Abstract
As more and more oilfields enter later stages of extraction, demulsification of water-in-oil (W/O) emulsions with high water content has become a challenging problem. To upgrade the current offshore oil treatment process, a compact and efficient demulsification treatment is highly desirable. In this paper, a novel enhanced treatment combining a direct current (DC) electric field and medium coalescence was proposed. Based on this idea, an electric-medium demulsifier was also designed for deep purification of W/O emulsions. The effects of operating conditions, emulsions characteristics and medium bed parameters on demulsification performance were investigated. The enhanced treatment showed better performance compared to electrostatic demulsification and medium coalescence alone, and was especially suitable for treating emulsions with strong emulsification. In short, at U = 3 kV, the demulsification efficiency increased by approximately 30% compared to that at U = 0 kV. This research provided a new approach for the treatment of W/O emulsions that has the advantages of wide operational flexibility, a tolerance for deteriorated characteristics and a rapid and thorough treatment process.
1. Introduction
The ocean has abundant oil and gas resources [1]. Offshore oil production met around 30% of the global oil supply in 2019 and has increased over the years [2]. However, as an oilfield enters its later extraction stage, the volume flow of produced liquid increases significantly to maintain the yield and the water content is also remarkably higher compared to that of the early period [3,4]. Moreover, the produced liquid usually represents a strong emulsification due to the aggravating trend of crude oil deterioration, surfactant existence and external energy input [5,6,7]. In offshore petroleum industries, the demulsification of W/O emulsions has become a challenging problem that needs to be solved urgently.
At present, the oil treatment applied on offshore platforms is a four-stage series process based on different demulsification technologies, including free water sedimentation separation, heater treatment, electrostatic demulsification and gravity sedimentation [8,9]. The produced liquid is first heated to 60 °C before being transported through single point mooring, and then treated by a serial stage demulsification process. The residence time for the first three stages of the process is about 60 min and gravity sedimentation is the last stage to ensure that the crude oil meets standards. Eventually, the conforming oil is regularly stored and transported elsewhere via shuttle tanker [10]. Such a series process leads to many problems, such as long residence time and large space occupation, and results in high investment, low productivity and extra energy consumption. Additionally, it is difficult for a series process to satisfy the treatment requirements of emulsions with large volumes and strong emulsification in the later extraction stage. To release the production capacity of old offshore platforms and save construction costs for new offshore platforms, it is essential to develop new demulsification technology to upgrade the four-stage series treatment.
In offshore petroleum extraction, electrostatic coalescers are used conventionally in standard-reaching treatment for dehydration, in line with desalting [11]. The electrostatic demulsification technique was first established 100 years ago through the pioneering work of Cottrell [12]. The applied electric field induces an attractive force between small droplets so that these droplets rapidly grow to a certain size for subsequent gravity sedimentation [13]. For electrostatic demulsification, two fundamental processes are dipole coalescence and migratory coalescence. The electric field causes a polarized force to act on the droplet surface, resulting in dipole interactions between adjacent droplets [14]. Apart from dipolar interactions, the electric field could compel water droplets to rapidly move along the electric field line by Coulombic force, which is called the electrohydrodynamic (EHD) effect [15]. The interconnection and movement of droplets provided the motivation for film drainage and coalescence. However, electrostatic demulsification has several problems in practical applications. To achieve a better treatment effect, the currently available electric coalescing equipment is designed to be large and bulky, with the aim of extending residence time for coalescence. In addition, emulsions with high water content tend to cause short circuits between electrodes and cause security incidents in industrial applications [16,17]. Several types of electric fields have already been proven to be useful for electrostatic demulsification, such as AC, pulsed AC, DC, and pulsed DC fields, as well as their combinations [18,19,20,21]. With some specific electrode structures, electric potential is high and changeable, which is important for the treatment of certain emulsions [22]. Determining the optimum electrode geometry and electric field parameters according to treatment requirements is essential, especially for the space and weight restrictions in offshore platforms [23].
Medium coalescence is a physical method based on the fluid flow properties of a surrounding fluid and the wettability of a medium surface [24]. Water droplets adhere, grow, and ultimately fall onto the medium surface. Droplet–medium collisions are preconditions for the above behaviors and research on demulsification should focus on increasing the probability of collisions [25,26]. For medium coalescers, a sufficient bed depth and narrow gaps between the medium are required to achieve efficient collisions. With the accumulation of droplets and other suspended solids in the gaps, the exposed surfaces gradually decrease, inevitably resulting in a large pressure drop [27]. Moreover, the separation performance of a medium coalescer is poor when processing fine droplets smaller than 5 μm due to the viscous effect and it is unable to adapt to complex flow fluctuation [28].
These demulsification methods are all restricted due to their limited throughput capacity and separation accuracy. The key to successful demulsification is rapid removal of large droplets and thorough removal of fine droplets, which is difficult to achieve with a single method. The future development trend lies in the combination of a multi-physical field, characterized by coupling electricity with centrifugal force, filtration, microwaves, and the addition of chemical agents to enhance the coalescence [29,30,31]. We believe that the use of electric fields and medium coalescence has appropriate compatibility. With the interception effect of the medium, droplets can coalesce on the medium surface without needing to form dipoles with each other, shortening the residence time. With the application of an electrical field to promote droplet–medium collisions, larger porosity and permeability channels can be used instead of the micropores, avoiding blockage after long-term operation. Underpinned by the above ideas, a medium bed demulsifier combined with an electric field was constructed in this work to enhance the demulsification of W/O emulsions. Key factors (operating conditions, emulsions characteristics and medium bed parameters) that affect the demulsification were investigated.
