Microplastics in the Deep: Comparing Dietary and Plastic Ingestion Data between Two Mediterranean Bathyal Opportunistic Feeder Species, Galeus melastomus, Rafinesque, 1810 and Coelorinchus caelorhincus (Risso, 1810), through Stomach Content Analysis
Abstract
:1. Introduction
2. Materials and Methods
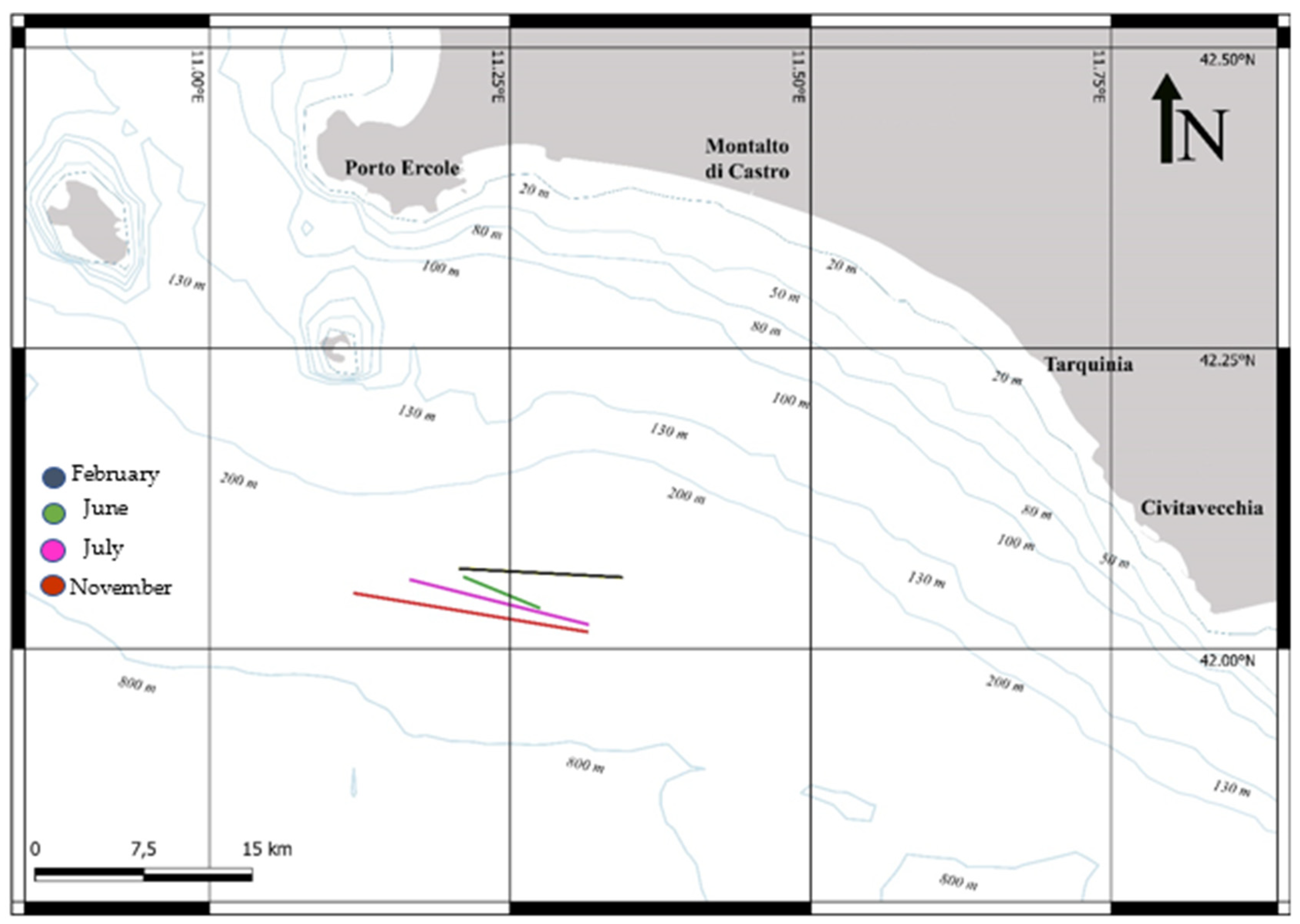
2.1. Sample Collection
2.2. Microplastics’ Identification and Validation
2.3. Secondary Contamination
2.4. Statistical Analyses
3. Results
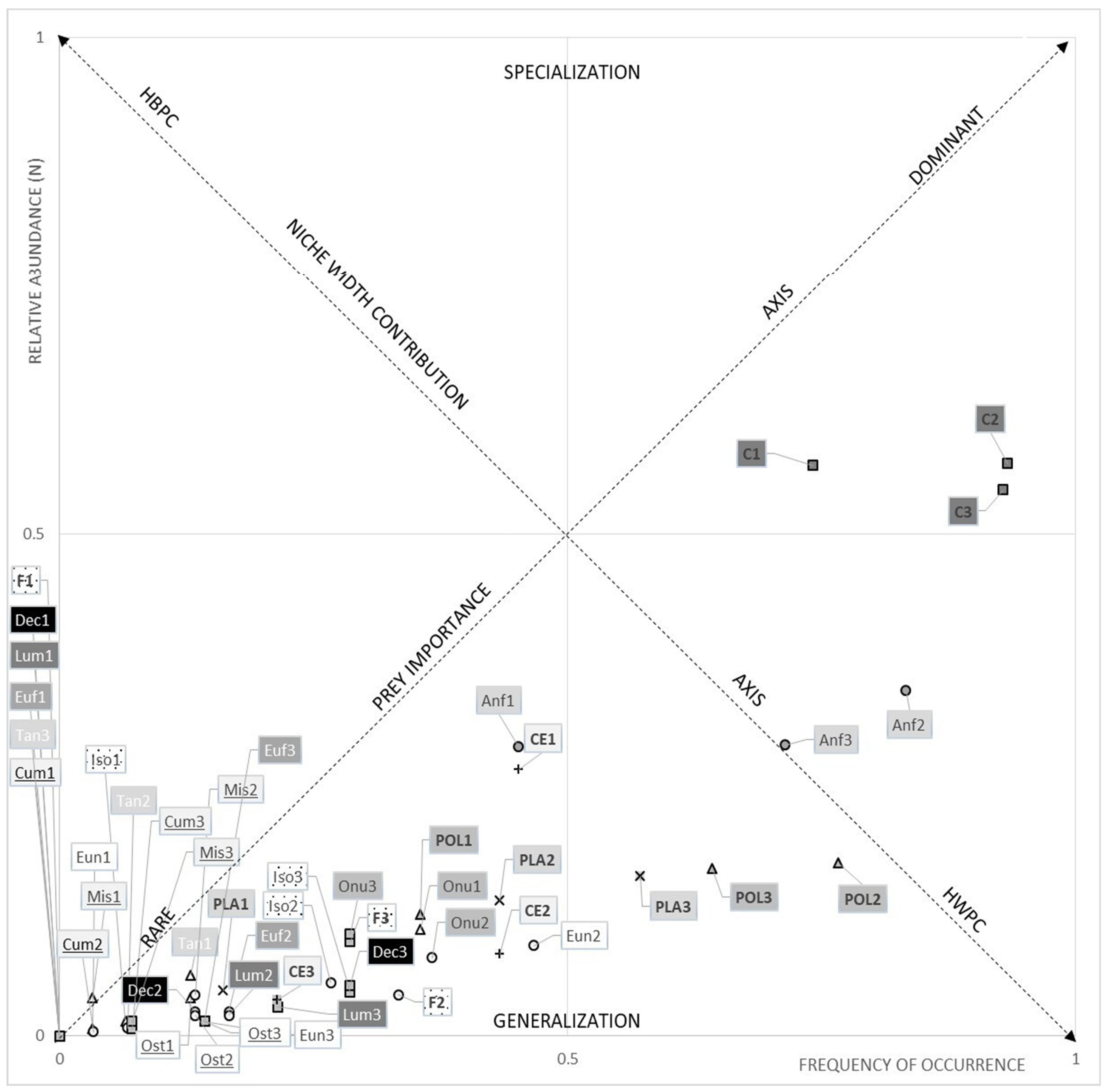
3.1. Dietary Data
3.1.1. The Hollow-Snout Grenadier
3.1.2. The Black Mouth Catshark
3.1.3. Diet Comparison between Species
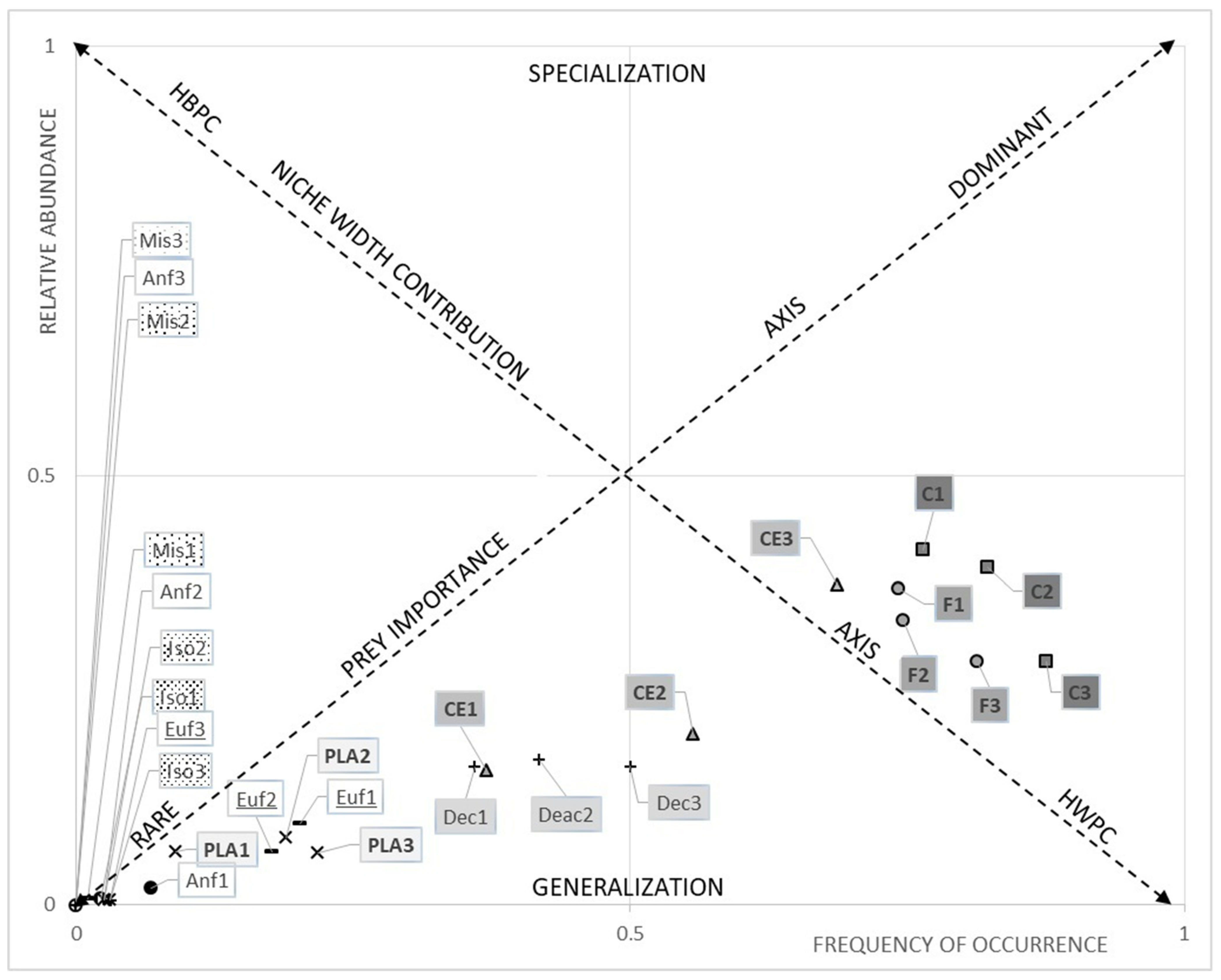
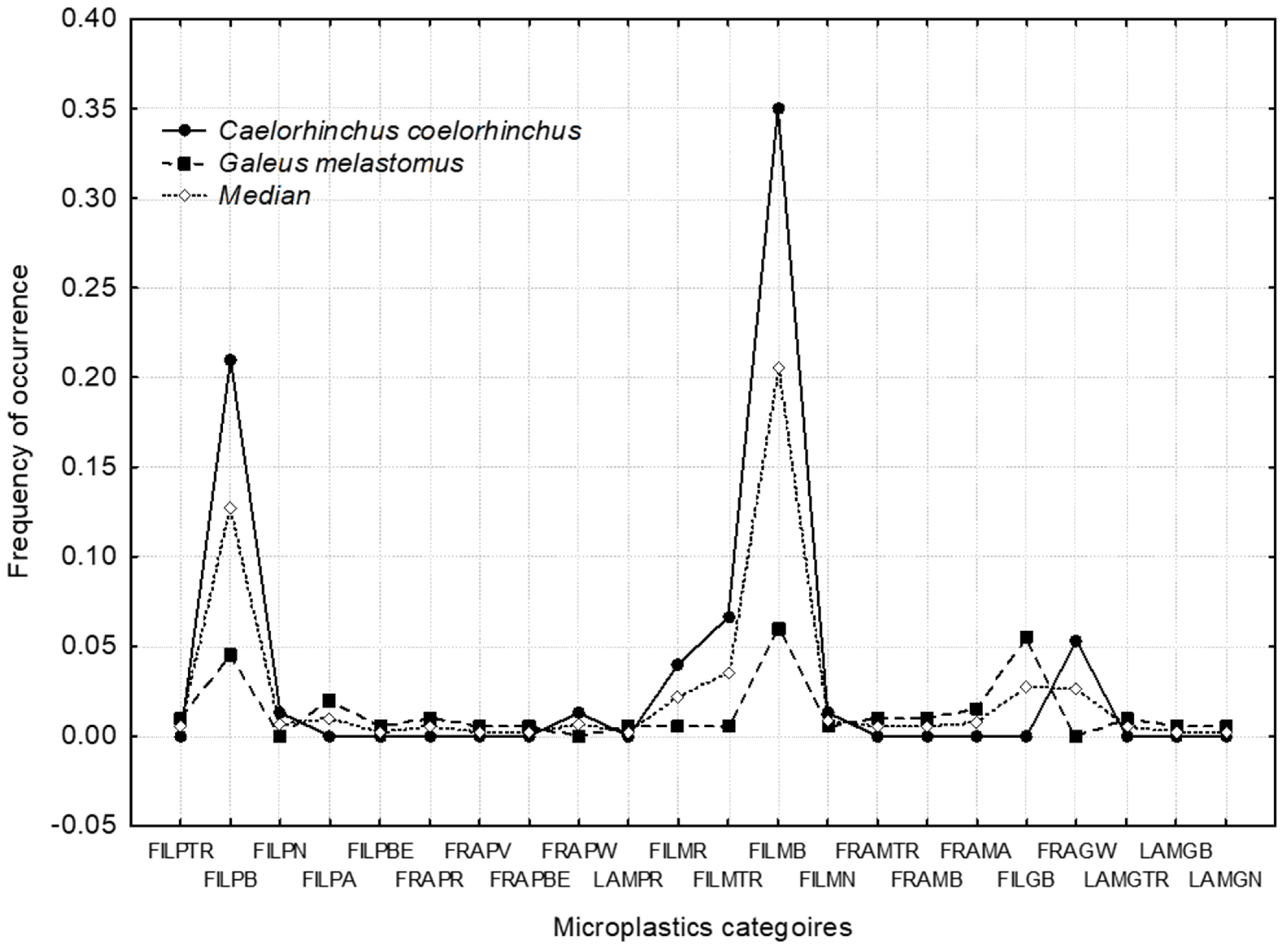
3.2. Microplastics Ingestion
3.2.1. Comparison between Species
3.2.2. Relationships between Microplastic Filaments and Polychaetes
3.2.3. Control for Secondary Contamination
4. Discussion
4.1. Dietary Data
4.2. Microplastics Ingestion
4.3. Data Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wen, X.; Huang, D.; Du, C.; Deng, R.; Zhou, Z.; Tao, J.; Li, R.; Zhou, W.; Wang, Z.; et al. Interactions between microplastics/nanoplastics and vascular plants. Environ. Pollut. 2021, 290, 117999. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Estarellas, F.; Deudero, S. Microplastics in the Mediterranean Sea: Deposition in coastal shallow sediments, spatial variation and preferential grain size. Mar. Environ. Res. 2016, 115, 1–10. [Google Scholar] [CrossRef]
- Onoja, S.; Nel, H.A.; Abdallah, M.A.E.; Harrad, S. Microplastics in freshwater sediments: Analytical methods, temporal trends, and risk of associated organophosphate esters as exemplar plastics additives. Environ. Res. 2022, 203, 111830. [Google Scholar] [CrossRef]
- Boucher, J.; Billard, G. The Mediterranean; (No. REPORT_SBM); IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Kane, I.A.; Clare, M.A. Dispersion, accumulation, and the ultimate fate of microplastics in deep-marine environments: A review and future directions. Front. Earth Sci. 2019, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Ru, J.; Huo, Y.; Yang, Y. Microbial degradation and valorization of plastic wastes. Front. Microbiol. 2020, 11, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, E.J.; Smith, K.L., Jr. Plastics on the Sargasso Sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- López-Martínez, S.; Morales-Caselles, C.; Kadar, J.; Rivas, M.L. Overview of global status of plastic presence in marine vertebrates. Glob. Change Biol. 2021, 27, 728–737. [Google Scholar] [CrossRef]
- Meaza, I.; Tojoda, J.H.; Wise, J.P. Microplastics in the sea turtles, marine mammals and humans: A one environmental health perspective. Front. Environ. Sci. 2021, 8, 575614. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Amélineau, F.; Bonnet, D.; Heitz, O.; Mortreux, V.; Harding, A.M.; Karnovsky, N.; Walkusz, W.; Fort, J.; Grémillet, D. Microplastic pollution in the Greenland Sea: Background levels and selective contamination of planktivorous diving seabirds. Environ. Pollut. 2016, 219, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Pellini, G.; Salvalaggio, V.; Fabi, G. Comparative effects of ingested PVC micro particles with and without adsorbed benzo (a) pyrene vs. spiked sediments on the cellular and sub cellular processes of the benthic organism Hediste diversicolor. Front. Mar. Sci. 2018, 5, 99. [Google Scholar] [CrossRef] [Green Version]
