Abstract
With global warming, in recent years, golden tides have frequently occurred off the coasts of China. Sargassum horneri, which attach to aquaculture rafts, can float and form small-scale golden tides after falling off. Temperature will affect the growth and reproduction of algae. In order to explore whether the temperature is the potential influence factor of the golden tide outbreak, in this study, the effects of global warming on the growth and proliferation of S. horneri in the mussel aquaculture area of Gouqi Island will be discussed. Samples of the macroalgae were collected monthly from August 2021 to July 2022 at various stages of its life cycle, and the relationship between algal growth and temperature was analyzed based on the concept of effective accumulated temperature, combined with the parameters of sea surface temperature, photosynthetic rate, growth rate, and growth cycle. Based on the continuous temperature variation observed, the growth cycle could be divided into five stages: the decreasing temperature period (October to November), the low-temperature adaptation period (December), the second suitable low-temperature period (January to February), and the high-temperature decay period (February to May). The effective accumulated temperature stored by S. horneri from 2021 to 2022 was 2772.4 °C·d; compared with previous studies, it decreased by about 800 °C·d. The winter temperature in 2022 was higher than the average temperature in the previous 5 years, allowing the macroalgae to enter the reproductive period in advance. This had a significant impact on the blooming time of golden tides and led to an earlier outbreak and extinction. Therefore, in the future, we can set up a monitoring system for the early warning of golden tides according to the change of SST in winter.
1. Introduction
Brown alga, Sargassum horneri, belongs to the family Sargassaceae, order Fucales, class Phaeophyceae [1,2,3,4]. The algal body has an attached apparatus and mainly grows on the reefs of subtidal zones where the tidal current is unobstructed and the wind and waves are less powerful. With the development of marine aquaculture, S. horneri has proliferated and is commonly found attached to coastal aquaculture rafts. At the same time, the vesicles that characterize its structure also allow it to float on the water surface during the growth stage [3]. These macroalgae primarily reproduce via sexual reproduction and, in part, also via vegetative reproduction [5,6]. S. horneri is widely distributed in China, from Dalian (Liaoning Province) in the north to Guangdong Province in the south [2,7,8,9]. It is an important species grown in seaweed fields, as it is the main, large brown alga found contiguously in the shallow, warm-temperate waters off the coast of China. In addition, it is also the single causative species of golden tides, the large algal blooms that have frequently occurred in the Yellow Sea and East China Sea in recent years [10,11,12] and that have caused significant economic losses to the seaweed aquaculture industry, tourism, and shipping in the coastal provinces of China [13,14,15]. The accumulation of large quantities of S. horneri along the coast will produce a foul smell and cause the proliferation of bacteria and parasites. If not treated in time, it will turn into a foul-smelling swamp that, when hypoxic, will produce the toxic gas hydrogen sulfide. It will have a major adverse impact on the coastal ecosystem and a negative impact on the lives of coastal residents [16]. In addition, a large amount of floating S. horneri can provide a habitat for fish to spawn and feed, and so, it can be used as feed for fishery aquaculture. Because of its unique adsorption effect on water pollutants, it can also be used as an environmentally friendly adsorption material [17,18]. However, a large amount of floating S. horneri will also form the golden tide. The golden tide brings huge economic losses; however, it also plays an important role in the restoration of marine ecology. As a result, S. horneri has gradually attracted widespread attention from the scientific community. However, previous research generally focuses on the exploration of the source of the golden tides and the study of the individual physiology of algae; it does not effectively combine the changes in environmental factors with the outbreak mechanism [6,7,8,9,10,11,12].
Temperature is an important abiotic factor that affects the biological activities of marine algae in nature and has a very significant impact on S. horneri. The metabolism, sexual maturity, growth and development stages, and natural distribution of this species are closely related to changes in seawater temperature [19,20]. According to the French forest scientist Dreomir, a plant needs to accumulate a certain amount of temperature to complete its life cycle; that is, a specific amount of daily average temperature must be accumulated in each growth stage. Once the required temperature is accumulated, the plant’s entire life cycle is completed. Based on Dreomir’s “effective accumulated temperature theory,” and assuming other environmental parameters are within the norm, temperature plays a leading role in the growth of S. horneri. The growth temperature needs to reach the biological lower limit for this macroalga, and a certain temperature accumulation is required to complete the entire life cycle.
From the beginning of the 20th century up to the 21st century, global warming events have frequently occurred, causing the global seawater temperature to constantly increase [21,22,23] and greatly affecting the marine ecosystem [24,25,26], including the growth of S. horneri. Within an appropriate temperature range, increasing temperatures promote the growth and proliferation of this species [27], but excessive temperatures have a negative impact on it, causing its decline and the disintegration of its biological structure [28]. Related to this, the algal field function will be degraded, and the frequency and scale of the golden tide outbreaks in the Yellow and East China Seas are constantly changing.