2. Experiment
2.1. Materials and Measurements
Distilled water was used as the dispersed phase and white oil was used as the continuous oil phase in the W/O emulsions. Important physical properties of the liquids used are listed in Table 1. The coalescence media were medium beds made up of glass sand (GS), glass (G), anthracite (A) and polystyrene (PS) particles. The G and PS particles were spheroidal, whereas the GS and A particles were irregularly shaped. The contact angle is an important parameter describing the wettability of solid surface and was measured by a contact angle meter (SL200B, KINO, Boston, MA, USA). The material was categorized as hydrophilic when the contact angle was over 90°; otherwise, it was categorized as hydrophobic. The contact angles of water droplets on the above materials in white oil were 104° (A), 85° (G), and 138° (PS) in Figure 1. The changes in droplet size distribution and water content in the inlet and export samples provided the major indexes to evaluate the treatment effect, which was measured with an electronic balance (ME204E, Mettler Toledo), a water analyzer (C20SD, Titroline KF Trace), a laser particle sizer (HIAC PODS, Beckman Coulter) and an optical microscope.

Table 1.
Physical properties of experimental liquids.
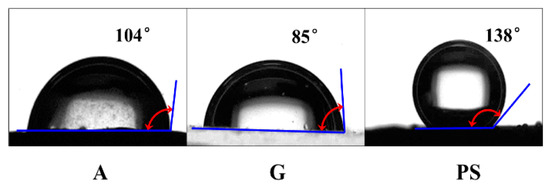
Figure 1.
The contact angles of water droplets on the surfaces of glass (G), anthracite (A) and polystyrene (PS) in white oil.
2.2. Equipmental Procedure
The experiment was performed on a laboratory scale. Figure 2a depicts a schematic of the experimental setup, which is composed of four major parts: the emulsion-generating device, the fluid circulating apparatus, the high-voltage DC power supply and the electric-medium demulsifier. The emulsion-generating device consisted of a peristaltic pump, a steam generator and two tanks for storing distilled water and emulsified oil. The hot steam transformed from distilled water was transported to the emulsified oil tank, producing a stable W/O emulsion. The degree of emulsion was determined by controlling the heating temperature and duration of steam emulsification. The fluid circulating apparatus was used to transport the emulsion into the demulsifier for demulsification.
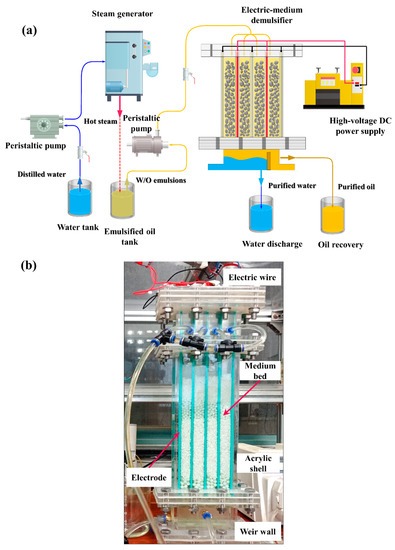
Figure 2.
(a) Schematic diagram of the experimental setup. (b) Photo of the demulsifier.
The shell of the electric-medium demulsifier, as shown in Figure 2b, was made of transparent acrylic for good visualization and insulation. The parallel electrodes were fixed against the interior wall and isolated from the emulsion by insulating paint. The distance between every electrode was 25 mm. A vertical weir wall was set under the medium bed, which was conducive to the formation of a clear oil–water interface and to oil–water recovery.
In the inner flow channel of the demulsifier, the emulsion flowed downward through the medium bed under the action of the electric field, in line with the coalescence process of water droplets. The emulsion continuously circulated during the operational process and samples were taken from the export for testing after each cycle.
The number of cycles N was calculated from Equation (1):
where v is the superficial velocity of the emulsion, t is the duration time of the demulsification and L is the depth of the medium bed.
The demulsification efficiency η was calculated from Equation (2):
where Cin is the influent water content of the prepared emulsion and CEf is the effluent water content of the outlet emulsion after cyclic treatment.
By raising the number of cycles N, the equivalent bed depth L* was increased, which was calculated from Equation (3):
where N is number of cycles and L is the depth of the packed medium bed. In this study, L was fixed at 10 cm.
2.3. Simulation Methods
To provide a detailed illustration of the coalescence process on the medium surface under an electric field, a numerical simulation was carried out. The simulation explored the dynamic behavior and adhesion pattern of a water droplet group in a medium bed.
