Environmental Impact on Harmful Species Pseudo-nitzschia spp. and Phaeocystis globosa Phenology and Niche
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Community Diversity and Structure
2.3. Spatial–Temporal Variation
2.4. Niche Analysis: The Relationship between the Community and Environmental Condition
2.5. Bloom-Detecting Algorithm
- The high points were above the value of 4, which correspond to the log10 of 10,000 cells·L. The threshold of 10,000 cells·L was used here in the study because, under this abundance threshold, the blooms of Pha and of Pse would not cause any HAB.
- The low points before and after the high points were inferior to 85% of the high point value. In this case, some humps can be merged as blooms can sometimes be bimodal.
- The merging of two humps would occur when the value of one of the lowest points do not fit the second condition. The merging of two humps cannot occur if the merging causes the increasing or decreasing phase of the bloom to be greater than 300 days.
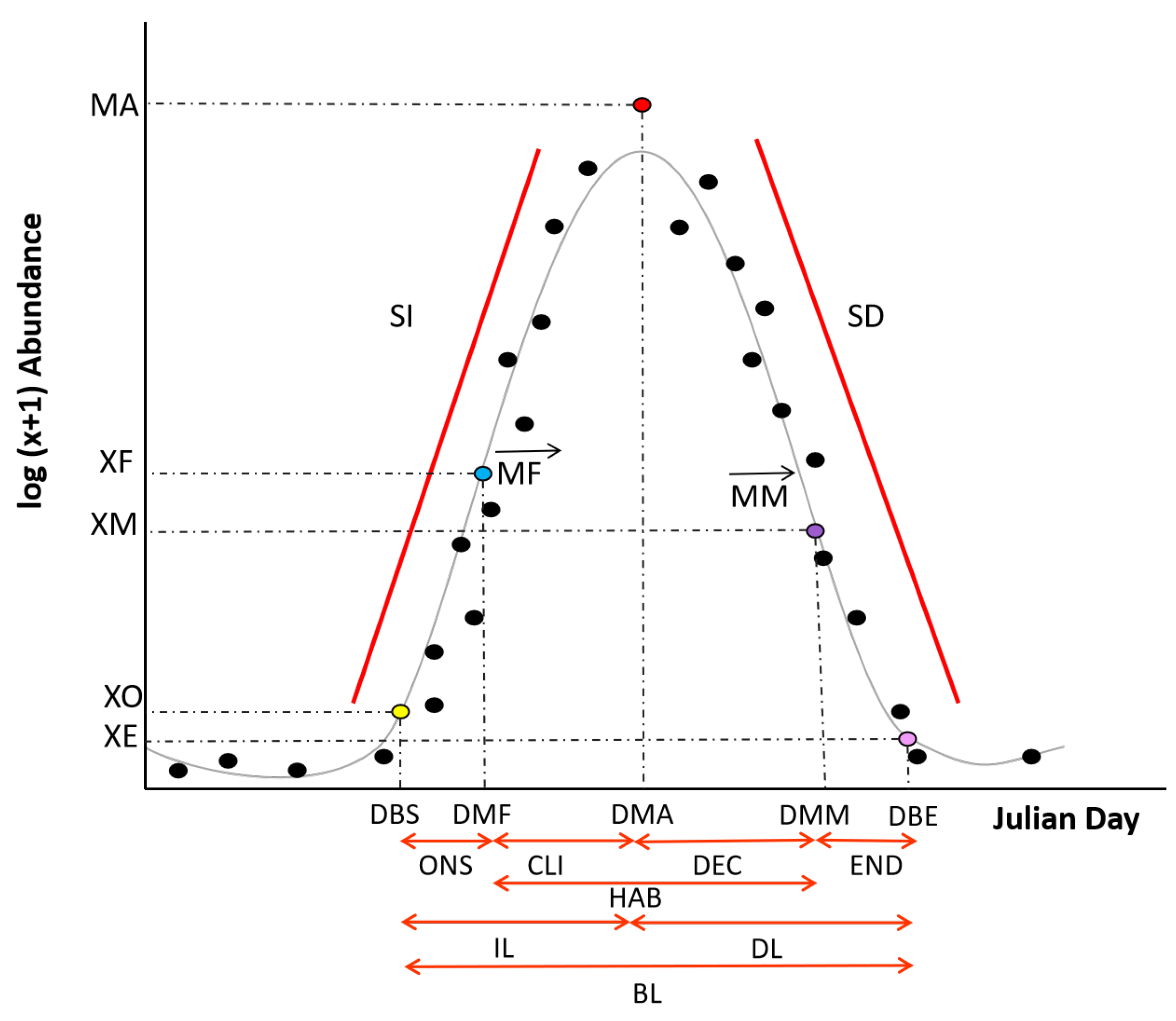
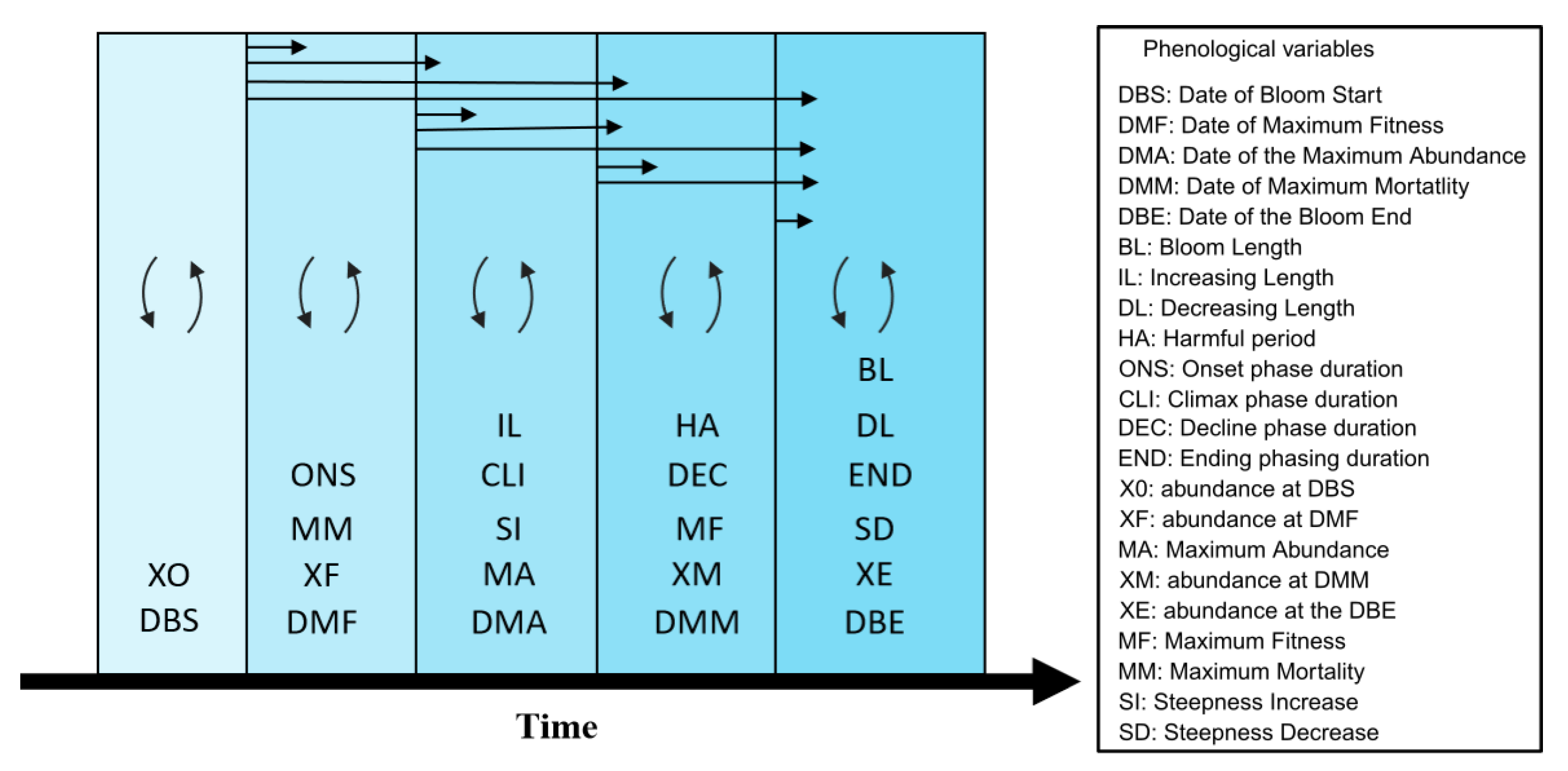
2.6. Phenological Variables
2.7. Temporal Continuum
2.8. Phenological Analyses
- can be estimated for all (continuous) random variables X and Y, regardless of their parametric distribution.
- (normalization).
- if and only if X and Y are independent (independence).
- if and only if Y is a function of X, so we have and in this case there is a complete dependence or full predictability of Y by X.
- We do not necessarily have (asymmetry).
- Scale changes do not affect (scale-invariance).
