Ghosts of the Holobiont: Borings on a Miocene Turtle Carapace from the Pisco Formation (Peru) as Witnesses of Ancient Symbiosis
Abstract
1. Introduction
2. Geological and Palaeontological Framework
3. Results
4. Other Turtle Barnacle Scars in the Fossil Record
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pokines, J.T.; Higgs, N. Macroscopic taphonomic alterations to human bone recovered from marine environments. J. Forensic Ident. 2015, 65, 953–984. [Google Scholar]
- Egeland, C.P.; Pickering, T.R. Cruel traces: Bone surface modifications and their relevance to forensic science. WIREs Forensic Sci. 2021, 3, e1400. [Google Scholar] [CrossRef]
- Brownstein, C.D. Trace fossils on dinosaur bones reveal ecosystem dynamics along the coast of eastern North America during the latest Cretaceous. PeerJ 2018, 6, e4973. [Google Scholar] [CrossRef]
- Collareta, A.; Tsai, C.-H.; Coletti, G.; Bosselaers, M. Thatchtelithichnus on a Pliocene grey whale mandible and barnacles as possible tracemakers. N. Jahrb. Geol. Palaontol. Abh. 2021, 302, 53–61. [Google Scholar] [CrossRef]
- Sacco, F. II delfino pliocenico di Camerano Casasco (Astigiana). Mem. Soc. Ital. Sci. 1893, 9, 1–15. [Google Scholar]
- Dennison, K.J.; Kieser, J.A.; Buckeridge, J.S.; Bishop, P.J. Post mortem cohabitation—Shell growth as a measure of elapsed time: A case report. Forensic Sci. Int. 2004, 139, 249–254. [Google Scholar] [CrossRef]
- Boessenecker, R.W. Taphonomic implications of barnacle encrusted sea lion bones from the middle Pleistocene Port Orford Formation, coastal Oregon. J. Paleontol. 2013, 87, 657–663. [Google Scholar] [CrossRef]
- Higgs, N.; Pokines, J.T. Marine environmental alterations to bone. In Manual of Forensic Taphonomy; Pokines, J.T., Symes, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 143–179. [Google Scholar]
- Tsai, C.H.; Collareta, A.; Bosselaers, M. A Pliocene gray whale (Eschrichtius sp.) from the eastern North Atlantic. Riv. Ital. Paleontol. Stratigr. 2020, 126, 189–196. [Google Scholar]
- Bosio, G.; Collareta, A.; Di Celma, C.; Lambert, O.; Marx, F.G.; de Muizon, C.; Gioncada, A.; Gariboldi, K.; Malinverno, E.; Malca, R.V.; et al. Taphonomy of marine vertebrates of the Pisco Formation (Miocene, Peru): Insights into the origin of an outstanding Fossil-Lagerstätte. PLoS ONE 2021, 16, e0254395. [Google Scholar] [CrossRef]
- Collareta, A.; Bianucci, G. The occurrence of the coronuloid barnacle Chelonibia Leach, 1817 as an encruster on mammalian bone in the central Mediterranean Sea. Acta Adriat. 2021, 62, 83–92. [Google Scholar] [CrossRef]
- Chan, B.K.K.; Dreyer, N.; Gale, A.S.; Glenner, H.; Ewers-Saucedo, C.; Pérez-Losada, M.; Kolbasov, G.A.; Crandall, K.A.; Høeg, J.T. The evolutionary diversity of barnacles, with an updated classification of fossil and living forms. Zool. J. Linn. Soc. 2021, 193, 789–846. [Google Scholar] [CrossRef]
- Darwin, C. A Monograph on The Sub-Class Cirripedia, with Figures of All the Species; The Balanidae, The Verrucidae; The Ray Society: London, UK, 1854. [Google Scholar]
- Fischer, P. Description d’un nouveau genre de Cirrhipèdes (Stephanolepas) parasite des tortues marines. Actes Soc. Linn. Bordeaux. 1886, 40, 193–196. [Google Scholar]
- Korschelt, E. Uber zwei parasitäre Cirripedien, Chelonibia und Dendrogaster, nebst Angaben uber die Beziehungen der Balanomorphen zu ihrer Unterlage. Zool. J. 1933, 64, 1–40. [Google Scholar]
- Hendrickson, J.R. The green sea turtle, Chelonia mydas (Linn.) in Malaya and Sarawak. In Proceedings of The Zoological Society of London; Blackwell Publishing Ltd.: Oxford, UK, 1958; Volume 130, pp. 455–535. [Google Scholar] [CrossRef]
- Davis, C.W. Studies on the Barnacles Epizoic on Marine Vertebrates. Ph.D. Thesis, California State University, Los Angeles, CA, USA, 1972. [Google Scholar]
- Herbst, L.H.; Jacobson, E.R. Diseases of marine turtles. In Biology and Conservation of Sea Turtles; Bjorndal, K.A., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1995; pp. 593–596. [Google Scholar]
- Green, D. Epizoites of Galapagos green turtles. In Proceedings of the 16th Annual Symposium on Sea Turtle Biology and Conservation, Hilton Head, SC, USA, 28 February–1 March 1996; Volume 412, p. 63. [Google Scholar]
- Frick, M.G.; Zardus, J.D.; Ross, A.; Senko, J.; Montano-Valdez, D.; Bucio-Pacheco, M.; Sosa-Cornejo, I. Novel records and observations of the barnacle Stephanolepas muricata (Cirripedia: Balanomorpha: Coronuloidea); including a case for chemical mediation in turtle and whale barnacles. J. Nat. Hist. 2011, 45, 629–640. [Google Scholar] [CrossRef]