This study explored the relationship between the proliferation of attached S. horneri and seawater temperature by monitoring the interannual variation in sea surface temperature (SST) in the area near Gouqi Island and the changes in the growth and proliferation of the macroalga in a local mussel farm, combined with the study of cumulative temperature. This relationship effectively reflected the impact of the rise in seawater temperature on the growth and reproduction of S. horneri within the context of global warming. This study will provide the theoretical basis for follow-up research on the assessment of risks associated with golden tides, early warning management, and analysis of outbreak mechanisms in China. The results will also assist in the development of seaweed fields and marine ranching planning and reveal the potential outbreak mechanism of the golden tide from the perspective of an algae physiology response to environmental factors.
2. Materials and Methods
2.1. Collection of S. horneri Samples
Gouqi Island (30°41′–30°44′ N, 122°44′–122°48′ E), which is part of the Maan Islands, Zhoushan Archipelago, is rich in marine fishery resources, and a large biomass of S. horneri is present around the island reefs [29]. The waters around the island represent one of the largest mussel aquaculture areas in China, with an area of over 6.6 km2, and a large number of S. horneri are attached to the aquaculture rafts. Macroalgal samples were collected every month from August 2021 to July 2022 (before the outbreak of the local golden tides) in the mussel culture area of Houtou Bay and Gouqi Island, and their growth status was monitored. As shown in Figure 1, three sampling points, namely A (30°43′40″, 122°45′51″), B (30°43′52”, 122°45′4″), and C (30°43′48″, 122°44′56″), were randomly set, and 30 samples were randomly collected from different culture cables each time.
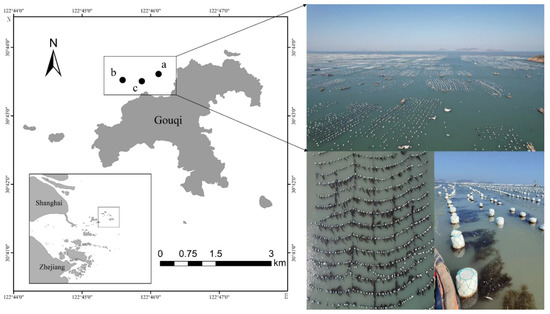
Figure 1.
Map showing the stations randomly selected for the sampling of attached S. horneri in the mussel culture area of Gouqi Island.
2.2. Monitoring of the Growth State of Attached S. horneri in the Aquaculture Area
The chlorophyll fluorescence parameters (PSII maximum fluorescence quantum yield (Fv/Fm)) were measured from the blades of the freshly collected samples using a DIVING- PAM-II/R (WALZ, Germany) instrument on-site. Then, the samples were placed in a low-temperature incubator (4 °C) and were covered with a wet towel soaked in seawater. They were taken to the laboratory within 24 h, and the water on their surface was dried with absorbent paper. Then, the plants were counted, and their growth stage, length, and weight (wet weight) were recorded. The appearance of vesicles and reproductive trays was monitored, and pictures were taken as records. Plant length (cm) was defined as the distance from the root fixator to the top of the algal body and was measured with a tape measure (Chenguang, China); plant weight (g) was measured using a weighing balance (Chenguang, China). The content of photosynthetic pigments (chlorophyll a and carotenoids) was measured from selected leaf portions of the algal body.
To determine chlorophyll a and carotenoids, first, nine samples were randomly selected from three points; then, 0.2 g of the algal body (fresh weight) was weighed and added to 2 mL of 90% acetone. The mixture was then ground into a homogenate and again added to acetone up to a volume of 10 mL. Extraction was carried out at 4 °C for 24 h in the dark and was followed by centrifugation (3–18K, Sigma, Germany) at 5000× g for 10 min. Then, the obtained supernatant was measured with a UV spectrophotometer (SPUV 2500, Spectrum, China) to test absorbances at 666, 653, and 470 nm. Chlorophyll a and carotenoids were calculated according to the following equations for element content [30]:
Chlorophyll a (mg · mg−1) = (15.65×A666 − 7.34 × A653) × V/(1000 × W)
Carotenoids (mg · mg−1) = (1000 × A470 + 1403.57 × A666 − 3473.87 × A653) × V/(221 × 1000 × W) (V: final volume after constant volume, W: the fresh weight of the algae)
The following equations were used to calculate the growth rate:
Vp (cm · d) = Lp/D
Vb (g · d) = Bb/D
(Vp: monthly growth in algal body length, Lp: difference in body length between the current and previous month, Vb: monthly growth in algal body biomass (wet weight), Bb: difference in biomass (wet weight) between the current and previous month, D: days in the current month)
2.3. Monitoring SST in the Area near Gouqi Island
From August 2021 to July 2022, the HOBO Bluetooth underwater light and temperature recorder (MX2202, ONSET, USA) was used in sampling area A (30°43’40”, 122°45’51”) in Houtouwan, Gouqi Island, to record changes in local SST. The data for this parameter for other years were obtained from the official website of NASA (https://oceancolor.gsfc.nasa.gov/l3/) (accessed on 1 July 2022).
2.4. Calculation of the Effective Accumulated Temperature
Enzymatic reactions in seaweeds are affected by temperature, which regulates growth and proliferation activities [31]. Within a suitable temperature range, the metabolic capacity of Sargassum spp. increases with the rise in temperature; in contrast, at higher or lower than optimum temperatures, this capacity decreases. If the temperature is lower than the biological lower limit for Sargassum spp. or higher than the biological upper limit, seaweeds enter a stagnation phase or decline and disintegrate [27]. In this study, the biological lower and upper limits for S. horneri were defined as 7.1 °C and 25.0 °C, respectively [3,32].
