Combined Influences of Light and Nitrogen Enrichment on the Physiological Performance of a Golden Tide Alga (Sargassum horneri)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Design
2.3. Measurement of Growth
2.4. Assessment of Photosynthetic Carbon Fixation
2.5. Identification of Photosynthetic Pigments
2.6. Estimate of Nitrogen Uptake Rate (NUR)
2.7. Evaluation of Soluble Carbohydrates and SOLUBLE Protein
2.8. Statistical Analyses
3. Result
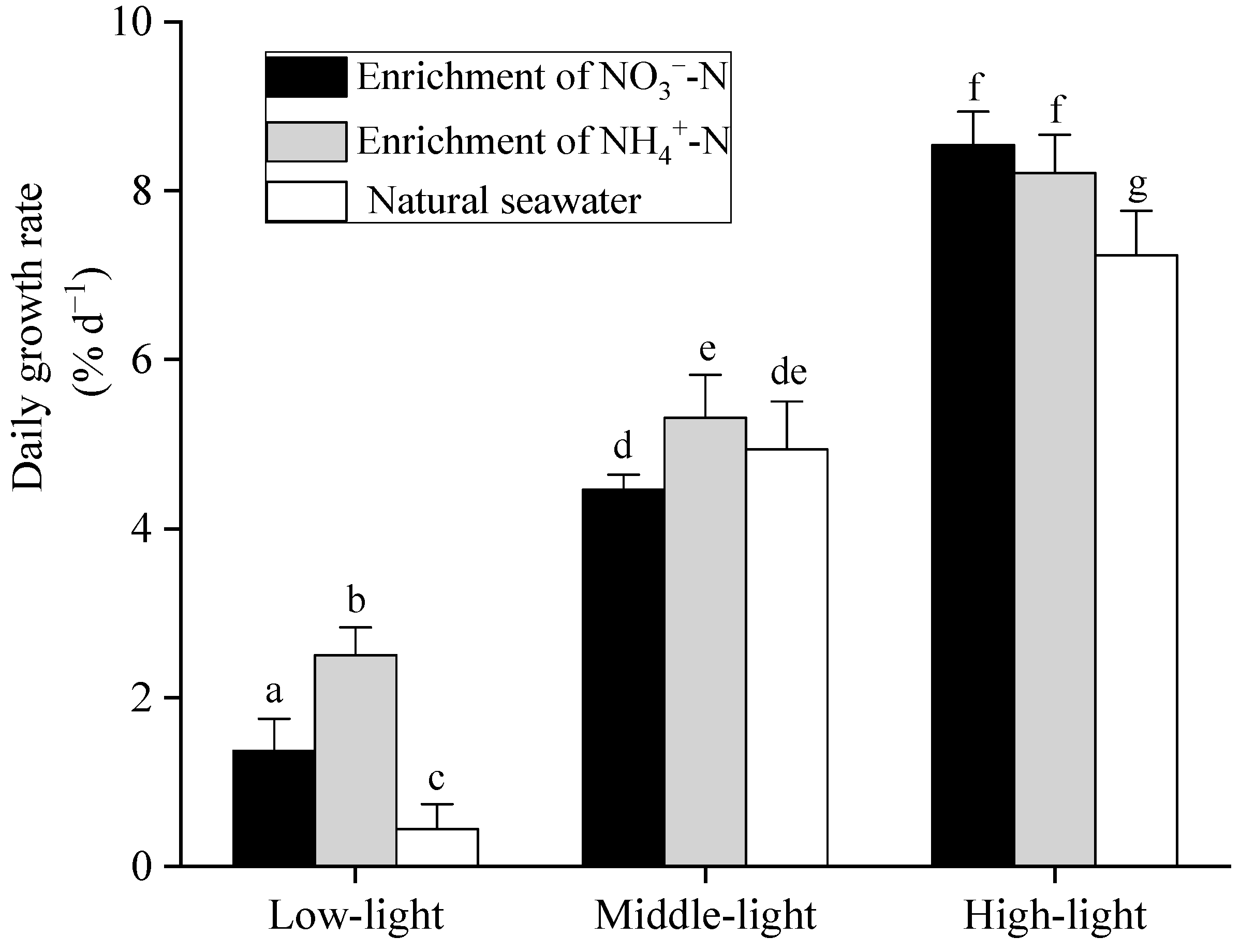
3.1. Growth Rate
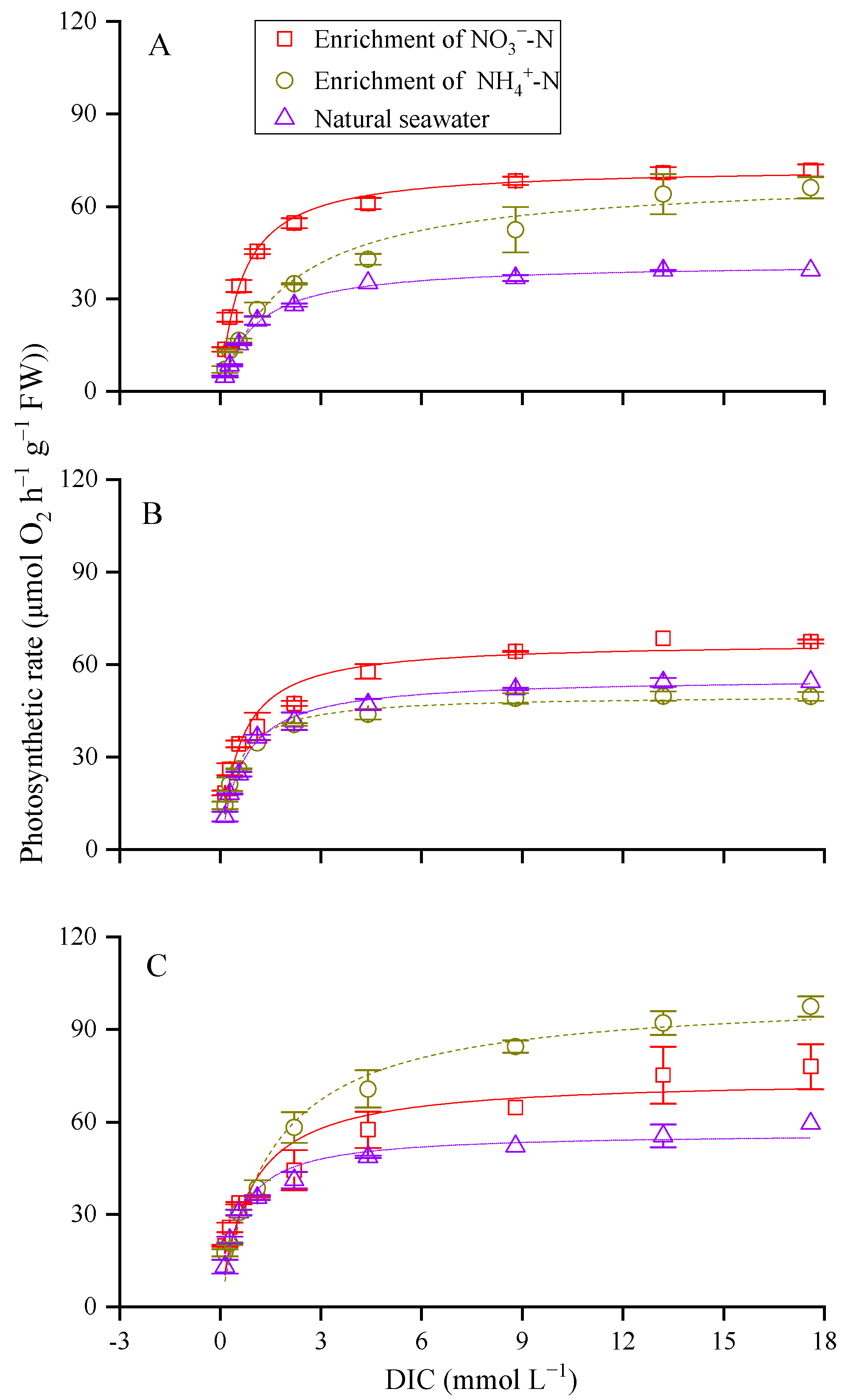
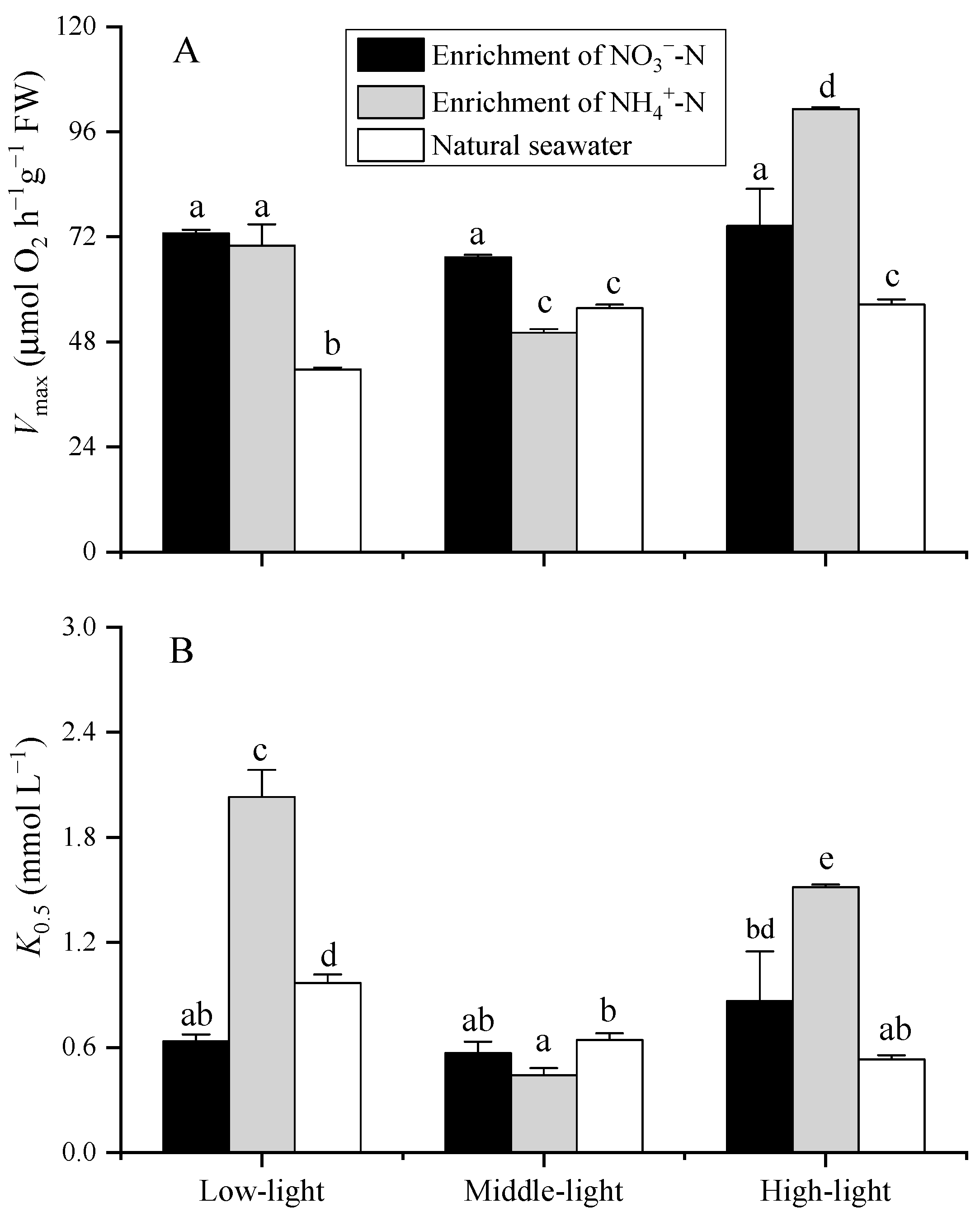
3.2. Photosynthetic Carbon Fixation
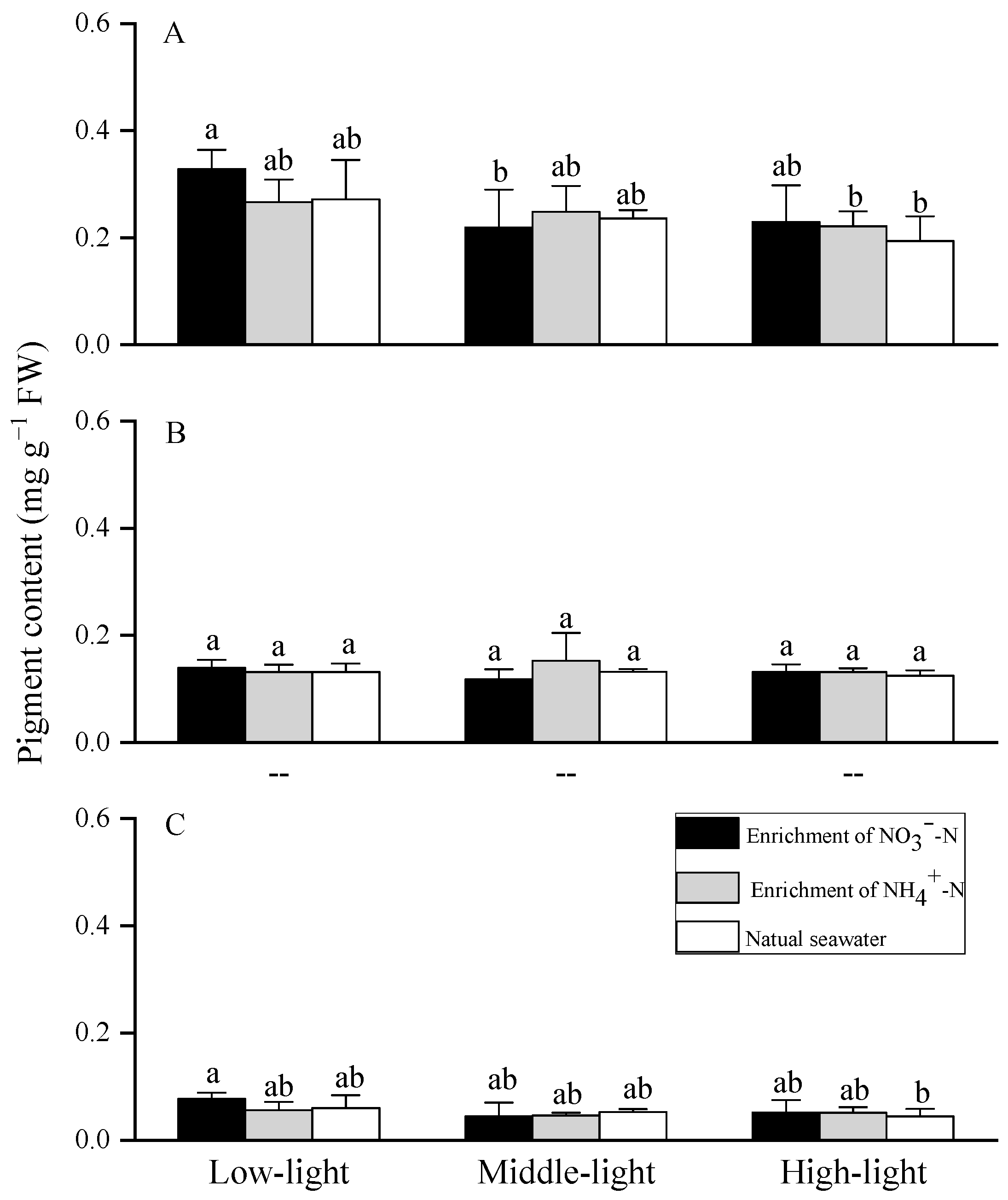
3.3. Photosynthetic Pigments
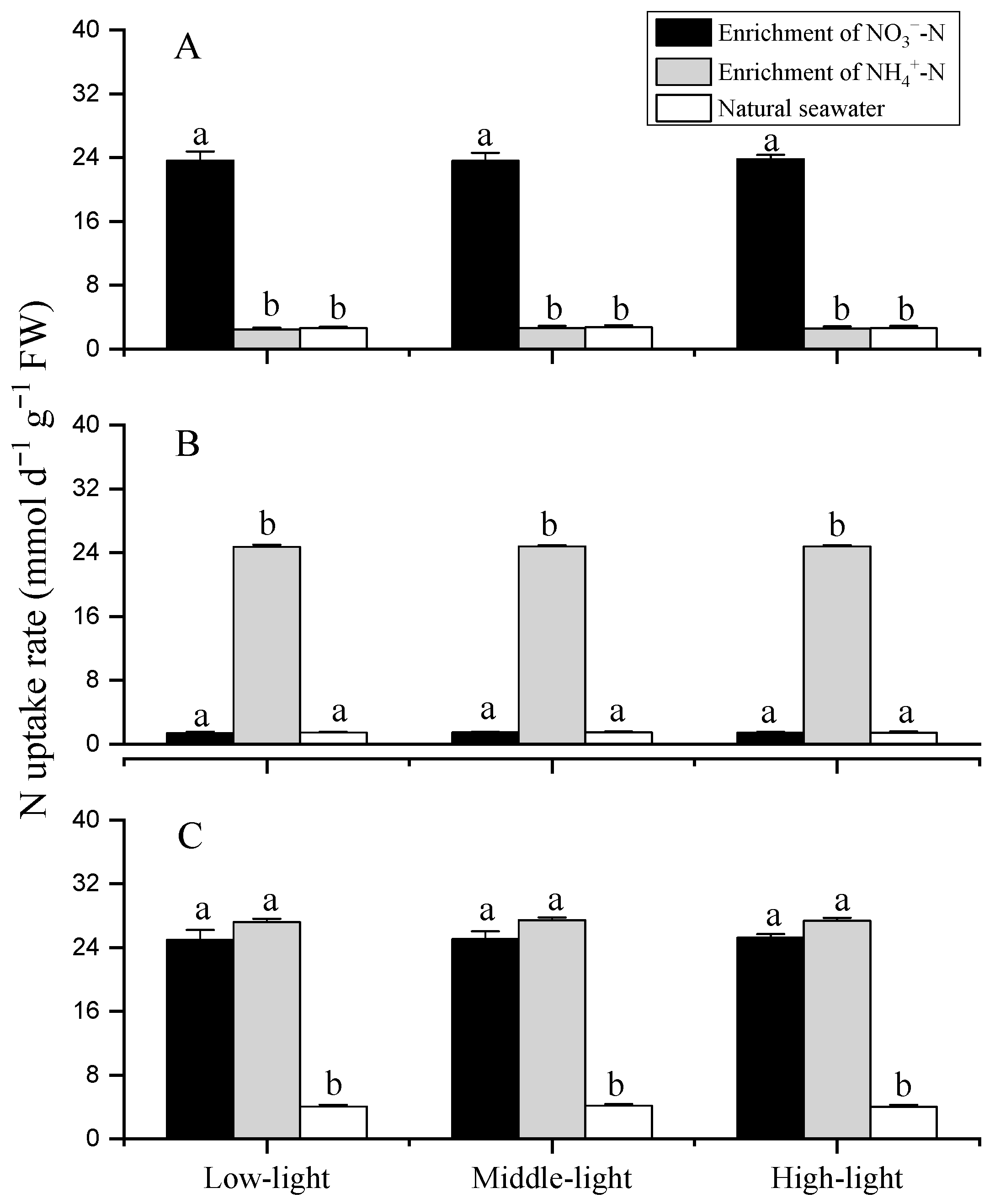
3.4. Nitrogen Uptake
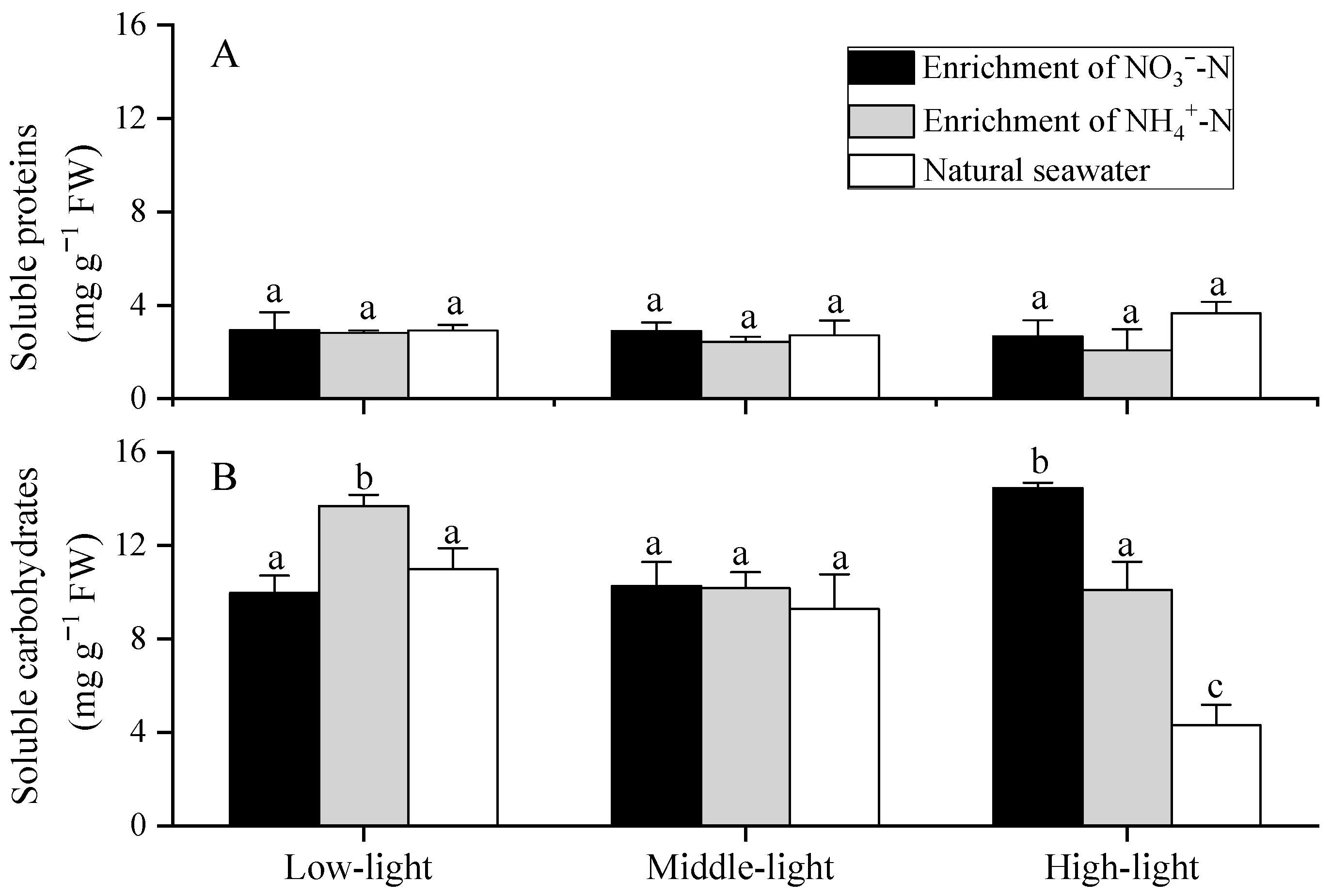
3.5. Soluble Protein and Soluble Carbohydrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.M.; Goodwin, D.S.; Siuda, A.N.S. Recent Sargassum inundation events in the Caribbean: Shipboard observations reveal dominance of a previously rare form. Oceanography 2015, 28, 8–10. [Google Scholar] [CrossRef]
- Acton, L.; Campbell, L.M.; Cleary, J.; Gray, N.J.; Halpin, P.N. What is the Sargasso Sea? The problem of fixing space in a fluid ocean. Polit. Geogr. 2019, 68, 86–100. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Hu, C.; Mikelsons, K.; Wang, M.; Lance, V. In search of floating algae and other organisms in global oceans and lakes. Remote Sens. Environ. 2020, 239, 111659. [Google Scholar] [CrossRef]