- Frick, M.G.; Pfaller, J.B. Sea turtle epibiosis. In The Biology of Sea Turtles; Wyneken, J., Lohmann, K.J., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 3, pp. 399–426. [Google Scholar]
- Hayashi, R.; Chan, B.K.; Simon-Blecher, N.; Watanabe, H.; Guy-Haim, T.; Yonezawa, T.; Levy, Y.; Shuto, T.; Achituv, Y. Phylogenetic position and evolutionary history of the turtle and whale barnacles (Cirripedia: Balanomorpha: Coronuloidea). Mol. Phylogenet. Evol. 2013, 67, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, J.-P.; Zonneveld, Z.E.E.; Bartels, W.S.; Gingras, M.K.; Head, J.J. Bone modification features resulting from barnacle attachment on the bones of loggerhead sea turtles (Caretta caretta), Cumberland Island, Georgia, USA: Implications for the paleoecological, and taphonomic analyses of fossil sea turtles. Palaios 2022, 37, 650–670. [Google Scholar] [CrossRef]
- Zardus, J.D. A Global Synthesis of the Correspondence Between Epizoic Barnacles and Their Sea Turtle Hosts. Integr. Org. Biol. 2021, 3, obab002. [Google Scholar] [CrossRef]
- Collareta, A.; Rasser, M.W.; Frey, E.; Harzhauser, M. Turtle barnacles have been turtle riders for more than 30 million years. PalZ 2022. [Google Scholar] [CrossRef]
- Harzhauser, M.; Newman, W.A.; Grunert, P. A new Early Miocene barnacle lineage and the roots of sea-turtle fouling Che-lonibiidae (Cirripedia, Balanomorpha). J. Syst. Palaeontol. 2011, 9, 473–480. [Google Scholar] [CrossRef]
- Collareta, A.; Merella, M.; Bosselaers, M.; Casati, S.; Di Cencio, A.; Bianucci, G. A Karethraichnus boring on a turtle shell bone from the Miocene of Italy is assessed as the attachment scar of a platylepadid symbiont. N. Jahrb. Geol. Palaontol. Abh. 2022, 303, 327–337. [Google Scholar] [CrossRef]
- Zonneveld, J.-P.; Bartels, W.S.; Gunnell, G.F.; McHugh, L.P. Borings in early Eocene turtle shell from the Wasatch Formation, South Pass, Wyoming. J. Paleontol. 2015, 89, 802–820. [Google Scholar] [CrossRef]
- Zonneveld, J.-P.; AbdelGawad, M.K.; Miller, E.R. Ectoparasite borings, mesoparasite borings, and scavenging traces in early Miocene turtle and tortoise shell: Moghra Formation, Wadi Moghra, Egypt. J. Paleontol. 2022, 96, 304–322. [Google Scholar] [CrossRef]
- Pilger, R. Plate reconstructions, aseismic ridges, and low-angle subduction beneath the Andes. GSA Bull. 1981, 92, 448–456. [Google Scholar] [CrossRef]
- Hsu, J.T. Quaternary uplift of the Peruvian coast related to the subduction of the Nazca Ridge: 13.5 to 15.6 degrees south latitude. Quat. Int. 1992, 15, 87–97. [Google Scholar] [CrossRef]
- Macharé, J.; Ortlieb, L. Plio-Quaternary vertical motions and the subduction of the Nazca Ridge, central coast of Peru. Tectonophysics 1992, 205, 97–108. [Google Scholar] [CrossRef]
- Hampel, A.; Kukowski, N.; Bialas, J.; Huebscher, C.; Heinbockel, R. Ridge subduction at an erosive margin: The collision zone of the Nazca Ridge in southern Peru. J. Geophys. Res. Solid Earth 2004, 109. [Google Scholar] [CrossRef]
- Dunbar, R.B.; Marty, R.C.; Baker, P.A. Cenozoic marine sedimentation in the Sechura and Pisco basins, Peru. Palaeogeogr. Palaeoclim. Palaeoecol. 1990, 77, 235–261. [Google Scholar] [CrossRef]
- DeVries, T.J. Oligocene deposition and Cenozoic sequence boundaries in the Pisco Basin (Peru). J. S. Am. Earth Sci. 1998, 11, 217–231. [Google Scholar] [CrossRef]
- DeVries, T.J. Eocene stratigraphy and depositional history near Puerto Caballas (East Pisco basin, Peru). Bol. Soc. Geol. Perú 2017, 112, 39–52. [Google Scholar]
- Di Celma, C.; Pierantoni, P.P.; Volatili, T.; Molli, G.; Mazzoli, S.; Sarti, G.; Ciattoni, S.; Bosio, G.; Malinverno, E.; Collareta, A.; et al. Towards deciphering the Cenozoic evolution of the East Pisco Basin (southern Peru). J. Maps 2022, 1–16. [Google Scholar] [CrossRef]
- Di Celma, C.; Malinverno, E.; Bosio, G.; Collareta, A.; Gariboldi, K.; Gioncada, A.; Molli, G.; Basso, D.; Varas-Malca, R.M.; Pierantoni, P.P.; et al. Sequence stratigraphy and paleontology of the upper Miocene Pisco Formation along the western side of the lower Ica valley (Ica Desert, Peru). Riv. Ital. Paleontol. Stratigr. 2017, 123, 255–274. [Google Scholar]
- Di Celma, C.; Malinverno, E.; Bosio, G.; Gariboldi, K.; Collareta, A.; Gioncada, A.; Landini, W.; Pierantoni, P.P.; Bianucci, G. Intraformational unconformities as a record of late Miocene eustatic falls of sea level in the Pisco Formation (southern Peru). J. Maps 2018, 14, 607–619. [Google Scholar] [CrossRef]
