Response of the Black Sea Zooplankton to the Marine Heat Wave 2010: Case of the Sevastopol Bay
Abstract
1. Introduction
2. Materials and Methods
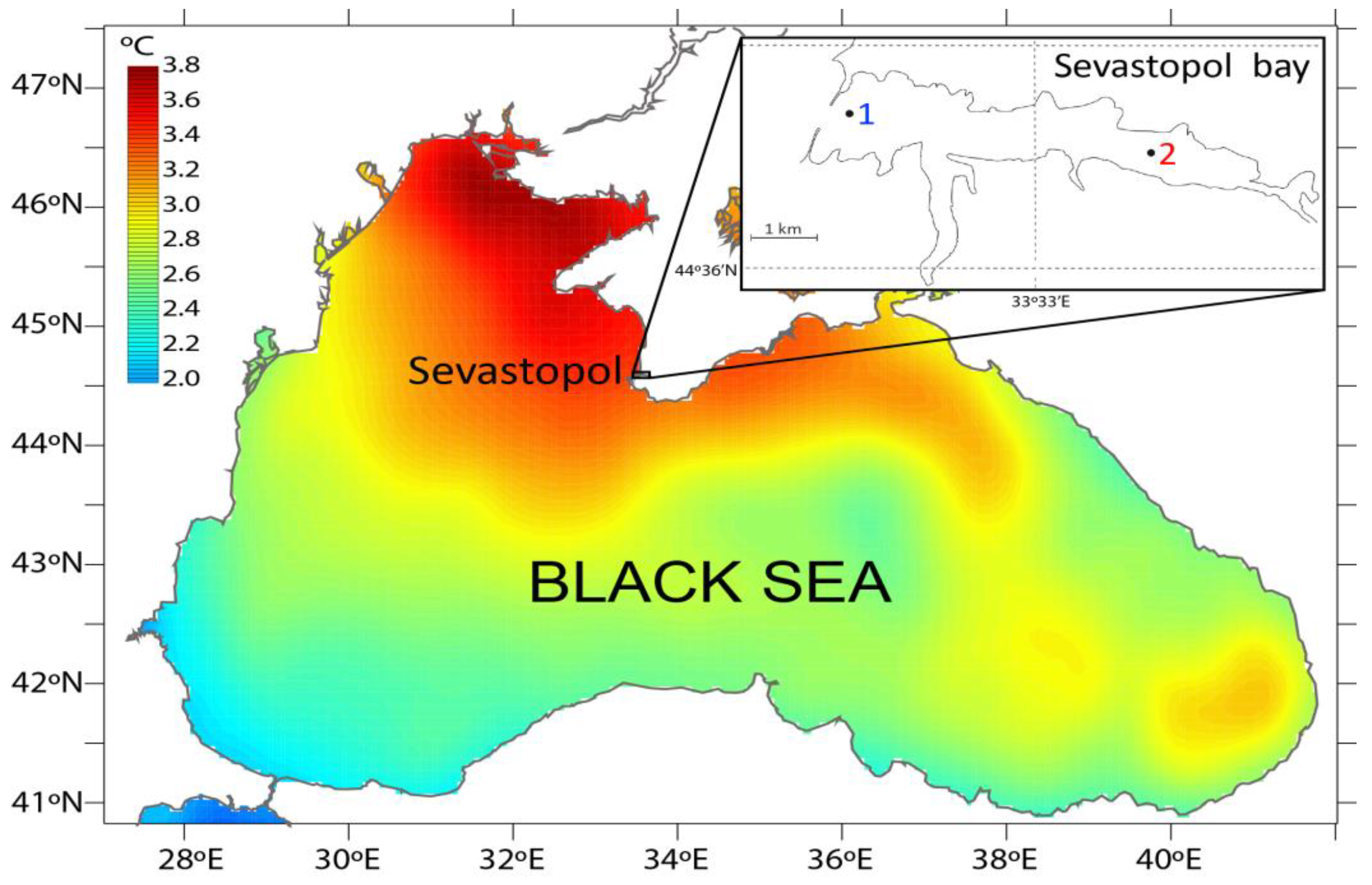
2.1. Area of Investigation