The effective accumulated temperature K was calculated based on Li et al. [33] using the following equation:
K (°C·d) = N (T-C)
(K: effective accumulated temperature, N: development period (i.e., the time required for growth and development), T: average temperature during the development period, C: development starting temperature (i.e., the biological lower limit temperature for S. horneri))
2.5. Data Processing and Analysis
The variations in biological parameters, chlorophyll content, photosynthetic parameters, SST, and effective accumulated temperature of copper algae were measured regularly and were visualized through variation curves generated using Origin software. One-way ANOVA analysis was performed in SPSS 20.0, and the significance level was set at 0.05.
3. Results
3.1. Variation in SST near Gouqi Island in 2021–2022 and in the Previous 5 Years
Gouqi Island is located in the East China Sea at the mouth of the Yangtze River and is affected by both the Taiwan warm current and the freshwater input from the Yangtze River Estuary. Figure 2 shows water temperature variation throughout the monitoring period (2021–2022): from August to September 2021, the SST near Gouqi Island ranged from 24 to 29 °C, and the average temperature exceeded the biological upper limit for S. horneri, reaching 26.8 °C; from October 2021, SST began to decrease, with the average dropping from 23.2 °C to 11 °C, and was the lowest in February 2022, with a variation range of 12–10 °C; from March 2022, it increased again, with the monthly average temperature reaching approximately 14 °C (Figure 2).
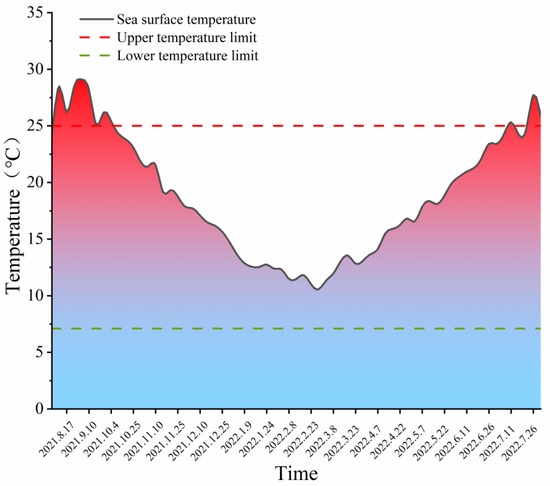
Figure 2.
Variation of SST in Gouqi Island from August 2021 to July 2022.
Figure 3 shows the variation in SST in the waters near Gouqi Island from 2017 to 2022 and indicates that this parameter increased each year during the winter period from December to February of the following year. The mean SST in December 2017 increased from 14.5 °C to 16.2 °C, and the temperatures in January and February of the following year increased from 10.5–9 °C to 12.7–11.5 °C, respectively. On average, the interannual monthly temperature increased by 2–3 °C, and the warm winter phenomenon became more and more obvious. The SST in winter and spring gradually increased every year; in March and April 2022, it increased from 11–16 °C to 14–17 °C, and in April 2022, the SST rose by about 1–2 °C.
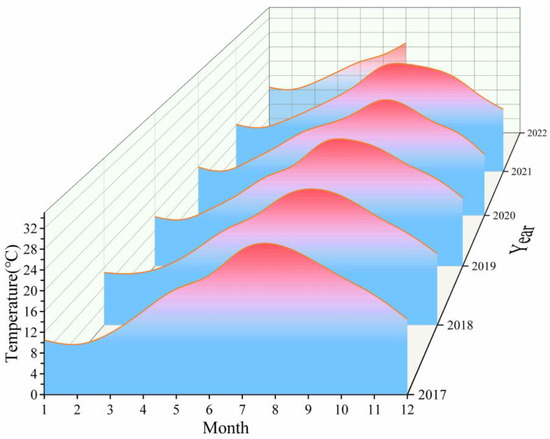
Figure 3.
Variation of SST at Gouqi Island from 2017 to 2022.
3.2. Morphological Characteristics of Attached S. horneri across Four Seasons between 2021 and 2022
Figure 4 shows the morphological characteristics of attached S. horneri in the mussel culture area of Gouqi Island from August 2021 to July 2022. The characteristics observed in August, October, December, and February were considered representative of the macroalga’s morphology in summer, autumn, winter, and spring, respectively.
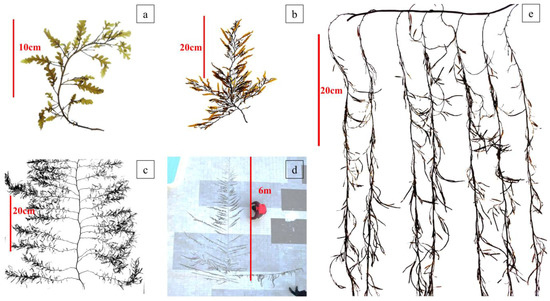
Figure 4.