2.9. Subniche Calculation: Relating the Community and Environmental Condition to Phenology
3. Results
3.1. Spatial–Temporal Variation
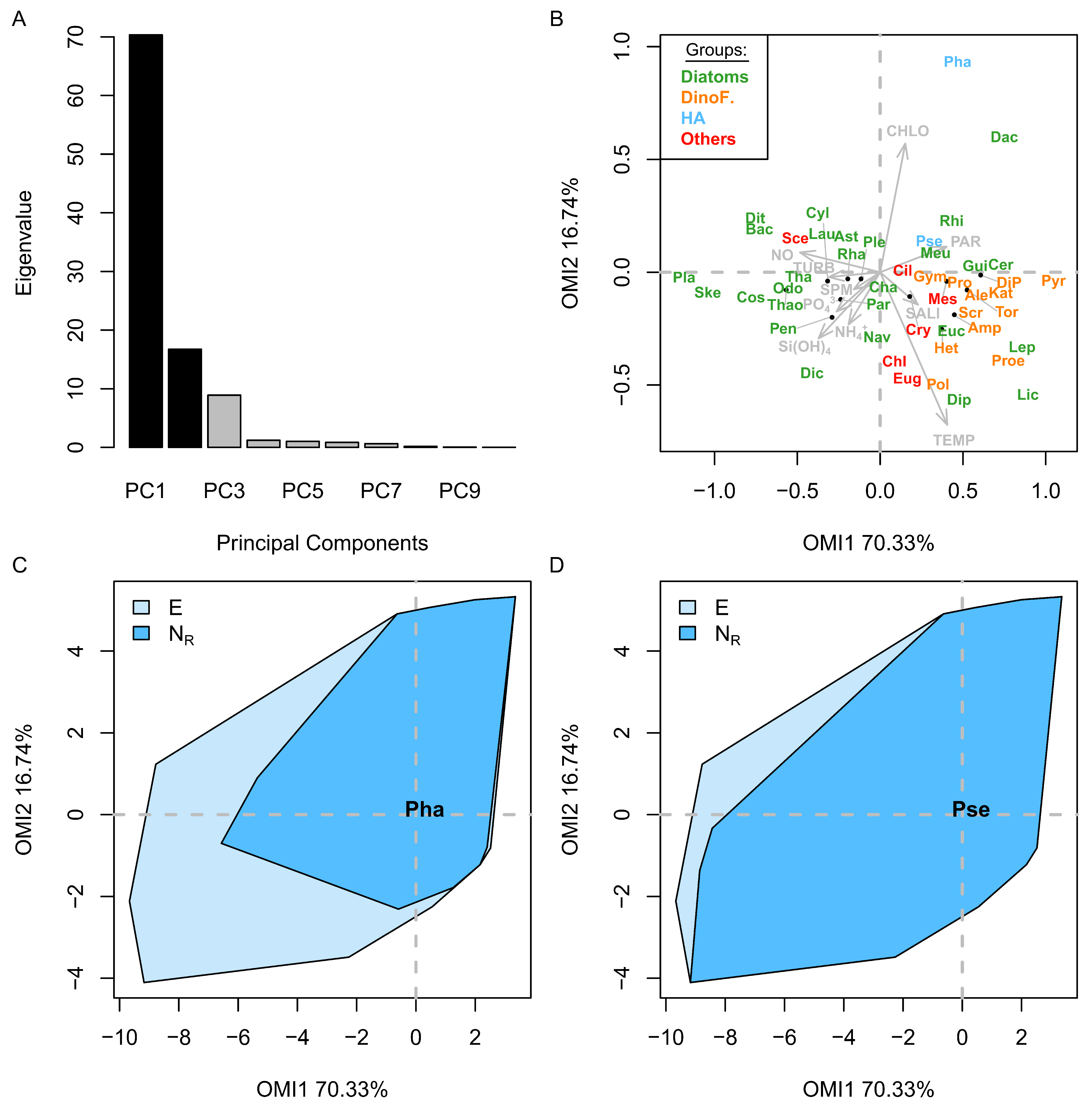
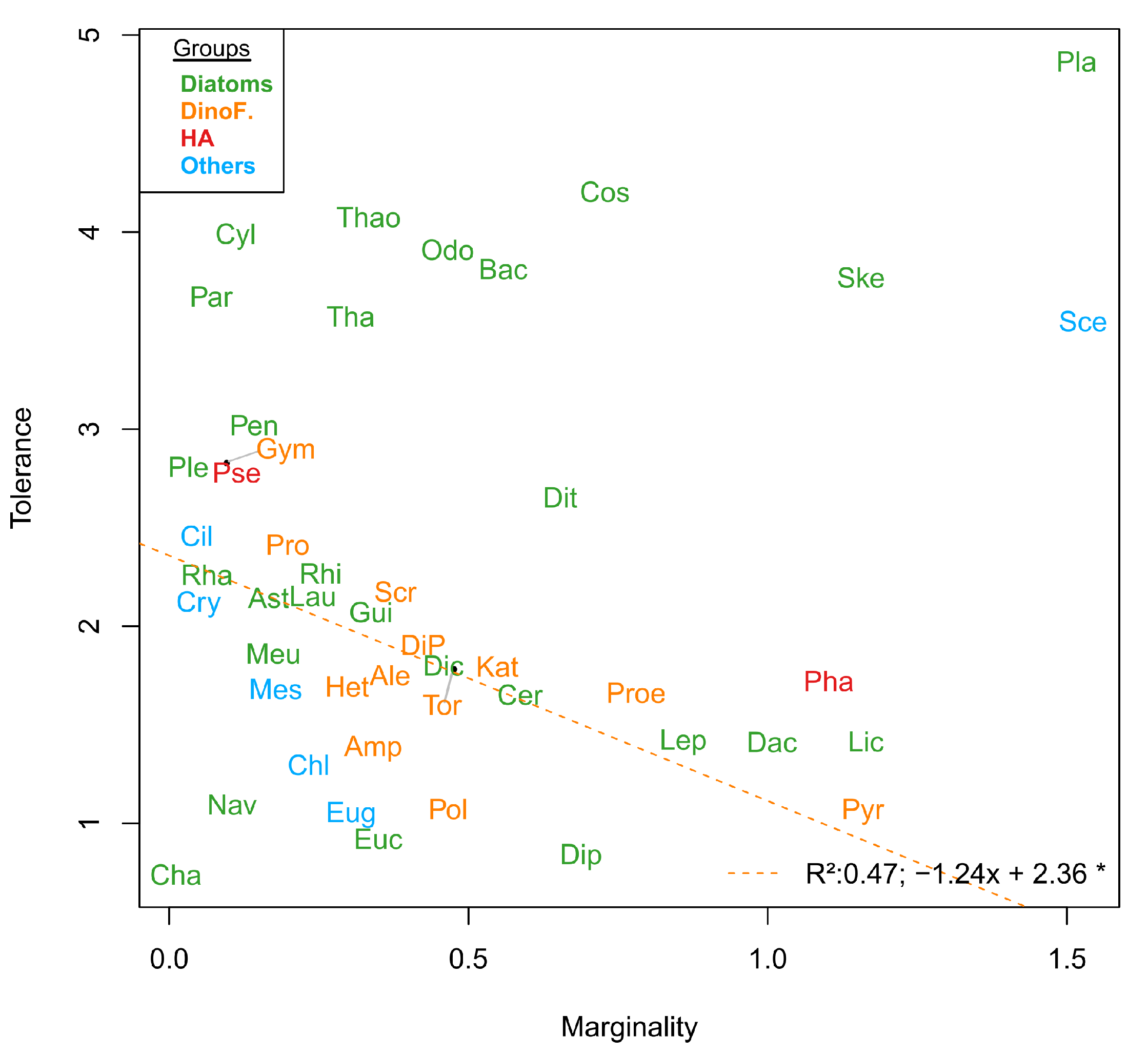
3.2. Niche
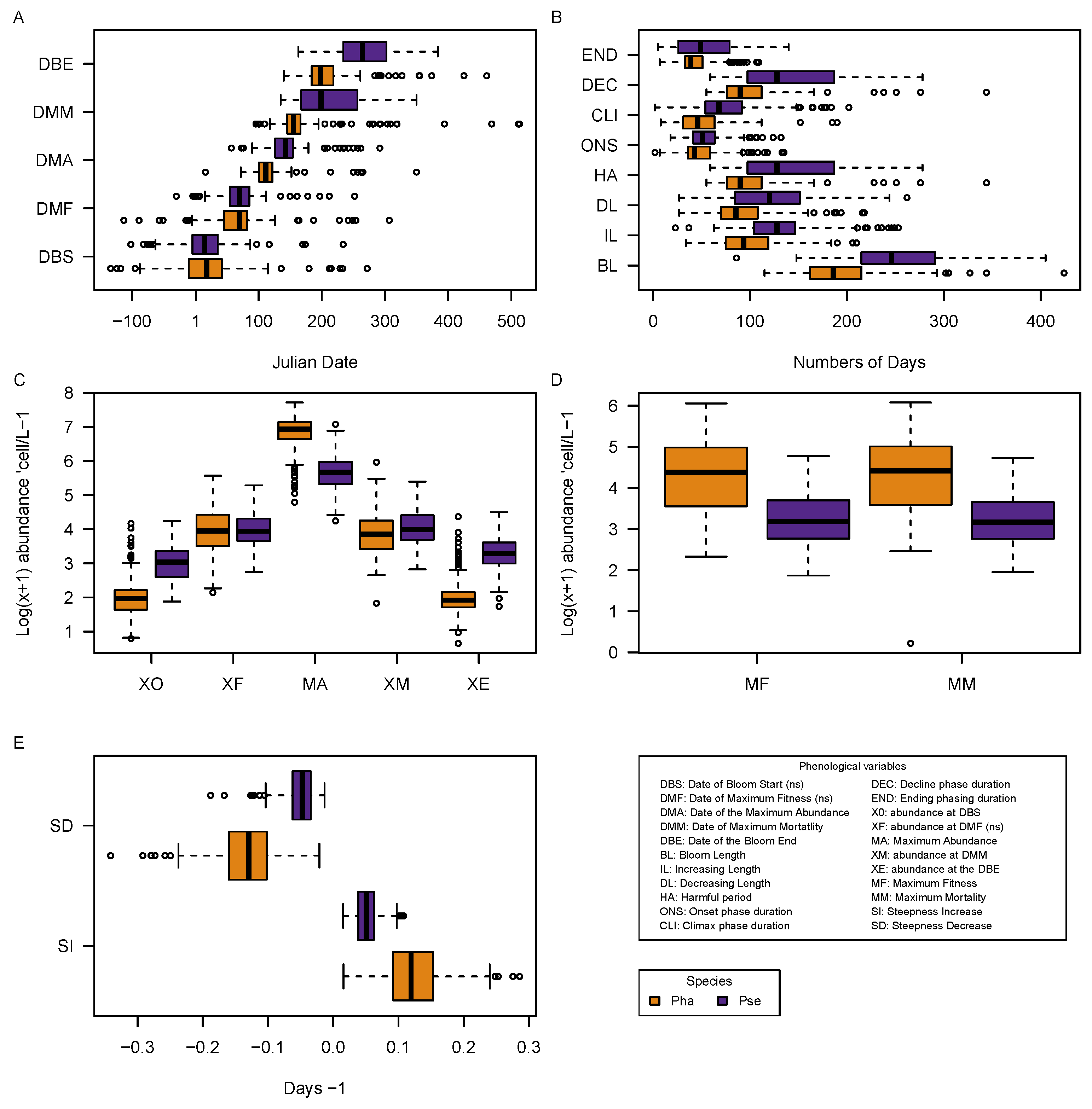
3.3. Phenological Data
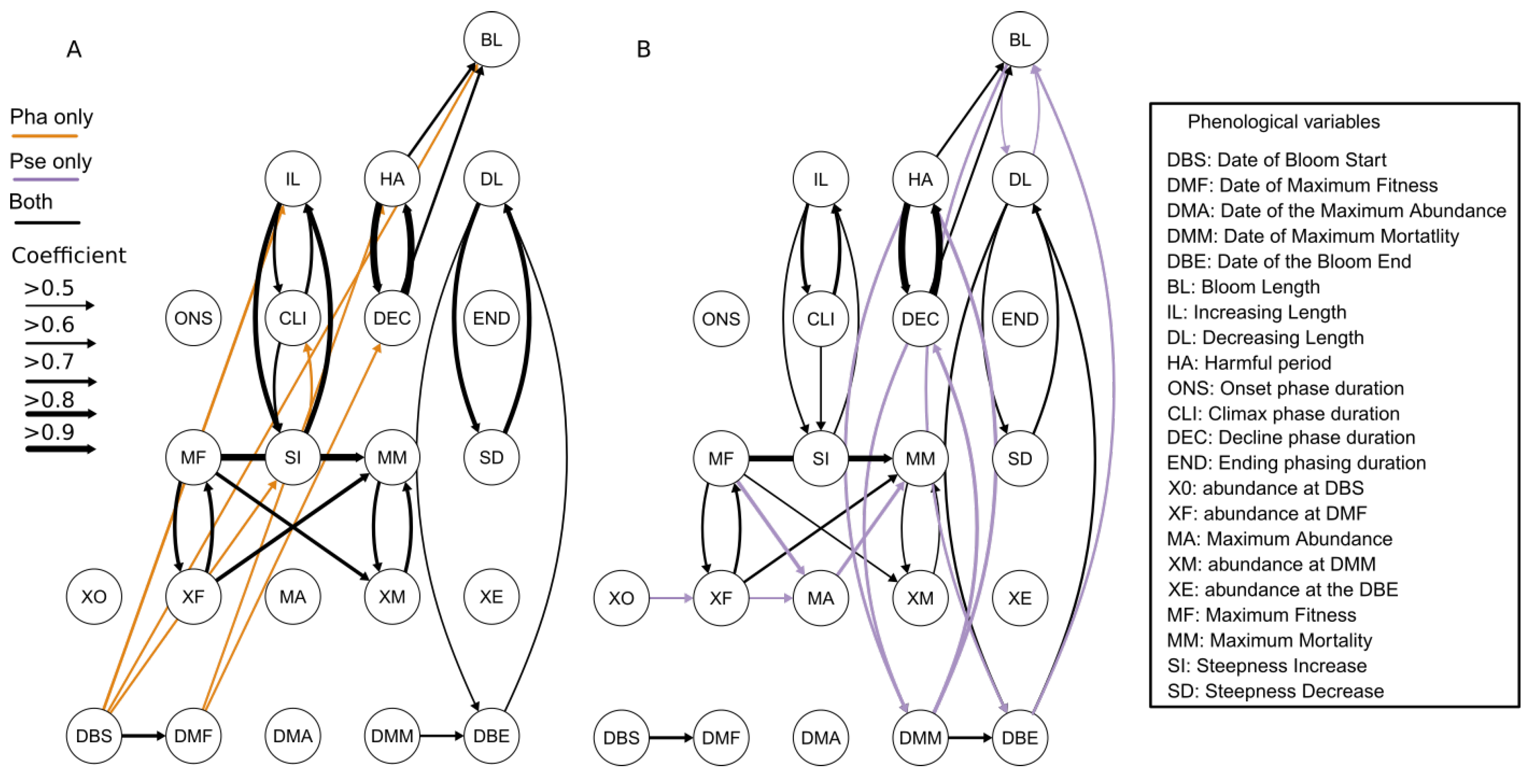
3.4. Relationships between Phenological Variables
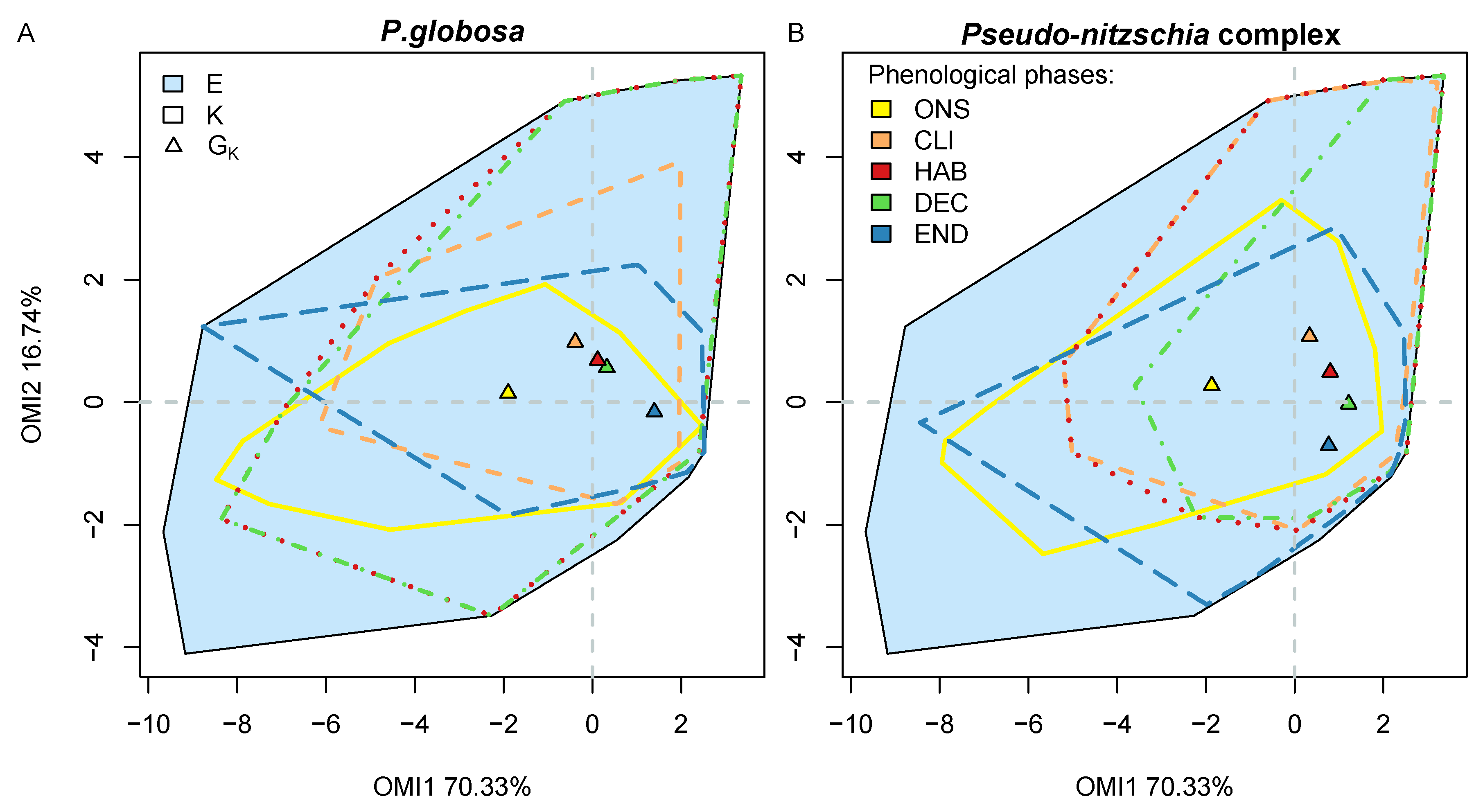
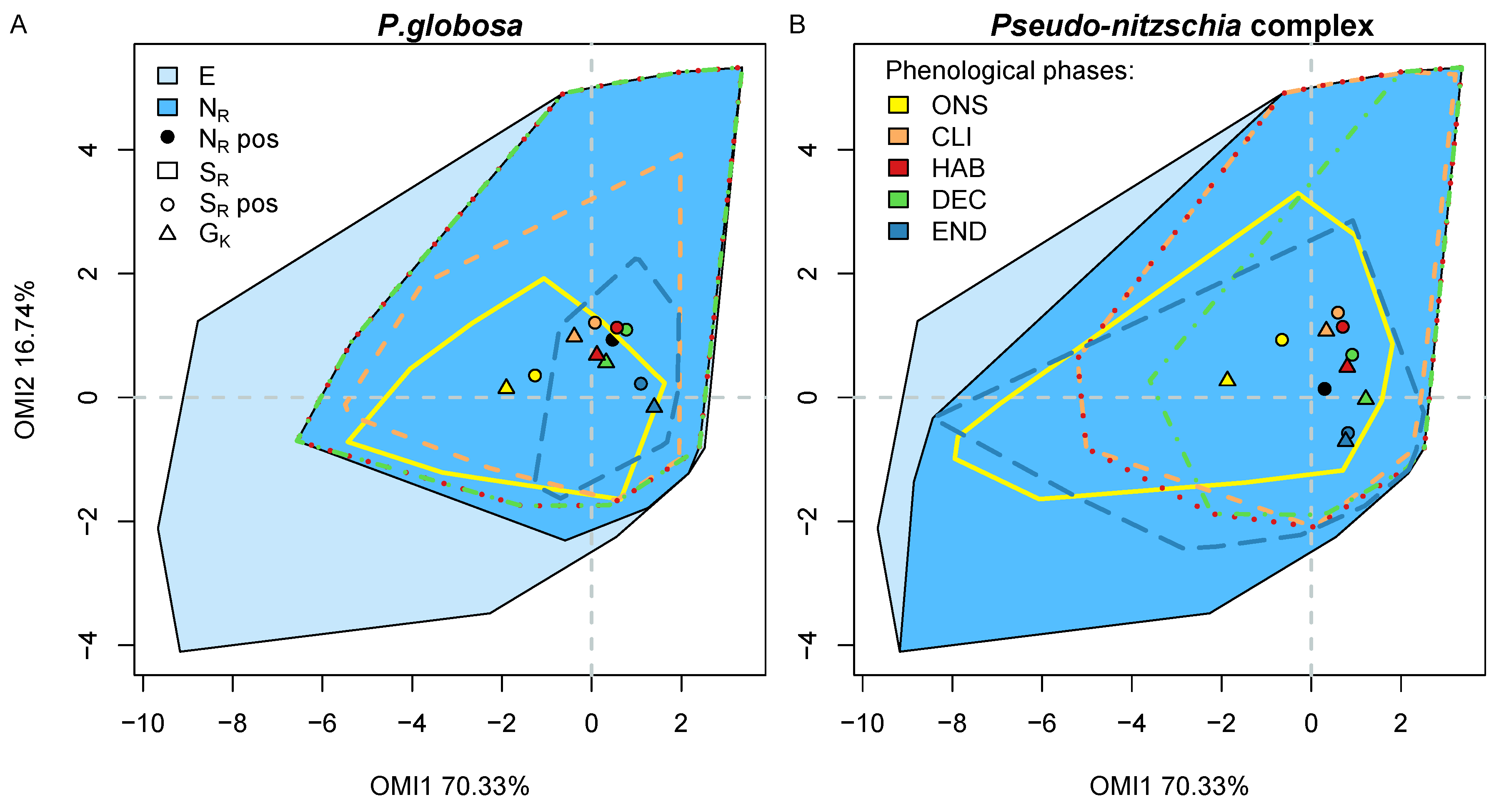
3.5. Phenological Subsets
3.6. Phenological Subniche
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Defriez, E.J.; Sheppard, L.W.; Reid, P.C.; Reuman, D.C. Climate change-related regime shifts have altered spatial synchrony of plankton dynamics in the North Sea. Glob. Chang. Biol. 2016, 22, 2069–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuatters-Gollop, A.; Edwards, M.; Helaouët, P.; Johns, D.G.; Owens, N.J.; Raitsos, D.E.; Schroeder, D.; Skinner, J.; Stern, R.F. The Continuous Plankton Recorder survey: How can long-term phytoplankton datasets contribute to the assessment of Good Environmental Status? Estuar. Coast. Shelf Sci. 2015, 162, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Wooster, W.S.; Zhang, C.I. Regime shifts in the North Pacific: Early indications of the 1976–1977 event. Prog. Oceanogr. 2004, 60, 183–200. [Google Scholar] [CrossRef]
- Drinkwater, K.F. The regime shift of the 1920s and 1930s in the North Atlantic. Prog. Oceanogr. 2006, 68, 134–151. [Google Scholar] [CrossRef]
- Dippner, J.W.; Möller, C.; Hänninen, J. Regime shifts in North Sea and Baltic Sea: A comparison. J. Mar. Syst. 2012, 105–108, 115–122. [Google Scholar] [CrossRef]
- Scharfe, M.; Wiltshire, K.H. Modeling of intra-annual abundance distributions: Constancy and variation in the phenology of marine phytoplankton species over five decades at Helgoland Roads (North Sea). Ecol. Model. 2019, 404, 46–60. [Google Scholar] [CrossRef]
- Atkinson, A.; Harmer, R.A.; Widdicombe, C.E.; McEvoy, A.J.; Smyth, T.J.; Cummings, D.G.; Somerfield, P.J.; Maud, J.L.; McConville, K. Questioning the role of phenology shifts and trophic mismatching in a planktonic food web. Prog. Oceanogr. 2015, 137, 498–512. [Google Scholar] [CrossRef]
- Cushing, D. Plankton Production and Year-class Strength in Fish Populations: An Update of the Match/Mismatch Hypothesis. In Advances in Marine Biology; Blaxter, J., Southward, A., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 26, pp. 249–293. [Google Scholar] [CrossRef]
