analyzed using SEM [40,41,42]. In terms of the SEM results (Figure 2), the SEM indicated that coral colony density had a positive effect on the number of turtle strandings. The green turtle is the most common species of sea turtle in Taiwan [3]. Because green turtles mainly feed on seagrass, seaweed, and in coral reef terrains [43,52,53], the highly significant positive correlation between “coral density” and “stranded sea turtle quantity” of 0.72 (Figure 2) revealed that suitable coral reef habitats attracted a large number of sea turtles, which may naturally result in larger numbers of stranded sea turtles (e.g., anthropogenic interactions). In other words, the majority of green turtles in this study were identified to be juveniles (CCL < 67 cm) to sub-adults (CCL 67–84 cm) (Table 2) and were more likely to be resident animals (neritic-stage) [32]. As such, they are more likely to be affected by anthropogenic activities due to their high dependence on coastal feeding grounds and frequent use of nearshore habitats [2,32].
Regarding fishing disturbance and stranding, the SEM (Figure 2) showed a significant effect of fishing disturbance on the number of stranded sea turtles (0.26). Previous literature has reported that bycatch, net entanglement, and collisions with ocean vessels can cause significant harm to sea turtles [28]. In addition, sea turtle strandings in the Mediterranean are also strongly linked to bycatch: according to a 14-year annual study [30], more than half of loggerhead sea turtle (C. caretta) strandings were the result of human activities such as fishing. In Turkey, strandings were primarily caused by bycatch and marine pollution [31]. Another study reported that the mortality of loggerhead, olive ridley, and leatherback sea turtles in southern Brazil was also associated with fishing activities in feeding habitats or in migratory corridors between breeding and feeding areas [32]. In a study conducted in Hawaii and the insular Pacific, the main reasons for sea turtle strandings were bycatch and collision (excluding FP) [33]. Bycatch was identified as a significant concern for sea turtles in Spain and Taiwan [30,34]. In Taiwan, a report titled “Twenty-three Years of Sea Turtle Stranding/Bycatch Research in Taiwan” indicated that eighty percent of the sea turtles that were stranded or caught had already perished [34]. One report by Chen et al. (2012) [46] indicates that fishery bycatch is likely the cause of stranded sea turtle mortality in Taiwan. In addition to coral colony density, ocean currents and wind may also influence the detection of sea turtle strandings. The lack of data on ocean currents and wind in our study is a limitation of this study.
With regard to heavy metals, we also observed higher concentrations of heavy metals in waters where there were more sea turtle strandings. Although this study cannot confirm whether high levels of heavy metals are harmful to the health of wild sea turtles, other researchers have raised concerns about heavy metal pollution in sea turtle habitats [2,9,54,55,56]. In fact, it has been suggested that sea turtles’ immune systems may be more susceptible to the harmful effects of heavy metals than those of other vertebrates [54,56]. As a consequence of the aforementioned circumstances, the current study focused on threats from environmental contaminants, a priority area of research for marine sea turtle conservation [4,57]. Fibropapillomatosis (FP), for example, is a tumor-forming disease that affects all species of sea turtles and is most commonly found in green turtles [5,6,11,12]. A higher prevalence of FP in sea turtles has been documented in highly contaminated marine environments or environments with poor water quality [48,49,58,59]. Reports of FP in sea turtles in Asia are still very limited. However, cases of FP have been discovered in Taiwanese waters in recent years [6,44,51]. Another issue related to environmental contaminants in marine ecology research is that the green turtles in Taiwan may have encountered coastal pollution containing antimicrobial agents or heavy metals when they migrated to nearshore feeding areas after recruitment [2]. As a consequence of the aforementioned circumstances, in order to benefit sea turtle conservation, future research should focus on how to prevent pollution in the main sea turtle activity areas in Taiwan.