2.2. SST Data
2.3. Sampling and Zooplankton Processing
2.4. Data Analysis
2.4.1. SST Data
2.4.2. Zooplankton
3. Results
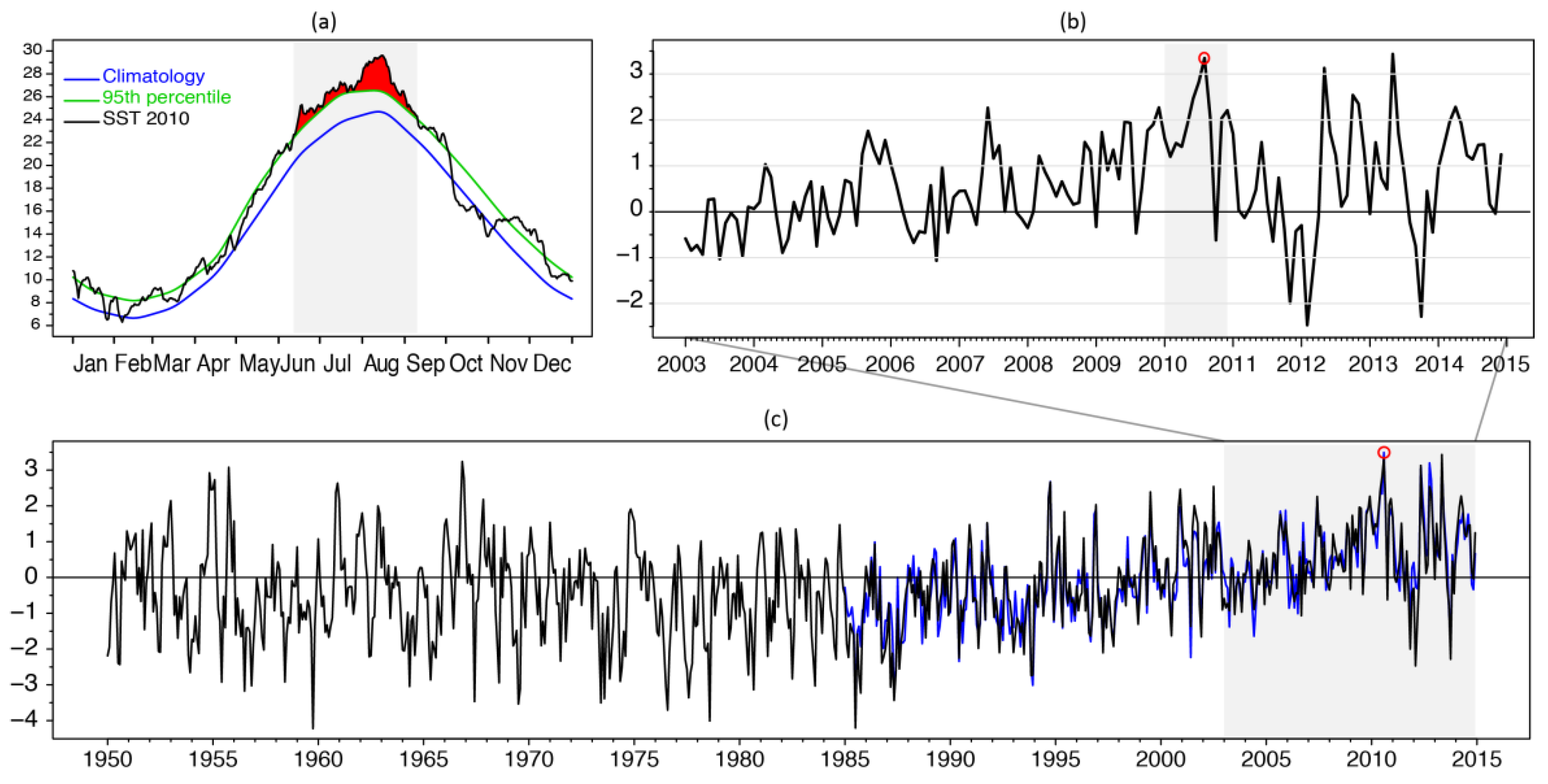
3.1. SST Variability and the Summer 2010 MHW