Morphological characteristics of S. horneri in the aquaculture area across seasons: (a) summer; (b) autumn; (c) winter; (d) spring; and (e) after March 2022.
The on-site monitoring and investigation showed that in August 2021, the germination of S. horneri in the aquaculture area was in the seedling stage, the plant was brown overall, the main branch was distinct, there were a few main lateral branches and blades, there were no obvious secondary lateral branches, and the blades were wide and serrated. The bottom of the root presented a colloidal crack-shaped retainer, which was used to attach to the breeding cable or mussel string, and only 26.08% of the algae showed a small number of vesicles, mainly located in the middle and top sections of the body. Most algae consist of only three parts: roots, stems, and blades. In October 2021, algal biomass increased, and 96% of the plants presented, on average, 171 vesicles each. In some algae, the main lateral branches at the center of the main branch were evenly arranged, and their length was uniform, forming a “net curtain” shape. Morphological changes tended to be stable in December; the blades were less serrated and became soft, and at the same time, the main lateral branches near the root grew longer and even approached or exceeded the length of the main branch. The length of the main lateral branches decreased, moving toward the top of the algal structure. Some algae formed an obvious secondary collateral structure, and the whole body was umbrella-shaped. In February 2022, 80% of the attached algae were in their reproductive stage and began to release sperm and eggs, their color gradually turning copper yellow. In addition, about 10% of their blades fell off, leaving only the vesicles and receptacles. In March 2022, S. horneri began to decline across the aquaculture area, and the decline continued until May.
3.3. Variation in the Monthly Photosynthetic Activity of Attached S. horneri from 2021 to 2022
From August to November 2021, the contents of chlorophyll a and carotenoids in the blades of attached S. horneri slowly increased; the former increased from 0.9 mg·mg−1 to 1.2 mg·mg−1, while the latter did not increase significantly and remained at about 0.3 mg·mg−1 (Figure 5). In December, chlorophyll a content decreased to 1.1 mg·mg−1, while carotenoids did not change significantly. In January 2022, both parameters increased significantly to 1.5 mg·mg−1 and approximately 0.5 mg·mg−1, respectively. In February 2022, the variation in chlorophyll content showed a downward trend again. The Fv/Fm value was relatively low from August to September 2021, ranging from 0.65 to 0.7, and it increased significantly to 0.75 from October to December. In January 2022, it further increased significantly to a maximum of 0.8 and decreased to 0.74 in January 2022. Due to the deterioration of algae in a large section of the aquaculture area in March 2022, the leaves detached from the rafts, and no samples could be collected for the determination of chlorophyll content and chlorophyll fluorescence parameters (Fv/Fm) after that month.
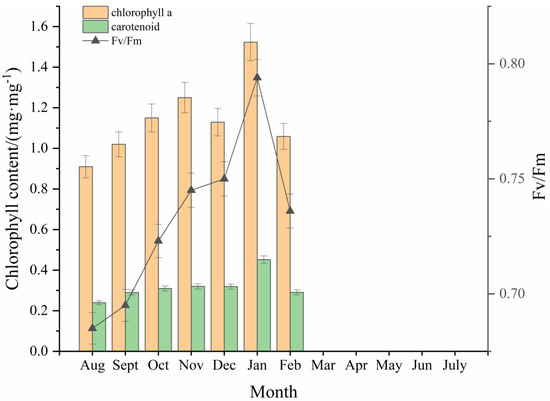
Figure 5.
Variation in chlorophyll A, carotenoid content, and Fv/Fm in the leaves of attached S. horneri.
3.4. Relationship between Growth Period and Effective Accumulated Temperature in Attached S. horneri
From August to September 2021, the SST in the area of Gouqi Island continued to rise from 24 °C to nearly 29 °C, the average exceeding the biological upper limit for S. horneri, and the growth of seedlings was slow. The algae’s length and average biomass increased from 15.3 cm to only 23.7 cm and from 3.6 g to 5.3 g (wet weight), respectively. At the end of August 2021, S. horneri individuals grew vesicles; from October to December 2021, the SST near Gouqi Island began to gradually decrease, dropping below the biological upper limit of 25 °C, and the algae’s growth rate began to increase continuously. Once adapted to the decrease in SST, the algae entered a period of rapid growth between January and February 2022; the maximum growth rates for individual biomass and plant length were reached in January and February 2022, respectively. In February, the algae present in the cultured area reached the growth “peaks,” with average and maximum plant lengths of 308 cm and 612 cm, respectively, and average and maximum individual biomasses of 511 g and 1265 g, respectively (Figure 6). During this period, S. horneri individuals matured and developed receptacle structures then began to release sperm and eggs and entered the reproductive period. However, a small number of algae have completed the reproduction process, and the leaves fall off and begin to enter the biological disintegration process. In March 2022, leaf and vesicle shedding occurred over a large section of the aquaculture area, leading to a dramatic reduction in algal biomass. The attached algae entered the stages of senescence and apoptosis, which lasted until May 2022.