Numerical computation was performed in a 2D domain with the aid of COMSOL Multiphysics commercial software. A laminar flow module (phase-field method) was utilized to simulate the flow condition of the continuous oil phase. An AC/DC module (Electrostatics method) was employed to model the function of the electric field. The dimension of the computational domain is depicted in Figure 3a and the physical properties of the phase used in the simulation were identical to those in Table 1. An unstructured mesh consisting of triangular elements was generated for pinpoint accuracy (as shown in Figure 3b), with encryption near the surface due to sharp gradient variation in the local area. Finally, after grid independence verification, the number of grids was determined to be 66,188, and the mesh quality attributes are listed in Table 2.
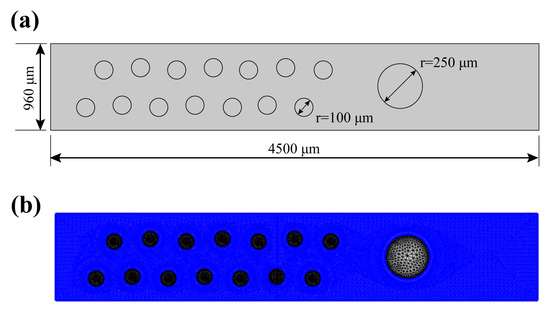
Figure 3.
(a) Geometric model. (b) Mesh distribution near and at boundaries.

Table 2.
Mesh quality statistics.
Boundary conditions were assumed to be a constant inlet velocity (0.02 m/s) and pressure outlet. Meanwhile, the voltage applied in the fluid region was 1.5 kV/cm and the under wall connected to ground had been considered to have zero potential.
The governing equation appropriate for the electrostatics interface is the Maxwell equation, calculated from Equation (4):
where is the vacuum relative dielectric constant, is the relative permittivity of the oil phase and is in terms of electric potential.
The electric force of the water–oil interface is given by the divergence of the Maxwell stress tensor in Equation (5):
The Maxwell stress tensor is given by Equation (6):
where is the electric field strength and is the electric displacement field strength:
3. Results
3.1. Demulsification Effect of the Enhanced Treatment
It was mentioned that the use of an electric field could enhance the demulsification effect of medium coalescence. A simulation was carried out to show the dynamic behavior of a water droplet group flowing through a medium under an electric field. The results in Figure 4 and Figure 5 are based on the simulation methods described in Section 2.3. For a single medium coalescence, the demulsification effect was inefficient. When the emulsion flowed through the medium bed, the viscous effect dominated the behavior of the water droplets in the emulsion. Few droplets collided with the medium surface while most droplets just escaped along the continuous phase streamlines in Figure 4a. When the droplet approached the medium, it deforms into an ellipsoid due to flow resistance and then gradually turns back to a sphere after flowing over the medium, as displayed in Figure 4b.
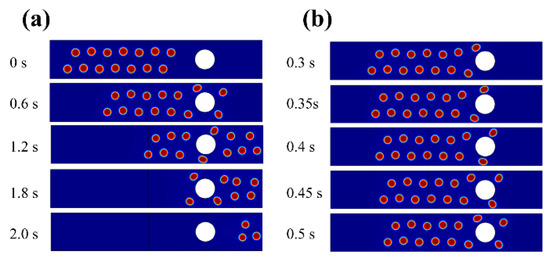
Figure 4.
Dynamic behavior of water droplets flowing through the medium without an applied electric field. (a) The whole process. (b) The specific behavior of droplet near the medium.
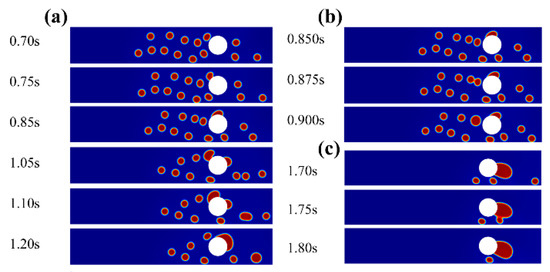
Figure 5.
Dynamic behaviour of water droplets flowing through the medium with an applied electric field. (a) The whole process. (b) The spreading behavior of adhered droplet by fluid drag force. (c) The direct capture behavior of merged droplet on medium surface.
The EHD effect, however, dominated in the enhanced treatment and drove the water droplets to overcome the viscous effect (Figure 5a). The droplet was forced to travel back and forth between the electrodes when a sufficient electric field was applied, contributing to the escape from continuous phase streamlines. At 0.70–1.05 s, a water droplet collided, adhered and spread rapidly on the medium surface. Subsequently, the adhered droplet continued to move along the surface by fluid drag force (Figure 5b). At 1.05–1.10 s, another droplet broke away from continuous phase streamlines and collided with the medium just like the previous one. At 1.10–1.20 s, the two adhered droplets formed a bridge joint and eventually merged into a bigger one. Furthermore, the somewhat big merged droplet with a large surface area could capture another fine droplet directly, improving the demulsification effect (Figure 5c).
In addition, the macroscopic demulsification effects of the electric field (EF), medium coalescence (GS), and enhanced combination (EF&GS) treatments were also compared in Figure 6. In this experiment, the voltage U applied for the electric field was 3 kV and the range of particle sizes applied for medium coalescence was 1.0–2.0 mm. Moreover, the superficial velocity v was fixed at v = 0.01 m/s and the equivalent bed depth L* was 10–50 cm.