Our findings suggest that offshore wind turbines do not have a significant impact on sea turtle strandings. Although our results cannot determine whether offshore wind turbines lead to physiological disturbances in sea turtles that cause them to leave an area, our results can serve as an important reference in future evaluations of wind turbine installations. Note that in this study, the offshore wind turbines located in Miaoli and Changhua, Taiwan are not situated in sea turtle foraging hotspots or significant migratory corridors for sea turtles. Satellite tracking studies to date do not provide conclusive evidence that the Miaoli and Changhua Sea are hot spots for sea turtle foraging, or that they are significant sea turtle migration corridors [3,10,27,45]. However, the impact of offshore wind turbines on sea turtles may include collisions with these reptiles caused by the working boats of wind turbines [18], undersea sounds created by wind turbines such as pile driving [19,20], and local magnetic disturbances generated by cables may have negative impacts on sea turtles [21,22,60,61]. Therefore, the potential impact of offshore wind turbines on sea turtles needs to be further investigated in the future. It is essential for sea turtle conservation management to document important foraging sites and the composition and numerical importance of foraging aggregations [62]. Further study of sea turtle movements (e.g., satellite tracking) in these areas should be pursued to characterize their main foraging areas and ultimately to assess their interactions with human activities in these habitats. Furthermore, as new molecular and genetic technologies (e.g., environmental DNA, eDNA) are developed, it is possible to adapt and optimize them for sea turtle conservation. For example, eDNA detection has complemented traditional in-water monitoring of sea turtles by allowing detection even when turtles have not been visually observed [63]. Therefore, eDNA techniques could be a viable and efficient alternative to traditional sea turtle monitoring methods.
5. Conclusions
In conclusion, to the best of our knowledge, this is the first study to unravel the complex relationships between environmental factors, anthropogenic interactions, and sea turtle strandings in Taiwan. The results of the SEM indicated that coral colony density, heavy metals, and fishing disturbance had a significant influence on sea turtle stranding events. As a result of the aforementioned circumstances, future analyses examining the impact of offshore wind turbines in significant sea turtle habitats ought to be conducted. Additionally, to conserve endangered sea turtles, we recommend applying PCA and SEM techniques to determine potential causes of sea turtle strandings and verify their direct and indirect effects in other areas of concern to establish upcoming environmental impact assessments.
Author Contributions
Conceptualization, T.-H.L. and W.-R.C.; methodology, T.-H.L. and W.-R.C.; software, W.-R.C.; validation, T.-H.L. and W.-R.C.; formal analysis, W.-R.C.; investigation, T.-H.L. and W.-R.C.; resources, T.-H.L. and P.-Y.W.; data curation, P.-Y.W. and W.-R.C.; writing—original draft preparation, T.-H.L. and W.-R.C.; writing—review and editing, T.-H.L.; visualization, P.-Y.W. and W.-R.C.; supervision, T.-H.L.; project administration, T.-H.L.; funding acquisition, T.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by intramural funding from the National Museum of Marine Biology and Aquarium to T.-H.L. (1121003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Ocean Conservation Administration in Taiwan authorized and funded the rehabilitation of stranded marine turtles rescued by NMMBA. This study was supported by intramural funding from the NMMBA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aguirre, A.A.; Lutz, P.L. Marine turtles as sentinels of ecosystem health: Is fibropapillomatosis an indicator? EcoHealth 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Tsai, M.A.; Chang, C.C.; Li, T.H. Antimicrobial-resistance profiles of gram-negative bacteria isolated from green turtles (Chelonia mydas) in Taiwan. Environ. Pollut. 2021, 277, 116870. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.W.; Fan, T.Y.; Ng, C.K.Y.; Cai, Y.; Balazs, G.H.; Li, T.H. Tale of the unlucky tags: The story of a rescued, rehabilitated, and released green sea turtle (Chelonia mydas) in southern Taiwan. Bull. Mar. Sci. 2017, 93, 689–690. [Google Scholar] [CrossRef]