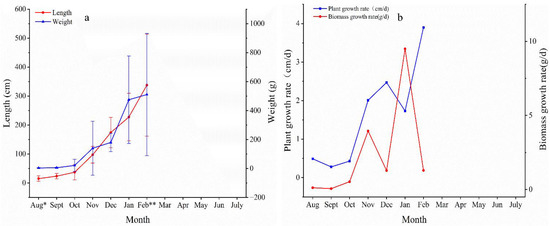
Figure 6.
Monthly variation in the plant length and individual biomass of S. horneri in the aquaculture area; (a) monthly variation in plant length and biomass; (b) rate of length and biomass variation. * Appearance of vesicles; ** appearance of receptacle.
During the life cycle of the attached S. horneri in the study area, the effective accumulated temperature increased from 645.1 °C·d in August 2021 to 2772.4 °C·d in February 2022. Throughout the life history of S. horneri from August 2021 to February 2022, the effective accumulated temperature continued to be stored (Figure 7). The analysis showed that this parameter increased with the growth cycle of S. horneri according to a cubic function. From August to October 2021, SST slowly decreased, and the increase in effective accumulated temperature remained basically stable; from November 2021 to February 2022, SST continued to decrease around Gouqi Island, and the effective accumulated temperature growth rate dropped sharply.
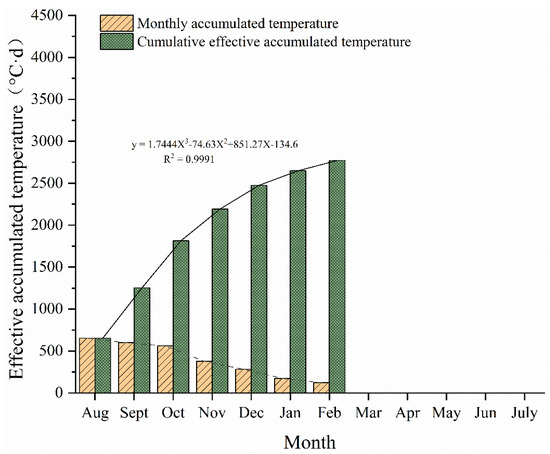
Figure 7.
Monthly variation in effective accumulated temperature.
4. Discussion and Conclusions
4.1. Temperature Adaptability of Attached S. horneri in the Mussel Culture Area near Gouqi Island
Temperature is an important abiotic factor that controls the growth rate, life cycle, and distribution of algae. S. horneri individuals at different growth stages respond differently to the same temperature, and their growth rates will also greatly differ. Similarly, under different temperatures, individuals at the same growth stage exhibit different levels of adaptability to this parameter [27,34]. From August to September 2021, the average SST reached 26–27 °C with peaks of 29 °C, which was far beyond the upper limit temperature for S. horneri. However, the chlorophyll content of the seedlings increased, together with the value of Fv/Fm, and biomass growth could still be maintained, indicating that photosynthesis was normal and the algae were at a low-speed growth stage. In March 2022, adult S. horneri entered the decline stage, which indicated that they had a considerably weaker heat tolerance ability compared to seedlings. From October 2021 to February 2022, the temperature continuously declined, and the variation in chlorophyll content and Fv/Fm showed a phased change together. In December 2021, when S. horneri entered the rapid growth stage, the content of chlorophyll a decreased after a relatively stable growth, while the content of carotenoids did not change. At this time, Fv/Fm stabilized, and the growth rate of the algal biomass was also lower than that in the previous month. Therefore, in December 2021, algal growth entered the “low-temperature adaptation period.” After this stage, in January 2022, a rapid increase in photosynthetic activity was observed, and S. horneri in the aquaculture area entered the rapid growth and reproduction period in February 2022.
Taxonomically, S. horneri can be divided into the “spring mature” and “autumn mature” types [35,36,37]. The former could be identified in the study area based on the algae’s reproductive characteristics and previous studies on attached S. horneri conducted in the local aquaculture area [10]. Based on the characteristics of algal growth and development and on the observed variations in SST, plant length, and individual biomass, the growth of attached S. horneri in the aquaculture area of Gouqi Island can be divided into five stages: (1) the first suitable high-temperature period (August to December), (2) the decreasing temperature period (October to November), (3) the low-temperature adaptation period (December), (4) the second suitable low-temperature period (January to February), and (5) the high-temperature decay period (February to May) (Figure 8).
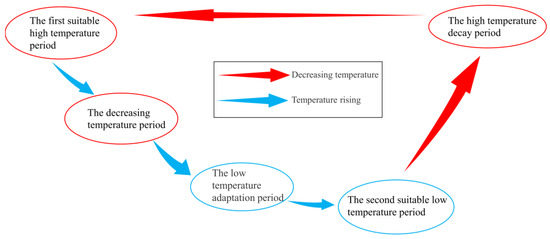
Figure 8.
Growth stages of S. horneri.
