Investigating the Role of Terminal Stolon of Marine Invasive Green Macroalga Caulerpa taxifolia in the Removal of Inorganic Nitrogen from Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Experimental Instruments
2.1.2. Experimental Samples and Pre-Treatment
2.2. Experimental Methods
2.2.1. Formulation for Culture Solution
2.2.2. Experimental Scheme Design
2.2.3. Analytical Methods
3. Results
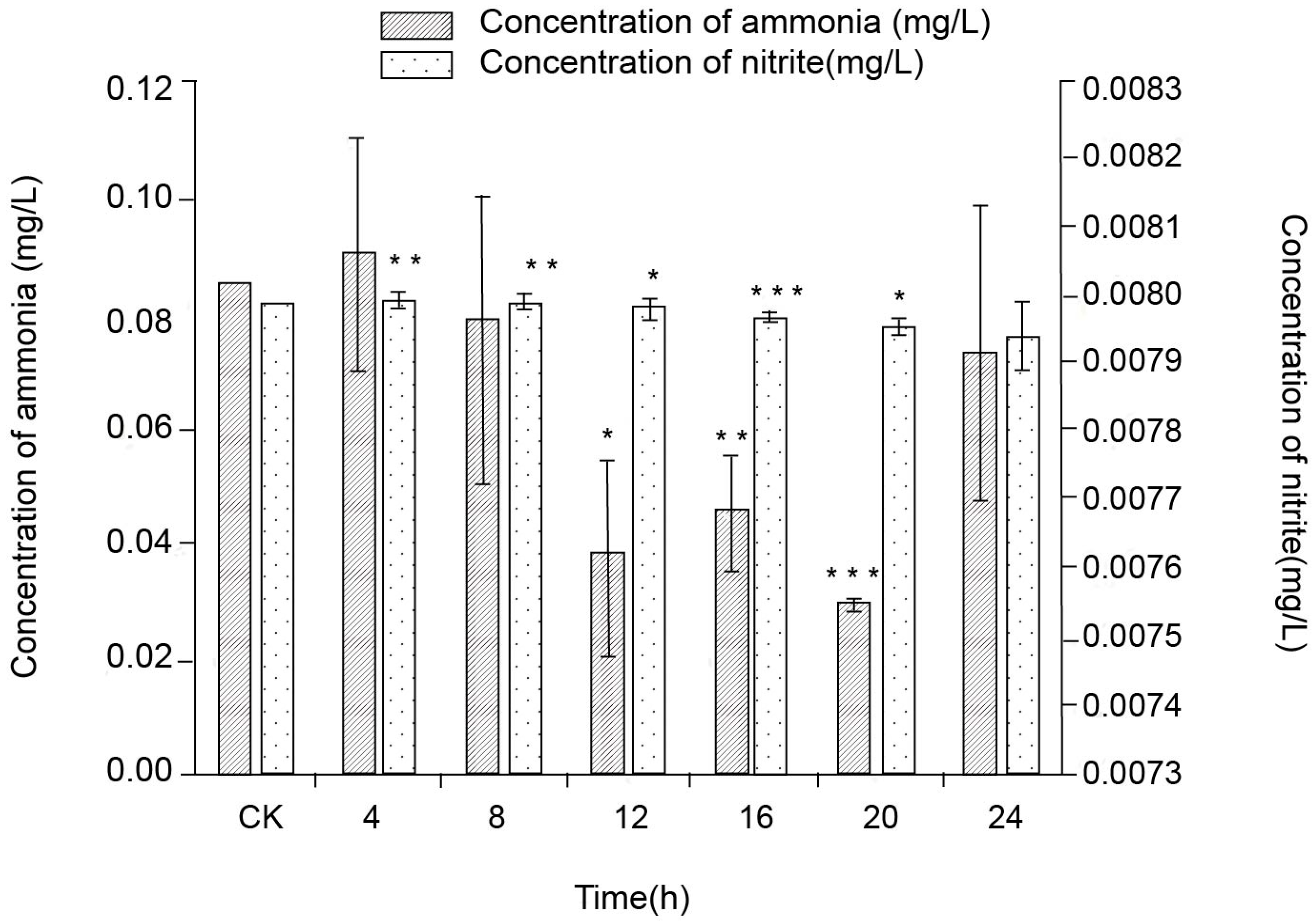
3.1. Effect of Cultured Time Interval on NH4-N and NO2-N Concentrations
3.1.1. Effect of Cultured Time Interval on NH4-N Concentration
3.1.2. Effect of Cultured Time Interval on NO2-N Concentration
3.1.3. Effect of Culture Time Interval on Different Forms of Nitrogen Concentration
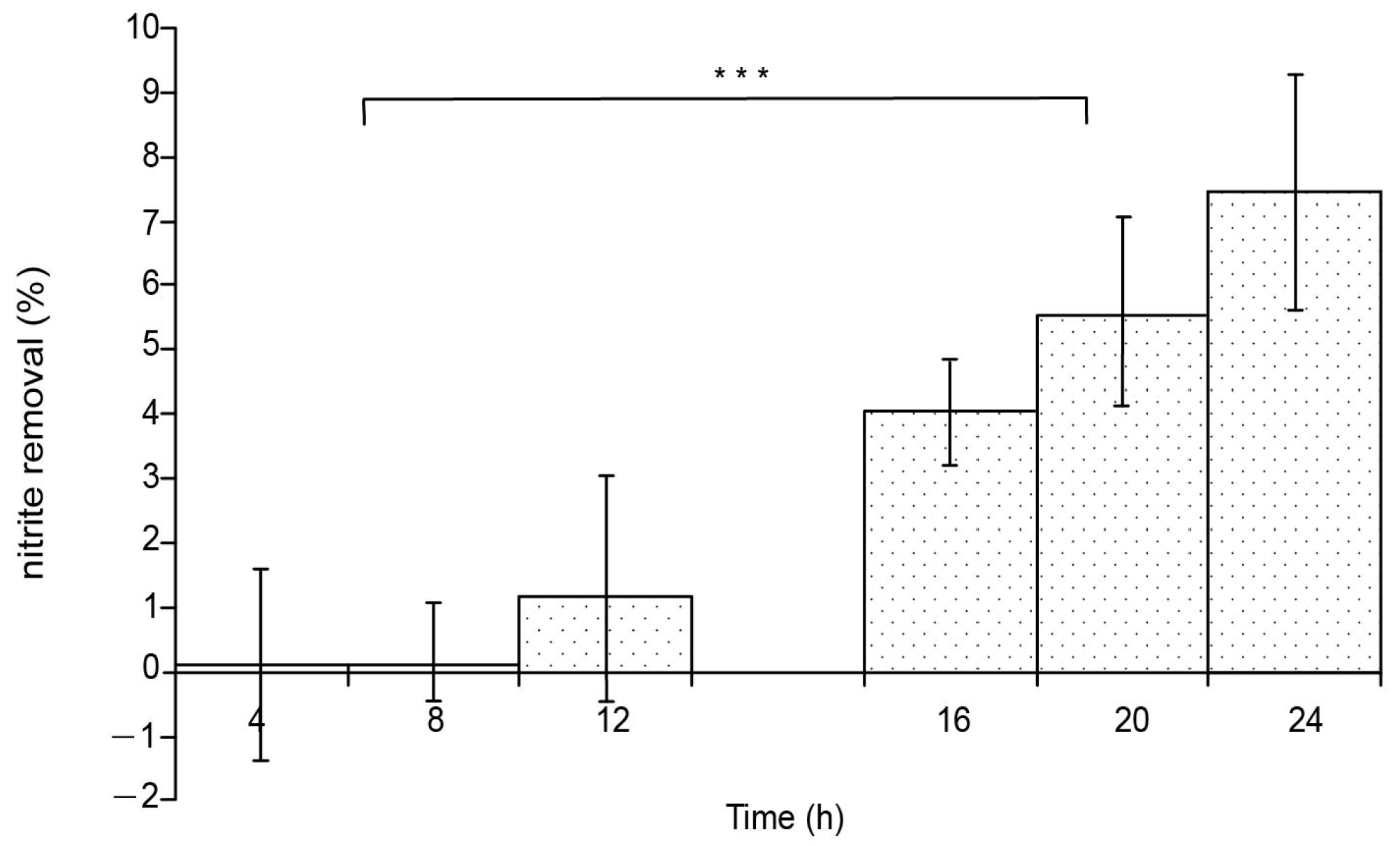
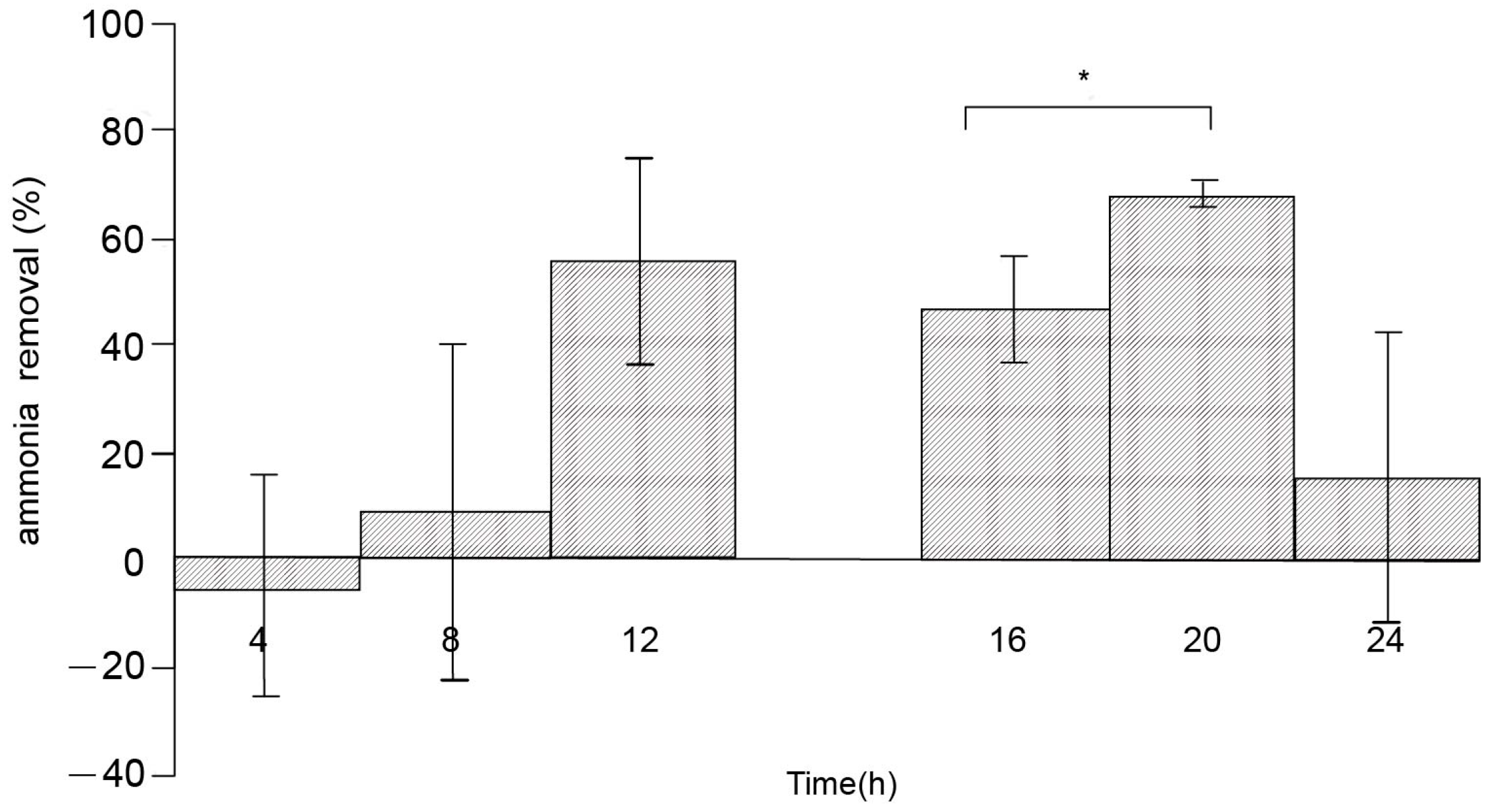
3.2. Effect of Culture Time Interval on NH4-N and NO2-N Removing Efficiency
3.2.1. Change in NO2-N Removing Efficiency
3.2.2. Change of NH4-N Removing Efficiency in Artificial Seawater
3.2.3. The Correlation Degree in NO2-N– and NH4-N–Removing Efficiencies
4. Discussion
4.1. Artificial Seawater
4.2. Initial Concentrations of NH4-N and NO2-N
4.3. Activity of Caulerpa taxifolia
4.4. Algal-Bacterial Symbiosis
4.5. Mechanism of NH4-N and NO2-N Removal
4.6. NH4-N Absorbency by Different Thallus Tissues
4.7. Light Influx and Dark Efflux of NH4-N
4.8. Dark Influx of NO2-N
4.9. Correlation between NH4-N Removing Efficiency and NO2-N Removing Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, J.X.; Chen, J.Y.; Xu, H.X.; Xu, K.D.; Zhu, W.B.; Liang, J. Strategic Thinking on Sustainability of Inshore Fishery Resources in China Based on the Concept of Green Development. Ocean Dev. Manag. 2021, 38, 11–17. [Google Scholar]