- Fu, F.X.; Tatters, A.O.; Hutchins, D.A. Global change and the future of harmful algal blooms in the ocean. Mar. Ecol. Prog. Ser. 2012, 470, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.; Glibert, P.; Burkholder, J. Harmful algal blooms and eutrophication: Nutrient sources, compositions, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.M. Turning back the harmful red tide. Nature 1997, 388, 513–514. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef] [Green Version]
- Lancelot, C.; Wassmann, P.; Barth, H. Ecology of Phaeocystis-dominated ecosystems. J. Mar. Syst. 1994, 5, 1–4. [Google Scholar] [CrossRef]
- Cadée, G.C.; Hegeman, J. Phytoplankton in the Marsdiep at the end of the 20th century; 30 years monitoring biomass, primary production, and Phaeocystis blooms. J. Sea Res. 2002, 48, 97–110. [Google Scholar] [CrossRef]
- Blauw, A.N.; Los, F.J.; Huisman, J.; Peperzak, L. Nuisance foam events and Phaeocystis globosa blooms in Dutch coastal waters analyzed with fuzzy logic. J. Mar. Syst. 2010, 83, 115–126. [Google Scholar] [CrossRef]
- Lancelot, C. The mucilage phenomenon in the continental coastal waters of the North Sea. Sci. Total Environ. 1995, 165, 83–102. [Google Scholar] [CrossRef]
- Verity, P.G.; Brussaard, C.P.; Nejstgaard, J.C.; van Leeuwe, M.A.; Lancelot, C.; Medlin, L.K. Current understanding of Phaeocystis ecology and biogeochemistry, and perspectives for future research. Biogeochemistry 2007, 83, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Gieskes, W.; Leterme, S.; Peletier, H.; Edwards, M.; Reid, P. Phaeocystis colony distribution in the North Atlantic Ocean since 1948, and interpretation of long-term changes in the Phaeocystis hotspot in the North Sea. Biogeochemistry 2007, 83, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, V.; Mathot, S.; Lancelot, C. Calculating carbon biomass of Phaeocystis sp. from microscopic observations. Mar. Biol. 1990, 107, 305–314. [Google Scholar] [CrossRef]
- Peperzak, L.; Poelman, M. Mass mussel mortality in The Netherlands after a bloom of Phaeocystis globosa (prymnesiophyceae). J. Sea Res. 2008, 60, 220–222. [Google Scholar] [CrossRef]
- Grattepanche, J.D.; Vincent, D.; Breton, E.; Christaki, U. Microzooplankton herbivory during the diatom–Phaeocystis spring succession in the eastern English Channel. J. Exp. Mar. Biol. Ecol. 2011, 404, 87–97. [Google Scholar] [CrossRef]