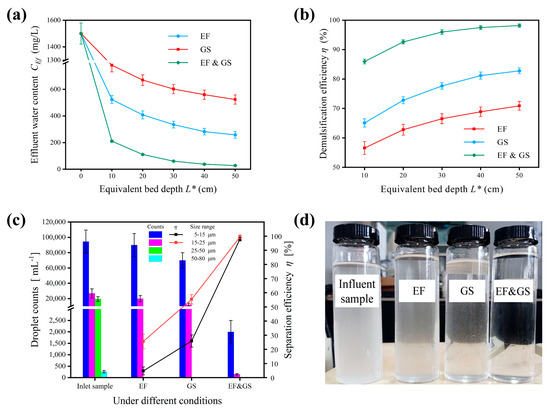
Figure 6.
Comparison of separation effects under a DC electric field, medium coalescence, and the enhanced treatment. (a) Effluent water content. (b) Separation efficiency. (c) Separation effect of droplets of different sizes. (d) Sample photos (at L* = 50 cm). Conditions: Cin = 1500 mg/L; v = 0.001 m/s; U = 3 kV or 0 kV; GS medium bed with size of 1.0–2.0 mm or no medium and L* = 10, 20, 30, 40, 50 cm.
The emulsion circulated continuously through the demulsifier. As the number of cycles N increased, the emulsion in the recovered oil tank became visibly clarified, indicating that the water droplets were basically separated and sedimented at the bottom. The sample photos taken under different treatments at N = 5 are displayed in Figure 6d and the turbidity of the emulsions implies the respective demulsification effect. Regarding this figure, the demulsification performance of the electric field (EF) was the worst, followed by medium coalescence (GS). In comparison, performance under the enhanced treatment (EF&GS) was significantly better, as the emulsion was almost transparent after treatment. The effluent water contents after treatment under the electric field, medium coalescence, and enhanced treatment were 524 mg/L, 258 mg/L, and 27.3 mg/L and the demulsification efficiencies were 70.9%, 82.8%, and 98.2%, respectively, as shown in Figure 6a,b. The key to successful demulsification depended on the thorough removal of fine droplets, as the separability of droplets weakened with droplet size. The enhanced treatment also demonstrated a remarkable ability to process fine droplets. When the droplet size to be processed decreased from 15–25 μm to 5–15 μm, the demulsification efficiencies of treatment with the electric field and medium coalescence decreased from 25.6% to 4.8%, 55.3% to 25.9%, respectively, which was a sharp drop (Figure 6d). The enhanced treatment only had a slight decrease from 99.4% to 97.9% under equal conditions. This suggested that the degree of emulsification had less impact with the enhanced treatment. In general, the complementary combination of an electric field and medium coalescence improved the demulsification effect, with higher efficiency and accuracy.
3.2. Effects of Operating Conditions
The operating conditions, including applied voltage and superficial velocity were discussed from the point view of practical application in Figure 7. The EHD effect intensified as the applied voltage increased, thereby increasing the collision probability between a droplet and the medium [32]. The demulsification performance was considerably improved by raising the voltage, as shown in Figure 7a. The effluent water content decreased from 1500 mg/L to 408 mg/L, 300 mg/L, 235 mg/L, 111 mg/L, 70.3 mg/L, and 34.1 mg/L for U = 0, 0.5, 1, 3, 5, and 7 kV, respectively. The corresponding demulsification efficiencies were 72.8%, 80.0%, 84.3%, 92.6%, 95.3%, and 97.7%. The efficiency rose rapidly with voltage at the beginning, but then relatively slowed down thereafter. At U = 0–3 kV, the efficiency increased by an average of 6.32% per 1 kV, while the average increase rate was only 1.28% per 1 kV at U = 3–7 kV. This was mainly related to the separability difference of droplets of different sizes. Larger droplets, which accounted for the majority of the water phase in the emulsion, were easier to drive even when subjected to a low voltage. In the later stage of the treatment, the dispersed large droplets were separated and the remaining droplets were too fine to be further processed. Further increasing the voltage was almost useless in terms of improving the demulsification of diluted emulsions. It is also worth pointing out that the excessive voltage may lead to breakup of droplets, which has a destructive influence on demulsification [33].
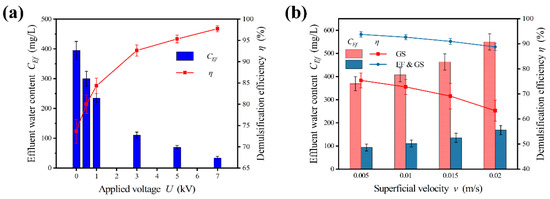
Figure 7.
Effects of operating condition parameters. (a) Effect of applied voltage. (b) Effects of superficial velocity. Conditions: Cin = 1500 mg/L; v = 0.001 m/s; U = 0–7 kV; GS medium bed with size of 1.0–2.0 mm and L* = 20 cm.
