Responses of Marine Diatom–Dinoflagellate Interspecific Competition to Different Phosphorus Sources
Abstract
1. Introduction
2. Methods and Materials
2.1. Algae and Culture Conditions
2.2. Experimental Design
2.3. Data Analysis
2.3.1. Growth Simulation in Bi-Algal Cultures
2.3.2. Statistical Analysis
3. Results
3.1. Growth Competition of S. costatum and P. donghaiense
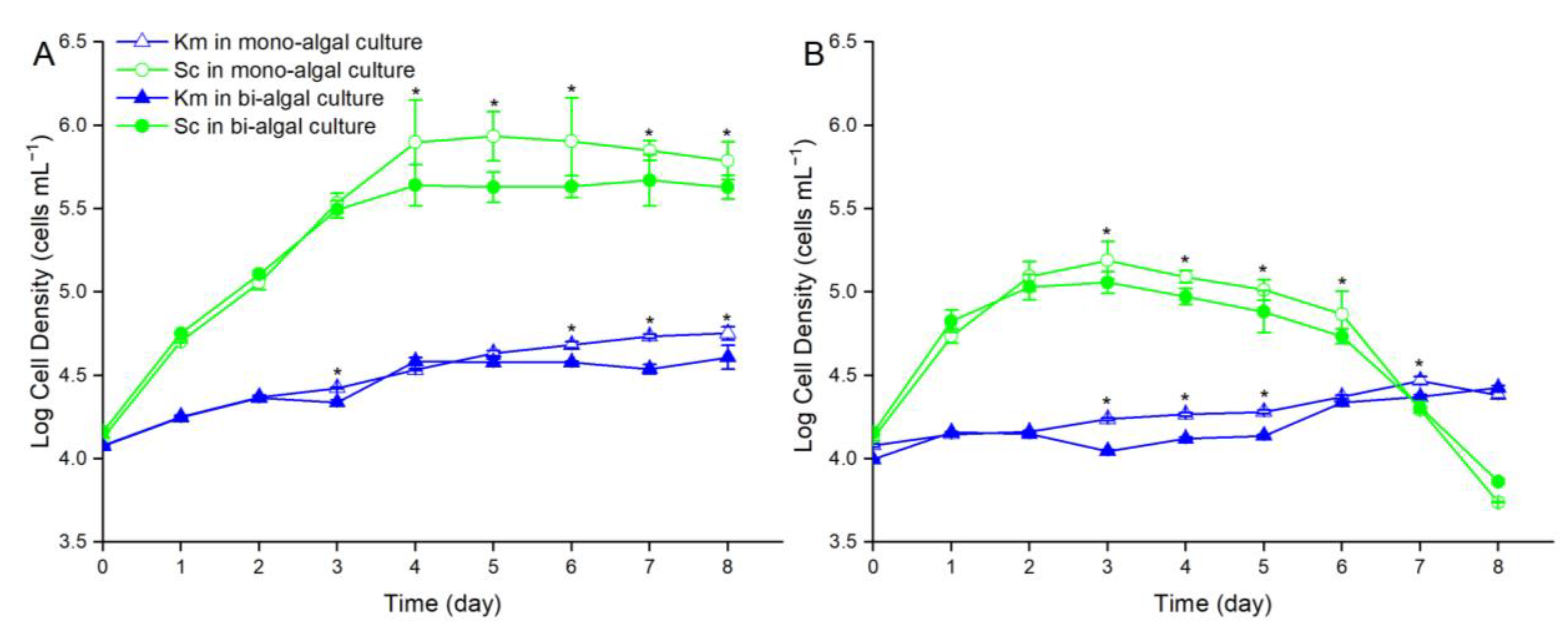
3.2. Growth Competition of S. costatum and K. mikimotoi
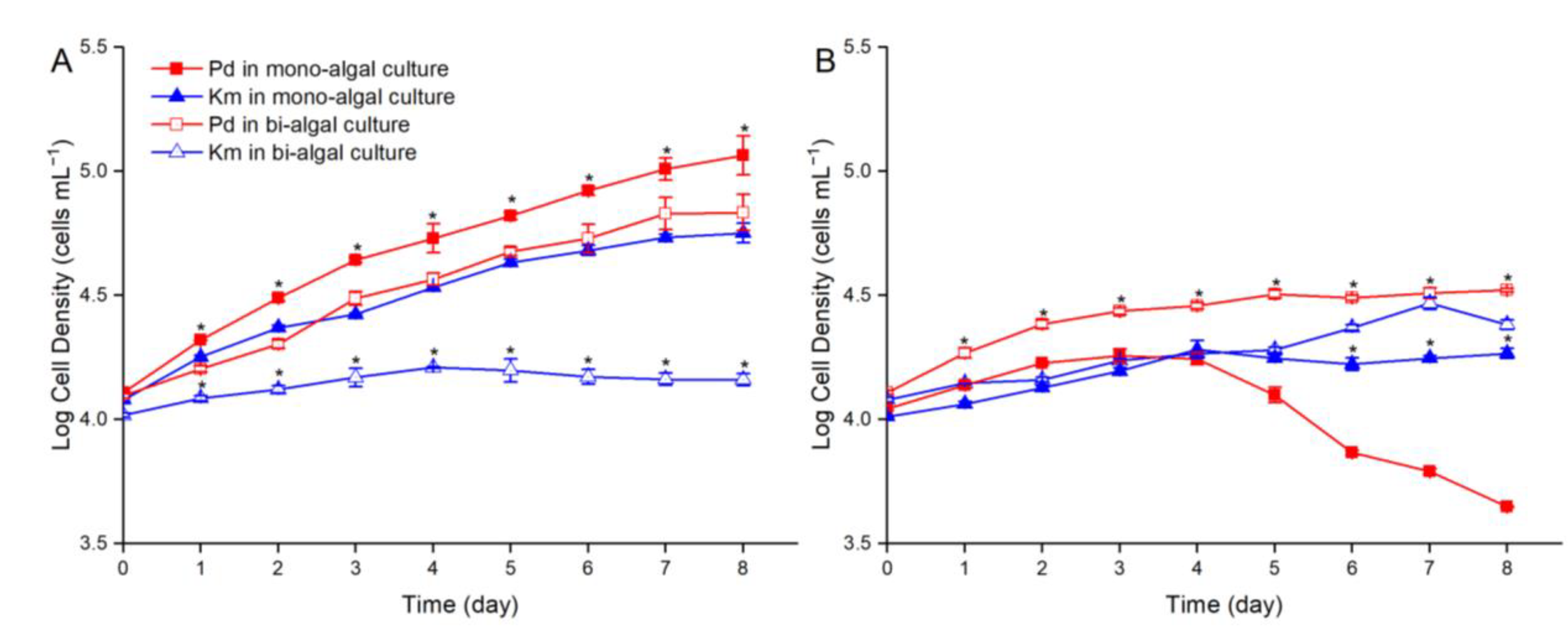
3.3. Growth Competition of P. donghaiense and K. mikimotoi
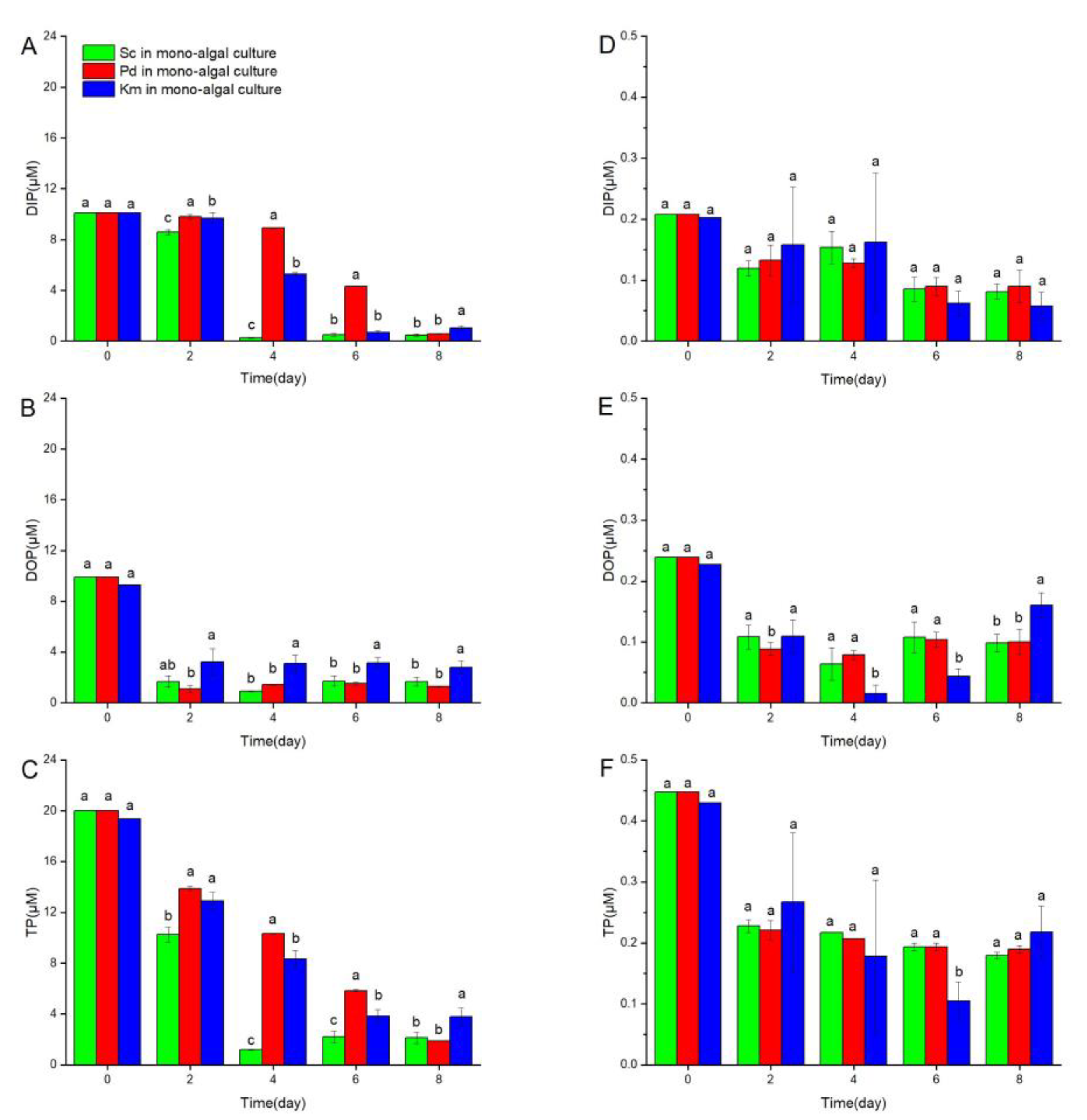
3.4. Changes in DIP, DOP, and TP
3.5. Changes in AP Activity
4. Discussion
4.1. Interspecific Competition Mechanism under P-Sufficient Condition
4.2. Interspecific Competition Mechanism under P Deficient Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, R.C.; Lu, S.H.; Qi, Y.Z.; Zhou, M.J. Progress and perspectives of harmful algal bloom studies in China. Oceanol. Limnol. Sin. 2020, 51, 768–788, (In Chinese with English abstract). [Google Scholar]
- Yu, Z.M.; Chen, N.S. Emerging trends in red tide and major research progresses. Oceanol. Limnol. Sin. 2019, 50, 474–486, (In Chinese with English abstract). [Google Scholar]
- Chen, N.S.; Chen, Y. Advances in the study of biodiversity of phytoplankton and red tide species in China (II): The East China Sea. Oceanol. Limnol. Sin. 2021, 52, 363–384, (In Chinese with English abstract). [Google Scholar]
- Bi, R.; Cao, Z.; Ismar-Rebitz, S.M.H.; Sommer, U.; Zhang, H.L.; Ding, Y.; Zhao, M.X. Responses of marine diatom-dinoflagellate competition to multiple environmental drivers: Abundance, elemental, and biochemical aspects. Front. Microbiol. 2021, 12, 731786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.P.; Zhang, Y.M.; Li, F.F.; Tan, L.J.; Wang, J.T. Nutrients structure changes impact the competition and succession between diatom and dinoflagellate in the East China Sea. Sci. Total Environ. 2017, 574, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.X.; Yu, R.C.; Zhou, M.J. Seasonal succession of microalgal blooms from diatoms to dinoflagellates in the East China Sea: A numerical simulation study. Ecol. Model. 2017, 360, 150–162. [Google Scholar] [CrossRef]
- Gu, H.F.; Wu, Y.R.; Lü, S.H.; Lu, D.D.; Tang, Y.Z.; Qi, Y.Z. Emerging harmful algal bloom species over the last four decades in China. Harmful Algae 2022, 111, 102059. [Google Scholar] [CrossRef]
- Smayda, T.J.; Reynolds, C.S. Community assembly in marine phytoplankton: Application of recent models to harmful dinoflagellate blooms. J. Plankton Res. 2001, 23, 447–461. [Google Scholar] [CrossRef]