- Hernández-Fariñas, T.; Soudant, D.; Barillé, L.; Belin, C.; Lefebvre, A.; Bacher, C. Temporal changes in the phytoplankton community along the French coast of the eastern English Channel and the southern Bight of the North Sea. ICES J. Mar. Sci. 2014, 71, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Sazhin, A.F.; Artigas, L.F.; Nejstgaard, J.C.; Frischer, M.E. The colonization of two Phaeocystis species (Prymnesiophyceae) by pennate diatoms and other protists: A significant contribution to colony biomass. In Phaeocystis, Major Link in the Biogeochemical Cycling of Climate-Relevant Elements; Springer: Dordrecht, The Netherlands, 2007; pp. 137–145. [Google Scholar] [CrossRef]
- Rousseau, V.; Lantoine, F.; Rodriguez, F.; LeGall, F.; Chrétiennot-Dinet, M.J.; Lancelot, C. Characterization of Phaeocystis globosa (Prymnesiophyceae), the blooming species in the Southern North Sea. J. Sea Res. 2013, 76, 105–113. [Google Scholar] [CrossRef]
- Delegrange, A.; Lefebvre, A.; Gohin, F.; Courcot, L.; Vincent, D. Pseudo-nitzschia sp. diversity and seasonality in the southern North Sea, domoic acid levels and associated phytoplankton communities. Estuar. Coast. Shelf Sci. 2018, 214, 194–206. [Google Scholar] [CrossRef] [Green Version]
- Barton, A.D.; Pershing, A.J.; Litchman, E.; Record, N.R.; Edwards, K.F.; Finkel, Z.V.; Kiørboe, T.; Ward, B.A. The biogeography of marine plankton traits. Ecol. Lett. 2013, 16, 522–534. [Google Scholar] [CrossRef]
- Beaugrand, G. Theoretical basis for predicting climate-induced abrupt shifts in the oceans. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130264. [Google Scholar] [CrossRef] [Green Version]
- Alves-de Souza, C.; Iriarte, J.L.; Mardones, J.I. Interannual Variability of Dinophysis acuminata and Protoceratium reticulatum in a Chilean Fjord: Insights from the Realized Niche Analysis. Toxins 2019, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasiewicz, S.; Breton, E.; Lefebvre, A.; Hernández-Fariñas, T.; Lefebvre, S. Realized niche analysis of phytoplankton communities involving HAB: Phaeocystis spp. as a case study. Harmful Algae 2018, 72, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun, P.; Vogt, M.; Payne, M.R.; Gruber, N.; O’Brien, C.J.; Buitenhuis, E.T.; Le Quéré, C.; Leblanc, K.; Luo, Y.W. Ecological niches of open ocean phytoplankton taxa. Limnol. Oceanogr. 2015, 60, 1020–1038. [Google Scholar] [CrossRef] [Green Version]