Unlike the application of voltage, the superficial velocity had the opposite effect, as shown in Figure 7b. Increasing superficial velocity reduced the residence time in the demulsifier and boosted the throughput of the equipment. The medium coalescence was sensitive to changes in superficial velocity. As superficial velocity increased, the viscosity effect of the fluid worsened [34]. The water droplet was washed away by fluid before it approached the medium. This decreased the probability of droplet–medium collision and lowered the effectiveness of demulsification. For medium coalescence alone, as the superficial velocity increased from 0.005 m/s to 0.02 m/s, the demulsification efficiency dropped from 75.4% to 63.3%, with a decrease of 4.03% per 0.005 m/s on average. The drop was sharp, especially when it exceeded 0.015 m/s. Applying an electric field could weaken the viscosity effect. The demulsification efficiency decreased slightly from 93.8% (0.005 m/s) to 88.8% (0.02 m/s) after an electric field with U = 3 kV was applied. In comparison to medium coalescence, the value of efficiency for the enhanced treatment was higher and the gradient of the decrease was lower. The enhanced treatment resisted fluctuations in the superficial velocity as well as in the flow rate and obtained a sufficient treatment effect with a large throughput.
3.3. Effects of W/O Emulsion Characteristics
The characteristics of the W/O emulsion to be processed including the initial content of water and surfactant were investigated in this section, as shown in Figure 8.
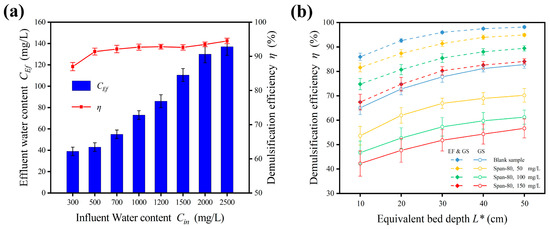
Figure 8.
Effects of W/O emulsion characteristics. (a) Effect of surfactant concentration. (b) Effects of influent water content. Conditions: Cin = 300–2500 mg/L; v = 0.001 m/s; U = 0 or 3 kV; GS medium bed with size of 1.0–2.0 mm and L* = 20 cm.
The water content of an emulsion fluctuates frequently in industrial production, therefore a study on water content was necessary. For emulsions with influent water content ranging from 300 to 2500 mg/L, the demulsification effect of the enhanced treatment was maintained at high levels, with an average efficiency of 92.09% (Figure 8a). The efficiency even increased slightly with a higher water content. When the influent water content was 2500 mg/L, the efficiency could reach up to 94.5%. There were two reasons accounting for this result. On the one hand, a higher water content meant a larger number of water droplets per unit volume in the emulsion. The possibility of droplets being captured by the medium increased. On the other hand, a higher water content meant a larger proportion of dispersed droplets. These droplets can be entirely separated; hence, the efficiency increased instead. The enhanced treatment revealed a high tolerance for water content and was adaptive to fluctuating water content.
Surfactants such as asphaltenes, resins, and waxes widely exist in the production of crude oil. Surfactants work by attaching themselves to the oil–water interface and change the interfacial tension during the coalescence process [35]. A nonionic oil-soluble surfactant, Span-80, was used to control the emulsification degree and investigate the effect of surfactant concentration, as shown in Figure 8b. For medium coalescence alone, the demulsification performance of the processing surfactant was poor. The efficiency changed from 65.1% (blank sample) to 53.7%, 46.7%, and 42.3% for surfactant concentrations of 50 mg/L, 100 mg/L, and 150 mg/L, respectively, at L* = 10 cm. The treatment effect deteriorated rapidly with surfactant concentration. Even though the equivalent depth L* was added up to 50 cm, the efficiency was increased by 16.5% (50 mg/L), 14.6% (100 mg/L) and 14.4% (150 mg/L), which was far below the efficiency of 82.8% of the blank sample. Hence, it is difficult to improve the demulsification effect by simply increasing the bed depth. In contrast, the efficiency of the enhanced treatment decreased from 86.0% (blank sample) to 81.6% (50 mg/L), 74.8% (100 mg/L), and 67.4% (150 mg/L) at L* = 10 cm. According to the data displayed, the advantage of the enhanced treatment is highlighted. For instance, the efficiency of the enhanced treatment at L* = 10 cm (67.43%) completely exceeded that of medium coalescence at L* = 50 cm (56.7%) for treating an emulsion of 150 mg/L. The enhanced treatment was particularly effective for processing an actual emulsion with strong emulsification.
3.4. Effects of Medium Bed Parameters
The investigation of the effect of medium bed parameters provided guidance for selecting the appropriate medium for treating the emulsion. The parameters of the medium bed, including medium size, bed depth, and wettability, were studied in this section.