- Zhou, B.; Sun, L.N.; Wang, Q.L.; Li, Y.Y.; Chai, M.; Xing, M.N.; Guo, M.T. Preliminary study on accounting of nitrogen and phosphorus fluxes into the sea in Tianjin coastal area. Mar. Environ. Sci. 2021, 40, 851–859. [Google Scholar]
- Dong, J.H. The analysis of <2013 China Fisheries statistical Yearbook>. China Fish. 2013, 7, 19–20. [Google Scholar]
- Jiang, L.X.; Pan, L.Q.; Xiao, G.Q. Effects of ammonia-N on immune parameters of white shrimp Litopenaeus vannamei. J. Fish. Sci. China 2005, 11, 537–541. [Google Scholar]
- Sun, G.M.; Tang, J.H. Toxicity Research of Ammonia Nitrogen and Nitrite Nitrogen to Penaeus vannamei. J. Aquac. 2002, 1, 22–24. [Google Scholar]
- Hong, M.L.; Chen, L.Q.; Gu, S.Z.; Liu, C.; Long, Z.Q.; Zhang, W. Effects of ammonia exposure on immunity indicators of haemolymph and histological structure of hepatopancreas in Chinese mitten crab (Eriocheir sinensis). J. Fish. Sci. China 2007, 14, 412–418. [Google Scholar]
- Wang, H.T.; Hu, D.G. Toxicity of nitrite to grass carp (Ctenopharyngodon idellus) in ponds and its way of prevention. J. Fish. China 1989, 13, 207–213. [Google Scholar]
- Huang, X.H.; Li, C.L.; Zheng, L.; Liu, C.W.; Zhou, J. The toxicity of NO2-N on Litopenaeus Vannamei and effects of NO2-N on factors relating to the anti-disease ability. Acta Hydrobiol. Sinica 2006, 30, 466–471. [Google Scholar]
- Lv, D.; Cheng, J.; Mo, W.; Tan, Y.H.; Sun, L.; Liao, Y.B. Pollution and Ecologial Restoration of Mariculture. Ocean Dev. Manag. 2019, 36, 43–48. [Google Scholar]