- Lusher, A.L.; Mchugh, M.; Thompson, R.C. Occurrence of microplastics in the gastro-intestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; d’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the Mediterranean mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, J.R.; Cole, M.; Lindeque, P.K.; Fileman, E.; Blackford, J.; Lewis, C.; Galloway, T.S. Marine microplastic debris: A targeted plan for understanding and quantifying interactions with marine life. Front. Ecol. Environ. 2016, 14, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.D.; Sinclair, M.; Levi, C.J.; Reeves, S.E.; Edgar, G.J. Ubiquity of microplastics in coastal seafloor sediments. Mar. Pollut. Bull. 2017, 121, 104–110. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Provencher, J.F.; Bond, A.L.; Avery-Gomm, S.; Borrelle, S.B.; Rebolledo, E.L.B.; Hammer, S.; Kühn, S.; Lavers, J.F.; Mallory, M.L.; Trevail, A.; et al. Quantifying ingested debris in marine megafauna: A review and recommendations for standardization. Anal. Methods 2017, 9, 1454–1469. [Google Scholar] [CrossRef]
- Sbrana, A.; Valente, T.; Scacco, U.; Bianchi, J.; Silvestri, C.; Palazzo, L.; de Lucia, G.A.; Valerani, C.; Ardizzone, G.; Matiddi, M. Spatial variability and influence of biological parameters on microplastic ingestion by Boops boops (L.) along the Italian coasts (Western Mediterranean Sea). Environ. Pollut. 2020, 263, 114429. [Google Scholar] [CrossRef]
- Ding, T.; Wei, L.; Hou, Z.; Li, J.; Zhang, C.; Lin, D. Microplastics altered contaminant behavior and toxicity in natural waters. J. Hazard. Mater. 2022, 425, 127908. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Li, H.-X.; Ma, L.-S.; Lin, L.; Ni, Z.-X.; Xu, X.-R.; Shi, H.-H.; Yan, Y.; Zheng, G.-M.; Rittschof, D. Microplastics in oysters Saccostrea cucullata along the Pearl River estuary, China. Environ. Pollut. 2018, 236, 619–625. [Google Scholar] [CrossRef]
- Marchant, D.J.; Jones, J.I.; Zemelka, G.; Eyice, O.; Kratina, P. Do microplastics mediate the effects of chemicals on aquatic organisms? Aquat. Toxicol. 2022, 242, 106037. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Casabianca, S.; Capellacci, S.; Giacobbe, M.G.; Dell’Aversano, C.; Tartaglione, L.; Varriale, F.; Narizzano, R.; Risso, F.; Moretto, P.; Dagnino, A.; et al. Plastic-associated harmful microalgal assemblages in marine environment. Environ. Pollut. 2019, 244, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, A.; Mancini, E.; Saraceni, P.R.; Della Ventura, G.; Scapigliati, G.; Picchietti, S. First evidence of in vitro cytotoxic effects of marine microlitter on Merluccius merluccius and Mullus barbatus, two Mediterranean commercial fish species. Sci. Total Environ. 2021, 813, 152618. [Google Scholar] [CrossRef]
- Carson, H.S. The incidence of plastic ingestion by fishes: From the prey’s perspective. Mar. Pollut. Bull. 2013, 74, 170–174. [Google Scholar] [CrossRef]
- Li, B.; Liang, W.; Liu, Q.-X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish ingest microplastics unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar] [CrossRef]
- Hamzah, S.R.; Anuar, S.T.; Khalik, W.M.A.W.M.; Kolandhasamy, P.; Ibrahim, Y.S. Ingestion of microplastics by the estuarine polychaete, Namalycastis sp. in the Setiu Wetlands, Malaysia. Mar. Pollut. Bull. 2021, 170, 112617. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Shim, W.J.; Han, G.M.; Song, Y.K.; Hong, S.H. Formation of microplastics by polychaetes (Marphysa sanguinea) inhabiting expanded polystyrene marine debris. Mar. Pollut. Bull. 2018, 131, 365–369. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, M.B.; Dos Santos, M.O.; de Farias Viegas, G.M.; Yapuchura Ocaris, E.R.; Barcellos Caniçali, F.; dos Reis Cozer, C.; Carvalho Zamprogno, G.; Paz Otegui, M.B. Quantitative evaluation of microplastics in colonies of Phragmatopoma caudata Krøyer in Mörch, 1863 (Polychaeta-Sabellariidae): Analysis in sandcastles and tissues and identification via Raman spectroscopy. Mar. Pollut. Bull. 2021, 165, 112127. [Google Scholar] [CrossRef]