- Houliez, E.; Lefebvre, S.; Dessier, A.; Huret, M.; Marquis, E.; Bréret, M.; Dupuy, C. Spatio-temporal drivers of microphytoplankton community in the Bay of Biscay: Do species ecological niches matter? Prog. Oceanogr. 2021, 194, 102558. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Visser, A.W.; MacKenzie, B.R.; Payne, M.R. Accuracy and precision in the calculation of phenology metrics. J. Geophys. Res. Ocean. 2014, 119, 8438–8453. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Edwards, M.; Richardson, A.J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 2004, 430, 881–884. [Google Scholar] [CrossRef]
- Greve, W.; Prinage, S.; Zidowitz, H.; Nast, J.; Reiners, F. On the phenology of North Sea ichthyoplankton. ICES J. Mar. Sci. 2005, 62, 1216–1223. [Google Scholar] [CrossRef]
- Ji, R.; Edwards, M.; Mackas, D.L.; Runge, J.A.; Thomas, A.C. Marine plankton phenology and life history in a changing climate: Current research and future directions. J. Plankton Res. 2010, 32, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Guallar, C.; Bacher, C.; Chapelle, A. Global and local factors driving the phenology of Alexandrium minutum (Halim) blooms and its toxicity. Harmful Algae 2017, 67, 44–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolinski, S.; Horn, H.; Petzoldt, T.; Lothar, P. Identifying cardinal dates in phytoplankton time series to enable the analysis of long-term trends. Oecologia 2007, 153, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fariñas, T.; Bacher, C.; Soudant, D.; Belin, C.; Barillé, L. Assessing phytoplankton realized niches using a French national phytoplankton monitoring network. Estuar. Coast. Shelf Sci. 2015, 159, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Husson, B.; Hernández-Fariñas, T.; Le Gendre, R.; Schapira, M.; Chapelle, A. Two decades of Pseudo-nitzschia spp. blooms and king scallop (Pecten maximus) contamination by domoic acid along the French Atlantic and English Channel coasts: Seasonal dynamics, spatial heterogeneity and interannual variability. Harmful Algae 2016, 51, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Dezecache, C.; Lefebvre, A. Toward Tracking the Dispersion and Advection of Harmful Algal Blooms (HAB) Based on Satellite Data; Technical Report; IFREMER/ODE/LITTORAL/LER.BL/19.10; IFREMER: Paris, France, 2019. [Google Scholar]
- REPHY Dataset French Observation and Monitoring Program for Phytoplankton and Hydrology in Coastal Waters. Metropolitan Data 2021. Available online: https://www.seanoe.org/data/00361/47248/ (accessed on 1 January 2021).