- North American Commission on Stratigraphic Nomenclature. North American stratigraphic code. AAPG Bull. 2005, 89, 1547–1591. [Google Scholar] [CrossRef]
- Travis, R.B.; Gonzales, G.; Pardo, A. Hydrocarbon potential of coastal basins of Peru: Hydrocarbons. In Circum-Pacific Energy and Mineral Resources, 1st ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1976; pp. 331–338. [Google Scholar]
- Thornburg, T.M.; Kulm, L.D. Sedimentary basins of the Peru continental margin: Structure, stratigraphy, and Cenozoic tectonics from 6 S to 16 S latitude. Nazca Plate Crustal Form. Andean Converg. 1981, 154, 393–422. [Google Scholar]
- Di Celma, C.; Malinverno, E.; Collareta, A.; Bosio, G.; Gariboldi, K.; Lambert, O.; Landini, W.; Gioncada, A.; Villa, I.M.; Coletti, G.; et al. Facies analysis, stratigraphy and marine vertebrate assemblage of the early Miocene Chilcatay Formation at Ullujaya (Pisco basin, Peru). J. Maps 2018, 14, 257–268. [Google Scholar] [CrossRef]
- Collareta, A.; Lambert, O.; Marx, F.G.; de Muizon, C.; Varas-Malca, R.; Landini, W.; Bosio, G.; Malinverno, E.; Gariboldi, K.; Gioncada, A.; et al. Vertebrate Palaeoecology of the Pisco Formation (miocene, Peru): Glimpses into the ancient Humboldt Current Ecosystem. J. Mar. Sci. Eng. 2021, 9, 1188. [Google Scholar] [CrossRef]
- Suess, E.; Von Huene, R.; Emeis, K.C.; Bourgois, J.; del C Cruzado Casteñeda, J.; De, P.; Eglinton, G.; Garrison, R.; Greenberg, M.; Herrera, P.E.; et al. Introduction, objectives, and principal results, Leg 112, Peru continental margin. Proc. Ocean Drill. Progr. Init. Rep. 1988, 112, 5–23. [Google Scholar]
- Brand, L.R.; Esperante, R.; Chadwick, A.V.; Porras, O.P.; Alomía, M. Fossil whale preservation implies high diatom accumulation rate in the Miocene–Pliocene Pisco Formation of Peru. Geology 2004, 32, 165–168. [Google Scholar] [CrossRef]
- de Muizon, C.; Devries, T.J. Geology and paleontology of late Cenozoic marine deposits in the Sacaco area (Peru). Geol. Rundsch. 1985, 74, 547–563. [Google Scholar] [CrossRef]
- de Muizon, C. Les Vertébrés de la Formation Pisco (Pérou). Troisième partie: Les Odontocètes (Cetacea, Mammalia) du Miocène. Bull. Inst. Fr. d’Études Andines 1988, 42, 1–244. [Google Scholar]
- Lambert, O.; Bianucci, G.; Post, K. A new beaked whale (Odontoceti, Ziphiidae) from the middle Miocene of Peru. J. Vertebr. Paleontol. 2009, 29, 910–922. [Google Scholar] [CrossRef]
- Lambert, O.; Bianucci, G.; Post, K.; de Muizon, C.; Salas-Gismondi, R.; Urbina, M.; Reumer, J. The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru. Nature 2010, 466, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Lambert, O.; Collareta, A.; Landini, W.; Post, K.; Ramassamy, B.; Di Celma, C.; Urbina, M.; Bianucci, G. No deep diving: Evidence of predation on epipelagic fish for a stem beaked whale from the Late Miocene of Peru. Proc. R. Soc. Lond. B Biol. Sci. 2015, 282, 20151530. [Google Scholar] [CrossRef] [PubMed]
- Lambert, O.; Bianucci, G.; de Muizon, C. Macroraptorial sperm whales (Cetacea, Odontoceti, Physeteroidea) from the Miocene of Peru. Zool. J. Linn. Soc. 2017, 179, 404–474. [Google Scholar] [CrossRef]
- Lambert, O.; Bianucci, G.; Urbina, M.; Geisler, J.H. A new inioid (Cetacea, Odontoceti, Delphinida) from the Miocene of Peru and the origin of modern dolphin and porpoise families. Zool. J. Linn. Soc. 2017, 179, 919–946. [Google Scholar] [CrossRef]
- Lambert, O.; Collareta, A.; Benites-Palomino, A.; Di Celma, C.; de Muizon, C.; Urbina, M.; Bianucci, G. A new small, mesorostrine inioid (Cetacea, Odontoceti, Delphinida) from four upper Miocene localities in the Pisco Basin, Peru. Pap. Palaeontol. 2021, 7, 1043–1064. [Google Scholar] [CrossRef]
- Bianucci, G.; Lambert, O.; Post, K. High concentration of long-snouted beaked whales (genus Messapicetus) from the Miocene of Peru. Palaeontology 2010, 53, 1077–1098. [Google Scholar] [CrossRef]
- Bianucci, G.; Di Celma, C.; Collareta, A.; Landini, W.; Post, K.; Tinelli, C.; de Muizon, C.; Bosio, G.; Gariboldi, K.; Gioncada, A.; et al. Fossil marine vertebrates of Cerro Los Quesos: Distribution of cetaceans, seals, crocodiles, seabirds, sharks, and bony fish in a late Miocene locality of the Pisco Basin, Peru. J. Maps 2016, 12, 1037–1046. [Google Scholar] [CrossRef]
- Bianucci, G.; Di Celma, C.; Landini, W.; Post, K.; Tinelli, C.; de Muizon, C.; Gariboldi, K.; Malinverno, E.; Cantalamessa, G.; Gioncada, A.; et al. Distribution of fossil marine vertebrates in Cerro Colorado, the type locality of the giant raptorial sperm whale Livyatan melvillei (Miocene, Pisco Formation, Peru). J. Maps 2016, 12, 543–557. [Google Scholar] [CrossRef]