- Lin, S.J.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wu, H.; Gao, L.; Shen, F.; Liang, X.S. Spatial distribution and physical controls of the spring algal blooming off the Changjiang River Estuary. Estuar. Coast. 2019, 42, 1066–1083. [Google Scholar] [CrossRef]
- Fang, T.H. Phosphorus speciation and budget of the East China Sea. Cont. Shelf Res. 2004, 24, 1285–1299. [Google Scholar] [CrossRef]
- Huang, B.Q.; Ou, L.J.; Wang, X.L.; Huo, W.Y.; Li, R.X.; Hong, H.S.; Zhu, M.Y.; Qi, Y.Z. Alkaline phosphatase activity of phytoplankton in East China Sea coastal waters with frequent HAB occurrences. Aquat. Microb. Ecol. 2007, 49, 195–206. [Google Scholar] [CrossRef]
- Chu, Q.; Liu, Y.; Shi, J.; Zhang, C.; Gong, X.; Yao, X.H.; Guo, X.Y.; Gao, H.W. Promotion effect of Asian dust on phytoplankton growth and potential dissolved organic phosphorus utilization in the South China Sea. J. Geophys. Res. Biogeosci. 2018, 123, 1101–1116. [Google Scholar] [CrossRef]
- Ou, L.J.; Wang, D.; Huang, B.Q.; Hong, H.S.; Qi, Y.Z.; Lu, S.H. Comparative study of phosphorus strategies of three typical harmful algae in Chinese coastal waters. J. Plankton Res. 2008, 30, 1007–1017. [Google Scholar] [CrossRef]
- Ou, L.J.; Qin, X.L.; Shi, X.Y.; Feng, Q.L.; Zhang, S.W.; Lu, S.H.; Qi, Y.Z. Alkaline phosphatase activities and regulation in three harmful Prorocentrum species from the coastal waters of the East China Sea. Microb. Ecol. 2020, 79, 459–471. [Google Scholar] [CrossRef]
- Vila-Costa, M.; Sebastián, M.; Pizarro, M.; Cerro-Gálvez, E.; Lundin, D.; Gasol, J.M.; Dachs, J. Microbial consumption of organophosphate esters in seawater under phosphorus limited conditions. Sci. Rep. 2019, 9, 233. [Google Scholar] [CrossRef]
- Huang, B.Q.; Ou, L.J.; Hong, H.S.; Luo, H.W.; Wang, D.Z. Bioavailability of dissolved organic phosphorus compounds to typical harmful dinoflagellate Prorocentrum donghaiense Lu. Mar. Pollut. Bull. 2005, 51, 838–844. [Google Scholar] [CrossRef]
- Ou, L.J.; Huang, X.Y.; Huang, B.Q.; Qi, Y.Z.; Lu, S.H. Growth and competition for different forms of organic phosphorus by the dinoflagellate Prorocentrum donghaiense with the dinoflagellate Alexandrium catenella and the diatom Skeletonema costatum. Hydrobiologia 2015, 754, 29–41. [Google Scholar] [CrossRef]
- Pang, Y.; Nie, R.; Lu, S.H. Effects of the different kinds of phosphorus sources on growth and alkaline phosphatase activity (APA) of Karenia mikimotoi Hansen. Mar. Sci. 2016, 40, 59–64, (In Chinese with English abstract). [Google Scholar]