- SRN Dataset Regional Observation and Monitoring Program for Phytoplankton and Hydrology in the Eastern English Channel. 2021. Available online: https://www.seanoe.org/data/00397/50832/ (accessed on 1 January 2021). [CrossRef]
- Le Borgne, P.; Legendre, G.; Marsouin, A. Validation of the OSI SAF radiative fluxes. In Proceedings of the 2006 EUMETSAT Conference, Helsinki, Finland, 12–16 June 2006. [Google Scholar]
- Le Borgne, P.; Legendre, G.; Marsouin, A. Operational SST retrieval from Msg/Seviri data. In Proceedings of the 2006 EUMETSAT Conference, Helsinki, Finland, 12–16 June 2006. [Google Scholar]
- Karasiewicz, S.; Chapelle, A.; Bacher, C.; Soudant, D. Harmful algae niche responses to environmental and community variation along the French coast. Harmful Algae 2020, 93, 101785. [Google Scholar] [CrossRef] [Green Version]
- Aminot, A.; Kérouel, R. Hydrologie des écosystèmes marins. In Paramètres et Analyses; Ifremer: Paris, France, 2004. [Google Scholar]
- Aminot, A.; Kérouel, R. Dosage automatique des nutriments dans les eaus marines. In Méthodes en Flux Continu; Ifremer: Paris, France, 2007. [Google Scholar]
- Grossel, H. Manuel d’observation et de déombrement du phytoplancton marin. In Document de Méthode REPHY; Ifremer: Nantes, France, 2006. [Google Scholar]
- Lefebvre, A.; Guiselin, N.; Barbet, F.; Artigas, F.L. Long-term hydrological and phytoplankton monitoring (1992–2007) of three potentially eutrophic systems in the eastern English Channel and the Southern Bight of the North Sea. ICES J. Mar. Sci. 2011, 68, 2029–2043. [Google Scholar] [CrossRef] [Green Version]
- Belin, C.; Neaud-Masson, N. Cahier de Procédures REPHY 2012–2013; Technical Report; Ifremer: Paris, France, 2012. [Google Scholar]
- Anneville, O.; Ginot, V.; Druart, J.C.; Angeli, N. Long-term study (1974–1998) of seasonal changes in the phytoplankton in Lake Geneva: A multi-table approach. J. Plankton Res. 2002, 24, 993–1007. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Pielou, E.C. The Measurement of Diversity in Different Types of Biological Collection. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5-7; CRAN: Online, 2020; Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 January 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.; Massey University: Auckland, New Zealand, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; Gimaret-Carpentier, C. Niche separation in community analysis: A new method. Ecology 2000, 81, 2914. [Google Scholar] [CrossRef]
- Reinsch, C.H. Smoothing by spline functions. Numer. Math. 1967, 10, 177–183. [Google Scholar] [CrossRef]
- Silverman, B. A Fast and Efficient Cross-Validation Method for Smoothing Parameter Choice in Spline Regression. J. Am. Stat. Assoc. 1984, 79, 584–589. [Google Scholar] [CrossRef]
- Simonoff, J.S. Smoothing Methods in Statistics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Christopoulos, D.T. On the efficient identification of an inflection point. Int. J. Math. Sci. Comput. 2016, 6, 13–20. [Google Scholar]
- Christopoulos, D.T. Developing methods for identifying the inflection point of a convex/concave curve. arXiv 2014, arXiv:1206.5478. [Google Scholar]
- Grüner, N.; Gebühr, C.; Boersma, M.; Feudel, U.; Wiltshire, K.H.; Freund, J.A. Reconstructing the realized niche of phytoplankton species from environmental data: Fitness versus abundance approach. Limnol. Oceanogr. Methods 2011, 9, 432–442. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 751. [Google Scholar]
- Trutschnig, W. On a strong metric on the space of copulas and its induced dependence measure. J. Math. Anal. Appl. 2011, 384, 690–705. [Google Scholar] [CrossRef] [Green Version]
- Junker, R.R.; Griessenberger, F.; Trutschnig, W. Estimating scale-invariant directed dependence of bivariate distributions. Comput. Stat. Data Anal. 2021, 153, 107058. [Google Scholar] [CrossRef]
- Karasiewicz, S.; Dolédec, S.; Lefebvre, S. Within outlying mean indexes: Refining the OMI analysis for the realized niche decomposition. PeerJ 2017, 5, e3364. [Google Scholar] [CrossRef] [Green Version]
- Cushing, D.H. The seasonal variation in oceanic production as a problem in population dynamics. ICES J. Mar. Sci. 1959, 24, 455–464. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol 1986, 106, 433–471. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Roelke, D.L.; Spatharis, S. Phytoplankton succession in recurrently fluctuating environments. PLoS ONE 2015, 10, e0121392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Lancelot, C.; Billen, G.; Sournia, A.; Weisse, T.; Colijn, F.; Veldhuis, M.J.W.; Davies, A.; Wassman, P. Phaeocystis blooms and nutrient enrichment in the continental coastal zones of the North Sea. Ambio 1987, 16, 38–46. [Google Scholar]
- Reid, P.C.; Lancelot, C.; Gieskes, W.W.C.; Hagmeier, E.; Weichart, G. Phytoplankton of the North Sea and its dynamics: A review. Neth. J. Sea Res. 1990, 26, 295–331. [Google Scholar] [CrossRef] [Green Version]
- Schoemann, V.; Wollast, R.; Chou, L.; Lancelot, C. Effects of photosynthesis on the accumulation of Mn and Fe by Phaeocystis colonies. Limnol. Oceanogr. 2001, 46, 1065–1076. [Google Scholar] [CrossRef]
- Veldhuis, M.J.W.; Colijn, F.; Admiraal, W. Phosphate Utilization in Phaeocystis-Pouchetii (Haptophyceae). Mar. Ecol.-Pubbl. Della Stn. Zool. Di Napoli I 1991, 12, 53–62. [Google Scholar] [CrossRef]
- Riegman, R.; Noordeloos, A.A.M. Phaeocystis blooms and eutrophication of the continental coastal zones of the North Sea. Mar. Biol. 1992, 112, 479–484. [Google Scholar] [CrossRef]
- Riegman, R. Nutrient-related selection mechanisms in marine phytoplankton communities and the impact of eutrophication on the planktonic food web. Water Sci. Technol. 1995, 32, 63–75. [Google Scholar] [CrossRef]
- Anderson, C.R.; Sapiano, M.R.; Prasad, M.B.K.; Long, W.; Tango, P.J.; Brown, C.W.; Murtugudde, R. Predicting potentially toxigenic Pseudo-nitzschia blooms in the Chesapeake Bay. J. Mar. Syst. 2010, 83, 127–140. [Google Scholar] [CrossRef]
- Thorel, M.; Claquin, P.; Schapira, M.; Le Gendre, R.; Riou, P.; Goux, D.; Le Roy, B.; Raimbault, V.; Deton-Cabanillas, A.F.; Bazin, P.; et al. Nutrient ratios influence variability in Pseudo-nitzschia species diversity and particulate domoic acid production in the Bay of Seine (France). Harmful Algae 2017, 68, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U. Are marine diatoms favoured by high Si: N ratios? Mar. Ecol. Prog. Ser. 1994, 115, 309–315. [Google Scholar] [CrossRef]
- Barbosa, A.B.; Domingues, R.B.; Galvão, H.M. Environmental Forcing of Phytoplankton in a Mediterranean Estuary (Guadiana Estuary, South-western Iberia): A Decadal Study of Anthropogenic and Climatic Influences. Estuaries Coasts 2010, 33, 324–341. [Google Scholar] [CrossRef]
- Čalić, M.; Carić, M.; Kršinić, F.; Jasprica, N.; Pećarević, M. Controlling factors of phytoplankton seasonal succession in oligotrophic Mali Ston Bay (south-eastern Adriatic). Environ. Monit. Assess. 2013, 185, 7543–7563. [Google Scholar] [CrossRef] [PubMed]
- Pulina, S.; Padedda, B.M.; Satta, C.T.; Sechi, N.; Lugliè, A. Long-term phytoplankton dynamics in a Mediterranean eutrophic lagoon (Cabras Lagoon, Italy). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2012, 146, 259–272. [Google Scholar] [CrossRef]