- Hamann, M.; Godfrey, M.H.; Seminoff, J.A.; Arthur, K.; Barata, P.C.R.; Bjorndal, K.A.; Bolten, A.B.; Broderick, A.C.; Campbell, L.M.; Carreras, C.; et al. Global research priorities for sea turtles: Informing management and conservation in the 21st century. Endanger. Species Res. 2010, 11, 245–269. [Google Scholar] [CrossRef]
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of fibropapillomatosis in green turtles (Chelonia mydas). Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef]
- Li, T.H.; Hsu, W.L.; Lan, Y.C.; Balazs, G.H.; Work, T.M.; Tseng, C.T.; Chang, C.C. Identification of Chelonid herpesvirus 5 (ChHV5) in endangered green turtles (Chelonia mydas) with fibropapillomatosis in Asia. Bull. Mar. Sci. 2017, 93, 1011–1022. [Google Scholar] [CrossRef]
- Perrault, J.R.; Stacy, N.I.; Lehner, A.F.; Mott, C.R.; Hirsch, S.; Gorham, J.C.; Buchweitz, J.P.; Bresette, M.J.; Walsh, C.J. Potential effects of brevetoxins and toxic elements on various health variables in Kemp’s ridley (Lepidochelys kempii) and green (Chelonia mydas) sea turtles after a red tide bloom event. Sci. Total Environ. 2017, 605–606, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.A.; Stockin, K.A. Anthropogenic impacts on green turtles Chelonia mydas in New Zealand. Endanger. Species Res. 2018, 37, 1–9. [Google Scholar] [CrossRef]
- Ng, C.K.Y.; Lam, J.C.W.; Zhang, X.H.; Gu, H.X.; Li, T.H.; Ye, M.B.; Xia, Z.R.; Zhang, F.Y.; Duan, J.X.; Wang, W.X.; et al. Levels of trace elements, methylmercury and polybrominated diphenyl ethers in foraging green turtles in the South China region and their conservation implications. Environ. Pollut. 2018, 234, 735–742. [Google Scholar] [CrossRef]
- Li, T.H.; Cai, Y.R.; Wu, P.Y.; Ng, C.K.Y.; Balazs, G.H. Lesson to learn from an endangered green turtle (Chelonia mydas): Marine debris ingestion, rehabilitation and satellite tracking. Indian J. Anim. Res. 2020, 1, 5. [Google Scholar] [CrossRef]
- Manes, C.; Herren, R.M.; Page, A.; Dunlap, F.D.; Skibicki, C.A.; Rollinson Ramia, D.R.; Farrell, J.A.; Capua, I.; Carthy, R.R.; Duffy, D.J. Green Turtle Fibropapillomatosis: Tumor Morphology and Growth Rate in a Rehabilitation Setting. Vet. Sci. 2023, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Yetsko, K.; Farrell, J.A.; Blackburn, N.B.; Whitmore, L.; Stammnitz, M.R.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Krstic, A.; et al. Molecular characterization of a marine turtle tumour epizootic, profiling external, internal and postsurgical regrowth tumours. Commun. Biol. 2021, 4, 152. [Google Scholar] [CrossRef]
- Lloret, J.; Turiel, A.; Solé, J.; Berdalet, E.; Sabatés, A.; Olivares, A.; Gili, J.M.; Vila-Subirós, J.; Sardá, R. Unravelling the ecological impacts of large-scale Offshore wind farms in the Mediterranean Sea. Sci. Total Environ. 2022, 824, 153803. [Google Scholar] [CrossRef] [PubMed]
- EEA. Europe’s Onshore and offshore Wind Energy Potential: An Assessment of Environmental and Economic Constraints; EEA Technical Report; No 6; EEA: Copenhagen, Denmark, 2009. [Google Scholar]
- Dannheim, J.; Bergström, L.; Birchenough, S.N.; Brzana, R.; Boon, A.R.; Coolen, J.W.; Dauvin, J.C.; De Mesel, I.; Derweduwen, J.; Gill, A.B.; et al. Benthic effects of offshore renewables: Identification of knowledge gaps and urgently needed research. ICES J. Mar. Sci. 2020, 77, 1092–1108. [Google Scholar] [CrossRef]
- Bennun, L.; van Bochove, J.; Ng, C.; Fletcher, C.; Wilson, D.; Phair, N.; Carbone, G. Mitigating Biodiversity Impacts Associated with Solar and Wind Energy Development: Guidelines for Project Developers; The Biodiversity Consultancy: Gland, Switzerland; IUCN: Cambridge, UK, 2021. [Google Scholar]