- Bianucci, G.; Di Celma, C.; Urbina, M.; Lambert, O. New beaked whales from the late Miocene of Peru and evidence for convergent evolution in stem and crown Ziphiidae (Cetacea, Odontoceti). PeerJ 2016, 4, e2479. [Google Scholar] [CrossRef]
- Bianucci, G.; Marx, F.G.; Collareta, A.; Di Stefano, A.; Landini, W.; Morigi, C.; Varola, A. Rise of the titans: Baleen whales became giants earlier than thought. Biol. Lett. 2019, 15, 20190175. [Google Scholar] [CrossRef] [PubMed]
- Brand, L.; Urbina, M.; Chadwick, A.; DeVries, T.J.; Esperante, R. A high resolution stratigraphic framework for the remarkable fossil cetacean assemblage of the Miocene/Pliocene Pisco Formation, Peru. J. S. Am. Earth Sci. 2011, 31, 414–425. [Google Scholar] [CrossRef]
- Esperante, R.; Brand, L.; Nick, K.E.; Poma, O.; Urbina, M. Exceptional occurrence of fossil baleen in shallow marine sediments of the Neogene Pisco Formation, Southern Peru. Palaeogeogr. Palaeoclim. Palaeoecol. 2008, 257, 344–360. [Google Scholar] [CrossRef]
- Esperante, R.; Brand, L.R.; Chadwick, A.V.; Poma, O. Taphonomy and paleoenvironmental conditions of deposition of fossil whales in the diatomaceous sediments of the Miocene/Pliocene Pisco Formation, southern Peru—A new fossil-lagerstätte. Palaeogeogr. Palaeoclim. Palaeoecol. 2015, 417, 337–370. [Google Scholar] [CrossRef]
- Parham, J.F.; Pyenson, N.D. New sea turtle from the Miocene of Peru and the iterative evolution of feeding ecomorphologies since the Cretaceous. J. Paleontol. 2010, 84, 231–247. [Google Scholar] [CrossRef]
- Collareta, A.; Landini, W.; Lambert, O.; Post, K.; Tinelli, C.; Di Celma, C.; Panetta, D.; Tripodi, M.; Salvadori, P.A.; Caramella, D.; et al. Piscivory in a Miocene Cetotheriidae of Peru: First record of fossilized stomach content for an extinct baleen-bearing whale. Sci. Nat. 2015, 102, 70. [Google Scholar] [CrossRef]
- Collareta, A.; Landini, W.; Chacaltana-Budiel, C.; Valdivia-Vera, W.; Altamirano-Sierra, A.; Urbina, M.; Bianucci, G. A well preserved skeleton of the fossil shark Cosmopolitodus hastalis from the late Miocene of Peru, featuring fish remains as fossilized stomach contents. Riv. Ital. Paleontol. Strat. 2017, 123, 11–22. [Google Scholar]
- Collareta, A.; Lambert, O.; de Muizon, C.; Palomino, A.M.B.; Urbina, M.; Bianucci, G. A new physeteroid from the late Miocene of Peru expands the diversity of extinct dwarf and pygmy sperm whales (Cetacea: Odontoceti: Kogiidae). Comptes Rendus Palevol 2020, 19, 79–100. [Google Scholar] [CrossRef]
- Collareta, A.; Di Celma, C.; Bosio, G.; Pierantoni, P.P.; Malinverno, E.; Lambert, O.; Marx, F.G.; Landini, W.; Urbina, M.; Bianucci, G. Distribution and paleoenvironmental framework of middle Miocene marine vertebrates along the western side of the lower Ica Valley (East Pisco Basin, Peru). J. Maps 2021, 17, 7–17. [Google Scholar] [CrossRef]
- Gariboldi, K.; Gioncada, A.; Bosio, G.; Malinverno, E.; Di Celma, C.; Tinelli, C.; Cantalamessa, G.; Landini, W.; Urbina, M.; Bianucci, G. The dolomite nodules enclosing fossil marine vertebrates in the East Pisco Basin, Peru: Field and petrographic insights into the Lagerstätte formation. Palaeogeogr. Palaeoclim. Palaeoecol. 2015, 438, 81–95. [Google Scholar] [CrossRef]
- Stucchi, M.; Emslie, S.D.; Varas-Malca, R.M.; Urbina, M. A new late Miocene condor (Aves, Cathartidae) from Peru and the origin of South American Condors. J. Vertebr. Paleontol. 2015, 35, e972507. [Google Scholar] [CrossRef]
- Stucchi, M.; Varas-Malca, R.M.; Urbina-Schmitt, M. New Miocene sulid birds from Peru and considerations on their Neogene fossil record in the Eastern Pacific Ocean. Acta Palaeontol. Pol. 2015. [Google Scholar] [CrossRef]
- Gioncada, A.; Collareta, A.; Gariboldi, K.; Lambert, O.; Di Celma, C.; Bonaccorsi, E.; Urbina, M.; Bianucci, G. Inside baleen: Exceptional microstructure preservation in a late Miocene whale skeleton from Peru. Geology 2016, 44, 839–842. [Google Scholar] [CrossRef]
- Gioncada, A.; Gariboldi, K.; Collareta, A.; Di Celma, C.; Bosio, G.; Malinverno, E.; Lambert, O.; Pike, J.; Urbina, M.; Bianucci, G. Looking for the key to preservation of fossil marine vertebrates in the Pisco Formation of Peru: New insights from a small dolphin skeleton. Andean Geol. 2018, 45, 379–398. [Google Scholar] [CrossRef]
- Gioncada, A.; Petrini, R.; Bosio, G.; Gariboldi, K.; Collareta, A.; Malinverno, E.; Bonaccorsi, E.; Di Celma, C.; Pasero, M.; Urbina, M.; et al. Insights into the diagenetic environment of fossil marine vertebrates of the Pisco Formation (late Miocene, Peru) from mineralogical and Sr-isotope data. J. S. Am. Earth Sci. 2018, 81, 141–152. [Google Scholar] [CrossRef]