The effect of medium size is shown in Figure 9a. The media used in this investigation were GS particles due to the effective regeneration of the particle medium after backwash, which is essential in practical applications [36]. The demulsification effect was remarkably enhanced with the decrease in medium size, regardless of whether the electric field was applied or not. In the absence of the electric field, the corresponding demulsification efficiencies were 78.1%, 72.8%, and 62.0% when treated with medium sizes of 0.5–1.0 mm, 1.0–2.0 mm, and 2.0–3.0 mm, respectively. Under an electric field of U = 5 kV, the corresponding efficiencies were 96.1% (0.5–1.0 mm GS), 95.3% (1.0–2.0 mm GS), and 89.9% (2.0–3.0 mm GS). A decrease in medium size resulted in a smaller porosity factor and larger specific surface area. Droplets were less likely to pass through the narrower gaps and the increase in specific surface area provided more sites for droplet–medium collisions, thus promoting coalescence. Since the usage of an electric field played a more important role than the medium, the efficiency of the media with varied diameters approached each other as the applied voltage increased. In the absence of an electric field, the difference in efficiency values between treatments with a medium size between 0.5–1.0 mm and 2.0–3.0 mm was 16.1%. However, the difference value was reduced to 6.2% after an electric field of U = 5 kV was applied. The large-sized medium could be used without much loss in efficiency thanks to the enhanced treatment, which reduced the gap of the demulsification effect between different sizes. Bed depth was also an important design parameter linearly related to residence time.
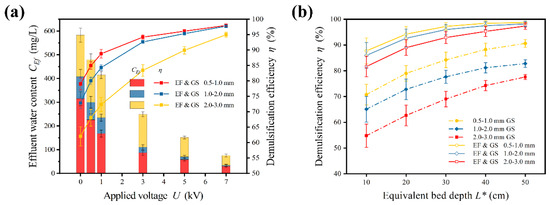
Figure 9.
Effects of medium bed parameters (a) Effect of medium particle size. (b) Effect of bed depth. Conditions: Cin = 1500 mg/L; v = 0.001 m/s; U = 0–7 kV; GS medium bed with size of 0.5–1.0 mm, 1.0–2.0 mm and 2.0–3.0 mm and L* = 20 cm.
The effect of larger bed depth and smaller medium size were similar, as shown in Figure 9b. An increase in bed depth meant an extension of the residence time, spontaneously improving the demulsification effect. Nonetheless, the demulsification effect was limited even at an excessively large bed depth. In the absence of electric field, as the bed depth increased from 10 cm to 50 cm, the efficiency increased from 65.1% to 82.8% (1.0–2.0 mm GS). The average increasing rates throughout first half (10–25 cm) and latter half (25–50 cm) were 0.63% and 0.17%, respectively. The rate of increase in efficiency gradually decreased with depth because the viscous effect was stronger at a greater depth. Hence, it is difficult to improve the demulsification effect simply by increasing the bed depth. On the contrary, increasing bed depth will inevitably result in a linear rise in the pressure drop.
The effect of medium wettability is displayed in Figure 10. Four kinds of materials, including GS, G, A and PS, were used in the experiment. GS and G were hydrophilic, whereas A and PS were hydrophobic. In the absence of an electric field, the corresponding efficiencies were 62.8% (PS), 66.7% (A), 70.1% (G), and 72.8% (GS). For medium coalescence, the hydrophilicity or hydrophobicity of the medium had a tremendous impact. The hydrophilic medium proved preferable for treating W/O emulsions [37]. This is because the droplets stuck together on the hydrophilic medium surface in the form of a film, which was conducive to coalescence. However, the effect of wettability was negligible in the enhanced treatment due to the dominant role of the electric field. At U = 7 kV, the efficiencies were 96.9% (PS), 97.2% (A), 97.4% (G), and 97.7% (GS). For the enhanced treatment, the demulsification effect of the hydrophilic medium was still slightly better than that of the hydrophobic medium. However, compared with the electric field, the wettability contributed very little. Curiously, the efficiency of GS was higher than that of G, even though they shared the same wettability. This occurred due to the higher shape factor of GS, which was more efficient at capturing droplets. The enhanced treatment narrowed the gap between different media and vastly expanded the kinds of media that could be used.
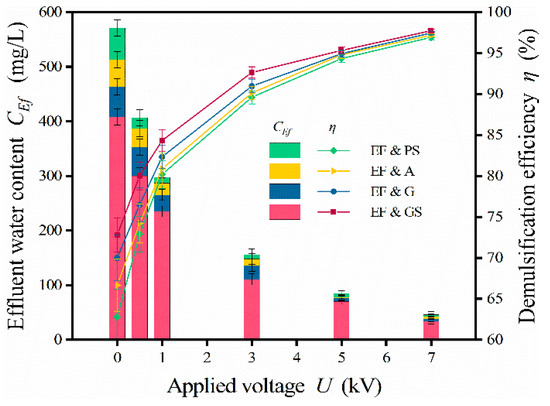
Figure 10.
Effect of wettability. Conditions: Cin = 1500 mg/L; v = 0.001 m/s; U = 0–7 kV; PS, A, G, and GS medium bed with size of 1.0–2.0 mm and L* = 20 cm.
4. Conclusions
It is expected that sufficient demulsification can be achieved after a single-pass operation in industrial applications. Due to the long procedure and limited space on offshore platforms, outdated oil-treatment facilities should be upgraded. The novel enhanced treatment proposed by this study provided a new approach to achieve fast and thorough demulsification at a lower cost. A comprehensive investigation has been conducted to investigate the effect of the enhanced treatment and the main conclusions are summarized as follows:
- (1)
- On a microscopic level, the EHD effect accelerated water droplets and increased the probability of droplet–medium collisions. In the macroscopic test, the enhanced treatment showed outstanding performance. The voltage of the electric field was a critical factor affecting demulsification and is dominant over the other parameters. As the voltage is increased, the demulsification effect improves, accompanied by a larger throughput and less residence time. In addition, short circuits between the electrodes will likely be avoided especially in practical applications.