- Taylor, M.L.; Gwinnett, C.; Robinson, L.F.; Woodall, L.C. Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 2016, 6, 33997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alomar, C.; Deudero, S.; Compa, M.; Guijarro, B. Exploring the relation between plastic ingestion in species and its presence in seafloor bottoms. Mar. Pollut. Bull. 2020, 160, 111641. [Google Scholar] [CrossRef]
- Gregory, M.R. Environmental implications of plastic debris in marine settings-entanglement, ingestion, smothering, hanger-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Battaglia, P.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Deudero, S.; Alomar, C. Revising interactions of plastics with marine biota: Evidence from the Mediterranean. In Marine litter in the Mediterranean and Black Seas; CIESM Workshop Monograph; CIESM: Monaco City, Monaco, 2014; Volume 46, p. 180. [Google Scholar]
- Buwono, N.R.; Risjani, Y.; Soegianto, A. Contamination of microplastics in Brantas River, East Java, Indonesia and its distribution in gills and digestive tracts of fish Gambusia affinis. Emerg. Contam. 2021, 7, 172–178. [Google Scholar] [CrossRef]
- Hu, L.; Chernick, M.; Lewis, A.M.; Ferguson, P.L.; Hinton, D.E. Chronic microfiber exposure in adult Japanese medaka (Oryzias latipes). PLoS ONE 2020, 15, e0229962. [Google Scholar] [CrossRef] [Green Version]
- Bray, L.; Digka, N.; Tsangaris, C.; Camedda, A.; Gambaiani, D.; de Lucia, G.A.; Matiddi, M.; Miaud, C.; Palazzo, L.; Pérez-del-Olmo, A.; et al. Determining suitable fish to monitor plastic ingestion trends in the Mediterranean Sea. Environ. Pollut. 2019, 247, 1071–1077. [Google Scholar] [CrossRef]
- Llorca, M.; Álvarez-Muñoz, D.; Ábalos, M.; Rodríguez-Mozaz, S.; Santos, L.H.M.L.M.; León, V.M.; Campillo, J.A.; Martínez-Gómez, C.; Abad, E.; Farré, M. Microplastics in Mediterranean coastal area: Toxicity and impact for the environment and human health. Trends Environ. Anal. Chem. 2020, 27, e00090. [Google Scholar] [CrossRef]
- UNEP. Marine Litter Assessment in the Mediterranean; United Nations Environment Program: Athens, Greece, 2015. [Google Scholar]
- Angiolillo, M.; di Lorenzo, B.; Farcomeni, A.; Bo, M.; Bavestrello, G.; Santangelo, G.; Cau, A.; Mastascusa, V.; Cau, A.; Sacco, F.; et al. Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar. Pollut. Bull. 2015, 92, 149–159. [Google Scholar] [CrossRef]
- Blašković, A.; Fastelli, P.; Čižmek, H.; Guerranti, C.; Renzi, M. Plastic litter in sediments from the Croatian marine protected area of the natural park of Telaščica bay (Adriatic Sea). Mar. Pollut. Bull. 2017, 114, 583–586. [Google Scholar] [CrossRef]
- Cannas, S.; Fastelli, P.; Guerranti, C.; Renzi, M. Plastic litter in sediments from the coasts of south Tuscany (Tyrrhenian Sea). Mar. Pollut. Bull. 2017, 119, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Cau, A.; Alvito, A.; Moccia, D.; Canese, S.; Pusceddu, A.; Rita, C.; Angiolillo, M.; Follesa, M.C. Submarine canyons along the upper Sardinian slope (Central Western Mediterranean) as repositories for derelict fishing gears. Mar. Pollut. Bull. 2017, 123, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Consoli, P.; Falautano, M.; Sinopoli, M.; Perzia, P.; Canese, S.; Esposito, V.; Battaglia, P.; Romeo, T.; Andaloro, F.; Galgani, F.; et