- Marx, F.G.; Collareta, A.; Gioncada, A.; Post, K.; Lambert, O.; Bonaccorsi, E.; Urbina, M.; Bianucci, G. How whales used to filter: Exceptionally preserved baleen in a Miocene cetotheriid. J. Anat. 2017, 231, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Marx, F.G.; Lambert, O.; de Muizon, C. A new Miocene baleen whale from Peru deciphers the dawn of cetotheriids. R. Soc. Open Sci. 2017, 4, 170560. [Google Scholar] [CrossRef]
- Ramassamy, B.; Lambert, O.; Collareta, A.; Urbina, M.; Bianucci, G. Description of the skeleton of the fossil beaked whale Messapicetus gregarius: Searching potential proxies for deep-diving abilities. Foss. Rec. 2018, 21, 11–32. [Google Scholar] [CrossRef]
- Bosio, G.; Malinverno, E.; Collareta, A.; Di Celma, C.; Gioncada, A.; Parente, M.; Berra, F.; Marx, F.G.; Vertino, A.; Urbina, M.; et al. Strontium Isotope Stratigraphy and the thermophilic fossil fauna from the middle Miocene of the East Pisco Basin (Peru). J. S. Am. Earth Sci. 2020, 97, 102399. [Google Scholar] [CrossRef]
- Bosio, G.; Gioncada, A.; Gariboldi, K.; Bonaccorsi, E.; Collareta, A.; Pasero, M.; Di Celma, C.; Malinverno, E.; Urbina, M.; Bianucci, G. Mineralogical and geochemical characterization of fossil bones from a Miocene marine Konservat-Lagerstätte. J. S. Am. Earth Sci. 2021, 105, 102924. [Google Scholar] [CrossRef]
- Boskovic, D.S.; Vidal, U.L.; Nick, K.E.; Esperante, R.; Brand, L.R.; Wright, K.R.; Sandberg, L.B.; Siviero, B.C. Structural and Protein Preservation in Fossil Whale Bones from The Pisco Formation (Middle-Upper Miocene), Peru. Palaios 2021, 36, 155–164. [Google Scholar] [CrossRef]
- Benites-Palomino, A.; Velez-Juarbe, J.; Altamirano-Sierra, A.; Collareta, A.; Carrillo-Briceño, J.D.; Urbina, M. Sperm whales (Physeteroidea) from the Pisco Formation, Peru, and their trophic role as fat sources for late Miocene sharks. Proc. R. Soc. Lond. B Biol. Sci. 2022, 289, 20220774. [Google Scholar] [CrossRef] [PubMed]
- Bianucci, G.; Collareta, A. An overview of the fossil record of cetaceans from the East Pisco Basin (Peru). Boll. Soc. PaleontoI. Ital. 2022, 61, 20. [Google Scholar]
- Ochoa, D.; Salas-Gismondi, R.; DeVries, T.J.; Baby, P.; de Muizon, C.; Altamirano, A.; Barbosa-Espitia, A.; Foster, D.A.; Quispe, K.; Cardich, J.; et al. Late Neogene evolution of the Peruvian margin and its ecosystems: A synthesis from the Sacaco record. Int. J. Earth Sci. 2021, 110, 995–1025. [Google Scholar] [CrossRef]
- DeVries, T.J.; Frassinetti, D. Range extensions and biogeographic implications of Chilean Neogene mollusks found in Peru. Bol. Mus. Nac. Hist. Nat. Chile 2003, 52, 119–135. [Google Scholar]
- DeVries, T.J.; Groves, L.T.; Urbina, M. A new early Miocene Muracypraea Woodring, 1957 (Gastropoda: Cypraeidae) from the Pisco Basin of southern Peru. Nautilus 2006, 120, 101–105. [Google Scholar]
- Bosio, G.; Bracchi, V.A.; Malinverno, E.; Collareta, A.; Coletti, G.; Gioncada, A.; Kočí, U.T.; DI Celma, C.; Bianucci, G.; Basso, D. Taphonomy of a Panopea Ménard de la Groye, 1807 shell bed from the Pisco Formation (Miocene, Peru). Comptes Rendus Palevol 2021, 20, 119–140. [Google Scholar] [CrossRef]
- Kočí, T.; Bosio, G.; Collareta, A.; Sanfilippo, R.; Ekrt, B.; Urbina, M.; Malinverno, E. First report on the cirratulid (Annelida, Polychaeta) reefs from the Miocene Chilcatay and Pisco Formations (East Pisco Basin, Peru). J. S. Am. Earth Sci. 2021, 107, 103042. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Kočí, T.; Bosio, G.; Collareta, A.; Ekrt, B.; Malinverno, E.; Di Celma, C.; Urbina, M.; Bianucci, G. An investigation of vermetid reefs from the Miocene of Peru, with the description of a new species. J. S. Am. Earth Sci. 2021, 108, 103233. [Google Scholar] [CrossRef]
- Coletti, G.; Bosio, G.; Collareta, A.; Buckeridge, J.; Consani, S.; El Kateb, A. Palaeoenvironmental analysis of the Miocene barnacle facies: Case studies from Europe and South America. Geol. Carpathica 2018, 69, 573–592. [Google Scholar] [CrossRef]
- Coletti, G.; Bosio, G.; Collareta, A.; Malinverno, E.; Bracchi, V.A.; Di Celma, C.; Basso, D.; Stainbank, S.; Spezzaferri, S.; Cannings, T.; et al. Biostratigraphic, evolutionary, and paleoenvironmental significance of the southernmost lepidocyclinids of the Pacific coast of South America (East Pisco Basin, southern Peru). J. S. Am. Earth Sci. 2019, 96, 102372. [Google Scholar] [CrossRef]