3.2. Species Composition and Ecological Groups of Zooplankton
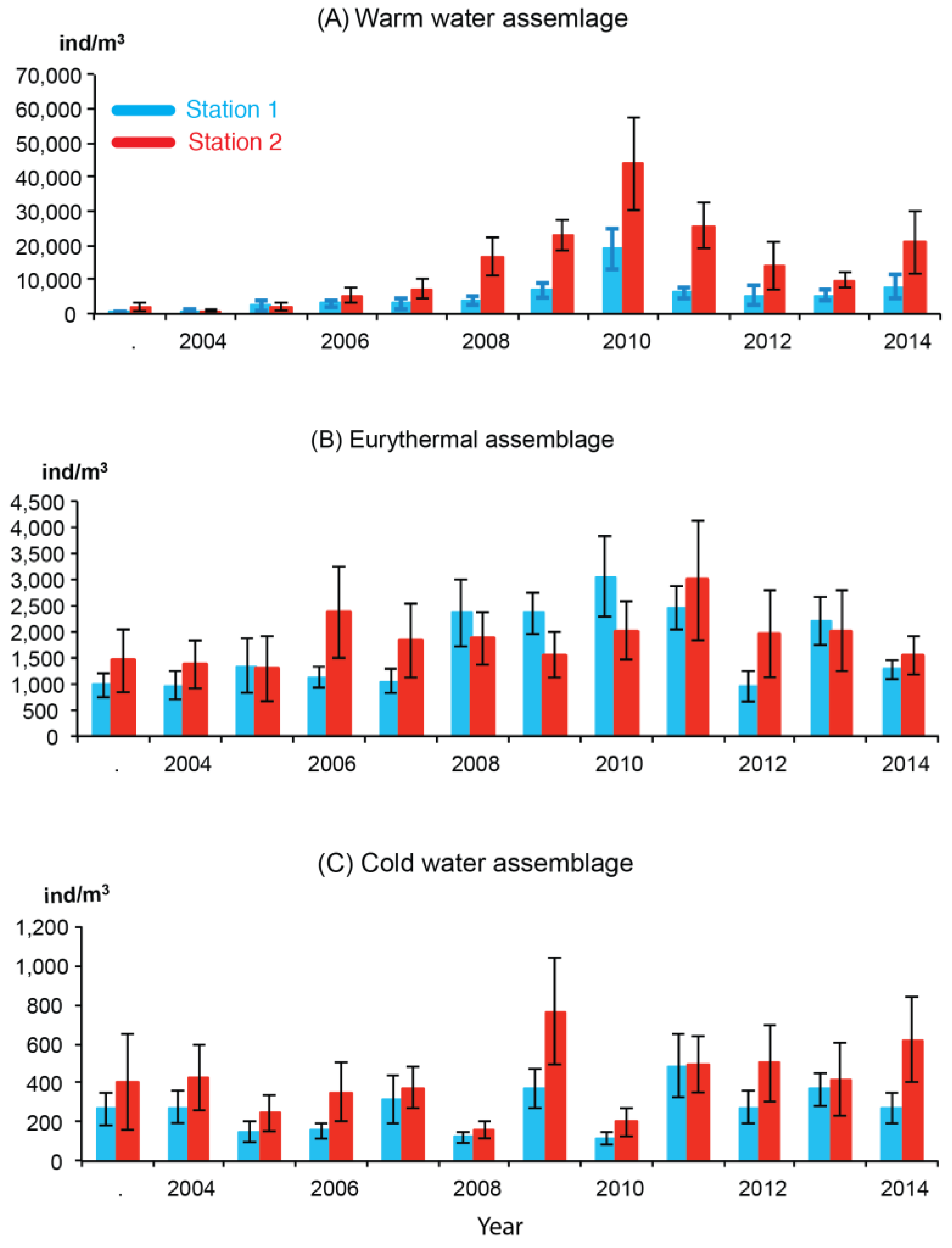