al. Composition and abundance of benthic marine litter in a coastal area of the central Mediterranean Sea. Mar. Pollut. Bull. 2018, 136, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Crocetta, F.; Riginella, E.; Lezzi, M.; Tanduo, V.; Balestrieri, L.; Rizzo, L. Bottom-trawl catch composition in a highly polluted coastal area reveals multifaceted native biodiversity and complex communities of fouling organisms on litter discharge. Mar. Environ. Res. 2020, 155, 104875. [Google Scholar] [CrossRef] [PubMed]
- Fastelli, P.; Blašković, A.; Bernardi, G.; Romeo, T.; Čižmek, H.; Andaloro, F.; .Russo, G.F.; .Guerranti, C.; Renzi, M. Plastic litter in sediments from a marine area likely to become protected (Aeolian Archipelago’s islands, Tyrrhenian Sea). Mar. Pollut. Bull. 2016, 113, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Fortibuoni, T.; Ronchi, F.; Mačić, V.; Mandić, M.; Mazziotti, C.; Peterlin, M.; Prevenios, M.; Prvan, M.; Somarakis, S.; Tutman, P.; et al. A harmonized and coordinated assessment of the abundance and composition of seafloor litter in the Adriatic-Ionian macroregion (Mediterranean Sea). Mar. Pollut. Bull. 2019, 139, 412–426. [Google Scholar] [CrossRef]
- Mancini, E.; Miccoli, A.; Piazzolla, D.; Saraceni, P.R.; Lezzi, M.; Tiralongo, F.; Bonifazi, A.; Picchietti, S.; Marcelli, M. Macrozoobenthic fauna associated with benthic marine litter (Northern Tyrrhenian Sea, Italy) and first report of two bryozoan species in Italian waters. Reg. Stud. Mar. Sci. 2021, 47, 101912. [Google Scholar] [CrossRef]
- Mistri, M.; Infantini, V.; Scoponi, M.; Granata, T.; Moruzzi, L.; Massara, F.; de Donati, M.; Munari, C. Small plastic debris in sediments from the Central Adriatic Sea: Types, occurrence and distribution. Mar. Pollut. Bull. 2017, 124, 435–440. [Google Scholar] [CrossRef]
- Palatinus, A.; Kovač Viršek, M.; Robič, U.; Grego, M.; Bajt, O.; Šiljić, J.; Suaria, G.; Liubartseva, S.; Coppini, G.; Peterlin, M. Marine litter in the Croatian part of the middle Adriatic Sea: Simultaneous assessment of floating and seabed macro and micro litter abundance and composition. Mar. Pollut. Bull. 2019, 139, 427–439. [Google Scholar] [CrossRef]
- Pasquini, G.; Ronchi, F.; Strafella, P.; Scarcella, G.; Fortibuoni, T. Seabed litter composition, distribution and sources in the Northern and Central Adriatic Sea (Mediterranean). Waste Manag. 2016, 58, 41–51. [Google Scholar] [CrossRef]
- Renzi, M.; Blašković, A.; Fastelli, P.; Marcelli, M.; Guerranti, C.; Cannas, S.; Barone, L.; Massara, F. Is the microplastic selective according to the habitat? Records in amphioxus sands, Mäerl bed habitats and Cymodocea nodosa habitats. Mar. Pollut. Bull. 2018, 130, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Strafella, P.; Fabi, G.; Spagnolo, A.; Grati, F.; Polidori, P.; Punzo, E.; Fortibuoni, T.; Marceta, B.; Raicevish, S.; Cvitkovic, I.; et al. Spatial pattern and weight of seabed marine litter in the northern and central Adriatic Sea. Mar. Pollut. Bull. 2015, 91, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Löhr, A.; Savelli, H.; Beunen, R.; Kalz, M.; Ragas, A.; van Belleghem, F. Solutions for global marine litter pollution. Curr. Opin. Environ. Sustain. 2017, 28, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Sheavly, S.B. Marine debris—An overview of a critical issue for our oceans. In Proceedings of the Sixth Meeting of the UN Open-Ended Informal Consultative Processes on Oceans & the Law of the Sea, Paris, France, 6–10 June 2005. [Google Scholar]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Galgani, F.; Hanke, G.; Maes, T. Global distribution, composition and abundance of marine litter. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 29–56. [Google Scholar] [CrossRef] [Green Version]