- Coletti, G.; Collareta, A.; Bosio, G.; Urbina, M.; Buckeridge, J. Perumegabalanus calziai gen. et sp. nov., a new intertidal megabalanine barnacle from the early Miocene of Peru. N. Jahrb. Geol. Palaontol. Abh. 2019, 294, 197–212. [Google Scholar] [CrossRef]
- Collareta, A.; Coletti, G.; Bosio, G.; Buckeridge, J.; de Muizon, C.; Devries, T.J.; Varas-Malca, R.M.; Altamirano-Sierra, A.; Urbina-Schmitt, M.; Bianucci, G. A new barnacle (Cirripedia: Neobalanoformes) from the early Miocene of Peru: Palaeoecological and palaeobiogeographical implications. N. Jahrb. Geol. Palaontol. Abh. 2019, 292, 321–338. [Google Scholar] [CrossRef]
- DeVries, T.J.; Jud, N.A. Lithofacies patterns and paleogeography of the Miocene Chilcatay and lower Pisco depositional sequences (East Pisco Basin, Peru). Bol. Soc. Geol. Perú 2018, 8, 124–167. [Google Scholar]
- Marocco, R.; de Muizon, C. Los vertebrados del Neogeno de la costa sur del Perú: Ambiente sedimentario y condiciones de fosilización. Bull. Inst. Fr. d’Études Andines 1988, 17, 105–117. [Google Scholar] [CrossRef]
- Göhlich, U.B. The oldest fossil record of the extant penguin genus Spheniscus–A new species from the Miocene of Peru. Acta Palaeontol. Pol. 2007, 52, 285–298. [Google Scholar]
- Lambert, O.; Bianucci, G.; Beatty, B.L. Bony outgrowths on the jaws of an extinct sperm whale support macroraptorial feeding in several stem physeteroids. Naturwissenschaften 2014, 101, 517–521. [Google Scholar] [CrossRef]
- Bosio, G.; Malinverno, E.; Villa, I.M.; Di Celma, C.; Gariboldi, K.; Gioncada, A.; Barberini, B.; Urbina, M.; Bianucci, G. Tephrochronology and chronostratigraphy of the Miocene Chilcatay and Pisco formations (East Pisco basin, Peru). Newsl. Stratigr. 2020, 53, 213–247. [Google Scholar] [CrossRef]
- Pritchard, P.C.H. A survey of neural bone variation among recent chelonian species, with functional interpretations. Acta Zool. Cracov. 1988, 31, 625–686. [Google Scholar]
- Bianucci, G.; Collareta, A.; Bosio, G.; Landini, W.; Gariboldi, K.; Gioncada, A.; Lambert, O.; Malinverno, E.; de Muizon, C.; Varas-Malca, R.; et al. Taphonomy and palaeoecology of the lower Miocene marine vertebrate assemblage of Ullujaya (Chilcatay Formation, East Pisco Basin, southern Peru). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 511, 256–279. [Google Scholar] [CrossRef]
- Wood, R.C.; Johnson-Gove, J.; Gaffney, E.S.; Maley, K.F. Evolution and phylogeny of the leatherback turtles (Dermochelyidae), with descriptions of new fossil taxa. Chelonian Conserv. Biol. 1996, 2, 266–286. [Google Scholar]
- Mikuláš, R.; Kadlecova, E.; Fejfar, O.; Dvorak, Z. Three New Ichnogenera of Biting and Gnawing Traces on Reptilian and Mammalian Bones: A Case Study from the Miocene of the Czech Republic. Ichnos 2006, 13, 113–127. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Perry, F.A. Mammalian bite marks on juvenile fur seal bones from the late Neogene Purisima Formation of Central California. Palaios 2011, 26, 115–120. [Google Scholar] [CrossRef]
- Zonneveld, J.P.; Bartels, W.S. The occurrence of bone modification features in the carapace and plastron of the extant red-eared slider Trachemys scripta elegans (Wied-Neuwied, 1839): Implications for paleoecological analyses of fossil turtle assemblages. Palaios 2022, 37, 499–519. [Google Scholar] [CrossRef]
- Sato, K.; Jenkins, R.G. Mobile home for pholadoid boring bivalves: First example from a Late Cretaceous sea turtle in Hokkaido Japan. Palaios 2020, 35, 228–236. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae Sive Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis, Editio Decima, Reformata; Laurentius Salvius: Stockholm, Sweden, 1758; pp. 1–824. [Google Scholar]
- Janssen, R.; Van Baal, R.R.; Schulp, A.S. Bone damage in Allopleuron hofmanni (Cheloniidae, Late Cretaceous). Neth. J. Geosci. 2013, 92, 153–157. [Google Scholar] [CrossRef]
- Gray, J.E. Synopsis Reptilium; or Short Descriptions of the Species of Reptiles. Part 1: Tortoises, Crocodiles and Enaliosaurians; Treuttel, Würtz & Company: London, UK, 1831; pp. 1–86. [Google Scholar]
- Weems, R.E.; Sanders, A.E. Oligocene pancheloniid sea turtles from the vicinity of Charleston, South Carolina, USA. J. Vertebr. Paleontol. 2014, 34, 80–99. [Google Scholar] [CrossRef]
- Weems, R.E. Middle Miocene sea turtles (Syllomus, Procolpochelys, Psephophorus) from the Calvert Formation. J. Paleontol. 1974, 48, 278–303. [Google Scholar]