- Xing, Q.; Guo, R.; Wu, L.; An, D.; Cong, M.; Qin, S.; Li, X. High-Resolution Satellite Observations of a New Hazard of Golden Tides Caused by Floating Sargassum in Winter in the Yellow Sea. IEEE Geosci. Remote Sens. Lett. 2017, 14, 1815–1819. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Wang, M.; Shang, S.; Wilson, C. Floating Algae Blooms in the East China Sea. Geophys. Res. Lett. 2017, 44, 11501–11509. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Arana, H.A.H.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; González-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- Caselle, J.E.; Davis, K.; Marks, L.M. Marine management affects the invasion success of a nonnative species in a temperate reef system in California, USA. Ecol. Lett. 2018, 21, 43–53. [Google Scholar] [CrossRef]
- Baker, P.; Minzlaff, U.; Schoenle, A.; Schwabe, E.; Hohlfeld, M.; Jeuck, A.; Brenke, N.; Prausse, D.; Rothenbeck, M.; Brix, S.; et al. Potential contribution of surface-dwelling Sargassum algae to deep-sea ecosystems in the southern North Atlantic. Deep-Sea Res. Part II 2018, 148, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.; Feagin, R. Sargassum as a natural solution to enhance dune plant growth. Environ. Manag. 2010, 46, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Harvey, P.J. Golden tides: Problem or golden opportunity? The valorization of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Cruz-Rivera, E.; Flores-Díaz, M.; Hawkins, A. A fish kill coincident with dense Sargassum accumulation in a tropical bay. Bull. Mar. Sci. 2015, 91, 455–456. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C. Annual growth and environmental relationships of the invasive species Sargassum muticum and Undaria pinnatifida in the lagoon of Venice. Estuar. Coast. Shelf Sci. 2013, 129, 162–172. [Google Scholar] [CrossRef]
- Oviatt, C.A.; Huizenga, K.; Rogers, C.S.; Miller, W.J. What nutrient sources support anomalous growth and the recent sargassum mass stranding on Caribbean beaches? A review. Mar. Pollut. Bull. 2019, 145, 517–525. [Google Scholar] [CrossRef]
- Brooks, M.; Coles, V.J.; Hood, R.R.; Gower, J.F.R. Factors controlling the seasonal distribution of pelagic sargassum. Mar. Ecol. Prog. Ser. 2018, 599, 1–18. [Google Scholar] [CrossRef]
- Louime, C.; Fortune, J.; Gervais, G. Sargassum invasion of coastal environments: A growing concern. Am. J. Environ. Sci. 2017, 13, 58–64. [Google Scholar] [CrossRef]
- Kouassi, M.; Zika, R.G. Light-induced destruction of the absorbance property of dissolved organic matter in seawater. Toxicol. Environ. Chem. 1992, 35, 195–211. [Google Scholar] [CrossRef]