- Savoca, M.S.; Tyson, C.W.; McGill, M.; Slager, C.J. Odours from marine plastic debris induce food search behaviours in a forage fish. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171000. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.A.; Robinson, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The vertical distribution and biological transport of marine microplastic across the epipelagic and mesopelagic water column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodall, L.C.; Sanchez–Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [Green Version]
- GESAMP. Guidelines for the Monitoring and Assessment of Plastic Litter in the Ocean; GESAMP Reports and Studies; GESAMP: London, UK, 2019; Volume 99, p. 130. [Google Scholar]
- Valente, T.; Sbrana, A.; Scacco, U.; Jacomini, C.; Bianchi, J.; Palazzo, L.; de Lucia, G.A.; SIlvestri, C.; Matiddi, M. Exploring microplastic ingestion by three deep-water elasmobranch species: A case study from the Tyrrhenian Sea. Environ. Pollut. 2019, 253, 342–350. [Google Scholar] [CrossRef]
- Valente, T.; Scacco, U.; Matiddi, M. Macro-litter ingestion in deep-water habitats: Is an underestimation occurring? Env. Res. 2020, 186, 109556. [Google Scholar] [CrossRef]
- Olaso, I.; Velasco, F.; Sánchez, F.; Serrano, A.; Rodríguez-Cabello, C.; Cendrero, O. Trophic relations of lesser-spotted catshark (Scyliorhinus canicula) and blackmouth catshark (Galeus melastomus) in the Cantabrian Sea. J. Northwest Atl. Fish. Sci. 2005, 35, 481–494. [Google Scholar] [CrossRef]
- Belluscio, A.; Scacco, U.; Colloca, F.; Carpentieri, P.; Ardizzone, G.D. Feeding strategies of two species of demersal chondrichthyans Galeus melastomus (Rafinesque, 1810) and Etmopterus spinax (Linneus, 1758), in the Central Tyrrhenian Sea. Biol. Mar. Mediterr. 2000, 7, 417–426. [Google Scholar]
- D’Iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and ecological aspects of the Blackmouth Catshark (Galeus melastomus Rafinesque, 1810) in the Southern Tyrrhenian Sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- D’Iglio, C.; Savoca, S.; Rinelli, P.; Spanò, N. Diet of the deep-sea shark Galeus melastomus Rafinesque, 1810, in the Mediterranean Sea: What we know and what we should know. Sustainability 2021, 13, 3962. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Mytilineou, C.; Lefkaditou, E.; Dokos, J.; Smith, C.J.; Siapatis, A.; Bekas, P.; Papadopoulou, K.-N. Diet and feeding strategy of blackmouth catshark Galeus melastomus. J. Fish Biol. 2013, 83, 1637–1655. [Google Scholar] [CrossRef]
- Rinelli, P.; Bottari, T.; Florio, G.; Romeo, T.; Giordano, D.; Greco, S. Observations on distribution and biology of Galeus melastomus (Chondrichthyes, Scyliorhinidae) in the southern Tyrrhenian Sea (central Mediterranean). Cybium 2005, 29, 41–46. [Google Scholar]
- Cohen, D.M. FAO Species Catalogue, Vol. 10: Gadiform Fishes of the World (Order Gadiformes): An Annotated and Illustrated Catalogue of Cods, Hakes, Grenadiers and Other Gadiform Fishes Known to Date; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990. [Google Scholar]
- Iwamoto, T. Coelorinchus caelorhincus. The IUCN Red List of Threatened Species. 2015. Available online: http://www.iucnredlist.org/details/198775/0 (accessed on 17 February 2022).