- Thackeray, S.; Jones, I.; Maberly, S. Long-term change in the phenology of spring phytoplankton: Species-specific responses to nutrient enrichment and climatic change. J. Ecol. 2008, 96, 523–535. [Google Scholar] [CrossRef]
- Winder, M.; Schindler, D.E. Climatic effects on the phenology of lake processes. Glob. Chang. Biol. 2004, 10, 1844–1856. [Google Scholar] [CrossRef] [Green Version]
- Winder, M.; Cloern, J.E. The annual cycles of phytoplankton biomass. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3215–3226. [Google Scholar] [CrossRef]
- Nejstgaard, J.C.; Tang, K.W.; Steinke, M.; Dutz, J.; Koski, M.; Antajan, E.; Long, J.D. Zooplankton grazing on Phaeocystis: A quantitative review and future challenges. Biogeochemistry 2007, 83, 147–172. [Google Scholar] [CrossRef] [Green Version]
- Long, J.D.; Smalley, G.W.; Barsby, T.; Anderson, J.T.; Hay, M.E. Chemical cues induce consumer-specific defenses in a bloom-forming marine phytoplankton. Proc. Natl. Acad. Sci. USA 2007, 104, 10512–10517. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Ou, L.; He, X.; Chen, D. Allocation costs associated with induced defense in Phaeocystis Globosa (Prymnesiophyceae): Eff. Nutr. Availability. Sci. Rep. 2015, 5, 10850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tett, A.P.B.; Joint, I.R.; Purdie, D.A.; Baars, M.; Oosterhuis, S.; Daneri, G.; Mills, D.K.; Plummer, D.; Pomroy, A.J.; Walne, A.W.; et al. Biological Consequences of Tidal Stirring Gradients in the North Sea [ and Discussion ] Biological consequences of tidal stirring gradients in the North Sea. Philos. Trans. Phys. Sci. Eng. 1993, 343, 493–508. [Google Scholar] [CrossRef]
- Tett, P.; Walne, A. Observations and simulations of hydrography, nutrients and plankton in the southern North Sea. Ophelia 1995, 42, 371–416. [Google Scholar] [CrossRef]
- Fiksen, Ø. The adaptive timing of diapause—A search for evolutionarily robust strategies in Calanus finmarchicus. ICES J. Mar. Sci. 2000, 57, 1825–1833. [Google Scholar] [CrossRef] [Green Version]
- Varpe, Ø.; Jørgensen, C.; Tarling, G.A.; Fiksen, Ø. The adaptive value of energy storage and capital breeding in seasonal environments. Oikos 2009, 118, 363–370. [Google Scholar] [CrossRef]
- Varpe, Ø.; Jørgensen, C.; Tarling, G.A.; Fiksen, Ø. Early is better: Seasonal egg fitness and timing of reproduction in a zooplankton life-history model. Oikos 2007, 116, 1331–1342. [Google Scholar] [CrossRef]
- Chapelle, A.; Le Gac, M.; Labry, C.; Siano, R.; Quere, J.; Caradec, F.; Le Bec, C.; Nezan, E.; Doner, A.; Gouriou, J. The Bay of Brest (France), a new risky site for toxic Alexandrium minutum blooms and PSP shellfish contamination. Harmful Algae News 2015, 51, 4–5. [Google Scholar]
- Belin, C.; Soudant, D.; Amzil, Z. Three decades of data on phytoplankton and phycotoxins on the French coast: Lessons from REPHY and REPHYTOX. Harmful Algae 2021, 102, 101733. [Google Scholar] [CrossRef]
- Pistocchi, R.; Pezzolesi, L.; Guerrini, F.; Vanucci, S.; Dell’Aversano, C.; Fattorusso, E. A review on the effects of environmental conditions on growth and toxin production of Ostreopsis ovata. Toxicon 2011, 57, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Batchelder, H.P. Forward-in-time-/backward-in-time-trajectory (FITT/BITT) modeling of particles and organisms in the coastal ocean. J. Atmos. Ocean. Technol. 2006, 23, 727–741. [Google Scholar] [CrossRef]
- Christensen, A.; Daewel, U.; Jensen, H.; Mosegaard, H.; John, M.S.; Schrum, C. Hydrodynamic backtracking of fish larvae by individual-based modelling. Mar. Ecol. Prog. Ser. 2007, 347, 221–232. [Google Scholar] [CrossRef]
- Speirs, D.C.; Gurney, W.S.; Holmes, S.J.; Heath, M.R.; Wood, S.N.; Clarke, E.D.; Harms, I.H.; Hirche, H.J.; McKenzie, E. Understanding demography in an advective environment: Modelling Calanus finmarchicus in the Norwegian Sea. J. Anim. Ecol. 2004, 73, 897–910. [Google Scholar] [CrossRef]

| Phylum | Code | Species |
|---|---|---|
| Ochrophyta | Ast | Asterionella sp., A. formosa, Asterionellopsis sp., mainly A. glacialis, Asteroplanus, A. karianus |
| Bac | Bacillaria sp., Bacillaria paxillifer, Bacillaria paxillifera | |
| Cer | Cerataulina pelagica | |
| Cha | Chaetoceros sp., C. affinis, C. castracanei, C. curvisetus, C. danicus, C. debilis, C. decipiens, C. densus, C. didymus, C. fragilis, C. lorenzianus, C. peruvianus, C. protuberans, C. pseudocurvisetus, C. rostratus, C. socialis, C. socialis f. radians, C. subtilis, C. wighamii | |
| Cos | Coscinodiscus sp., Stellarima sp. | |
| Cyl | Cylindrotheca sp., Cylindrotheca closterium, Cylindrotheca gracilis, Nitzschia longissima, Hantzschia sp. | |
| Dac | Dactyliosolen sp., mainly D. fragilissimus | |
| Dic | Dictyocha sp., mainly Dictyocha fibula | |
| Dip | Diploneis sp. | |
| Dit | Ditylum sp., mainly D. brightwellii | |
| Euc | Eucampia sp., mainly E. zodiacus, Climacodium sp. | |
| Gui | Guinardia sp., G. flaccida, mainly G. striata and G. delicatula | |
| Lau | Lauderia sp., L. annulata, Schroederella sp. | |
| Lep | Leptocylindrus sp., L. danicus, L. curvatus, L. minimus | |
| Lic | Licmophora spp. | |
| Meu | Meuniera sp., Meuniera membranacea | |
| Nav | Navicula sp., N. cryptocephala, N. gregaria, N. pelagica, Fallacia sp., Haslea sp., H. wawrikae, Lyrella sp., Petroneis sp. | |
| Odo | Odontella sp., O. aurita, O. granulata, O. mobiliensis, O. regia, O. Sinensis | |
| Par | Paralia sulcata | |
| Pen | Unidentified taxa of the Order Pennales | |
| Pla | Brockmanniella sp., Brockmanniella brockmannii, Plagiogramma sp. | |
| Ple | Pleurosigma sp., Gyrosigma sp. | |
| Pse | Pseudo-nitzschia spp. | |
| Rha | Rhaphoneis sp., Delphineis sp. | |
| Rhi | Rhizosolenia sp., R. hebetata, R. imbricata, R. styliformis, R. setigera, R. setigera f. pungens, Neocalyptrella robusta | |
| Ske | Skeletonema sp., mainly Skeletonema costatum | |
| Tha | Thalassiosira sp., T. angulata, T. antarctica, T. gravida, T. levanderi, T. minima, T. nordenskioeldii, T. rotula, T. subtilis, Porosira sp. | |
| Thao | Thalassionema sp., mainly T. nitzschioides, Thalassiothrix sp., Lioloma sp. | |
| Myzozoa | Ale | mainly Alexandrium minutum, A. margalefii, A. ostenfeldii, A. pseudogonyaulax |
| Amp | Amphidinium sp., Amphidinium carterae, Amphidinium operculatum, Amphidinium crassum | |
| DiP | Diplopsalis sp., Diplopelta sp., Diplopsalopsis sp., Preperidinium sp., Oblea sp. | |
| Gym | Gymnodinium sp., G. catenatum, Gyrodinium sp., G. spirale | |
| Het | Heterocapsa sp., H. niei, H. triquetra | |
| Kat | Katodinium sp. | |
| Pol | Polykrikos sp., P. schwarzii | |
| Pro | Protoperidinium sp., mainly P. bipes, P. conicum, P. depressum, P. diabolum, P. longipes, P. steinii, P. pyriforme, Archaeperidinium minutum, Peridinium sp., P. quiquecorne | |
| Proe | Prorocentrum sp., P. arcuatum, P. balticum, P. cordatum, P. compressum, P. gibbosum, P. gracile, P. micans, P. triestinum | |
| Pyr | All Taxa of the Pyrocystaceae familly | |
| Scr | mainly Scrippsiella sp., Ensiculifera sp., Pentapharsodinium sp., Bysmatrum sp. | |
| Tor | Torodinium robustum | |
| Haptophyte | Pha | Phaeocystis sp. |
| Euglenophyte | Eug | Euglena sp., Eutreptia sp., Eutreptiella sp. |
| Cryptophyta | Cry | All Taxa of the order Cryptomonadales |