- Zhao, Y.F.; Yu, Z.M.; Song, X.X.; Cao, X.H. Effects of different phosphorus substrates on the growth and phosphatase activity of Skeletonema costatum and Prorocentrum donghaiense. Chin. J. Envir. Sci. 2009, 30, 693–699, (In Chinese with English abstract). [Google Scholar]
- Li, M.Z.; Li, L.; Shi, X.G.; Lin, L.X.; Lin, S.J. Effects of phosphorus deficiency and adenosine 5′-triphosphate (ATP) on growth and cell cycle of the dinoflagellate Prorocentrum donghaiense. Harmful Algae 2015, 47, 35–41. [Google Scholar] [CrossRef]
- Luo, H.; Lin, X.; Li, L.; Lin, L.X.; Zhang, C.; Lin, S.J. Transcriptomic and physiological analyses of the dinoflagellate Karenia mikimotoi reveal non-alkaline phosphatase-based molecular machinery of ATP utilization. Environ. Microbiol. 2017, 19, 4506–4518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Lin, S.J.; Liu, D.Y. Transcriptomic and physiological responses of Skeletonema costatum to ATP utilization. Environ. Microbiol. 2020, 22, 1861–1869. [Google Scholar] [CrossRef]
- Wang, J.T.; Zhang, Y.W.; Li, H.; Cao, J. Competitive interaction between diatom Skeletonema costatum and dinoflagellate Prorocentrum donghaiense in laboratory culture. J. Plankton Res. 2013, 35, 367–378. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Xu, Z.J.; Li, R.X.; Wang, Z.L.; Shi, X.Y. Interspecies competition for nutrients between Prorocentrum donghaiense Lu and Skeletonema costatum (Grey.) Cleve in mesocosm experiments. Acta Oceanol. Sin. 2009, 28, 72–82. [Google Scholar]
- Hu, H.H.; Zhang, J.; Chen, W.D. Competition of bloom-forming marine phytoplankton at low nutrient concentrations. J. Environ. Sci. 2011, 23, 656–663. [Google Scholar] [CrossRef]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J.; Rhee, G.Y. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part 2. Crit. Rev. Microbiol. 1984, 11, 13–81. [Google Scholar] [CrossRef]
- Ou, L.J.; Huang, B.Q.; Hong, H.S.; Qi, Y.Z.; Lu, S.H. Comparative alkaline phosphatase characteristics of the typical harmful algal bloom species Prorocentrum donghaiense, Alexandrium catenella and Skeletonema costatum. J. Phycol. 2010, 46, 260–265. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, R.X.; Zhu, M.Y.; Chen, B.Z.; Hao, Y.J. Study on population growth processes and interspecific competition of Prorocentrum donghaiense and Skeletonema costatum in semi-continuous dilution experiment. Adv. Mar. Sci. 2006, 24, 495–503, (In Chinese with English abstract). [Google Scholar]