- Madurell, T.; Cartes, J.E. Trophic relationships and food consumption of slope dwelling macrourids from bathyal Ionian Sea (eastern Mediterranean). Mar. Biol. 2006, 148, 1325–1338. [Google Scholar] [CrossRef]
- McLellan, T. Feeding strategies of the macrourids. Deep-Sea Res. 1977, 24, 1019–1036. [Google Scholar] [CrossRef]
- García–Ruiz, C.; Hidalgo, M.; Carpentieri, P.; Fernandez-Arcaya, U.; Gaudio, P.; González, M.; Jadaud, A.; Mulas, A.; Peristeraki, P.; Rueda, J.L.; et al. Spatio–temporal patterns of macrourid fish species in the northern Mediterranean Sea. Sci. Mar. 2019, 83 (Suppl. S1), 117–127. [Google Scholar] [CrossRef]
- Alomar, C.; Deudero, S. Evidence of microplastic ingestion in the shark Galeus melastomus Rafinesque, 1810 in the continental shelf off the western Mediterranean Sea. Environ. Pollut. 2017, 223, 223–229. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Mytilineou, C.; Smith, C.J.; Papadopoulou, K.-N. Plastic debris ingested by deep–water fish of the Ionian Sea (Eastern Mediterranean). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2013, 74, 11–13. [Google Scholar] [CrossRef]
- Sala, A. Final Project Report Technical Specifications of Mediterranean Trawl Gears (myGears); European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Tiralongo, F. Sfruttamento della Risorsa Biologica Associata Alla Pesca a Strascico Nell’area di Civitavecchia e Impatto Sulle Comunità Demersali e Bentoniche. Ph.D. Thesis, University of Tuscia, Viterbo, Italy, 2016. [Google Scholar]
- Tiralongo, F.; Mancini, E.; Ventura, D.; De Malerbe, S.; Paladini De Mendoza, F.; Sardone, M.; Arciprete, R.; Massi, D.; Fiorentino, F.; Minervini, R. Commercial catches and discards composition in the central Tyrrhenian Sea: A multispecies quantitative and qualitative analysis from shallow and deep bottom trawling. Med. Mar. Sci. 2021, 22, 521–531. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis: A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Hermsen, E.; Mintenig, S.M.; Besseling, E.; Koelmann, A.A. Quality Criteria for the Analysis of Microplastic in Biota Samples: A Critical Review. Environ. Sci. Eng. 2018, 52, 10230–10240. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Hanke, G.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.C.; van Franeker, J.; Vlachogianni, T.; et al. Guidance on Monitoring for Marine Litter in European Seas; MSFD Technical Subgroup on Marine Litter (TSG-ML); European Commission, Joint Research Centre Scientific and Policy, Institute for Environment and Sustainability Reports: Brussels, Belgium, 2013. [Google Scholar]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [Green Version]
- Löder, M.G.J.; Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef] [Green Version]
- Torre, M.; Digka, N.; Anastasopoulou, A.; Tsangaris, C.; Mytilineou, C. Anthropogenic microfibres pollution in marine biota. A new and simple methodology to minimize airborne contamination. Mar. Pollut. Bull. 2016, 113, 55–61. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Gabler, H.M.; Staldvik, F.J. A new approach to graphical analysis of feeding strategy from stomach contents data - modifications of the Costello (1990) method. J. Fish Biol. 1996, 48, 607–614. [Google Scholar] [CrossRef]
- Costello, M.J. Predator feeding strategy and prey importance: A new graphical analysis. J. Fish Biol. 1990, 36, 261–263. [Google Scholar] [CrossRef]
- StatSoft Inc. STATISTICA (Data Analysis Softare System), Version 6; StatSoft Inc.: Tulsa, OK, USA, 2001; p. 150. [Google Scholar]
- Sever, T.M.; Filiz, H.; Bayhan, B.; Taskavak, E.; Bilge, G. Food habits of the hollowsnout grenadier, Caelorinchus caelorhincus (Risso, 1810), in the Aegean Sea, Turkey. Belg. J. Zool. 2008, 138, 81. [Google Scholar]
- Thomas, S.M.; Harrod, C.; Hayden, B.; Malinen, T.; Kahilainen, K.K. Ecological speciation in a generalist consumer expands the trophic niche of a dominant predator. Sci. Rep. 2017, 7, 8765. [Google Scholar] [CrossRef] [Green Version]
- Costello, M.J.; Chaudhary, C. Marine biodiversity, biogeography, deep–sea gradients, and conservation. Curr. Biol. 2017, 27, R511–R527. [Google Scholar] [CrossRef]
- Warrant, E.J.; Locket, N.A. Vision in the deep sea. Biol. Rev. 2004, 79, 671–712. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, D.; Knörzer, A.; Espenhahn, S.; Hilbig, R.; Haas, U.; Blum, M. Sensitivity differences in fish offer near–infrared vision as an adaptable evolutionary trait. PLoS ONE 2013, 8, e64429. [Google Scholar] [CrossRef] [Green Version]
- Thurman, H.V.; Burton, E.A. Introductory Oceanography, 9th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Barnes, R.D. Invertebrate Zoology; Holt-Saunders International: Philadelphia, PA, USA, 1982; pp. 469–525. [Google Scholar]
- Langeneck, J.; Busoni, G.; Aliani, S.; Lardicci, C.; Castelli, A. Distribution and diversity of polychaetes along a bathyal escarpment in the western Mediterranean Sea. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2019, 144, 85–94. [Google Scholar] [CrossRef]
- Possatto, F.E.; Barletta, M.; Costa, M.F.; do Sul, J.A.I.; Dantas, D.V. Plastic debris ingestion by marine catfish: An unexpected fisheries impact. Mar. Pollut. Bull. 2011, 62, 1098–1102. [Google Scholar] [CrossRef]
- Ferreira, G.V.; Barletta, M.; Lima, A.R. Use of estuarine resources by top predator fishes. How do ecological patterns affect rates of contamination by microplastics? Sci. Total Environ. 2019, 655, 292–304. [Google Scholar] [CrossRef]