- Wang, L.; Wang, W.J.; Yang, X.C.; Sun, L.D.; Yang, J.L. Monitoring and Evaluation of Main Pollutants in the Tail Water of Marine Aquaculture Plants in Yantai Area. J. Ludong Univ. 2022, 38, 114–119. [Google Scholar]
- Gao, L.P.; Liu, Z.H.; Li, S.X. Experimental study on treatment of mariculture wastewater by immobilized bacteria-algae. Ind. Water Wastewater 2021, 52, 11–15, 24. [Google Scholar]
- Li, R.X.; Nie, W.J.; Li, B.; Li, J.L.; Wang, X.Y.; Qiao, P.; Fu, R. Purification Effects of Tetragonia tetragonoides on Seawater with Different Salinity and Eutrophication Degrees. Shandong Agric. Sci. 2022, 54, 99–105. [Google Scholar]
- Li, X.; Jiao, Z.K.; Qi, T.H.; Yang, L.J.; Zhao, Y.; Song, C.C.; Yang, Y.Y. Study on the Removal Effect of Canna coccinea Mill on Ammonia Nitrogen, Nitrate and Nitrite Nitrogen in Marineculture Tail Water. J. Aquac. 2022, 43, 57773–57789. [Google Scholar]
- Wang, C.Q.; Xiang, Z.W.; Huang, B.S.; Wang, Z.Q.; Wang, J.Y.; Li, B.S. Effects of Three Varieties of Halophytes on the Purification of Aquaculture Waste Water. J. Guangdong Ocean Univ. 2022, 42, 25–32. [Google Scholar]
- Wang, S.Q.; Zhang, E.D.; Wang, S.H. Research of Removal Capacity of Ammonia-Nitrogen and Phosphate-Phosphorus from Simulated Mariculture Wastewater by Two Species of Marine Microalgae. Tianjin Agric. Sci. 2022, 28, 86–90. [Google Scholar]
- Ye, B.C.; Lv, B.; Zhou, J.C.; Zhang, X.Z.; Zheng, X.; Gu, Z.F. Purification Effect of Two Oxidation Methods on Simulated Aquaculture Tail Water. J. Guangdong Ocean Univ. 2022, 42, 33–38. [Google Scholar]