3.3. Interannual Variation in Abundance
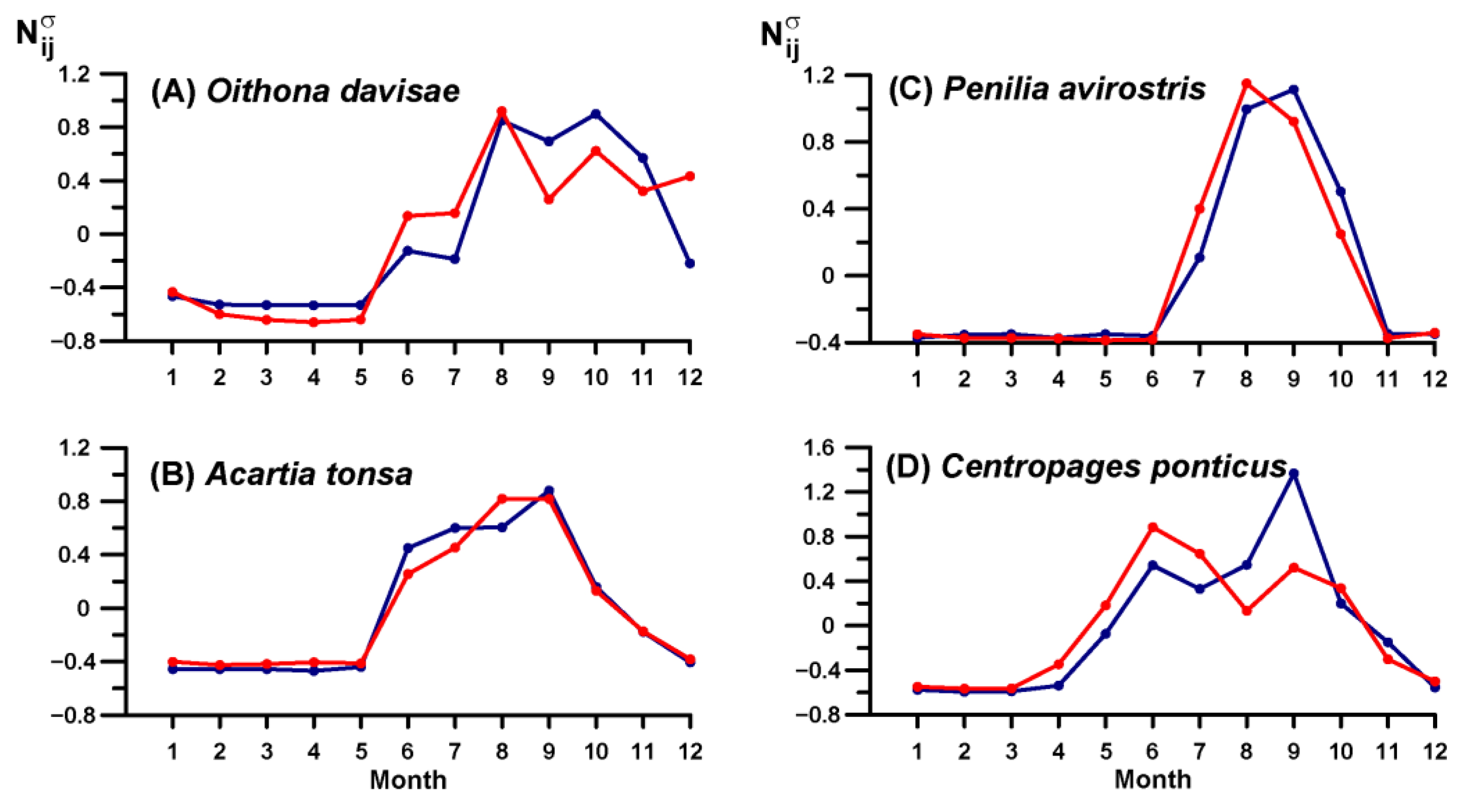