- (2)
- The enhanced treatment can adapt to fluctuation in the water content as well as to strong emulsification. When the influent water content ranged from 300 to 2500 mg/L, the average demulsification efficiency was about 92%. The enhanced treatment also showed high tolerance for flow rate. A superficial velocity of 0.015 m/s was appropriate, under which the balance between throughput and efficiency was mainly achieved.
- (3)
- The effect of wettability was much weaker than that of voltage; hence, the wettability parameter could be neglected when designing the medium. A smaller medium size and larger bed depth of the medium bed were favorable for demulsification, but this trend decreased gradually in the later stage of treatment. Higher voltage could weaken the effect of the medium and reduce the difference between medium parameters.
In general, the combined treatment has the advantages of wide operational flexibility, a tolerance for deteriorated characteristics and a rapid and thorough treatment process. It is expected to be employed in technology upgrades on space-constrained offshore platforms. To promote industrialization, in-depth research on parameter optimization according to actual requirements and safe utilization of electric power should be further explored.
Author Contributions
S.F.: Conceptualization, Methodology, Validation, Software, Writing—original draft. S.W.: Formal Analysis, Visualization. Y.L. (Yudong Li): Data curation, Investigation. X.Y.: Resources, Supervision. Y.Y.: Resources, Validation. Y.L. (Yiqian Liu): Writing—original draft, Visualization, Investigation. H.L.: Conceptualization, Funding acquisition, Methodology, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (Grant No. 52000072, 52025103) and the Shanghai Rising-Star Program (Grant No. 22QA1402600).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Watson, S.M. Greenhouse gas emissions from offshore oil and gas activities—Relevance of the Paris Agreement, Law of the Sea, and Regional Seas Programmes. Ocean. Coast. Manag. 2020, 185, 104942. [Google Scholar] [CrossRef]
- Yacovitch, T.I.; Daube, C.; Herndon, S. Methane Emissions from Offshore Oil and Gas Platforms in the Gulf of Mexico. Environ. Sci. Technol. 2020, 54, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Less, S.; Vilagines, R. The electrocoalescers’ technology: Advances, strengths and limitations for crude oil separation. J. Pet. Sci. Eng. 2012, 81, 57–63. [Google Scholar] [CrossRef]
- Yue, X.; Fu, D.; Zhang, T.; Yang, D.; Qiu, F. Superhydrophobic Stainless-Steel Mesh with Excellent Electrothermal Properties for Efficient Separation of Highly Viscous Water-in-Crude Oil Emulsions. Ind. Eng. Chem. Res. 2020, 59, 17918–17926. [Google Scholar] [CrossRef]
- Sousa, A.M.; Pereira, M.; Matos, H. Oil-in-water and water-in-oil emulsions formation and demulsification. J. Pet. Sci. Eng. 2022, 210, 110041. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Barbosa, Y.M.; Silva, J.; Junior, S.D.O. A novel methodology for the design and optimisation of oil and gas offshore platforms. Energy 2019, 185, 158–175. [Google Scholar] [CrossRef]
- Yonguep, E.; Kapiamba, K.F.; Kabamba, K.J.; Chowdhury, M. Formation, stabilization and chemical demulsification of crude oil-in-water emulsions: A review. Pet. Res. 2022. [Google Scholar] [CrossRef]
- Nam, B.W.; Kim, Y.; Hong, S. Time-domain simulation of berthing problem between FPSO and shuttle tanker in waves. Appl. Ocean. Res. 2016, 58, 49–61. [Google Scholar] [CrossRef]
- Ye, G.; Lü, X.; Peng, F.; Han, P.; Shen, X. Pretreatment of Crude Oil by Ultrasonic-electric United Desalting and Dewatering. Chin. J. Chem. Eng. 2008, 16, 564–569. [Google Scholar] [CrossRef]
- White, H.J. Centenary of Frederick Gardner Cottrell. J. Electrost. 1977, 4, 1–34. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M.; Sharif, A.O.; Williams, T.J. Electrostatic enhancement of coalescence of water droplets in oil: A review of the current understanding. Chem. Eng. J. 2001, 84, 173–192. [Google Scholar] [CrossRef]
- Guo, C.; He, L. Coalescence behaviour of two large water-drops in viscous oil under a DC electric field. J. Electrost. 2014, 72, 470–476. [Google Scholar] [CrossRef]
- Khorshidi, B.; Jalaal, M.; Esmaeilzadeh, E.; Mohammadi, F. Characteristics of deformation and electrical charging of large water drops immersed in an insulating liquid on the electrode surface. J. Colloid Interface Sci. 2010, 352, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Eow, J.S.; Ghadiri, M. Electrostatic enhancement of coalescence of water droplets in oil: A review of the technology. Chem. Eng. J. 2002, 85, 357–368. [Google Scholar] [CrossRef]