- Tedetti, M.; Sempéré, R. Penetration of ultraviolet radiation in the marine environment. A review. Photochem. Photobiol. 2006, 82, 389–397. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, K.; Li, G.; Helbling, E.W. Seasonal impacts of solar UV radiation on the photosynthesis of phytoplankton assemblages in the coastal water of the South China Sea. Photochem. Photobiol. 2010, 86, 586–592. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, H.; Zhan, D.; Sun, F.; Sun, J.; Wang, G. Combined effects of light intensity and NH4+-enrichment on growth, pigmentation, and photosynthetic performance of Ulva prolifera (Chlorophyta). Chin. J. Oceanol. Limnol. 2014, 32, 1016–1023. [Google Scholar] [CrossRef]
- Erin Cox, T.; Smith, C.M. Photosynthetic rapid light curves for Padina sanctaecrucis vary with irradiance, aerial exposure, and tides in Hawaii’s micro-intertidal zones. Mar. Biol. 2015, 162, 1061–1076. [Google Scholar] [CrossRef]
- Yakovleva, I.M.; Titlyanov, E.A. Effect of high visible and UV irradiance on subtidal Chondrus crispus: Stress, photoinhibition and protective mechanisms. Aquat. Bot. 2001, 71, 47–61. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Huovinen, P.; Matos, J.; Pinto, I.S.; Figuero, F.L. The role of ammonium in photoprotection against high irradiance in the red alga Grateloupia lanceola. Aquat. Bot. 2006, 84, 308–316. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, K. Impacts of UV radiation on growth and photosynthetic carbon acquisition in Gracilaria lemaneiformis (Rhodophyta) under phosphorus-limited and replete conditions. Funct. Plant Biol. 2009, 36, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, K. NH4+ enrichment and UV radiation interact to affect the photosynthesis and nitrogen uptake of Gracilaria lemaneiformis (Rhodophyta). Mar. Pollut. Bull. 2012, 64, 99–105. [Google Scholar] [CrossRef]