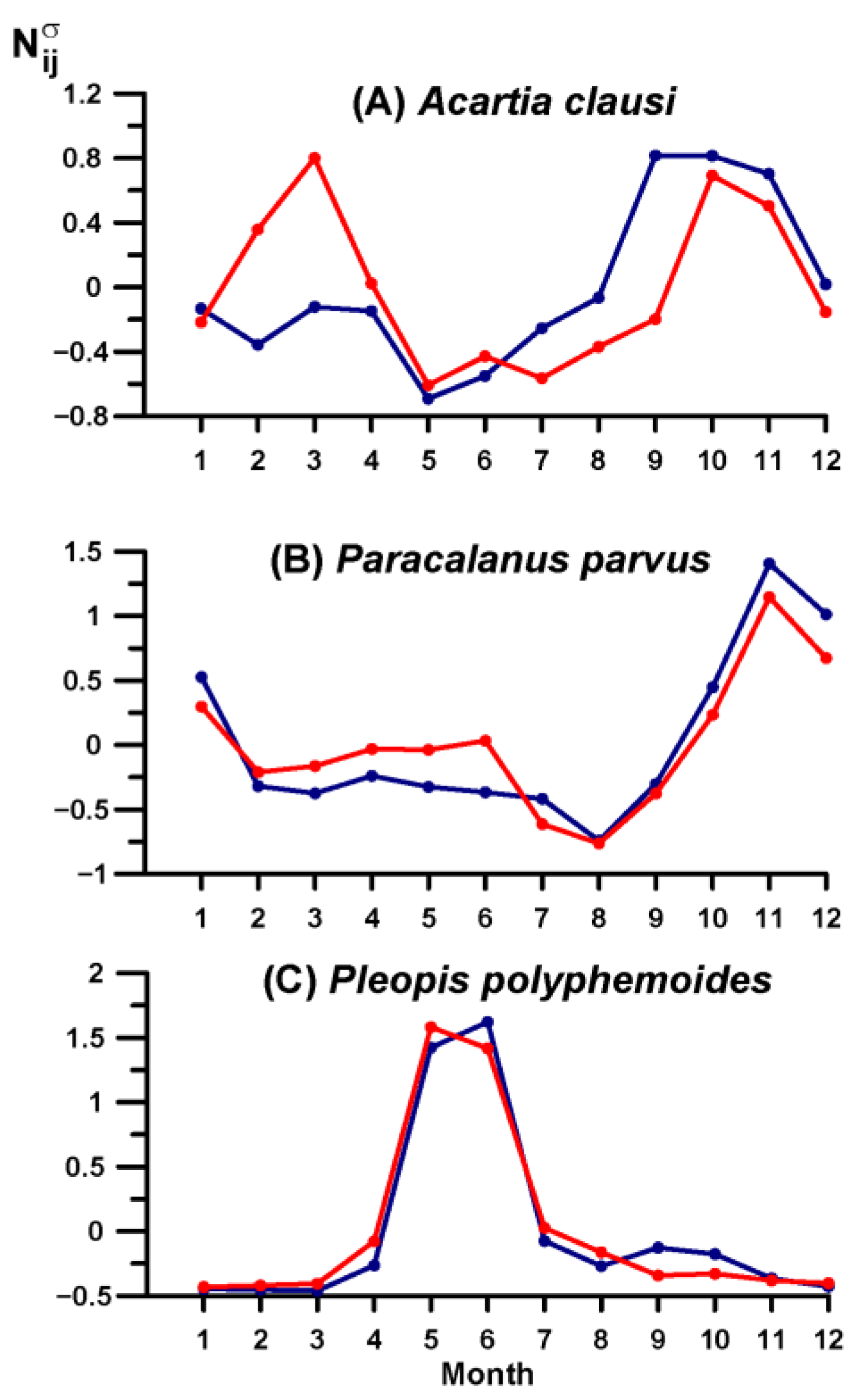
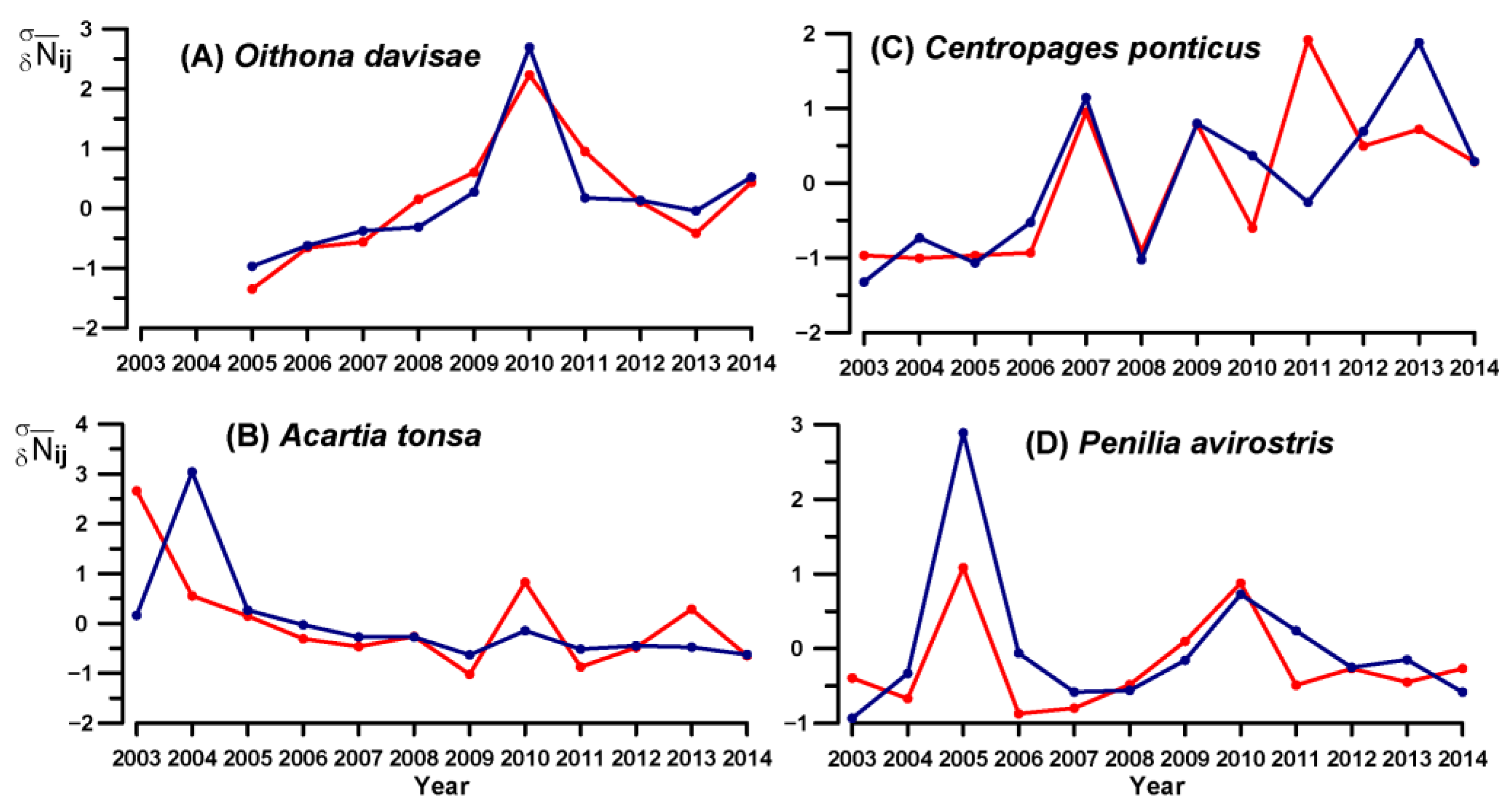
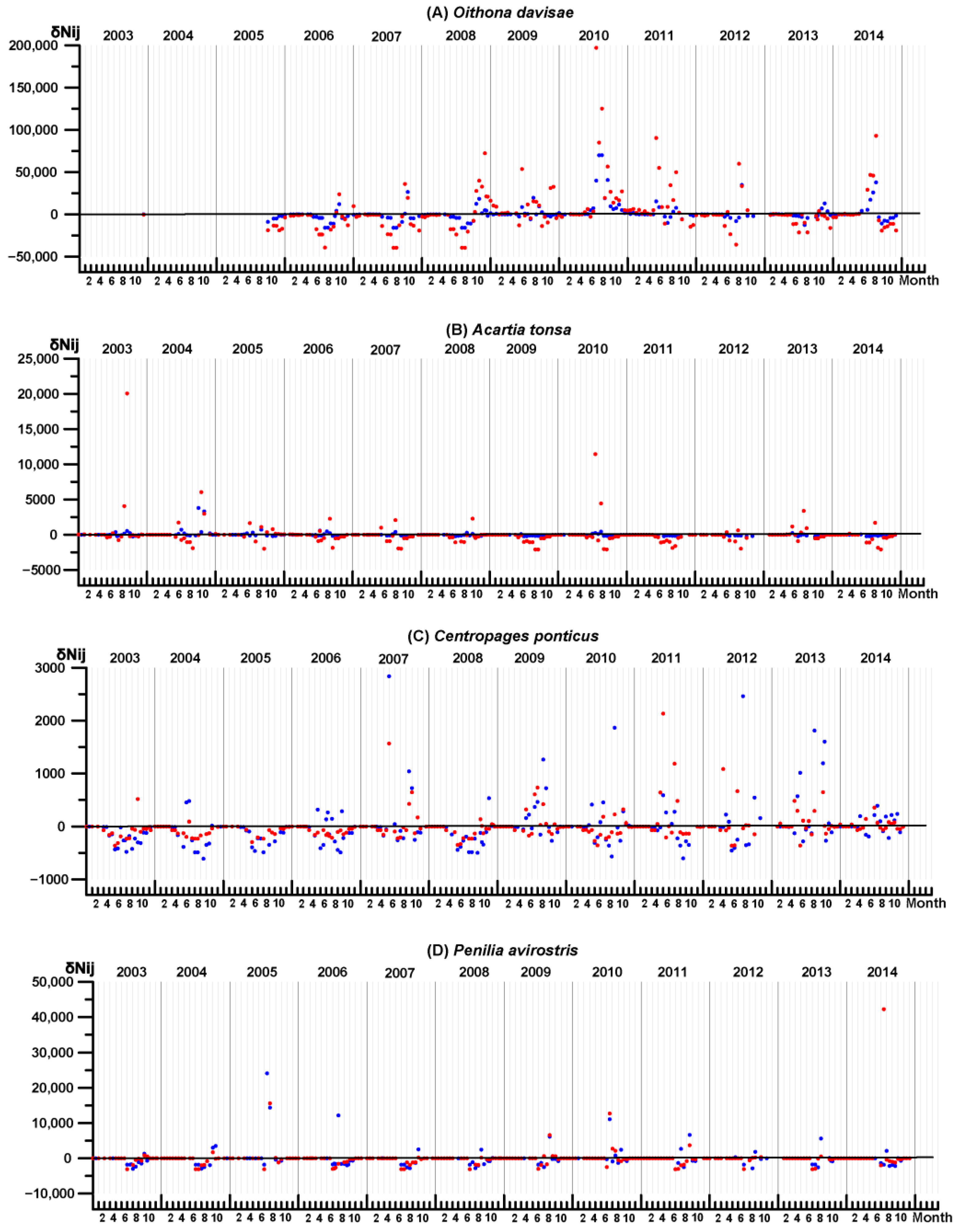