- Gabyshev, D.N.; Fedorets, A.A. Electrically induced coalescence of droplet clusters in external electric fields. J. Electrost. 2021, 112, 103596. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Cai, X.; Huang, S.; Ji, Y. Research on electrostatic coalescence of water-in-crude-oil emulsions under high frequency/high voltage AC electric field based on electro-rheological method. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 246–256. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.; He, L.; Luo, X.; Lü, Y. Coalescence characteristics of droplets at oil–water interface subjected to different electric waveforms. Chem. Eng. Process.—Process Intensif. 2018, 134, 28–37. [Google Scholar] [CrossRef]
- Wang, B.-B.; Wang, X.-D.; Wang, T.-H.; Lu, G.; Yan, W.-M. Electro-coalescence of two charged droplets under constant and pulsed DC electric fields. Int. J. Heat Mass Transf. 2016, 98, 10–16. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Ghadiri, M.; Buckley, M. Electro-coalescence of water drops in oils under pulsatile electric fields. Chem. Eng. Sci. 2014, 120, 130–142. [Google Scholar] [CrossRef]
- Yang, D.; Feng, Y.; Wang, B.; Liu, Y.; Zheng, Y.; Sun, X.; Peng, J.; Feng, M.; Wang, D. An asymmetric AC electric field of triboelectric nanogenerator for efficient water/oil emulsion separation. Nano Energy 2021, 90, 106641. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M. Drop–drop coalescence in an electric field: The effects of applied electric field and electrode geometry. Colloids Surf. A Physicochem. Eng. Asp. 2003, 219, 253–279. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.-Q.; Cai, J.-B.; Xu, X.; Xie, L.-S.; Yang, Q.; Li, Y.-X.; Zhu, K. Treatment of offshore oily produced water: Research and application of a novel fibrous coalescence technique. J. Pet. Sci. Eng. 2019, 178, 602–608. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, Z.; Luo, H.; Bai, Z.; Zhang, B. Application of a combined fibrous coalescence technique in deep purification separation of composite ionic liquid alkylation reaction products. J. Clean. Prod. 2020, 261, 121290. [Google Scholar] [CrossRef]
- Liao, Y.; Lucas, D. A literature review on mechanisms and models for the coalescence process of fluid particles. Chem. Eng. Sci. 2010, 65, 2851–2864. [Google Scholar] [CrossRef]
- Liu, B.; Wei, Q.; Ma, H.; Chen, L.; Chang, Y.; Chen, J.; Dai, L.; Sun, Y.; Lu, H.; Wang, H.; et al. Cooperative physical separation of oil and suspended solids from methanol-to-olefin wastewater: A pilot study. J. Environ. Manag. 2022, 311, 114841. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Pan, Z.; Dai, P.; Yang, Q.; Lu, H. Treatment of electric desalting wastewater by swirling flotation coupled with medium coalescence. J. Environ. Chem. Eng. 2021, 9, 106055. [Google Scholar] [CrossRef]
- Parvasi, P.; Hesamedini, A.K.; Jahanmiri, A.; Rahimpour, M.R. A Comparative Study on Droplet Coalescence in Heavy Crude Oil Emulsions Subjected to Microwave and Ultrasonic Fields. Sep. Sci. Technol. 2013, 48, 1591–1601. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Pan, Z. Experimental Study on the Performance of a Novel Compact Electrostatic Coalescer with Helical Electrodes. Energies 2021, 14, 1733. [Google Scholar] [CrossRef]
- Gong, H.; Yu, B.; Dai, F.; Peng, Y. Influence of electric field on water-droplet separated from emulsified oil in a double-field coupling device. Colloids Surf. A Physicochem. Eng. Asp. 2018, 550, 27–36. [Google Scholar] [CrossRef]
- Hase, M.; Watanabe, S.; Yoshikawa, K. Rhythmic motion of a droplet under a dc electric field. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2006, 74 Pt 2, 046301. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M. Motion, deformation and break-up of aqueous drops in oils under high electric field strengths. Chem. Eng. Process. Process Intensif. 2003, 42, 259–272. [Google Scholar] [CrossRef]
- Agarwal, S.; Von Arnim, V.; Stegmaier, T.; Planck, H.; Agarwal, A. Effect of Fibrous Coalescer Geometry and Operating Conditions on Emulsion Separation. Ind. Eng. Chem. Res. 2013, 52, 13164–13170. [Google Scholar] [CrossRef]
- Luo, X.; Yan, H.; Huang, X.; Yang, D.; Wang, J.; He, L. Breakup characteristics of aqueous droplet with surfactant in oil under direct current electric field. J. Colloid Interface Sci. 2017, 505, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Sobolciak, P.; Popelka, A.; Tanvir, A.; Al-Maadeed, M.A.; Adham, S.; Krupa, I. Materials and Technologies for the Tertiary Treatment of Produced Water Contaminated by Oil Impurities through Nonfibrous Deep-Bed Media: A Review. Water 2020, 12, 3419. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Q.; Xu, X.; Wang, H.-L. Effect of the Mixed Oleophilic Fibrous Coalescer Geometry and the Operating Conditions on Oily Wastewater Separation. Chem. Eng. Technol. 2016, 39, 255–262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).