- Wang, S.H.; Liu, Y.; Li, S.; Liu, H.; Gu, J.; Yang, G.; Wang, J.; Wang, H. Effects of iron-loaded activated carbon on adsorption and removal of low concentration phosphate in water. J. Dalian Ocean Univ. 2022, 37, 276–284. [Google Scholar]
- Fei, X. Solving the coastal eutrophication problem by large scale seaweed cultivation. Hydrobiologia 2004, 512, 145–151. [Google Scholar] [CrossRef]
- Tang, K.; You, X.; Lin, Y.; Chen, M.; Shen, D.; Lin, S. A study on bioremediation of eutrophication of mariculture waters by Gracilaria lemaneaformis. Acta Ecol. Sin. 2005, 25, 3044–3051. [Google Scholar]
- Liu, B.; Wang, C.Y.; Zhang, H.R.; Geng, M.Y.; Guan, H.S. New progress in the research on bioactivities and applications of alginate, a kind of marine algal polysaccharide. China J. Mar. Drugs 2004, 21, 36–41. [Google Scholar]
- Lincoln, T.; Eduardo, Z.; Ian, M.M.; Angus, M. Plant Physiology and Development, 6th ed.; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 2015. [Google Scholar]
- Williams, S.L.; Schroeder, S.L. Eradication of the invasive seaweed Caulerpa taxifolia by chlorine bleach. Mar. Ecol. Prog. Ser. 2004, 272, 69–76. [Google Scholar] [CrossRef]
- Thibaut, T.; Meinesz, A.; Coquillard, P. Biomass seasonality of Caulerpa taxifolia in the Mediterranean Sea. Aquat. Bot. 2004, 80, 291–297. [Google Scholar] [CrossRef]
- Dalton, R. Action urged to combat killer algae. Nature 2001, 412, 260. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.W.J. California’s Reaction to Caulerpa taxifolia: A Model for Invasive Species Rapid Response. Biol. Invasions 2005, 7, 1003–1016. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D.; Ceccherelli, G.; Cinelli, F. Comparative study of the growth of the two co-occurring introduced green algae Caulerpa taxifolia and Caulerpa racemosa along the Tuscan coast (Italy, western Mediterranean). Cryptogam. Algol. 2001, 22, 459–466. [Google Scholar] [CrossRef]