| Ciliophora | Mes | Mesodinium sp., mainly Mesodinium rubrum |
| Cil | Unidentified taxa of the Phylum Ciliophora | |
| Chlorophyta | Sce | Scenedesmus sp., mainly Scenedesmus quadricauda, Desmodesmus communis |
| Chl | Unidentified taxa of the Order Chlorophyceae |
| Variables | Months | Years | Stations | M:Y | M:S | Y:S | M:Y:S |
|---|---|---|---|---|---|---|---|
| Environmental | |||||||
| CHLO | 0.238 * | 0.039 * | 0.039 * | 0.248 * | 0.028 | 0.03 | 0.239 |
| SPM | 0.045 * | 0.015 * | 0.06 * | 0.176 * | 0.07 * | 0.049 | 0.485 |
| NH | 0.236 * | 0.057 * | 0.094 * | 0.152 * | 0.1 * | 0.049 * | 0.23 |
| NO | 0.456 * | 0.054 * | 0.122 * | 0.108 * | 0.045 * | 0.028 * | 0.126 |
| PAR | 0.638 * | 0.007 * | 0.002 | 0.095 * | 0.007 | 0.013 | 0.114 |
| PO | 0.251 * | 0.14 * | 0.022 * | 0.197 * | 0.016 | 0.067 * | 0.186 |
| SALI | 0.022 * | 0.164 * | 0.387 * | 0.108 * | 0.021 * | 0.05 * | 0.193 * |
| Si(OH) | 0.41 * | 0.028 * | 0.122 * | 0.15 * | 0.073 * | 0.03 * | 0.153 * |
| TEMP | 0.897 * | 0.021 * | 0.002 * | 0.039 * | 0.011 * | 0.002 | 0.013 |
| TURB | 0.08 * | 0.011 * | 0.328 * | 0.129 * | 0.098 * | 0.045 * | 0.251 * |
| Community | |||||||
| H | 0.405 * | 0.045 * | 0.004 | 0.155 * | 0.025 | 0.03 | 0.199 |
| J | 0.401 * | 0.039 * | 0.008 * | 0.16 * | 0.026 | 0.032 | 0.194 |
| S | 0.127 * | 0.233 * | 0.068 * | 0.149 * | 0.055 * | 0.052 * | 0.223 |
| Phylum | Code | Inertia | OMI | Tol | Rtol | p Value |
|---|---|---|---|---|---|---|
| Ochrophyta | Ast | 10.606 | 0.166 | 2.144 | 8.297 | 0.001 |
| Bac | 12.439 | 0.558 | 3.812 | 8.069 | 0.001 | |
| Cer | 9.719 | 0.586 | 1.653 | 7.48 | 0.001 | |
| Cha | 8.764 | 0.011 | 0.74 | 8.014 | 0.001 | |
| Cos | 13.068 | 0.728 | 4.205 | 8.135 | 0.001 | |
| Cyl | 11.327 | 0.111 | 3.978 | 7.239 | 0.001 | |
| Dac | 8.983 | 1.007 | 1.412 | 6.565 | 0.001 | |
| Dic | 10.308 | 0.458 | 1.8 | 8.049 | 0.001 | |
| Dip | 8.176 | 0.688 | 0.829 | 6.659 | 0.001 | |
| Dit | 11.105 | 0.653 | 2.654 | 7.798 | 0.001 | |
| Euc | 7.967 | 0.349 | 0.921 | 6.696 | 0.001 | |
| Gui | 8.138 | 0.337 | 2.072 | 5.729 | 0.001 | |
| Lau | 9.58 | 0.229 | 2.157 | 7.194 | 0.001 | |
| Lep | 7.963 | 0.859 | 1.411 | 5.693 | 0.001 | |
| Lic | 6.473 | 1.164 | 1.415 | 3.894 | 0.001 | |
| Meu | 7.702 | 0.174 | 1.861 | 5.667 | 0.001 | |
| Nav | 10.727 | 0.105 | 1.095 | 9.527 | 0.001 | |
| Odo | 12.803 | 0.465 | 3.911 | 8.426 | 0.001 | |
| Par | 10.781 | 0.07 | 3.673 | 7.039 | 0.001 | |
| Pen | 11.198 | 0.142 | 3.02 | 8.036 | 0.001 | |
| Pla | 15.986 | 1.516 | 4.866 | 9.604 | 0.001 | |
| Ple | 10.957 | 0.032 | 2.808 | 8.117 | 0.001 | |
| Pse | 8.871 | 0.112 | 2.797 | 5.962 | 0.001 | |
| Rha | 9.898 | 0.063 | 2.261 | 7.574 | 0.001 | |
| Rhi | 8.738 | 0.253 | 2.268 | 6.217 | 0.001 | |
| Ske | 12.15 | 1.156 | 3.77 | 7.225 | 0.001 | |
| Tha | 11.09 | 0.303 | 3.572 | 7.215 | 0.001 | |
| Thao | 11.728 | 0.333 | 4.076 | 7.319 | 0.001 | |
| Myzozoa | Ale | 6.473 | 0.369 | 1.751 | 4.353 | 0.001 |
| Amp | 7.378 | 0.341 | 1.378 | 5.659 | 0.001 | |
| DiP | 8.121 | 0.398 | 1.86 | 5.863 | 0.001 | |
| Gym | 8.745 | 0.096 | 2.828 | 5.821 | 0.001 | |
| Het | 8.951 | 0.313 | 1.686 | 6.951 | 0.001 | |
| Kat | 7.62 | 0.548 | 1.795 | 5.277 | 0.001 | |
| Pol | 8.877 | 0.466 | 1.072 | 7.339 | 0.001 | |
| Pro | 7.671 | 0.198 | 2.414 | 5.059 | 0.001 | |
| Proe | 7.261 | 0.78 | 1.662 | 4.819 | 0.001 | |
| Pyr | 5.945 | 1.159 | 1.058 | 3.727 | 0.001 | |
| Scr | 8.705 | 0.376 | 2.147 | 6.183 | 0.001 | |
| Tor | 7.429 | 0.476 | 1.782 | 5.171 | 0.001 | |
| Haptophyta | Pha | 8.485 | 1.102 | 1.722 | 5.661 | 0.001 |
| Euglenozoa | Eug | 10.791 | 0.304 | 1.042 | 9.445 | 0.001 |
| Cryptophyta | Cry | 9.207 | 0.049 | 2.112 | 7.045 | 0.001 |
| Mes | 6.876 | 0.178 | 1.681 | 5.017 | 0.001 | |
| Ciliophora | Cil | 9.567 | 0.046 | 2.459 | 7.062 | 0.001 |
| Sce | 19.656 | 1.527 | 3.548 | 14.582 | 0.001 | |
| Chlorophyta | Chl | 11.366 | 0.233 | 1.297 | 9.836 | 0.001 |
| Station | Pse | Pha | Sum |
|---|---|---|---|
| DK1 | 14 | 19 | 33 |
| DK3 | 17 | 16 | 33 |
| DK4 | 12 | 16 | 28 |
| BL1 | 16 | 20 | 36 |
| BL2 | 14 | 19 | 33 |
| BL3 | 12 | 17 | 29 |
| S1 | 13 | 19 | 32 |
| S2 | 12 | 17 | 29 |
| S3 | 15 | 22 | 37 |
| Mim | 17 | 18 | 35 |
| Bif | 17 | 21 | 38 |
| All | 159 | 204 | 363 |
| Taxa | K (n) | CHLO | SPM | NH | NO | PAR | PO | SALI | Si(OH) | TEMP | TURB | A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pha | ONS (317) | 1.7 * | 13.3 * | 1.2 | 21.5 * | 79.2 * | 0.7 * | 33.4 * | 8.4 * | 8.1 * | 9.4 * | 26.8 |
| CLI (292) | 6.1 * | 6.9 | 0.8 * | 13.4 * | 153.6 | 0.4 * | 33.6 | 3 * | 8.2 * | 6.2 | 30.2 | |
| HAB (1010) | 8 * | 9.7 | 0.9 * | 8.6 | 183.5 * | 0.4 * | 33.6 * | 3.2 * | 11 * | 7.8 * | 60.1 | |
| DEC (718) | 8.8 * | 10.8 | 1 * | 6.7 * | 195.7 * | 0.4 * | 33.5 * | 3.3 * | 12.2 * | 8.4 * | 58.6 | |
| END (385) | 5 | 3.9 * | 0.8 * | 2.4 * | 243.3 * | 0.3 * | 33.9 * | 1.7 * | 16.1 * | 3.2 * | 28.1 | |
| Pse | ONS (283) | 2.3 * | 17.8 * | 1.2 | 20.4 * | 88.2 * | 0.7 * | 33.6 * | 8 * | 7.6 * | 11.6 * | 32.2 |
| CLI (480) | 9.1 * | 6.9 * | 0.6 * | 7.8 * | 188.3 * | 0.3 * | 33.9 * | 2.2 * | 9.7 * | 5.9 | 41.1 | |
| HAB (1020) | 7.3 * | 5.8 * | 0.7 * | 4.8 * | 206.2 * | 0.3 * | 34 * | 2 * | 12.5 | 4.8 * | 43.4 | |
| DEC (540) | 5.7 * | 4.8 * | 0.8 * | 2.2 * | 222.2 * | 0.3 * | 34.1 * | 1.8 * | 15.1 * | 3.8 * | 29.8 | |
| END (332) | 3.7 * | 8.3 | 1.2 | 4.1 * | 188.2 * | 0.4 | 33.9 * | 4.1 | 17 * | 5.8 | 36.5 |
| Taxa | Phase | Inertia | WitOMIGK | Tol | SB | p Value | H | S | J |
|---|---|---|---|---|---|---|---|---|---|
| Pha | ONS | 9.444 | 0.594 | 1.718 | 6.665 | <0.001 | 1.797 | 19 | 0.617 |
| CLI | 5.769 | 0.295 | 1.863 | 3.438 | <0.001 | 1.253 | 20 | 0.421 | |
| HAB | 7.536 | 0.429 | 1.747 | 1.774 | <0.001 | 1.096 | 20 | 0.369 | |
| DEC | 8.035 | 0.547 | 1.677 | 1.774 | <0.001 | 1.032 | 19 | 0.348 | |
| END | 5 | 0.407 | 0.552 | 16.596 | <0.001 | 1.399 | 20 | 0.475 | |
| Pse | ONS | 8.807 | 0.102 | 2.651 | 4.172 | <0.001 | 1.786 | 20 | 0.604 |
| CLI | 7.046 | 0.005 | 1.825 | 0 | <0.001 | 0.988 | 20 | 0.329 | |
| HAB | 6.536 | 0.003 | 1.642 | 0 | <0.001 | 1.159 | 20 | 0.384 | |
| DEC | 5.246 | 0.006 | 1.174 | 0 | <0.001 | 1.311 | 21 | 0.433 | |
| END | 6.222 | 0.022 | 1.675 | 4.02 | <0.001 | 1.594 | 22 | 0.522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karasiewicz, S.; Lefebvre, A. Environmental Impact on Harmful Species Pseudo-nitzschia spp. and Phaeocystis globosa Phenology and Niche. J. Mar. Sci. Eng. 2022, 10, 174. https://doi.org/10.3390/jmse10020174
Karasiewicz S, Lefebvre A. Environmental Impact on Harmful Species Pseudo-nitzschia spp. and Phaeocystis globosa Phenology and Niche. Journal of Marine Science and Engineering. 2022; 10(2):174. https://doi.org/10.3390/jmse10020174
Chicago/Turabian StyleKarasiewicz, Stéphane, and Alain Lefebvre. 2022. "Environmental Impact on Harmful Species Pseudo-nitzschia spp. and Phaeocystis globosa Phenology and Niche" Journal of Marine Science and Engineering 10, no. 2: 174. https://doi.org/10.3390/jmse10020174
APA StyleKarasiewicz, S., & Lefebvre, A. (2022). Environmental Impact on Harmful Species Pseudo-nitzschia spp. and Phaeocystis globosa Phenology and Niche. Journal of Marine Science and Engineering, 10(2), 174. https://doi.org/10.3390/jmse10020174