- ICES. Workshop on socio-economic implications of offshore wind on fishing communities (WKSEIOWFC). ICES Sci. Rep. 2021, 3, 33. [Google Scholar]
- Foley, A.M.; Stacy, B.A.; Hardy, R.F.; Shea, C.P.; Minch, K.E.; Schroeder, B.A. Characterizing watercraft-related mortality of sea turtles in Florida. J. Wildl. Manag. 2019, 83, 1057–1072. [Google Scholar] [CrossRef]
- Lavender, A.L.; Bartol, S.M.; Bartol, I.K. A two-method approach for investigating the hearing capabilities of loggerhead sea turtles (Caretta caretta). In Proceedings of the 31st Annual Symposium on Sea Turtle Biology and Conservation, San Diego, CA, USA, 10–16 April 2011. [Google Scholar]
- Nelms, S.E.; Piniak, W.E.D.; Weir, C.R.; Godley, B.J. Seismic surveys and marine turtles: An underestimated global threat? Biol. Conserv. 2016, 193, 49–65. [Google Scholar] [CrossRef]
- Irwin, W.P.; Lohmann, K.J. Disruption of magnetic orientation in hatchling loggerhead sea turtles by pulsed magnetic fields. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 475–480. [Google Scholar] [CrossRef]
- Lohmann, K.J.P.; Luschi, P.; Hays, G.C. Goal navigation and island-finding in sea turtles. J. Exp. Mar. Biol. Ecol. 2008, 356, 83–95. [Google Scholar] [CrossRef]
- Renaud, M.L.; Carpenter, J.A. Movements and submergence patterns of loggerhead turtles (Caretta caretta) in the Gulf of Mexico determined through satellite telemetry. Bull. Mar. Sci. 1994, 55, 1–15. [Google Scholar]
- Hochscheid, S.; Bentivegna, F.; Hamza, A.; Hays, G.C. When surfacers do not dive: Multiple significance of extended surface times in marine turtles. J. Exp. Biol. 2010, 213, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Iverson, A.R.; Fujisaki, I.; Lamont, M.M.; Hart, K.M. Loggerhead sea turtle (Caretta caretta) diving changes with productivity, behavioral mode, and sea surface temperature. PLoS ONE 2019, 14, e0220372. [Google Scholar] [CrossRef] [PubMed]
- Clukey, K.E.; Lepczyk, C.A.; Balazs, G.H.; Work, T.M.; Lynch, J.M. Investigation of plastic debris ingestion by four species of sea turtles collected as bycatch in pelagic Pacific longline fisheries. Mar. Pollut. Bull. 2017, 120, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.Y.; Gu, H.X.; Li, T.H.; Ye, M.B.; Xia, Z.R.; Zhang, F.Y.; Duan, J.X.; Hsu, C.K.; Balazs, G.H.; Murphy, M.B. Insights into identifying habitat hot spots and migratory corridors of green turtles in the South China region. Aquatic. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1181–1191. [Google Scholar] [CrossRef]
- Shigenaka, G. Oils and Sea Turtle: Biology, Planning, and Response; Office of Response and Restoration, NOAA Ocean Service: Washington, DC, USA, 2010.
- Li, T.H.; Wu, P.Y.; Chen, I.C. Lethal Lesions Caused by Propeller Cuts on the Endangered Green Turtle Chelonia mydas on Liuchiu Island, Taiwan. Indian J. Anim. 2022, 1, 3. [Google Scholar] [CrossRef]
- Tomás, J.; Gozalbes, P.; Raga, J.A.; Godley, B.J. Bycatch of loggerhead sea turtles: Insights from 14 years of stranding data. Endanger. Species Res. 2008, 5, 161–169. [Google Scholar] [CrossRef]
- Sönmez, B. Sixteen year (2002–2017) record of sea turtle strandings on Samandağ Beach, the eastern Mediterranean coast of Turkey. Zool. Stud. 2018, 57, 53. [Google Scholar]
- Guimarães, S.M.; de Almeida, L.G.; Nunes, L.A.; Lacerda, P.D.; de Amorim, C.E.S.; Burato, M.; Baldassin, P.; Werneck, M.R. Distribution and potential causes of sea turtles stranding in the state of Rio de Janeiro, southern Brazil. Herpetol. Conserv. Biol. 2021, 16, 225–237. [Google Scholar]
- Work, T.M.; Balazs, G.H.; Summers, T.M.; Hapdei, J.R.; Tagarino, A.P. Causes of mortality in green turtles from Hawaii and the insular Pacific exclusive of fibropapillomatosis. Dis. Aquat. Organ. 2015, 115, 103–110. [Google Scholar] [CrossRef]
- Cheng, I.J.; Wang, H.Y.; Hsieh, W.Y.; Chan, Y.T. Twenty-three years of sea turtle stranding/bycatch research in Taiwan. Zool. Stud. 2019, 58, 44. [Google Scholar]
- Chen, Z.L. 108 Annual Coral Reef Ecosystem Survey Project; Ocean Conservation Division, Ocean Affairs Council: Kaohsiung City, Taiwan, 2019.