- Cuny, P.; Serve, L.; Jupin, H.; Boudouresque, C.F. Water soluble phenolic compounds of the marine phanerogam Posidonia oceanica in a Mediterranean area colonised by the introduced chlorophyte Caulerpa taxifolia. Aquat. Bot. 1995, 52, 237–242. [Google Scholar] [CrossRef]
- PERES, J.; PICARD, J. Causes of decrease and disappearance of the seagrass Posidonia oceanica on the French Mediterranean coast. Aquat. Bot. 1975, 1, 133–139. [Google Scholar] [CrossRef]
- Ding, L.P.; Huang, B.X.; Luan, R.X. New classification system of marine green algae of China. Guangxi Sci. 2015, 22, 201–210. [Google Scholar]
- Liu, R.; Li, Y.M.; Wang, Y.X.; Ding, L.P.; Huang, B.X.; Yang, N.; Chen, Y.W. Biogeographical distribution, biological characteristics and biological invasion of Caulerpa (Chlorophyta) seaweeds. J. Anhui Agric. Sci. 2019, 47, 95–98, 104. [Google Scholar]
- Harrison, P.J.; Waters, R.; Taylor, F.J. A broad spectrum artificial sea water medium for coastal and open ocean phytoplankton. J. Phycol. 1980, 16, 28–35. [Google Scholar] [CrossRef]
- GB 17378.4-2007; The Specification for Marine Monitoring—Part 4: Seawater Analysis. National Standard of the People’s Republic of China. Issued by the General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and Standardization Administration of China. Available online: https://www.chinesestandard.net/PDF/English.aspx/GB17378.4-2007 (accessed on 10 November 2022).