4.2. Analysis of the Effect of Global Warming on the Growth and Reproduction of Attached S. horneri near Gouqi Island Based on the Effective Accumulated Temperature Theory
From August 2021 to February 2022, the effective accumulated temperature required by S. horneri to complete its life cycle in the aquaculture area of Gouqi Island was about 2700–2800 °C·d. A previous study on subtidal S. horneri at this site conducted between 2013 and 2014 [33] found that the effective accumulated temperature needed was 3600–3700 °C·d, about 800 °C·d more than the temperature observed in the present study. Ding Xiaowei et al. reported that the required temperature from 2017 to 2018 was 3300–3400 °C·d [10]. The receptacle structure of S. horneri is formed around April [38], and the effective accumulated temperature required to enter the flowering period is calculated to be about 2900–3000 °C·d, about 200 °C·d more than the values observed in 2022. As previously mentioned, plants need to achieve a certain amount of effective accumulated temperature to complete their life cycle. The reason for the great difference in effective accumulated temperature required for the same developmental stage may be explained by global warming.
From December 2021 to January–February 2022, the water temperature around Gouqi Island was, on average, 3–4 °C higher than that observed from December 2013 to January–February 2014 and 1–2 °C higher than that from December 2018 to January–February 2019. An obvious “warm winter” climate is now established, and its intensity increases each year. Consequently, S. horneri, which should still be in the “low-temperature adaptation period” at this time, activates gene regulation and enzymes under favorable temperature conditions. This enhanced activity shortens the “low-temperature adaptation period,” causing the algae to enter the “low-temperature reproductive period” in advance, which promotes the formation of the receptacle. The continuous high temperature for an extended period of time also makes the reproductive period earlier than it should be. In this study, it was observed that the release of sperm and eggs, which normally occurs around May, was advanced to February. A small number of S. horneri individuals completed the reproductive phase and began to disintegrate and die over a large section of the aquaculture area. The algae entered the “high-temperature decline period” about 3 months earlier than expected. Therefore, the continuous high temperatures in winter caused by global warming may cause the algae to complete their life cycle in advance before reaching the effective accumulated temperature required for the growth stage, fitting their entire life cycle into a much shorter period. Even the population that was originally classified as “spring-mature” will form a new “winter-mature” population due to this dramatic temperature variation.
4.3. Golden Tides and the Early Warning Problem
The seasonal variation in and succession of algal growth are limited by seawater temperature [39,40,41,42]. The reproductive period varies [43,44], and changes in SST control the biogeographical distribution of marine algae [45,46]. Due to global warming, the temperature of coastal seawater in China increases each year, leading to changes in the growth and proliferation of S. horneri. Similarly, the time point of the golden tide bloom may also be advanced because the life cycle is compressed. The occurrence of golden tides in the Yellow and East China Sea will also change due to the early maturity stage because only the S. horneri populations that reach a certain growth level begin to decline, detach from the matrix, and move to a free-floating state [10]. Therefore, by monitoring seawater temperature and algal growth, it is possible to predict the outbreaks and declines of golden tides, effectively avoiding a number of economic losses associated with them. First of all, in the waters around Gouqi Island, the annual golden tide outbreak causes a large amount of algae to accumulate on the coastal beaches, which affects the income of the local tourism season and brings great trouble to local tourism practitioners. In addition, a large amount of accumulated algae will sink to the seabed after rotting, which will cause a devastating blow to the local benthos and seriously threaten the ecosystem safety of Gouqi Island. Finally, the economic losses caused by the collapse of laver and other cultivation rafts during the golden tide outbreak are numerous. Therefore, it is particularly important to establish a reliable and mature early warning mechanism for the outbreak of golden tides.
At the same time, by monitoring temperature variation in different sea areas and the growth of local S. horneri populations and combining the data on the occurrence and frequency of golden tides in the Yellow and East China Seas, it is possible to identify the potential sources of golden tides in these regions and provide a scientific basis to trace this harmful phenomenon. In addition, the temperature early warning system based on the results of this study can be used not only for the monitoring of golden tides but also for the research into other harmful algal blooms, such as green tides and red tides, all of which have certain scientific research value.