- Chou, W.R.; Hsieh, H.Y.; Hong, G.K.; Ko, F.C.; Meng, P.J.; Tew, K.S. Verification of an Environmental Impact Assessment Using a Multivariate Statistical Model. J. Mar. Sci. Eng. 2022, 10, 1023. [Google Scholar] [CrossRef]
- Burstyn, I. Principal component analysis is a powerful instrument in occupational hygiene inquiries. Ann. Occup. Hyg. 2004, 48, 655–661. [Google Scholar] [PubMed]
- Chou, W.R.; Fang, L.S.; Wang, W.H.; Tew, K.S. Environmental influence on coastal phytoplankton and zooplankton diversity: A multivariate statistical model analysis. Environ. Monit. Assess. 2012, 184, 5679–5688. [Google Scholar] [CrossRef]
- Malaeb, Z.A.; Summers, J.K.; Pugesek, B.H. Using structural equation modeling to investigate relationships among ecological variables, Environ. Ecol. Stat. 2000, 7, 93–111. [Google Scholar] [CrossRef]
- Shipley, B. Cause and Correlation in Biology: A User’s Guide to Path Analysis, Structural Equations and Causal Inference; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Fan, Y.; Chen, J.; Shirkey, G.; John, R.; Wu, S.R.; Park, H.; Shao, C. Applications of structural equation modeling (SEM) in ecological studies: An updated review. Ecol. Process. 2016, 5, 19. [Google Scholar] [CrossRef]
- Bjorndal, K.A. Foraging ecology and nutrition of sea turtles. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; p. 34. [Google Scholar]
- Li, T.H.; Chang, C.C. The impact of fibropapillomatosis on clinical characteristics, blood gas, plasma biochemistry, and hematological profiles in juvenile green turtles (Chelonia mydas). Bull. Mar. Sci. 2020, 96, 723–734. [Google Scholar] [CrossRef]
- Ng, C.K.Y.; Matsuzawa, Y. Sea Turtles in the East Asia Region; MTSG Annual Regional Report; The Marine Turtle Specialist Group: Ross, CA, USA, 2020. [Google Scholar]
- Chen, H.; Kuo, R.J.; Chang, T.C.; Hus, C.K.; Bray, R.A.; Cheng, I.J. Fluke (Spirorchiidae) infections in sea turtles stranded on Taiwan: Prevalence and pathology. J. Parasitol. 2012, 98, 437–439. [Google Scholar] [CrossRef]
- Liu, T.K.; Chen, P.; and Chen, H.Y. Comprehensive assessment of coastal eutrophication in Taiwan and its implications for management strategy. Bull. Mar. Sci. 2015, 97, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Van Houtan, K.S.; Hargrove, S.K.; Balazs, G.H. Land use, macroalgae, and a tumour-forming disease in marine turtles. PLoS ONE 2010, 5, e12900. [Google Scholar] [CrossRef]
- Van Houtan, K.S.; Smith, C.M.; Dailer, M.L.; Kawachi, M. Eutrophication and the dietary promotion of sea turtle tumours. PeerJ. 2014, 2, e602. [Google Scholar] [CrossRef] [PubMed]
- Dujon, A.M.; Schofield, G.; Venegas, R.M.; Thomas, F.; Ujvari, B. Turtles in the cancer risk landscape: A global meta-analysis of fibropapillomatosis prevalence and associated risk factors. Sea Pathogens. 2021, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Lei, I.I.; Byadgi, O.V.; Chen, I.C.; Tsai, M.A. Evidence of chelonid herpesvirus 5 infection in green turtle (Chelonia mydas) indicated a possible tumorigenesis activation by transcriptome analysis. Front. Mar. Sci. 2023, 10, 1185111. [Google Scholar] [CrossRef]
