Filtration Rates and Scaling in Demosponges
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Scaling in Single-Osculum Single-Module Demosponges
3.2. Scaling in Multi-Oscula Multi-Modular Demosponges
3.3. Scaling in Single-Osculum Multi-Modular Demosponges
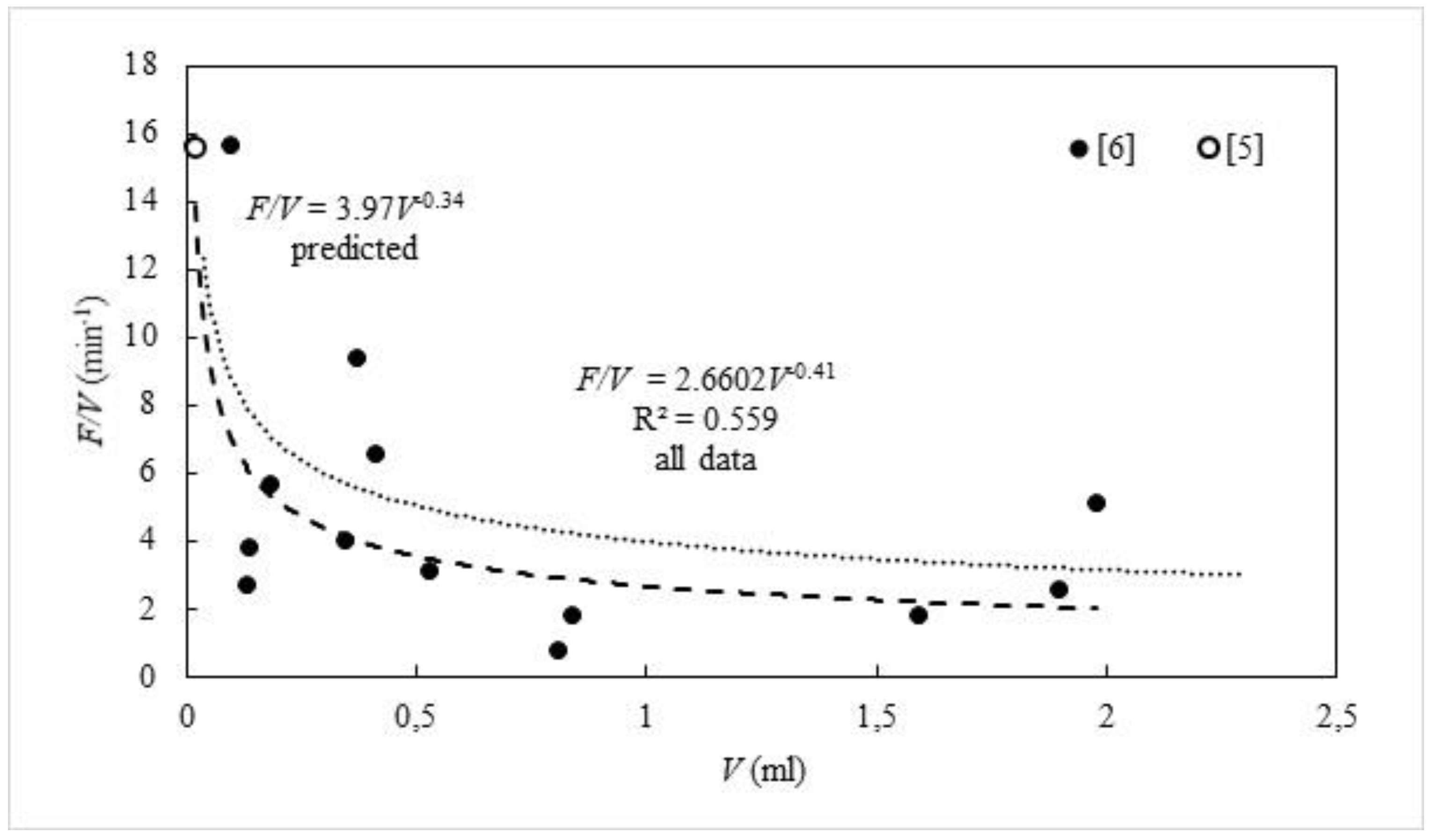
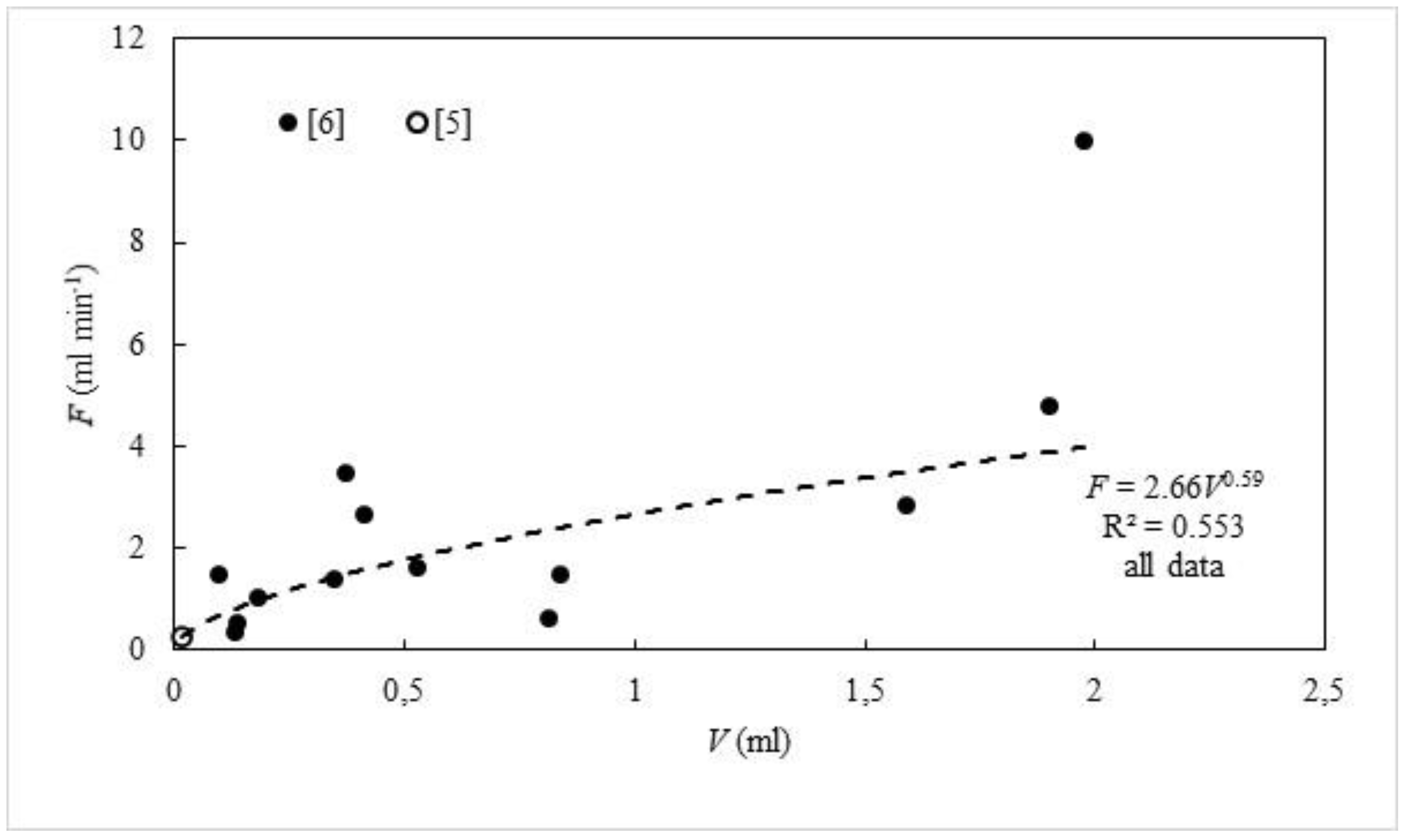
4. Filtration Rate, OSA, and Size
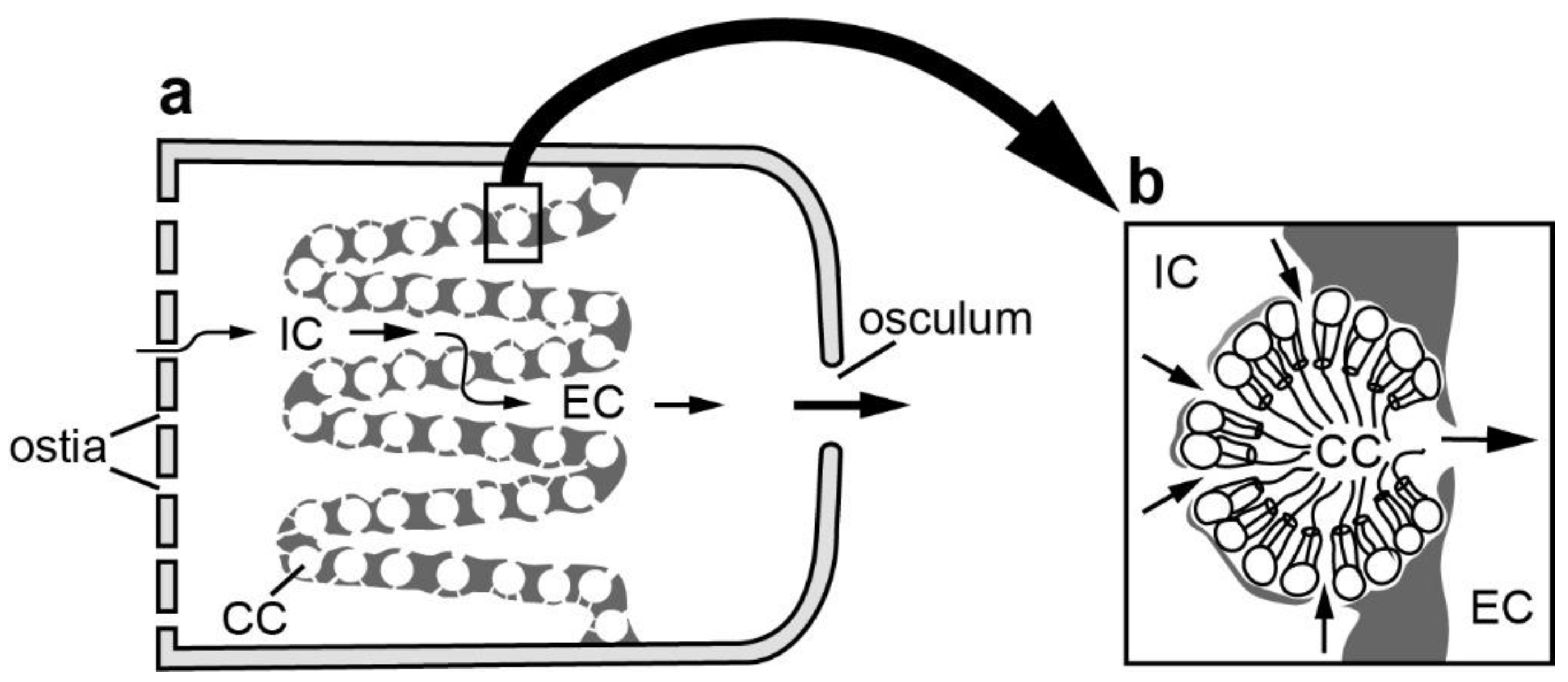
5. Density and Filtration Rates of Choanocyte Cambers in a Single-Osculum Module
5.1. Filtration Rates
5.2. Closing Remarks: Towards a Better Understanding
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Voogd, N.J.; Alvarez, B.; Boury-Esnault, N.; Carballo, J.L.; Cárdenas, P.; Díaz, M.-C.; Dohrmann, M.; Downey, R.; Hajdu, E.; Hooper, J.N.A.; et al. World Porifera Database. 2022. Available online: https://www.marinespecies.org/porifera (accessed on 28 March 2022). [CrossRef]
- Fry, W.G. The sponge as a population: A biometric approach. Symp. Zool. Soc. Lond. 1970, 25, 135–162. [Google Scholar]