Author Contributions
Data curation, T.W., L.X., M.Z. (Minmin Zhuang), P.H. and J.Z.; Formal analysis, T.W. and X.S.; Funding acquisition, P.H., Y.Q. and J.Z.; Methodology, Z.Z. and M.Z. (Meijing Zhang); Writing—original draft, T.W. and L.X.; Writing—review and editing, P.H., J.Z., J.P., J.L., W.D., Z.Z. and M.Z. (Minmin Zhuang). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFC3106001), the Natural Science Foundation of Shanghai (21ZR1427400), and the Project of Shanghai Municipal Bureau of Oceanography (2022-03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tseng, C.E.U. Common Seaweeds of China; Science Press: Beijing, China, 1983; p. 316. [Google Scholar]
- Zeng, C.K.; Lu, B.R. Records of China Seaweed (Vol. 3 Phaeophyta Vol. 2 Chaetoceros); Science Press: Beijing, China, 2000; pp. 43–44. [Google Scholar]
- Sun, J.Z.; Chen, W.D.; Zhuang, D.G.; Zheng, H.Y.; Lin, L.; Pang, S.J. In situ ecological studies of the subtidal brown alga Sargasssum horneri at Nanji Island of China. South China Fish. Sci. 2008, 3, 58–63. [Google Scholar] [CrossRef]
- Sun, J.Z.; Zhuang, D.G.; Yang, J.B.; Chen, W.D.; Wang, T.G.; Pang, S.J. Primary study on enhancement technique of Sargassum horneri around Nanji Island. Mod. Fish. Inf. 2010, 25, 23–27. [Google Scholar] [CrossRef]
- Sun, J.Z.; Zhuang, D.G.; Chen, W.D.; Zheng, H.Y.; Lin, L.; Pang, S.J. Studies on sexual reproduction and seedling production of the brown alga Sargassum horneri. South China Fish. Sci. 2008, 2, 6–14. [Google Scholar] [CrossRef]
- Li, D.P.; Li, H.; Zhang, G.J.; Ma, Z.L.; Xin, M.L.; Ding, G.; Wu, H.Y.; Guo, W. Studies on the reproduction and development of Sargassum horneri. Mar. Sci. 2018, 42, 40–45. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Wang, M.; Shang, S.; Wilson, C. Floating Algae Blooms in the East China Sea. Geophys. Res. Lett. 2017, 44, 11501–11509. [Google Scholar] [CrossRef]
- Zhang, P.; Cai, Y.F.; Wang, T.G.; Zhong, C.H.; Xie, Q.L.; Chen, S.B.; Wang, N. AFLP analysis of different geographic populations of Sargassum horneri along the coast of Zhejiang Province. Acta Agric. Zhejiangensis 2015, 27, 1586–1592. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Chen, J.J.; Zhu, Q.; Wu, X.K.; Luo, Q.J.; Yang, R.; Chen, H.M. Analysis of pigments in floating and fixed Sargassum horneri by high performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry. J. Nucl. Agric. Sci. 2019, 33, 1173–1180. [Google Scholar] [CrossRef]
- Ding, X.W.; Zhang, J.H.; Zhuang, M.M.; Kang, X.Y.; Zhao, X.H.; He, P.M.; Liu, S.R.; Liu, J.F.; Wen, Y.; Shen, H.; et al. Growth of Sargassum horneri distribution properties of golden tides in the Yangtze Estuary and adjacent waters. Mar. Fish. 2019, 41, 188–196. [Google Scholar] [CrossRef]
- Wu, Z.L.; Cheng, L.R.; Wang, K.; Zhang, S.Y.; Bi, Y.X. Morphological characteristics of vesicle of Sargassum horneri and its relationship to environmental factors in Gouqi Island. J. Fish. China 2020, 44, 793–804. [Google Scholar] [CrossRef]
- Zhang, J.H.; Ding, X.W.; Zhuang, M.M.; Wang, S.Y.; Chen, L.; Shen, H.; He, P.M. An increase in new Sargassum (Phaeophyceae) blooms along the coast of the East China Sea and Yellow Sea. Phycologia 2019, 58, 374–381. [Google Scholar] [CrossRef]
- Xing, Q.; Guo, R.; Wu, L.; An, D.; Cong, M.; Qin, S.; Li, X. High-Resolution Satellite Observations of a New Hazard of Golden Tides Caused by Floating Sargassum in Winter in the Yellow Sea. IEEE Geosci. Remote Sens. Lett. 2017, 14, 1815–1819. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- State Oceanic Administration. Bulletin of China Marine Ecological Environment Status. 2017; pp. 55–56. Available online: http://gc.mnr.gov.cn/201806/t20180619_1797652.html. (accessed on 1 July 2022).
- Hiraoka, M.; Masao, O.; Shigeo, K.; Goro, Y. Crossing test among floating Ulva thalli forming ′green tide′ in Japan. Hydrobiologia 2004, 512, 239–245. [Google Scholar] [CrossRef]
- Abdelrhman, A.M.; Mohamed, A.; Mohamed, A.A.; Zaka, Z.S.; Hany, N.; Mohamed, A.A.Z.; Norhan, H.A.; Sherine, R.A.; Ehab, E.; Hien, V.D.; et al. Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquac. Rep. 2022, 25, 101212. [Google Scholar] [CrossRef]
- Abdallah, T.M.; Ahmed, E.A.; Khamael, M.A.; Hossam, S.E.; Khaled, M.A.R.; Mohamed, A. Dried Brown Seaweed’s Phytoremediation Potential for Methylene Blue Dye Removal from Aquatic Environments. Polymers 2022, 14, 1375. [Google Scholar] [CrossRef]
- Goro, Y.; Koji, Y.; Toshinobu, T. Growth and maturation of two populations of Sargassum horneri (Fucales, Phaeophyta) in Hiroshima Bay, the Seto Inland Sea. Fish. Sci. 2001, 67, 1023–1029. [Google Scholar] [CrossRef]
- Ogawa, H. The maturation and early development of Sargassaceous plants and the effects of environment to them. Gekkan Kaiyo-Kagaku 1985, 17, 26–31. [Google Scholar]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global climate projections. In Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 749–846. [Google Scholar]