- Cao, J.; Wang, J.T. The Inhibitory degree between Skeletonema costatum and dinoflagllate Prorocentrum donghaiense at different concentrations of phosphate and nitrate/phosphate ratios. J. Ocean Univ. China. 2012, 11, 153–158. [Google Scholar] [CrossRef]
- Li, R.X.; Zhu, M.Y.; Wang, Z.L.; Shi, X.Y.; Chen, B.Z. Mesocosm experiment on competition between two HAB species in East China Sea. Chin. J. Appl. Ecol. 2003, 14, 1049–1054, (In Chinese with English abstract). [Google Scholar]
- Si, D.; Zhang, N.H.; Liu, G.Q.; Xue, J.Z.; Wu, H.X. Inter-specific competition of Karenia mikimotoi and Skeletonema costatum under different nitrogen and phosphorus ratio. J. Oceanol. Limnol. 2016, 5, 118–124. [Google Scholar]
- Huang, K.X.; Xie, Y.H.; Lu, S.H. The interspecific competition between Karenia mikimotoi and Prorocentrum donghaiense in co-cultural systems. Ecol. Sci. 2009, 28, 118–122, (In Chinese with English abstract). [Google Scholar]
- Shen, A.L.; Yuan, X.W.; Liu, G.P.; Li, D.J. Growth interactions between the bloom-forming dinoflagellates Prorocentrum donghaiense and Karenia mikimotoi under different temperature. Thalassas 2014, 30, 33–45. [Google Scholar]
- Wang, A.J.; Wang, X.L.; Han, X.R.; Li, Y.B.; Zhu, C.J. Effects of solar radiation on the growth and succession of harmful algae in the East China Sea in spring. Mar. Environ. Sci 2008, 27, 144–148, (In Chinese with English abstract).. [Google Scholar]
- Uchida, T.; Toda, S.; Matsuyama, Y.; Yamaguchi, M.; Kotani, Y.; Honjo, T. Interactions between the red tide dinoflagellates Heterocapsa circularisquama and Gymnodinium mikimotoi in laboratory culture. J. Exp. Mar. Biol. Ecol. 1999, 241, 285–299. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals, 1st ed.; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; Part I; pp. 29–60. [Google Scholar]
- Ou, L.J.; Huang, B.Q.; Lin, L.Z.; Hong, H.S.; Zhang, F.; Chen, Z.Z. Phosphorus stress of phytoplankton in the Taiwan Strait determined by bulk and single-cell alkaline phosphatase activity assays. Mar. Ecol. Prog. Ser. 2006, 327, 95–106. [Google Scholar] [CrossRef][Green Version]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Jeffries, D.S.; Dieken, F.P.; Jones, D.E. Performance of the autoclave digestion method for total phosphorus analysis. Water Res. 1979, 13, 275–279. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.M.; Song, X.X.; Zhang, S.D. Interactions between the bloom-forming dinoflagellates Prorocentrum donghaiense and Alexandrium tamarense in laboratory cultures. J. Sea Res. 2006, 56, 17–26. [Google Scholar] [CrossRef]