- Ereskovskii, A.V. Problems of coloniality, modularity, and individuality in sponges and special features of their mor-phogeneses during growth and asexual reproduction. Russ. J. Mar. Biol. 2003, 29, 46–56. [Google Scholar] [CrossRef]
- Reiswig, H.M. The aquiferous systems of three marine demospongiae. J. Morph. 1975, 145, 493–502. [Google Scholar] [CrossRef]
- Kumala, L.; Riisgård, H.U.; Canfield, D.E. Osculum dynamics and filtration activity studied in small single-osculum explants of the demosponge Halichondria panicea. Mar. Ecol. Prog. Ser. 2017, 572, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.; Riisgård, H.U.; Larsen, P.S. Exhalant jet speed of single-osculum explants of the demosponge Halichondria panicea and basic properties of the sponge-pump. J. Exp. Mar. Biol. Ecol. 2019, 511, 82–90. [Google Scholar] [CrossRef]
- Kealy, R.A.; Busk, T.; Goldstein, J.; Larsen, P.S.; Riisgård, H.U. Hydrodynamic characteristics of aquiferous modules in the demosponge Halichondria panicea. Mar. Biol. Res. 2019, 15, 531–540. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Thomassen, S.; Jakobsen, H.; Weeks, J.; Larsen, P.S. Suspension feeding in marine sponges Halichondria panicea and Haliclona urceolus: Effects of temperature on filtration rate and energy cost of pumping. Mar. Ecol. Prog. Ser. 1993, 96, 177–188. [Google Scholar] [CrossRef]
- Larsen, P.S.; Riisgård, H.U. The sponge pump. J. Theor. Biol. 1994, 168, 53–63. [Google Scholar] [CrossRef]
- Thomassen, S.; Riisgård, H.U. Growth and energetics of the sponge Halichondria panicea. Mar. Ecol. Prog. Ser. 1995, 128, 239–246. [Google Scholar] [CrossRef]
- McMurray, S.E.; Blum, J.E.; Pawlik, J.R. Redwood of the reef: Growth and age of the giant barrel sponge Xestospongia muta in the Floridas Keys. Mar. Biol. 2008, 155, 159–171. [Google Scholar] [CrossRef]
- McMurray, S.E.; Pawlik, J.R.; Finelli, C.M. Trait-mediated ecosystem impacts: How morphology and size affect pumping rates of the Caribbean giant barrel sponge. Aquat. Biol. 2014, 23, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Reiswig, H.M. In situ pumping activities of tropical Demospongiae. Mar. Biol. 1971, 9, 38–50. [Google Scholar] [CrossRef]
- Strehlow, B.W.; Jørgensen, D.; Webster, N.S.; Pineda, M.C.; Duckworth, A. Using a thermistor flowmeter with attached video camera for monitoring sponge excurrent speed and oscular behaviour. PeerJ 2016, 4, e2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morganti, T.M.; Ribes, M.; Moskovich, R.; Weisz, J.B.; Yahel, G.; Coma, R. In situ pumping rate of 20 marine demosponges is a function of osculum area. Front. Mar. Sci. 2021, 8, 583188. [Google Scholar] [CrossRef]
- Larsen, P.S.; Riisgård, H.U. Pumping rate and size of demosponges-towards an understanding using modeling. J. Mar. Sci. Eng. 2021, 9, 1308. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 2 March 2022).