- Wang, B.; Xin, M.; Wei, Q.; Xie, L. A historical overview of coastal eutrophication in the China Seas. Mar. Pollut. Bull. 2018, 136, 394–400. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Li, Q.; Liu, Y.; Song, J.; Zhang, Y. Environmental response to long-term mariculture activities in the Weihai coastal area, China. Sci. Total Environ. 2017, 601–602, 22–31. [Google Scholar] [CrossRef]
- Meng, W.; Feagin, R.A. Mariculture is a double-edged sword in China. Estuar. Coast. Shelf Sci. 2019, 222, 147–150. [Google Scholar] [CrossRef]
- Pustizzi, F.; MacIntyre, H.; Warner, M.E.; Hutchins, D.A. Interaction of nitrogen source and light intensity on the growth and photosynthesis of the brown tide alga Aureococcus anophagefferens. Harmful Algae 2004, 3, 343–360. [Google Scholar] [CrossRef]
- Liang, Y.; Beardall, J.; Heraud, P. Effects of nitrogen source and UV radiation on the growth, chlorophyll fluorescence and fatty acid composition of Phaeodactylum tricormutum and Chaetoceros muelleri (Bacillatiophyceae). J. Photochem. Photobiol. B 2006, 82, 161–172. [Google Scholar] [CrossRef]
- Feng, P.; Xu, Z.; Qin, L.; Alam, M.A.; Wang, Z.; Zhu, S. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef]