- Karl, H.V.; Lindow, B.E.; Tütken, T. Miocene leatherback turtle material of the genus Psephophorus (Testudines: Dermochelyoidea) from the Gram Formation (Denmark). Stud. Palaeocheloniol. 2012, 9, 205–216. [Google Scholar]
- Misuri, A. Sopra un nuovo chelonio del calcare miocenico di Lecce (Euclastes melii Misuri). Palaeontogr. Ital. 1910, 16, 119–136. [Google Scholar]
- Zug, G.R.; Ray, C.E.; Bohaska, D.J. Turtles of the Lee Creek Mine (Pliocene: North Carolina). Smithson. Contrib. Paleobiol. 2001, 90, 203–218. [Google Scholar]
- Hay, O.P. Characteristics of sundry fossil vertebrates; Part VII, New fossil turtle from Eocene marl of South Carolina. Pan Am. Geol. 1923, 39, 105–109. [Google Scholar]
- Leidy, J. Several fossils from the Greensand of New Jersey. Proc. Acad. Nat. Sci. Phila. 1851, 5, 329–330. [Google Scholar]
- Bronn, H.G. Italiens Tertiär-Gebilde und Deren Organischen Einschlüsse. In Vier Abhandlungen; Groos: Heidelberg, Germany, 1831; pp. 1–176. [Google Scholar]
- Epibiont Research Cooperative. A synopsis of the literature on the turtle barnacles (Cirripedia: Balanomorpha: Coronuloidea) 1758–2007. ERC Spec. Publ. 2007, 1, 62. [Google Scholar]
- Lydekker, R.A. Catalogue of the Fossil Reptilia and Amphibia in the British Museum (Natural History). III, Chelonia; British Museum (Natural History): London, UK, 1889; pp. 1–239. [Google Scholar]
- Meyer, H.v. Mittheilungen an Prof. Bronn gerichtet. N. Jb. Miner. Geogn. Geol. Petrefakt. 1843, 698–704. [Google Scholar]
- Lapparent de Broin, F. The European turtle fauna from the Triassic to the present. Dumerilia 2001, 4, 155–218. [Google Scholar]
- Meyer, H.v. Letter on various fossils. N. Jahrb. Miner. Geogn. Geol. Petrefakt. 1846, 462–476. [Google Scholar]
- Frick, M.G.; Williams, K.L.; Robinson, M. Epibionts associated with nesting loggerhead sea turtles (Caretta caretta) in Georgia, USA. Herpetol. Rev. 1998, 29, 211–214. [Google Scholar]
- Pilsbry, H.A. The sessile barnacles (Cirripedia) contained in the collections of the US National Museum; including a monograph of the American species. Bull. U. S. Natl. Mus. 1916, 93, 1–366. [Google Scholar] [CrossRef]
- Gruvel, J.A. Revision des Cirripèdes appartenant à la collection du Muséum d’Histoire Naturelle (Operculés). II. Partie systématique. Nouv. Arch. Mus. d’Hist. Nat. Ser. IV 1903, 45, 95–170. [Google Scholar]
- Cheang, C.C.; Tsang, L.M.; Chu, K.H.; Cheng, I.-J.; Chan, B.K. Host-Specific Phenotypic Plasticity of the Turtle Barnacle Chelonibia testudinaria: A Widespread Generalist Rather than a Specialist. PLoS ONE 2013, 8, e57592. [Google Scholar] [CrossRef] [PubMed]
- Zardus, J.D.; Lake, D.T.; Frick, M.G.; Rawson, P.D. Deconstructing an assemblage of “turtle” barnacles: Species assignments and fickle fidelity in Chelonibia. Mar. Biol. 2014, 161, 45–59. [Google Scholar] [CrossRef]
- Chesi, F.; Delfino, M.; Varola, A.; Rook, L. Fossil sea turtles (Chelonii, Dermochelyidae and Cheloniidae) from the Miocene of Pietra Leccese (late Burdigalian–early Messinian), Southern Italy. Geodiversitas 2007, 29, 321–333. [Google Scholar]
- Blick, J.P.; Zardus, J.D.; Dvoracek, D. The sea turtle barnacle, Chelonibia testudinaria (Cirripedia: Balanomorpha: Coronuloidea), from pre-Columbian deposits on San Salvador, Bahamas. Caribb. J. Sci. 2010, 46, 228–239. [Google Scholar] [CrossRef]
- Collareta, A.; Bosselaers, M.; Bianucci, G. Jumping from turtles to whales: A Pliocene fossil record depicts an ancient dispersal of Chelonibia on mysticetes. Riv. Ital. Paleontol. Strat. 2016, 122. [Google Scholar] [CrossRef]
- Collareta, A. Discovery of complemental males in a Pliocene accumulation of Chelonibia testudinaria (Linnaeus, 1758), with some notes on the evolution of androdioecy in turtle barnacles. N. Jahrb. Geol. Palaontol. Abh. 2020, 297, 193–203. [Google Scholar] [CrossRef]
- Collareta, A.; Harzhauser, M.; Rasser, M.W. New and overlooked occurrences of the rarely reported protochelonibiine “turtle” barnacles from the Oligocene and Miocene of Europe. PalZ 2022, 96, 197–206. [Google Scholar] [CrossRef]
- Collareta, A.; Newman, W.A.; Bosio, G.; Coletti, G. A new chelonibiid from the Miocene of Zanzibar (Eastern Africa) sheds light on the evolution of shell architecture in turtle and whale barnacles (Cirripedia: Coronuloidea). Integr. Zool. 2022, 17, 24–43. [Google Scholar] [CrossRef]
- Perreault, R.T.; Collareta, A.; Buckeridge, J.S. A new species of the archaic “turtle barnacle” genus Protochelonibia (Coronuloidea, Chelonibiidae) from the upper Rupelian Chickasawhay Formation of Mississippi (U.S.A.). N. Jahrb. Geol. Pal. Abh. 2022, 305, 225–235. [Google Scholar] [CrossRef]