- Tameishi, M.; Yamasaki, Y.; Nagasoe, S.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Allelopathic effects of the dinophyte Prorocentrum minimum on the growth of the bacillariophyte Skeletonema costatum. Harmful Algae 2009, 8, 421–429. [Google Scholar] [CrossRef]
- Qiu, Y.C.; Yamasaki, Y.; Shimasaki, Y.; Gunjikake, H.; Matsubara, T.; Nagasoe, S.; Etoh, T.; Matsui, S.; Honjo, T.; Oshima, Y. Growth interactions between the raphidophyte Chattonella antiqua and the dinoflagellate Akashiwo sanguinea. Harmful Algae 2011, 11, 81–87. [Google Scholar] [CrossRef]
- Wang, F.; Ge, W.; Chai, C.; Wang, J.Y.; Zhao, X.F. Effects of nutritional condition on the competitive parameters of Prorocentrum donghaiense and Skeletonema costatum. Chin. J. Appl. Ecol. 2012, 23, 1393–1399, (In Chinese with English abstract). [Google Scholar]
- Wang, R.; Xue, Q.N.; Wang, J.T.; Tan, L.J. Competitive interactions between two allelopathic algal species: Heterosigma akashiwo and Phaeodactylum tricornutum. Mar. Biol. Res. 2020, 16, 32–43. [Google Scholar] [CrossRef]
- Legovi, T.; Cruzado, A. A model of phytoplankton growth on multiple nutrients based on the Michaelis-Menten-Monod uptake, Droop's growth and Liebig’s law. Ecol. Modell. 1997, 99, 19–31. [Google Scholar] [CrossRef]
- Shi, X.G.; Lin, X.; Li, L.; Li, M.Z.; Palenik, B.; Lin, S.J. Transcriptomic and microRNAomic profiling reveals multi-faceted mechanisms to cope with phosphate stress in a dinoflagellate. ISME J. 2017, 11, 2209–2218. [Google Scholar] [CrossRef]
- Eckford-Soper, L.K.; Daugbjerg, N. Interspecific competition study between Pseudochattonella farcimen and P. verruculosa (Dictyochophyceae)-Two ichthyotoxic species that co-occur in Scandinavian waters. Microb. Ecol. 2017, 73, 259–270. [Google Scholar] [CrossRef]
- Zheng, C.Z.; Zuo, L.M.; Ma, W.; Zhu, Q.; Wang, H.H.; Lü, S.H.; Chen, H.; Huang, K.X. Interactions among Aureococcus anophagefferens, Skeletonema costatum, and Chattonella marina under different temperatures. J. Trop. Oceanogr. 2021, 40, 124–131, (In Chinese with English abstract). [Google Scholar]
- Shen, A.L.; Ma, Z.L.; Jiang, K.J.; Li, D.J. Effects of temperature on growth, photophysiology, Rubisco gene expression in Prorocentrum donghaiense and Karenia mikimotoi. Ocean Sci. J. 2016, 51, 581–589. [Google Scholar] [CrossRef]
- Yao, W.M.; Pan, X.D.; Hua, D.D. The preliminary study of the causes of Karenia mikimotoi red tide near Zhejiang. J. Hydroecol. 2007, 27, 57–58, (In Chinese with English abstract). [Google Scholar]