- Thayer, G.W.; Bjorndal, K.A.; Ogden, J.C.; Williams, S.L.; Zieman, J.C. Role of larger herbivores in seagrass communities. Estuaries 1984, 7, 351–376. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Hoey, A.S.; Bellwood, D.R. The Role of Turtles as Coral Reef Macroherbivores. PLoS ONE 2012, 7, e39979. [Google Scholar] [CrossRef]
- Day, R.D.; Segars, A.L.; Arendt, M.D.; Lee, A.M.; Peden-Adams, M.M. Relationship of blood mercury levels to health parameters in the loggerhead sea turtle (Caretta caretta). Environ. Health Perspect. 2007, 115, 1421–1428. [Google Scholar] [CrossRef]
- Day, R.D.; Keller, J.M.; Harms, C.A.; Segars, A.L.; Cluse, W.M.; Godfrey, M.H.; Lee, A.M.; Peden-Adams, M.; Thorvalson, K.; Dodd, M.; et al. Comparison of mercury burdens in chronically debilitated and healthy loggerhead sea turtles (Caretta caretta). J. Wildl. Dis. 2010, 46, 111–117. [Google Scholar] [CrossRef]
- Camacho, M.; Orós, J.; Boada, L.D.; Zaccaroni, A.; Silvi, M.; Formigaro, C.; López, P.; Zumbado, M.; Luzardo, O.P. Potential adverse effects of inorganic pollutants on clinical parameters of loggerhead sea turtles (Caretta caretta): Results from a nesting colony from Cape Verde, West Africa. Mar. Environ. Res. 2013, 92, 15–22. [Google Scholar] [CrossRef]
- Rees, A.F.; Alfaro-Shigueto, J.; Barata, P.C.R.; Bjorndal, K.A.; Bolten, A.B.; Bourjea, J.; Broderick, A.C.; Campbell, L.M.; Cardona, L.; Carreras, C.; et al. Are we working towards global research priorities for management and conservation of sea turtles? Endanger. Species Res. 2016, 31, 337–382. [Google Scholar] [CrossRef]
- Formia, A.; Deem, S.; Billes, A.; Ngouessono, S.; Parnell, R.; Collins, T.; Sounguet, G.-P.; Gibudi, A.; Villarubia, V.A.; Balazs, G.H.; et al. Fibropapillomatosis confirmed in Chelonia mydas in the gulf of Guinea, West Africa. Mar. Turtle Newslett. 2007, 116, 20. [Google Scholar]
- Manes, C.; Pinton, D.; Canestrelli, A.; Capua, I. Occurrence of fibropapillomatosis in green turtles (Chelonia mydas) in relation to environmental changes in coastal ecosystems in Texas and Florida: A retrospective study. Animals 2022, 12, 1236. [Google Scholar] [CrossRef]
- Klimley, A.P.; Putman, N.F.; Keller, B.A.; Noakes, D. A call to assess the impacts of electromagnetic fields from subsea cables on the movement ecology of marine migrants. Conserv. Sci. Pract. 2021, 3, e436. [Google Scholar] [CrossRef]
- Yalçin-Özdilek, S.; Yalçin, S. Wind Energy Plants and Possible Effects on Samandag Sea Turtles. Mar. Turtle Newsletter. 2012, 133, 7–9. [Google Scholar]
- Catry, P.; Senhoury, C.; Sidina, E.; Bar, N.E.; Bilal, A.S.; Ventura, E.; Godley, B.J.; Pires, A.J.; Regalla, A.; Patricio, A.R. Satellite tracking and field assessment highlight major foraging site for green turtles in the Banc d’Arguin, Mauritania. Biol. Conserv. 2023, 277, 109823. [Google Scholar] [CrossRef]
- Farrell, J.A.; Whitmore, L.; Mashkour, N.; Rollinson Ramia, D.R.; Thomas, R.S.; Eastman, C.B.; Burkhalter, B.; Yetsko, K.; Mott, C.; Wood, L.; et al. Detection and population genomics of sea turtle species via noninvasive environmental DNA analysis of nesting beach sand tracks and oceanic water. Mol. Ecol. Resour. 2022, 22, 2471–2493. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).