- Ross, A. Chelonibia in the Neogene of Florida. Q. J. Florida Acad. Sci. 1963, 26, 221–233. [Google Scholar]
- Mimoto, K. Cirripedian fossils from the Pleistocene deposits of the southwestern part of Kochi Prefecture Shikoku. Fossils 1991, 51, 15–23. [Google Scholar]
- Collareta, A.; Reitano, A.; Rosso, A.; Sanfilippo, R.; Bosselaers, M.; Bianucci, G.; Insacco, G. The oldest platylepadid turtle barnacle (Cirripedia, Coronuloidea): A new species of Platylepas from the Lower Pleistocene of Italy. Eur. J. Taxon. 2019, 516, 1–17. [Google Scholar] [CrossRef]
- Karasawa, H.; Kobayashi, N. Cirripedes from the middle Pleistocene Atsumi Group, Japan, with a reevaluation of the genus Adna Sowerby, 1823 (Balanoidea: Pyrgomatidae). Bull. Mizunami Fossil Mus. 2022, 49, 67–93. [Google Scholar]
- Fabricius, J.C. Tillaeg-til Conchylie-Slaegterne Lepas, Pholas, Mya og Solen. Skr. Naturhist. Selsk. 1798, 4, 34–51. [Google Scholar]
- Linnaeus, C. Systema Naturae Sive Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis, Editio Duodecima, Reformata; Laurentius Salvius: Stockholm, Sweden, 1766; pp. 1–1327. [Google Scholar]
- Pinou, T.; Domenech, F.; Lazo-Wasem, E.A.; Majewska, R.; Pfaller, J.B.; Zardus, J.D.; Robinson, N.J. Standardizing sea turtle epibiont sampling: Outcomes of the epibiont workshop at the 37th International Sea Turtle Symposium. Mar. Turtle Newsl. 2019, 157, 22–32. [Google Scholar]
- Collareta, A.; Bosselaers, M. A new record of Gunnellichnus moghraensis from the Middle Miocene of Belgium, with some remarks on the origin of this seemingly uncommon ichnospecies. N. Jahrb. Geol. Palaontol. Abh. 2022, 305, 237–243. [Google Scholar] [CrossRef]
- Khakhina, L. Evolutionary Significance of Symbiosis; Development of the Symbiogenesis Concept. Symbiosis 1992, 14, 217–228. [Google Scholar]
- Sloan, K.; Zardus, J.D.; Jones, M.L. Substratum fidelity and early growth in Chelonibia testudinaria, a turtle barnacle especially common on debilitated loggerhead (Caretta caretta) sea turtles. Bull. Mar. Sci. 2014, 90, 581–597. [Google Scholar] [CrossRef]
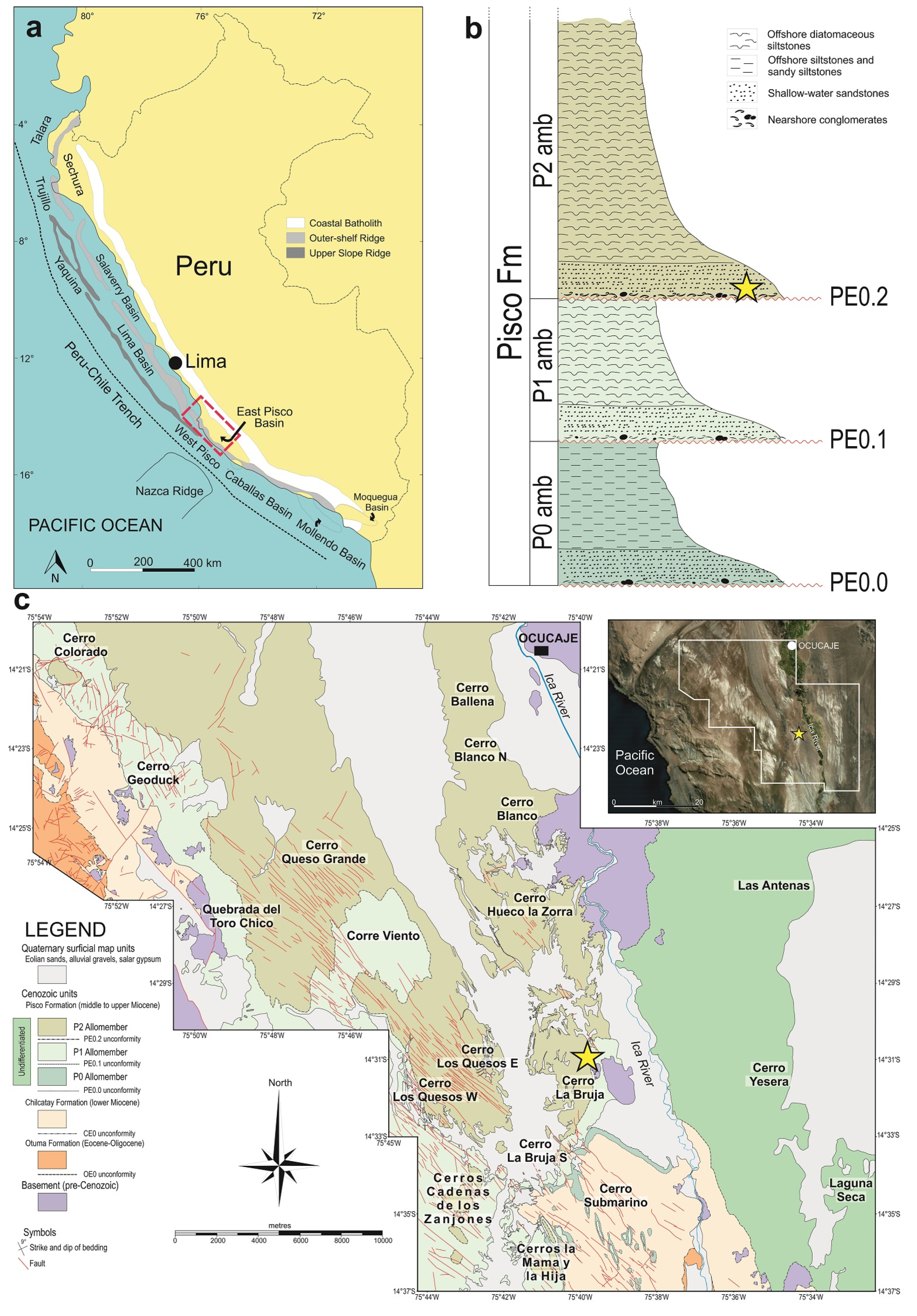
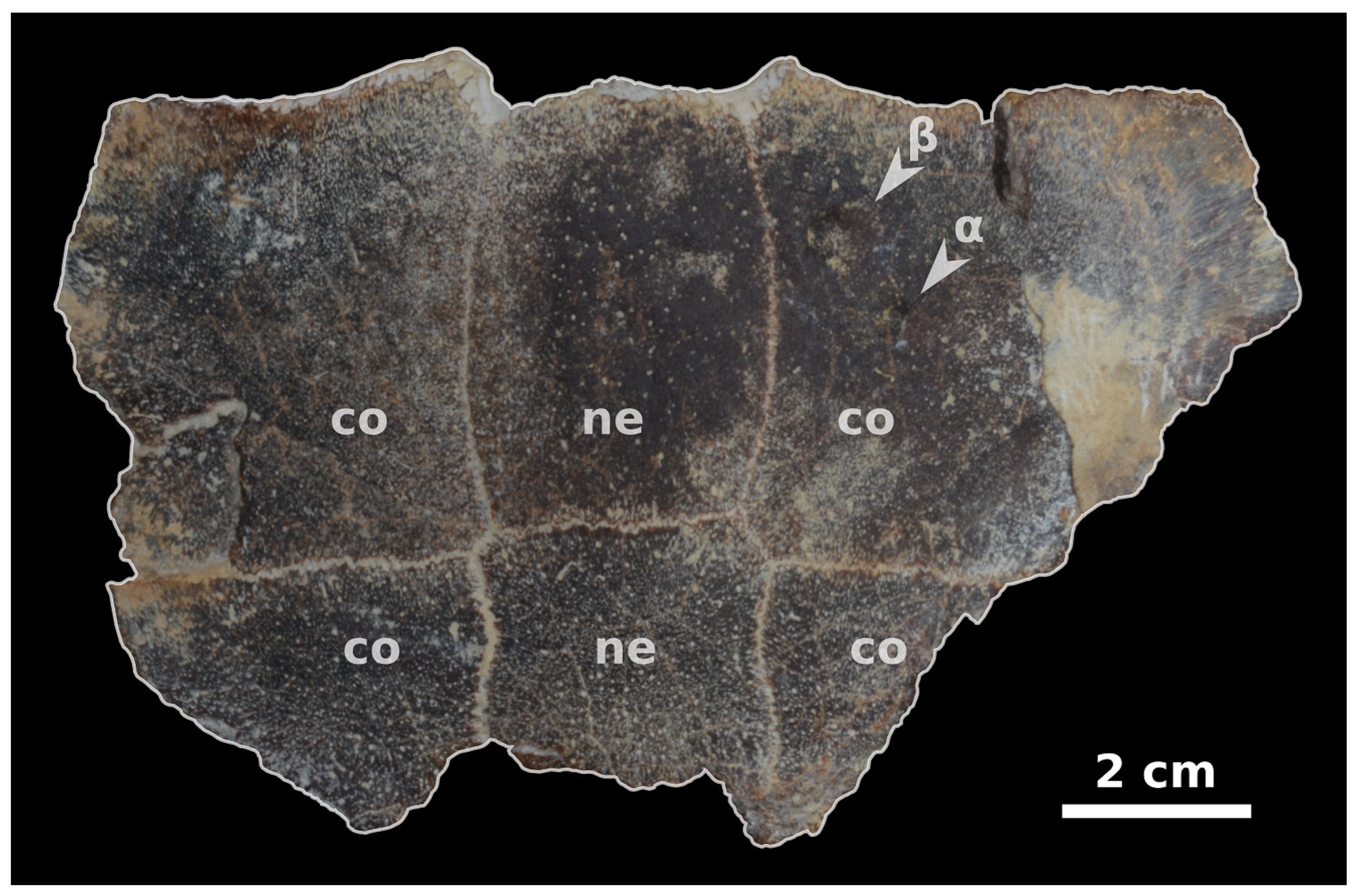


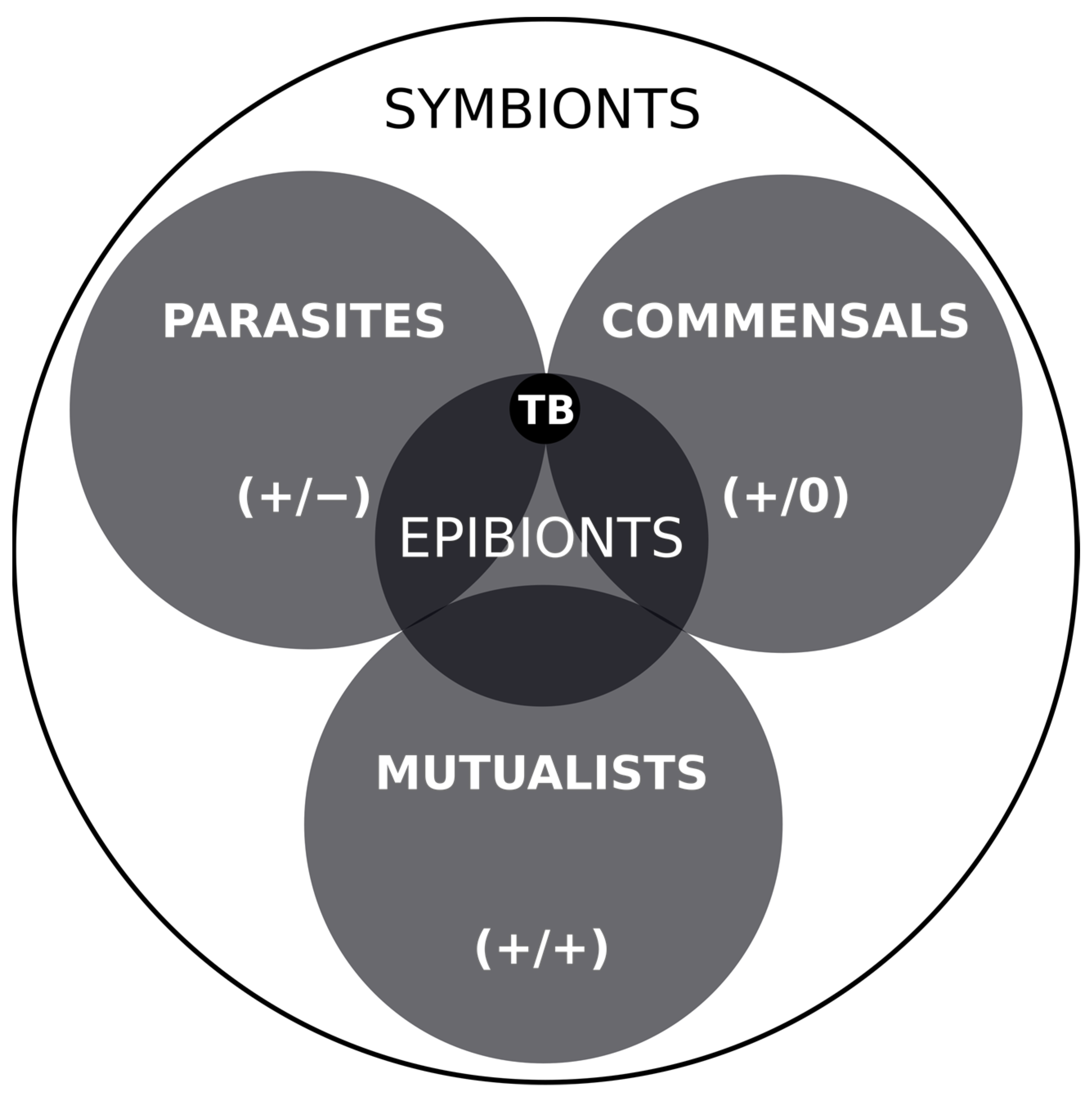
| Locality | Formation (Fm) and Age | Host Taxon | Inferred Epibiont Taxon | Key References |
|---|---|---|---|---|
| South Carolina, U.S. East Coast | Chandler Bridge Fm, Rupelian | Carolinochelys wilsoni | Platylepadidae | Weems and Sanders [107] Collareta et al. [27] |
| U.S. East Coast | Calvert Fm, Middle Miocene | Procolpochelys grandaeva | Balanidae? | Weems [108] |
| Maryland, U.S. East Coast | Calvert Fm, Middle Miocene | Trachyaspis lardyi | Platylepadidae? | Weems [108] Collareta et al. [27] |
| Southern Denmark | Gram Fm, Tortonian | Psephophorus polygonus | Chelonibiidae? | Karl et al. [109] Zonneveld et al. [23] |
| Southern Italy | Pietra leccese Fm, Miocene | Cheloniidae indet. (“Euclastes” melii) | Platylepadidae | Misuri [110] Hayashi et al. [22] |
| Central Italy | Arenaria di Ponsano Fm, Tortonian | Cheloniidae indet. | Platylepadidae | Collareta et al. [27] |
| Southern Peru | Pisco Fm, Tortonian | Cheloniidae indet. | Platylepadidae | this work |
| North Carolina, U.S. East Coast | Yorktown Fm, Pliocene | Caretta patriciae | Platylepadidae | Zug [111] this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collareta, A.; Varas-Malca, R.; Bosio, G.; Urbina, M.; Coletti, G. Ghosts of the Holobiont: Borings on a Miocene Turtle Carapace from the Pisco Formation (Peru) as Witnesses of Ancient Symbiosis. J. Mar. Sci. Eng. 2023, 11, 45. https://doi.org/10.3390/jmse11010045
Collareta A, Varas-Malca R, Bosio G, Urbina M, Coletti G. Ghosts of the Holobiont: Borings on a Miocene Turtle Carapace from the Pisco Formation (Peru) as Witnesses of Ancient Symbiosis. Journal of Marine Science and Engineering. 2023; 11(1):45. https://doi.org/10.3390/jmse11010045
Chicago/Turabian StyleCollareta, Alberto, Rafael Varas-Malca, Giulia Bosio, Mario Urbina, and Giovanni Coletti. 2023. "Ghosts of the Holobiont: Borings on a Miocene Turtle Carapace from the Pisco Formation (Peru) as Witnesses of Ancient Symbiosis" Journal of Marine Science and Engineering 11, no. 1: 45. https://doi.org/10.3390/jmse11010045
APA StyleCollareta, A., Varas-Malca, R., Bosio, G., Urbina, M., & Coletti, G. (2023). Ghosts of the Holobiont: Borings on a Miocene Turtle Carapace from the Pisco Formation (Peru) as Witnesses of Ancient Symbiosis. Journal of Marine Science and Engineering, 11(1), 45. https://doi.org/10.3390/jmse11010045