- Renzi, M.; Specchiulli, A.; Blašković, A.; Manzo, C.; Mancinelli, G.; Cilenti, L. Marine litter in stomach content of small pelagic fishes from the Adriatic Sea: Sardines (Sardina pilchardus) and anchovies (Engraulis encrasicolus). Environ. Sci. Pollut. Res. 2019, 26, 2771–2781. [Google Scholar] [CrossRef]
- Rikardsen, A.H.; Sandring, S. Diet and size-selective feeding by escaped hatchery rainbow trout Oncorhynchus mykiss (Walbaum). ICES J. Mar. Sci. 2005, 63, 460–465. [Google Scholar] [CrossRef]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- Dantas, D.V.; Barletta, M.; Da Costa, M.F. The seasonal and spatial patterns of ingestion of polyfilament nylon fragments by estuarine drums (Sciaenidae). Environ. Sci. Pollut. Res. 2012, 19, 600–606. [Google Scholar] [CrossRef]
- Davison, P.; Asch, R.G. Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. Mar. Ecol. Prog. Ser. 2011, 432, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.A.; Barletta, M.; Costa, M.F. Ingestion of nylon threads by Gerreidae while using a tropical estuary as foraging grounds. Aquat. Biol. 2012, 17, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.D.A.; de Carvalho-Souza, G.F. Are we eating plastic–ingesting fish. Mar. Pollut. Bull. 2016, 103, 109–114. [Google Scholar] [CrossRef]
- De Sá, L.C.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar] [CrossRef]
- Fernández–Ojeda, C.; Muniz, M.C.; Cardoso, R.P.; Dos Anjos, R.M.; Huaringa, E.; Nakazaki, C.; Henostroza, A.; Garcés–Ordóñez, O. Plastic debris and natural food in two commercially important fish species from the coast of Peru. Mar. Pollut. Bull. 2021, 173, 113039. [Google Scholar] [CrossRef]
- Savoca, M.S.; McInturf, A.G.; Hazen, E.L. Plastic ingestion by marine fish is widespread and increasing. Glob. Change Biol. 2021, 27, 2188–2199. [Google Scholar] [CrossRef]
- Collignon, A.; Hecq, J.-H.; Glagani, F.; Voisin, P.; Collard, F.; Goffart, A. Neustonic microplastic and zooplankton in the North Western Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Iannilli, V.; Pasquali, V.; Setini, A.; Corami, F. First evidence of microplastics ingestion in benthic amphipods from Svalbard. Environ. Res. 2019, 179, 108811. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef] [Green Version]
- Jones-Williams, K.; Galloway, T.; Cole, M.; Stowasser, G.; Waluda, C.; Manno, C. Close encounters-microplastic availability to pelagic amphipods in sub-antarctic and antarctic surface waters. Environ. Int. 2020, 140, 105792. [Google Scholar] [CrossRef]
- Piazzolla, D.; Cafaro, V.; Mancini, E.; Scanu, S.; Bonamano, S.; Marcelli, M. Preliminary investigation of microlitter pollution in low-energy hydrodynamic basins using Sabella spallanzanii (Polychaeta: Sabellidae) tubes. Bull. Environ. Contam. Toxicol. 2020, 104, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Meiburg, E.; Kneller, B. Turbidity currents and their deposits. Annu. Rev. Fluid Mech. 2010, 42, 135–156. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.E. Primary sedimentary structures formed by turbidity currents and related resedimentation mechanisms. In Primary Sedimentary Structures and Their Hydrodynamic Interpretation; SEPM Special Publication No. 12; Middleton, G.V., Ed.; SEPM Society for Sedimentary Geology: Broken Arrow, OK, USA, 1965; pp. 192–219. [Google Scholar]
- Chassignet, E.P.; Xu, X.; Zavala-Romero, O. Tracking marine litter with a global ocean model: Where does it go? Where does it come from? Front. Mar. Sci. 2021, 8, 414. [Google Scholar] [CrossRef]
- Deudero, S.; Alomar, C. Mediterranean marine biodiversity under threat: Reviewing influence of marine litter on species. Mar. Pollut. Bull. 2015, 98, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human health and ocean pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef]
- Bonamano, S.; Piazzolla, D.; Scanu, S.; Mancini, E.; Madonia, A.; Piermattei, V.; Marcelli, M. Modelling approach for the evaluation of burial and erosion processes on Posidonia oceanica meadows. Estuar. Coast. Shelf Sci. 2021, 254, 107321. [Google Scholar] [CrossRef]
- Piazzolla, D.; Scanu, S.; Frattarelli, F.M.; Mancini, E.; Tiralongo, F.; Brundo, M.V.; Tibullo, D.; Pecoraro, R.; Copat, C.; Ferrante, M.; et al. Trace-metal enrichment and pollution in coastal sediments in the Northern Tyrrhenian Sea, Italy. Arch. Environ. Contam. Toxicol. 2015, 69, 470–481. [Google Scholar] [CrossRef]
- Scanu, S.; Piazzolla, D.; Frattarelli, F.M.; Mancini, E.; Tiralongo, F.; Brundo, M.V.; Tibullo, D.; Pecoraro, R.; Copat, C.; Ferrante, M.; et al. Mercury enrichment in sediments of the coastal area of northern Latium, Italy. Bull. Environ. Contam. Toxicol. 2016, 96, 630–637. [Google Scholar] [CrossRef]
- Piazzolla, D.; Cafaro, V.; de Lucia, G.A.; Mancini, E.; Scanu, S.; Bonamano, S.; Piermattei, V.; Vianello, V.; Della Ventura, G.; Marcelli, M. Microlitter pollution in coastal sediments of the northern Tyrrhenian Sea, Italy: Microplastics and fly-ash occurrence and distribution. Estuar. Coast. Shelf Sci. 2020, 241, 106819. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Agriculture; Food and Agriculture Office of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Kuczenski, B.; Vargas Poulsen, C.; Gilman, E.L.; Musyl, M.; Geyer, R.; Wilson, J. Plastic gear loss estimates from remote observation of industrial fishing activity. Fish Fish. 2022, 23, 22–33. [Google Scholar] [CrossRef]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2017, 9, 1346–1360. [Google Scholar] [CrossRef] [Green Version]