- Li, Y.B. The Study of the Seasonal Occurrence Mechanism of HABs in the Changjiang Estuary and Its Adjacent Sea. Ph.D. Dissertation, Ocean University of China, Qingdao, China, 8 June 2008. (In Chinese with English abstract). [Google Scholar]
- Coello-Camba, A.; Agustí, S.; Vaqué, D.; Holding, J.; Arrieta, J.M.; Wassmann, P.; Duarte, C.M. Experimental assessment of temperature thresholds for Arctic phytoplankton communities. Estuar. Coasts 2015, 38, 873–885. [Google Scholar] [CrossRef]
- Armstrong, R.A. A hybrid spectral representation of phytoplankton growth and zooplankton response: The “control rod” model of plankton interaction. Deep Sea Res. Part II. 2003, 50, 2895–2916. [Google Scholar] [CrossRef]
- Tubay, J.M.; Ito, H.; Uehara, T.; Kakishima, S.; Morita, S.; Togashi, T.; Tainaka, K.; Niraula, M.P.; Casareto, B.E.; Suzuki, Y.; et al. The paradox of enrichment in phytoplankton by induced competitive interactions. Sci. Rep. 2013, 3, 2835. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Glibert, P.M.; Zhou, M.J.; Lu, S.H.; Lu, D.D. Relationships between nitrogen and phosphorus forms and ratios and the development of dinoflagellate blooms in the East China Sea. Mar. Ecol. Prog. Ser. 2009, 383, 11–26. [Google Scholar] [CrossRef]
- Yu, R.C.; Zhang, Q.C.; Kong, F.Z.; Zhou, Z.X.; Chen, Z.F.; Zhao, Y.; Geng, G.X.; Dai, L.; Yan, T.; Zhou, M.J. Status, impacts and long-term changes of harmful algal blooms in the sea area adjacent to the Changjiang River Estuary. Oceanol. Limnol. Sin. 2017, 48, 1178–1186, (In Chinese with English abstract). [Google Scholar]
- Shen, A.L.; Ishizaka, J.; Yang, M.M.; Ouyang, L.L.; Yin, Y.E.; Ma, Z.L. Changes in community structure and photosynthetic activities of total phytoplankton species during the growth, maintenance, and dissipation phases of a Prorocentrum donghaiense bloom. Harmful Algae 2019, 82, 35–43. [Google Scholar] [CrossRef]
- Nicholson, D.; Dyhrman, S.; Chavez, F.; Paytan, A. Alkaline phosphatase activity in the phytoplankton communities of Monterey Bay and San Francisco Bay. Limnol. Oceanogr. 2006, 51, 874–883. [Google Scholar] [CrossRef]
- Reynolds, C.S. Functional morphology and the adaptive strategies of freshwater phytoplankton. In Growth and Reproductive Strategies of Freshwater Phytoplankton, 2nd ed.; Sandgren, C.D., Ed.; Cambridge University Press: Cambridge, UK, 1988; Volume 10, pp. 388–433. [Google Scholar]
- Reynolds, C.S. Swings and roundabouts: Engineering the environment of algal growth. In Urban Watershed Regeneration, Problems and Prospects, 1st ed.; White, K.H., Bellinger, E.G., Saul, A.J., Symes, M., Hendry, K., Eds.; Ellis Horwood: Chichester, UK, 1993; pp. 330–349. [Google Scholar]