- Provasoli, L. Media and prospects for the cultivation of marine algae. In Cultures and Collections of Algae; Watanabe, A., Hattori, A., Eds.; Japanese Society of Plant Physiologists: Kyoto, Japan, 1968; pp. 63–67. [Google Scholar]
- Spanò, N.; Paola, D.D.; Albano, M.; Manganaro, A.; Sanfilippo, M.; D’Iglio, C.; Capillo, G.; Savoca, S. Growth performance and bioremediation potential of Gracilaria gracilis (Steentoft, L.M. Irvine & Farnham, 1995). Int. J. Environ. Stud. 2022, 79, 748–760. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, G.; Xu, J.; Wu, H. Physiological response of a golden tide alga (Sargassum muticum) to the interaction of ocean acidification and phosphorus enrichment. Biogeosciences 2017, 14, 671–681. [Google Scholar] [CrossRef]
- Caemmerer, S.V.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanzen. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual for Chemical and Biologtcal Methods for Seawater Analysis, 1st ed.; Pergamon Press: New York, NY, USA, 1984; p. 173. ISBN 9781483293394. [Google Scholar]
- Lobban, C.S.; Chapman, D.J.; Kremer, B.P. Experimental Phycology: A Laboratory Manual; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Kochert, G. Carbohydrate determination by the phenol-sulfuric acid method. In Handbook of Phycological Methods: Physiological and Biochemical Methods; Hellebust, J.A., Graigie, J.S., Eds.; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ferrario-Méry, S.; Murchie, E.; Hirel, B.; Galtier, N.; Quick, W.P.; Foyer, C.H. Manipulation