- Fry, W.G. Taxonomy, the individual and the sponge. Biology and Systematics of Colonial organisms. Syst. Ass. Spec. 1979, 11, 49–80. [Google Scholar]
- Pinheiro, U.S.; Hajdu, E. Shallow-water Aplysina Nardo (Aplysinidaee Verongida, Demospongiae) from the São Sebastião Channel and its environs (Tropical southwestern Atlantic), with the description of a new species and a literature review of other brazilian records of genus. Rev. Bras. Zool. 2001, 18 (Suppl. S1), 143–160. [Google Scholar] [CrossRef]
- Morganti, T.M.; Ribes, M.; Yahel, G.; Coma, R. Size is the major determinant of pumping rates in marine sponges. Front. Physiol. 2019, 10, 1474. [Google Scholar] [CrossRef] [Green Version]
- Reiswig, H.M. Population dynamics of three Jamaican Demospongiae. Bull. Mar. Sci. 1973, 23, 191–226. [Google Scholar]
- Reiswig, H.M. Water transport, respiration and energetics of three tropical marine sponges. J. Exp. Mar. Biol. Ecol. 1974, 14, 231–249. [Google Scholar] [CrossRef]
- Southwell, M.W.; Weisz, J.B.; Martens, C.S.; Lindquist, N. In situ fluxes of dissolved inorganic nitrogen from the sponge community on Conch Reef, Key Largo, Florida. Limnol. Oceanogr. 2008, 53, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Dahihande, A.S.; Thakur, N.L. Temperature- and size-associated differences in the skeletal structures and osculum cross-sectional area influence the pumping rate of contractile sponge Cinachyrella cf. cavernosa. Mar. Ecol. 2019, 40, e12565. [Google Scholar] [CrossRef]
- Barthel, D. On the ecophysiology of the sponge Halichondria panicea in Kiel Bight. I. Substrate specificity, growth and reproduction. Mar. Ecol. Prog. Ser. 1986, 32, 291–298. [Google Scholar] [CrossRef]
- Lüskow, F.; Riisgård, H.U.; Solovyeva, V.; Brewer, J.R. Seasonal changes in bacteria and phytoplankton biomass control the condition index of the demosponge Halichondria panicea in temperate Danish waters. Mar. Ecol. Prog. Ser. 2019, 608, 119–132. [Google Scholar] [CrossRef]
- Barthel, D. Influence of different current regimes on the growth form of Halichondria panicea Pallas. In Fossil and Recent Sponges; Reitner, J., Keupp, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 387–394. [Google Scholar]
- Imsiecke, G. Ingestion, digestion, and egestion in Spongilla lacustris (Porifera, Spongillidae) after pulse feeding with Chlamydomonas reinhardtii (Volvocales). Zoomorphology 1993, 113, 233–244. [Google Scholar] [CrossRef]
- Weissenfels, N. The filtration apparatus for food collection in freshwater sponges (Porifera, Spongillidae). Zoomorphology 1992, 112, 51–55. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Kumala, L.; Charitonidou, K. Using the F/R-ratio for an evaluation of the ability of the demosponge Halichondria panicea to nourish solely on phytoplankton versus free-living bacteria in the sea. Mar. Biol. Res. 2016, 12, 907–916. [Google Scholar] [CrossRef]
- Ludeman, D.A.; Reidenbach, M.A.; Leys, S.P. The energetic cost of filtration by demosponges and their behavioural response to ambient currents. J. Exp. Biol. 2017, 220, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Asadzadeh, S.S.; Larsen, P.S.; Riisgård, H.U.; Walther, J.H. Hydrodynamics of the leucon sponge pump. JRSI 2019, 16, 20180630. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riisgård, H.U.; Larsen, P.S. Filtration Rates and Scaling in Demosponges. J. Mar. Sci. Eng. 2022, 10, 643. https://doi.org/10.3390/jmse10050643
Riisgård HU, Larsen PS. Filtration Rates and Scaling in Demosponges. Journal of Marine Science and Engineering. 2022; 10(5):643. https://doi.org/10.3390/jmse10050643
Chicago/Turabian StyleRiisgård, Hans Ulrik, and Poul S. Larsen. 2022. "Filtration Rates and Scaling in Demosponges" Journal of Marine Science and Engineering 10, no. 5: 643. https://doi.org/10.3390/jmse10050643
APA StyleRiisgård, H. U., & Larsen, P. S. (2022). Filtration Rates and Scaling in Demosponges. Journal of Marine Science and Engineering, 10(5), 643. https://doi.org/10.3390/jmse10050643