- Fliermans, C.B.; Schmidt, E.L. Autoradiography and immunofluorescence combined for autecological study of single cell activity with Nitrobacter as a model system. Appl. Microbiol. 1975, 30, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, Y.F.; Ye, C.P. Environmental pollution and countermeasures in mariculture. Trans. Oceanol. Limnol. 2006, 3, 111–118. [Google Scholar]
- Luo, H.M.; Cheng, Y.X.; Li, L.W. The reason, damage and approach of high value of nitrite, ammonia, hydrogen sulfide, pH in aquaculture water. Fish. Guide Be Rich 2005, 14, 34. [Google Scholar]
- Yao, C.F. Beware of nitrite when farming fish. J. Beijing Fish. 2006, 2, 44. [Google Scholar]
- Delbridge, L.M.; Coulburn, J.; Fagerberg, W.R.; Tisa, L.S. Community profiles of bacterial endosymbionts in four species of Caulerpa. Symbiosis 2004, 37, 335–344. [Google Scholar]
- Chen, W.M. Effects of Nitrite on Physiological Characteristics of Microcystis aeruginosa. Ph.D. Thesis, Nankai University, Tianjin, China, 2009. [Google Scholar]
- O’Kelley, J.; Becker, G.E.; Nason, A. Characterization of the particulate nitrite oxidase and its component activities from the chemoautotroph Nitrobacter agilis. Biochim. Biophys. Acta Bioenerg. 1970, 205, 409–425. [Google Scholar] [CrossRef]
- Chisholm, J.R.; Moulin, P. Stimulation of nitrogen fixation in refractory organic sediments by Caulerpa taxifolia (Chlorophyta). Limnol. Oceanogr. 2003, 48, 787–794. [Google Scholar] [CrossRef]
- Malta, E.; Ferreira, D.; Vergara, J.J.; Pérez-Lloréns, J.L. Nitrogen load and irradiance affect morphology, photosynthesis and growth of Caulerpa prolifera (Bryopsidales: Chlorophyta). Mar. Ecol. Prog. Ser. 2005, 298, 101–114. [Google Scholar] [CrossRef]
- Eyre, B.D.; Ferguson, A.J. Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae-and macroalgae-dominated warm-temperate Australian lagoons. Mar. Ecol. Prog. Ser. 2002, 229, 43–59. [Google Scholar] [CrossRef]
- Hein, M.; Pedersen, M.F.; Sand-Jensen, K. Size-dependent nitrogen uptake in micro-and macroalgae. Mar. Ecol. Prog. Ser. 1995, 118, 247–253. [Google Scholar] [CrossRef]
- Eyre, B.D.; Maher, D.T.; Oakes, J.M.; Erler, D.V.; Glasby, T.M. Differences in benthic metabolism, nutrient fluxes, and denitrification in Caulerpa taxifolia communities compared to uninvaded bare sediment and seagrass (Zostera capricorni) habitats. Limnol. Oceanogr. 2011, 56, 1737–1750. [Google Scholar] [CrossRef]
- Alleman, J.E.; Keramida, V.; Pantea-Kiser, L. Light induced Nitrosomonas inhibition. Water Res. 1987, 21, 499–501. [Google Scholar] [CrossRef]
| Group | CK | Experiment | |||||
|---|---|---|---|---|---|---|---|
| B0 | E1 | E2 | E3 | E4 | E5 | E6 | |
| Light Period | Dark Period | ||||||
| Culture time interval/h | 4 | 4 | 8 | 12 | 16 | 20 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Wang, Z.; Chu, Y.; Ding, L. Investigating the Role of Terminal Stolon of Marine Invasive Green Macroalga Caulerpa taxifolia in the Removal of Inorganic Nitrogen from Seawater. J. Mar. Sci. Eng. 2023, 11, 43. https://doi.org/10.3390/jmse11010043
Huang B, Wang Z, Chu Y, Ding L. Investigating the Role of Terminal Stolon of Marine Invasive Green Macroalga Caulerpa taxifolia in the Removal of Inorganic Nitrogen from Seawater. Journal of Marine Science and Engineering. 2023; 11(1):43. https://doi.org/10.3390/jmse11010043
Chicago/Turabian StyleHuang, Bingxin, Zhan Wang, Yue Chu, and Lanping Ding. 2023. "Investigating the Role of Terminal Stolon of Marine Invasive Green Macroalga Caulerpa taxifolia in the Removal of Inorganic Nitrogen from Seawater" Journal of Marine Science and Engineering 11, no. 1: 43. https://doi.org/10.3390/jmse11010043
APA StyleHuang, B., Wang, Z., Chu, Y., & Ding, L. (2023). Investigating the Role of Terminal Stolon of Marine Invasive Green Macroalga Caulerpa taxifolia in the Removal of Inorganic Nitrogen from Seawater. Journal of Marine Science and Engineering, 11(1), 43. https://doi.org/10.3390/jmse11010043