- Ji, Y.; Xu, Z.; Zou, D.; Gao, K. Ecophysiological responses of marine macroalgae to climate change factors. J. Appl. Phycol. 2016, 28, 2953–2967. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; Gouezo, M.; Tilbrook, B.; Dove, S.; Anthony, K.R.N. High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 2011, 14, 156–162. [Google Scholar] [CrossRef]
- Wernberg, T.; Bennett, S.; Babcock, R.; De Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.; Hovey, R.; et al. Climate-driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Levac, E.; Guelphen, L.; Pohle, G.; Chmura, G. The effect of global climate change on the future distribution of economically important macroalgae (seaweeds) in the Northwest Atlantic. Facets 2018, 3, 275–286. [Google Scholar] [CrossRef]
- Zhang, J. Indoor Cultivation of the Brown Alga Sargassum Vachellianum and Sargassum horneri: Morphological Observation and Techniques of Artificial Seeding. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2012; pp. 50–51. [Google Scholar]
- Wang, Y.; Zhong, Z.H.; Qin, S.; Li, J.L.; Li, J.J.; Liu, Z.Y. Effects of temperature and light on growth rate and photosynthetic characteristics of Sargassum horneri. J. Ocean Univ. China 2021, 20, 101–110. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Liu, H.S.; Zhang, J.W. Research on the benthic algae distribution around Gouqi Island using digital echo sounding system and interpolation. J. Shanghai Ocean. Univ. 2012, 21, 445–451. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Zhao, S.J. Marine Algae Ecology; Ocean Press: Beijing, China, 2014; p. 164. [Google Scholar]
- Wu, Z.L.; Zhang, S.Y. Effect of typhoon on the distribution of macroalgae in the seaweed beds of Gouqi Island, Zhejiang Province. J. Agric. Sci. Technol. 2019, 21, 159–168. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, S.Y.; Wang, K.; Chen, L.R.; Chen, T.H. Effect of annual temperature variation on growth of Sargassum horneri in Gouqi Island. Oceanol. Limnol. Sin. 2020, 51, 1136–1143. [Google Scholar]
- Choi, H.G.; Lee, K.H.; Yoo, H.I.; Kang, P.J.; Kim, Y.S.; Nam, K.W. Physiological differences in the growth of Sargassum horneri between the germling and adult stages. J. Appl. Phycol. 2008, 5, 29–735. [Google Scholar] [CrossRef]
- Okuda, T. Monoecism and autumn-fruiting of S. horneri. Jpn. J. Phycol. 1987, 37, 279–283. [Google Scholar]
- Okuda, T.; Satoh, Y. Conceptacular development in Sargassum filicinum and autumnal S. horneri (Phaeophyceae). Jpn. J. Phycol. 1989, 37, 279–283. [Google Scholar]
- Uchida, T.; Arima, S. Crossing experiments between autumn and spring fruiting types of Sargassum horneri (Phaeophyta). Nippon. Suisan Gakkaishi 1993, 59, 1685–1688. [Google Scholar] [CrossRef][Green Version]
- Chen, L.R.; Zhang, S.Y.; Chen, Y.; Zhao, X.; Zhou, X.J.; Chen., Y.Z. Life history and morphology of Sargassum horneri from the Sargassum seaweed bed of Gouqi Island. J. Fish. China 2015, 39, 1218–1229. [Google Scholar]
- McCourt, R.M. Seasonal patterns of abundance, distributions, and phenology in relation to growth strategies of three Sargassum species. J. Exp. Mar. Biol. Ecol. 1984, 74, 141–156. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Ragaza, A.R. Sargassum studies in Currimao, Ilocos Norte, Northern Philippines I. Seasonal variations in the biomass of Sargassum carpophyllum J. Agardh, Sargassum ilicifolium (Turner) C. Agardh and Sargassum siliquosum J. Agardh(Phaeophyta, Sargassaceae). Bot. Marina 1999, 42, 321–325. [Google Scholar] [CrossRef]
- Díaz-Villa, T.; Sansón, M.; Afonso-Carrillo, J. Seasonal variations in growth and reproduction of Sargassum orotavicum (Fucales, Phaeophyceae) from the Canary Islands. Bot. Marina 2005, 48, 18–29. [Google Scholar] [CrossRef]
- Rivera, M.; Scrosati, R. Population dynamics of Sargassum lapazeanum (Fucales, Phaeophyta) from the Gulf of California, Mexico. Phycologia 2006, 45, 178–189. [Google Scholar] [CrossRef]
- Taylor, W.R. Marine Algae of the Eastern Tropical and Subtropical Coasts of the Americas; University of Michigan Press: Ann Arbor, MI, USA, 1960; p. 167. [Google Scholar]
- Deysher, L.E. Reproductive phenology of newly introduced populations of the brown alga, Sargassum muticum (Yendo) Fensholt. Hydrobiologia 1984, 116–117, 403–407. [Google Scholar] [CrossRef]
- Ang, P.O. Phenology of Sargassum spp. in Tung Ping Chau Marine Park, Hong Kong SAR, China. J. Appl. Phycol. 2006, 18, 629–636. [Google Scholar] [CrossRef]
- Komatsu, T.; Fukuda, M.; Mikami, A.; Mizuno, S.; Kantachumpoo, A.; Tanoue, H.; Kawamiya, M. Possible change in distribution of seaweed, Sargassum horneri, in northeast Asia under A2 scenario of global warming and consequent effect on some fish. Mar. Pollut. Bull. 2014, 85, 317–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).