- Galgani, F.; Fleet, D.; van Franeker, J.A.; Katsanevakis, S.; Maes, T.; Mouat, J.; Oosterbaan, L.; Poitou, I.; Hanke, G.; Thompson, R.; et al. Marine Strategy Framework Directive-Task Group 10 Report Marine Litter Do Not Cause Harm to the Coastal and Marine Environment; Report on the Identification of Descriptors for the Good Environmental Status of European Seas Regarding Marine Litter under the Marine Strategy Framework Directive; Office for Official Publications of the European Communities: Luxembourg, 2010. [Google Scholar] [CrossRef]
- ISPRA. Proposte per la Definizione del Buono Stato Ambientale e dei Traguardi Ambientali; Descrittore 10: Rifiuti Marini; ISPRA: Rome, Italy, 2013. [Google Scholar]
- MSFD GES Technical Subgroup on Marine Litter. Marine Litter Technical Recommendations 493 for the Implementation of MSFD Requirements; EUR 25009 EN—2011; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]

| Categories | C. caelorhincus | G. melastomus | |
|---|---|---|---|
| Shape | Filament | 56 | 42 |
| Fragment | 5 | 11 | |
| Lamina | 0 | 5 | |
| Color | Red | 3 | 4 |
| Trasparent | 5 | 7 | |
| Blue | 46 | 35 | |
| Black | 2 | 2 | |
| White | 5 | 0 | |
| Light Blue | 0 | 7 | |
| Green | 0 | 1 | |
| Beige | 0 | 2 | |
| Dimension | Smaller microplastics (0.33–1.00 mm) | 18 | 21 |
| Medium microplastics (1.01–4.75 mm) | 39 | 22 | |
| Mesoplastics (4.76–200 mm) | 4 | 15 | |
| Color | Red | Transparent | Blue | Black | Light Blue | White | Green | Beige | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | GM | CC | GM | CC | GM | CC | GM | CC | GM | CC | GM | CC | GM | CC | GM | CC |
| Shape and Dimension | ||||||||||||||||
| Small Filament | 0; 0 | 0; 0 | 2; 0.01 | 0; 0 | 9; 0.045 | 16; 0.21 | 0; 0 | 1; 0.013 | 4; 0.02 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 1; 0.005 | 0; 0 |
| Medium Filament | 1; 0.005 | 3; 0.04 | 1; 0.005 | 5; 0.066 | 12; 0.06 | 30; 0.35 | 1; 0.005 | 1; 0.013 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 |
| Large Filament | 0; 0 | 0; 0 | 0;0 | 0; 0 | 11; 0.055 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 |
| Small Fragment | 2; 0.01 | 0; 0 | 0;0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 1; 0.013 | 1; 0.005 | 0; 0 | 1; 0.005 | 0; 0 |
| Medium Fragment | 0; 0 | 0; 0 | 2; 0.01 | 0; 0 | 2; 0.01 | 0; 0 | 0; 0 | 0; 0 | 3; 0.015 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 |
| Large Fragment | 0; 0 | 0; 0 | 0;0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 4; 0.053 | 0; 0 | 0; 0 | 0; 0 | 0; 0 |
| Small Lamina | 1; 0.005 | 0; 0 | 0;0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 |
| Medium Lamina | 0; 0 | 0; 0 | 0;0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 |
| Large Lamina | 0; 0 | 0; 0 | 2; 0.01 | 0; 0 | 1; 0.005 | 0; 0 | 1; 0.005 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 |
| Fish | Crustaceans | Polychaetes | Cephalopods | Microplastics | |
|---|---|---|---|---|---|
| Fish | 1.00 | n.m. | n.m. | −1.00 | n.m. |
| Crustaceans | n.m. | 1.00 | 0.20 | 0.04 | −0.09 |
| Polychaetes | n.m. | 0.20 | 1.00 | −0.51 | −0.31 |
| Cephalopods | −1.00 | 0.04 | −0.51 | 1.00 | 0.11 |
| Microplastics | 1.00 | −0.09 | –0.31 | 0.11 | 1.00 |
| Microplastics | Polychaetes | Onuphidae | Eunicidae | Lumbrinereidae | |
|---|---|---|---|---|---|
| Microplastics | 1.00 | −0.31 | −0.69 | −1.00 | n.m. |
| Polychaetes | −0.31 | 1.00 | 0.86 | −0.22 | 1.00 |
| Onuphidae | −0.69 | 0.86 | 1.00 | n.m | n.m. |
| Eunicidae | −1.00 | −0.22 | n.m. | 1.00 | n.m |
| Lumbrinereidae | n.m. | 1.00 | n.m. | n.m. | 1.00 |
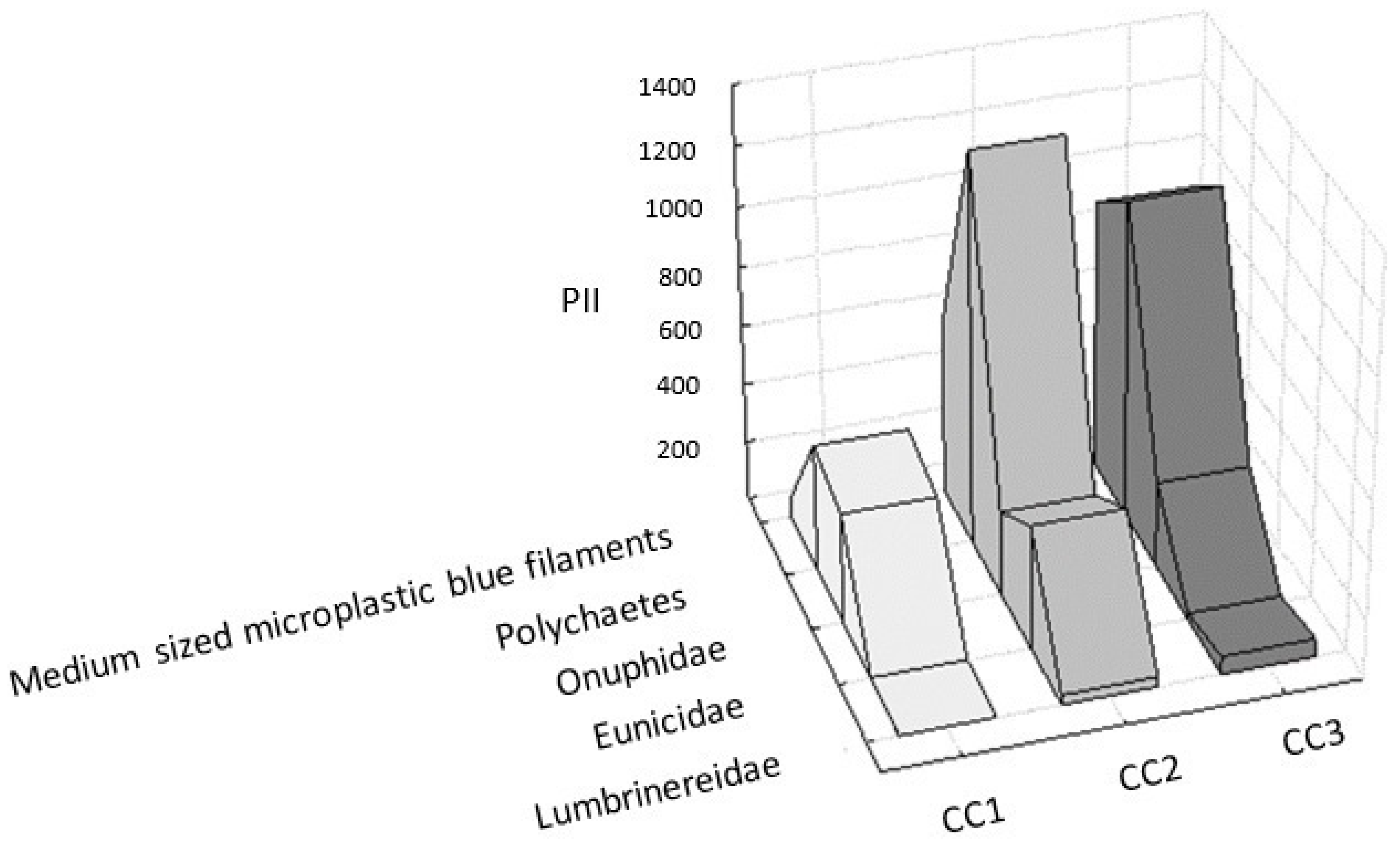
| Mean Rank | Sum of Ranks | Mean PII | s.d (±) | |
|---|---|---|---|---|
| Medium–sized microplastic blue filaments | 3.66 | 11.00 | 523.45 | 422.32 |
| Polichaetes | 5.00 | 15.00 | 940.40 | 459.03 |
| Onuphidae | 3.00 | 9.00 | 317.24 | 51.23 |
| Eunicidae | 2.00 | 6.00 | 147.97 | 236.42 |
| Lumbrineridae | 1.33 | 4.00 | 32.09 | 31.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scacco, U.; Mancini, E.; Marcucci, F.; Tiralongo, F. Microplastics in the Deep: Comparing Dietary and Plastic Ingestion Data between Two Mediterranean Bathyal Opportunistic Feeder Species, Galeus melastomus, Rafinesque, 1810 and Coelorinchus caelorhincus (Risso, 1810), through Stomach Content Analysis. J. Mar. Sci. Eng. 2022, 10, 624. https://doi.org/10.3390/jmse10050624
Scacco U, Mancini E, Marcucci F, Tiralongo F. Microplastics in the Deep: Comparing Dietary and Plastic Ingestion Data between Two Mediterranean Bathyal Opportunistic Feeder Species, Galeus melastomus, Rafinesque, 1810 and Coelorinchus caelorhincus (Risso, 1810), through Stomach Content Analysis. Journal of Marine Science and Engineering. 2022; 10(5):624. https://doi.org/10.3390/jmse10050624
Chicago/Turabian StyleScacco, Umberto, Emanuele Mancini, Federica Marcucci, and Francesco Tiralongo. 2022. "Microplastics in the Deep: Comparing Dietary and Plastic Ingestion Data between Two Mediterranean Bathyal Opportunistic Feeder Species, Galeus melastomus, Rafinesque, 1810 and Coelorinchus caelorhincus (Risso, 1810), through Stomach Content Analysis" Journal of Marine Science and Engineering 10, no. 5: 624. https://doi.org/10.3390/jmse10050624
APA StyleScacco, U., Mancini, E., Marcucci, F., & Tiralongo, F. (2022). Microplastics in the Deep: Comparing Dietary and Plastic Ingestion Data between Two Mediterranean Bathyal Opportunistic Feeder Species, Galeus melastomus, Rafinesque, 1810 and Coelorinchus caelorhincus (Risso, 1810), through Stomach Content Analysis. Journal of Marine Science and Engineering, 10(5), 624. https://doi.org/10.3390/jmse10050624