of the pathways of sucrose biosynthesis and nitrogen assimilation in transformed plants to improved photosynthesis and productivity. In A Molecular Approach to Primary Metabolism in Higher Plants; Foyer, C.H., Quick, W.P., Eds.; Taylor & Francis: London, UK, 1997; pp. 125–153. [Google Scholar]
- Bao, Y.; Huo, Y.; Duan, Y.; He, P.; Wu, M.; Yang, N.; Sun, B. Growth and nutrient uptake of Myriophyllum spicatum under different nutrient conditions and its potential ecosystem services in an enclosed sea area in the East China Sea. Mar. Pollut. Bull. 2020, 151, 110801. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Interactions between NH4+ and NO3- uptake and assimilation: Comparison of diatoms and dinoflagellates at several growth temperature. Mar. Biol. 1999, 133, 541–551. [Google Scholar] [CrossRef]
- Lobban, C.S.; Harrison, P.J.; Duncan, M.J. The Physiological Ecology of Seaweeds, 1st ed.; Cambridge University Press: New York, NY, USA, 1985; pp. 75–109. ISBN 0-521-26508-8. [Google Scholar]
- Chen, L.; Xing, R.; Jiang, A.; Yao, Y.; Zhou, G. Effects of nitrogen source and N/P on growth and photosynthesis in the invasive marine macroalga Chaetomorpha valida. Environ. Sci. Pollut. Res. 2020, 27, 24272–24283. [Google Scholar] [CrossRef]