- Reynolds, C.S. Successional change in the planktonic vegetation: Species, structures, scales. In The Molecular Ecology of Aquatic Microbes, 1st ed.; Joint, I., Ed.; Springer: Berlin, Germany, 1995; Volume 38, pp. 115–132. [Google Scholar]
- Lu, S.H.; Li, Y. Nutritional storage ability of four harmful algae from the East China Sea. Chin. J. Process Eng. 2006, 6, 439–444, (In Chinese with English abstract). [Google Scholar]
- Rao, N.N.; Gomez-Garcia, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Wang, J.T.; Tan, L.J.; Cao, J.; Li, H. Effect of allelopathy on the competition and succession of Skeletonema costatum and Prorocentrum donghaiense. Mar. Biol. Res. 2015, 11, 1093–1099. [Google Scholar] [CrossRef]
- Xia, W. Study on molecular response mechanism of Prorocentrum donghaiense Lu and Karenia mikimotoi Hansen grown into nitrogen limitation or phosphorus limitation. Master’s Thesis, Jinan University, Guangzhou, China, 1 May 2016. (In Chinese with English abstract). [Google Scholar]
- Granéli, E.; Hansen, P.L. Alleopthy in harmful algae: A mechanism to compete for resources. In Ecology of Harmful Algae, 1st ed.; Granéli, E., Turner, J.T., Eds.; Springer: Berlin, Germany, 2006; Volume 189, pp. 189–201. [Google Scholar]
- Wang, H.; Niu, X.Q.; Feng, X.Q.; Gonçalves, R.J.; Guan, W.C. Effects of ocean acidification and phosphate limitation on physiology and toxicity of the dinoflagellate Karenia mikimotoi. Harmful Algae 2019, 87, 101621. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.X.; Zhuang, Y.Q.; Wang, Z.; Ou, L.J.; Cen, J.Y.; Lu, S.H.; Qi, Y.Z. Bioavailability of organic phosphorus compounds to the harmful dinoflagellate Karenia mikimotoi. Microorganisms 2021, 9, 1961. [Google Scholar] [CrossRef] [PubMed]
- Poulson-Ellestad, K.L.; Jones, C.M.; Roy, J.; Viant, M.R.; Fernández, F.M.; Kubanek, J.; Nunn, B.L. Metabolomics and proteomics reveal impacts of chemically mediated competition on marine plankton. Proc. Natl. Acad. Sci. USA 2014, 111, 9009–9014. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Yan, T.; Yu, R.C.; Zhou, M.J. A review of Karenia mikimotoi: Bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 2019, 90, 101702. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Burkholder, J.M.; Kana, T.M. Recent insights about relationships between nutrient availability, forms, and stoichiometry, and the distribution, ecophysiology, and food web effects of pelagic and benthic Prorocentrum species. Harmful Algae 2012, 14, 231–259. [Google Scholar] [CrossRef]