- Dai, G.; Deblois, C.P.; Liu, S.; Juneau, P.; Qiu, B. Differential sensitivity of five cyanobacterial strains to ammonium toxicity and its inhibitory mechanism on the photosynthesis of rice-field cyanobacterium Ge–Xian–Mi (Nostoc). Aquat. Toxicol. 2008, 89, 113–121. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, F.; Jiang, H.; Ma, Y.; Cui, C.; Qin, H.; Liu, L.; Zang, S.; Xing, H.; Xu, Z.; Wu, H. Combined Influences of Light and Nitrogen Enrichment on the Physiological Performance of a Golden Tide Alga (Sargassum horneri). J. Mar. Sci. Eng. 2022, 10, 1195. https://doi.org/10.3390/jmse10091195
Yan F, Jiang H, Ma Y, Cui C, Qin H, Liu L, Zang S, Xing H, Xu Z, Wu H. Combined Influences of Light and Nitrogen Enrichment on the Physiological Performance of a Golden Tide Alga (Sargassum horneri). Journal of Marine Science and Engineering. 2022; 10(9):1195. https://doi.org/10.3390/jmse10091195
Chicago/Turabian StyleYan, Fang, Huichao Jiang, Yuanqing Ma, Cuiju Cui, Huawei Qin, Lijuan Liu, Shasha Zang, Hongyan Xing, Zhiguang Xu, and Hongyan Wu. 2022. "Combined Influences of Light and Nitrogen Enrichment on the Physiological Performance of a Golden Tide Alga (Sargassum horneri)" Journal of Marine Science and Engineering 10, no. 9: 1195. https://doi.org/10.3390/jmse10091195
APA StyleYan, F., Jiang, H., Ma, Y., Cui, C., Qin, H., Liu, L., Zang, S., Xing, H., Xu, Z., & Wu, H. (2022). Combined Influences of Light and Nitrogen Enrichment on the Physiological Performance of a Golden Tide Alga (Sargassum horneri). Journal of Marine Science and Engineering, 10(9), 1195. https://doi.org/10.3390/jmse10091195