- Ji, X.Q.; Han, X.T.; Zheng, L.; Yang, B.J.; Yu, Z.M.; Zou, J.Z. Allelopathic interactions between Prorocentrum micans and Skeletonema costatum or Karenia mikimotoi in laboratory cultures. Chin. J. Oceanol. Limnol. 2011, 29, 840–848. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.T.; Xue, Q.N.; Sha, X.Y.; Tan, l.j.; Xin Guo, X. Allelopathic interactions between Skeletonema costatum and Alexandrium minutum. Chem. Ecol. 2017, 33, 485–498. [Google Scholar] [CrossRef]



| Species | Maximum Biomass (K) (Cells mL−1) | Growth Rate (r) (d−1) | Interaction Rate | |
|---|---|---|---|---|
| α or β | A or B (mL Cell−1 s−1) | |||
| Sc | 848,978 | 2.298 | 6.740 | 1.481 × 10−5 |
| Pd | 173,978 | 0.378 | 0.320 | 6.963 × 10−7 |
| Sc | 848,978 | 2.298 | 12.577 | 2.762 × 10−5 |
| Km | 77,077 | 0.355 | 0.087 | 4.013 × 10−7 |
| Pd | 173,978 | 0.378 | 2.735 | 5.950 × 10−6 |
| Km | 77,077 | 0.355 | 1.306 | 6.010 × 10−6 |
| Species | Maximum Biomass (K) (Cells mL−1) | Growth Rate (r) (d−1) | Interaction Rate | |
|---|---|---|---|---|
| α or β | A or B (mL Cell−1 s−1) | |||
| Sc | 162,903 | 1.818 | 2.729 | 3.045 × 10−5 |
| Pd | 32,733 | 0.677 | 0.059 | 1.217 × 10−6 |
| Sc | 162,903 | 1.818 | 5.499 | 6.136 × 10−5 |
| Km | 41,390 | 0.193 | 0.076 | 3.547 × 10−7 |
| Pd | 32,733 | 0.677 | 1.549 | 3.205 × 10−5 |
| Km | 41,390 | 0.193 | 1.200 | 5.609 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, A.; Liu, H.; Xin, Q.; Hu, Q.; Wang, X.; Chen, J. Responses of Marine Diatom–Dinoflagellate Interspecific Competition to Different Phosphorus Sources. J. Mar. Sci. Eng. 2022, 10, 1972. https://doi.org/10.3390/jmse10121972
Shen A, Liu H, Xin Q, Hu Q, Wang X, Chen J. Responses of Marine Diatom–Dinoflagellate Interspecific Competition to Different Phosphorus Sources. Journal of Marine Science and Engineering. 2022; 10(12):1972. https://doi.org/10.3390/jmse10121972
Chicago/Turabian StyleShen, Anglu, Hongyue Liu, Quandong Xin, Qingjing Hu, Xinliang Wang, and Jufa Chen. 2022. "Responses of Marine Diatom–Dinoflagellate Interspecific Competition to Different Phosphorus Sources" Journal of Marine Science and Engineering 10, no. 12: 1972. https://doi.org/10.3390/jmse10121972
APA StyleShen, A., Liu, H., Xin, Q., Hu, Q., Wang, X., & Chen, J. (2022). Responses of Marine Diatom–Dinoflagellate Interspecific Competition to Different Phosphorus Sources. Journal of Marine Science and Engineering, 10(12), 1972. https://doi.org/10.3390/jmse10121972