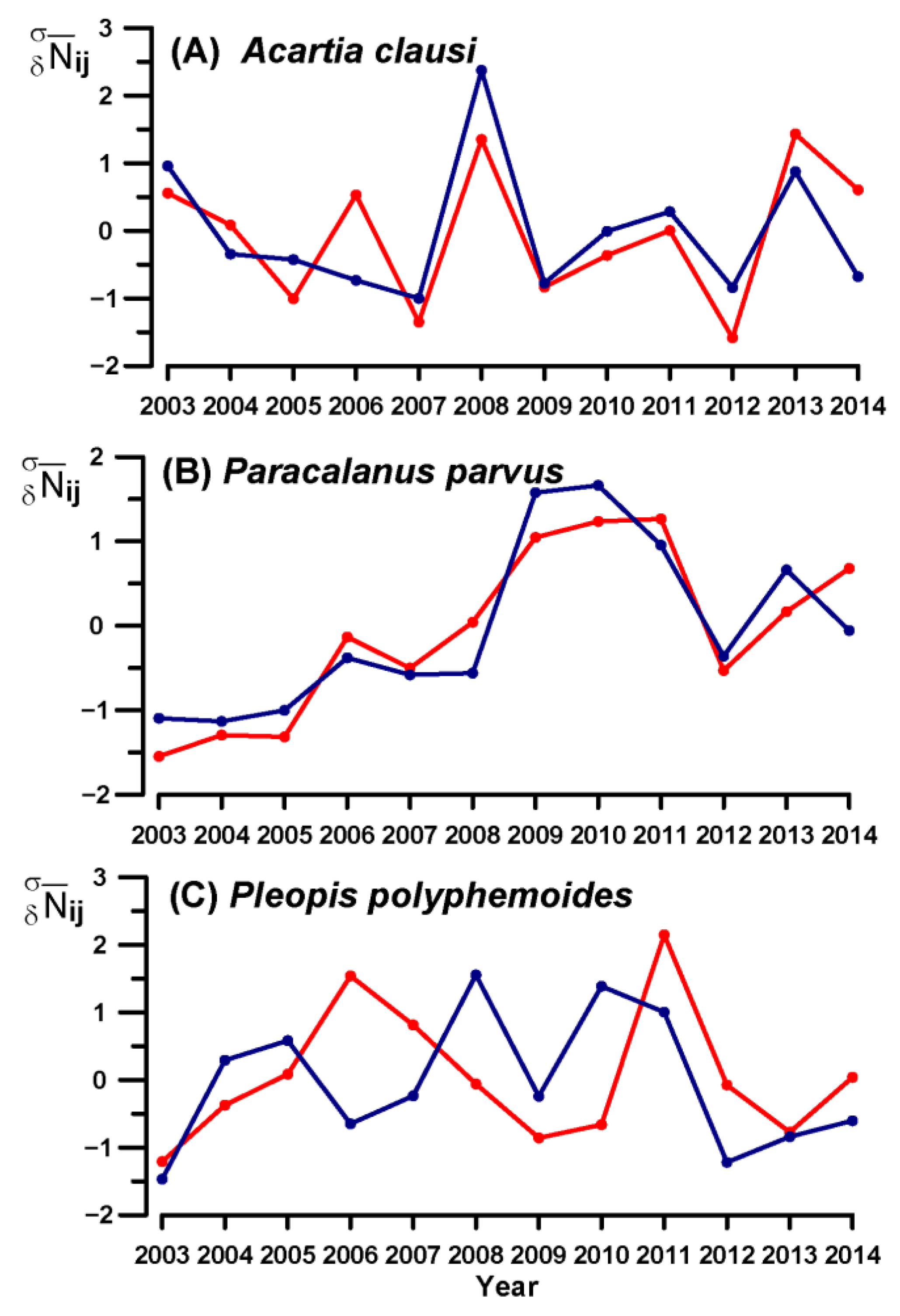
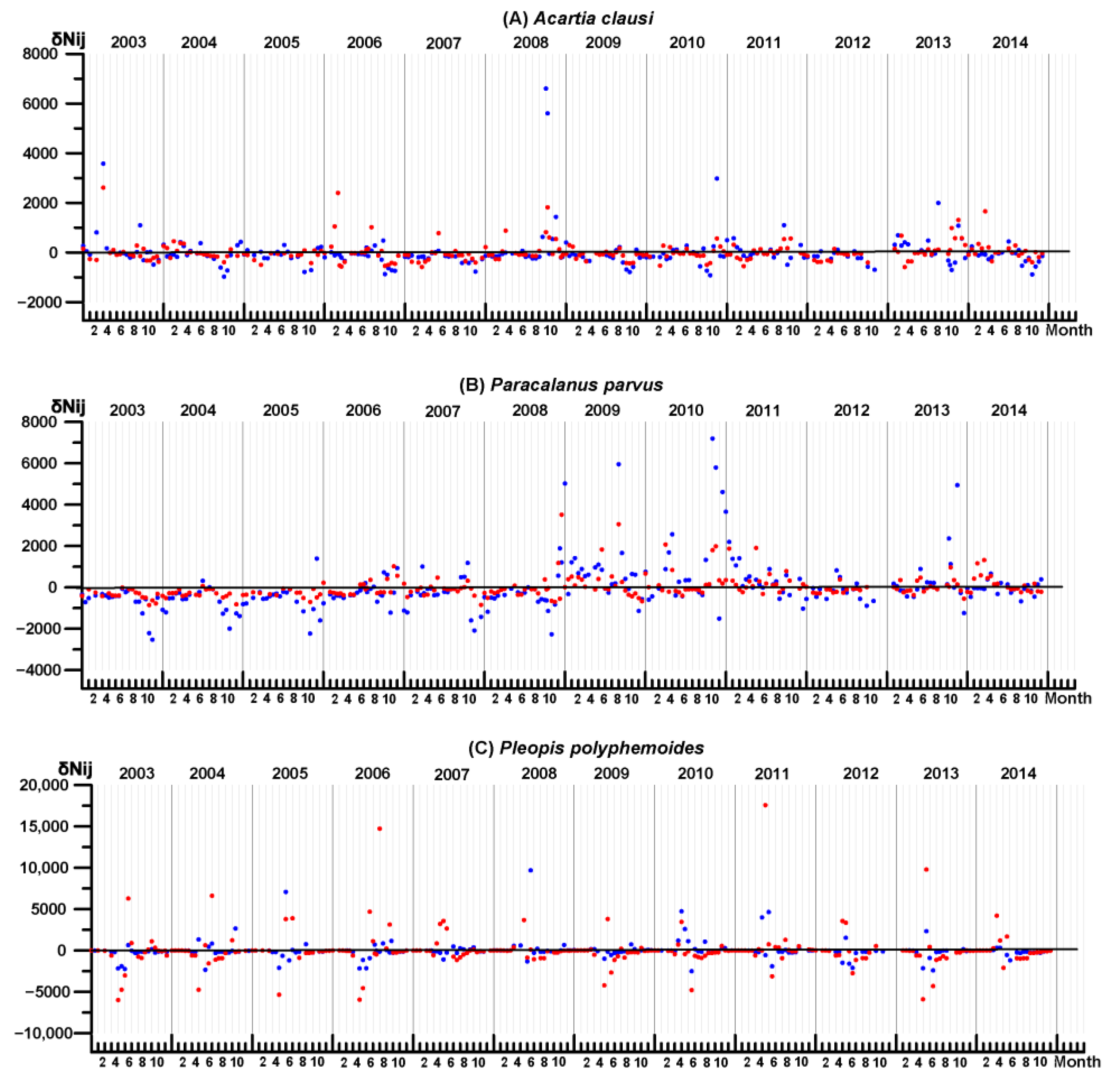
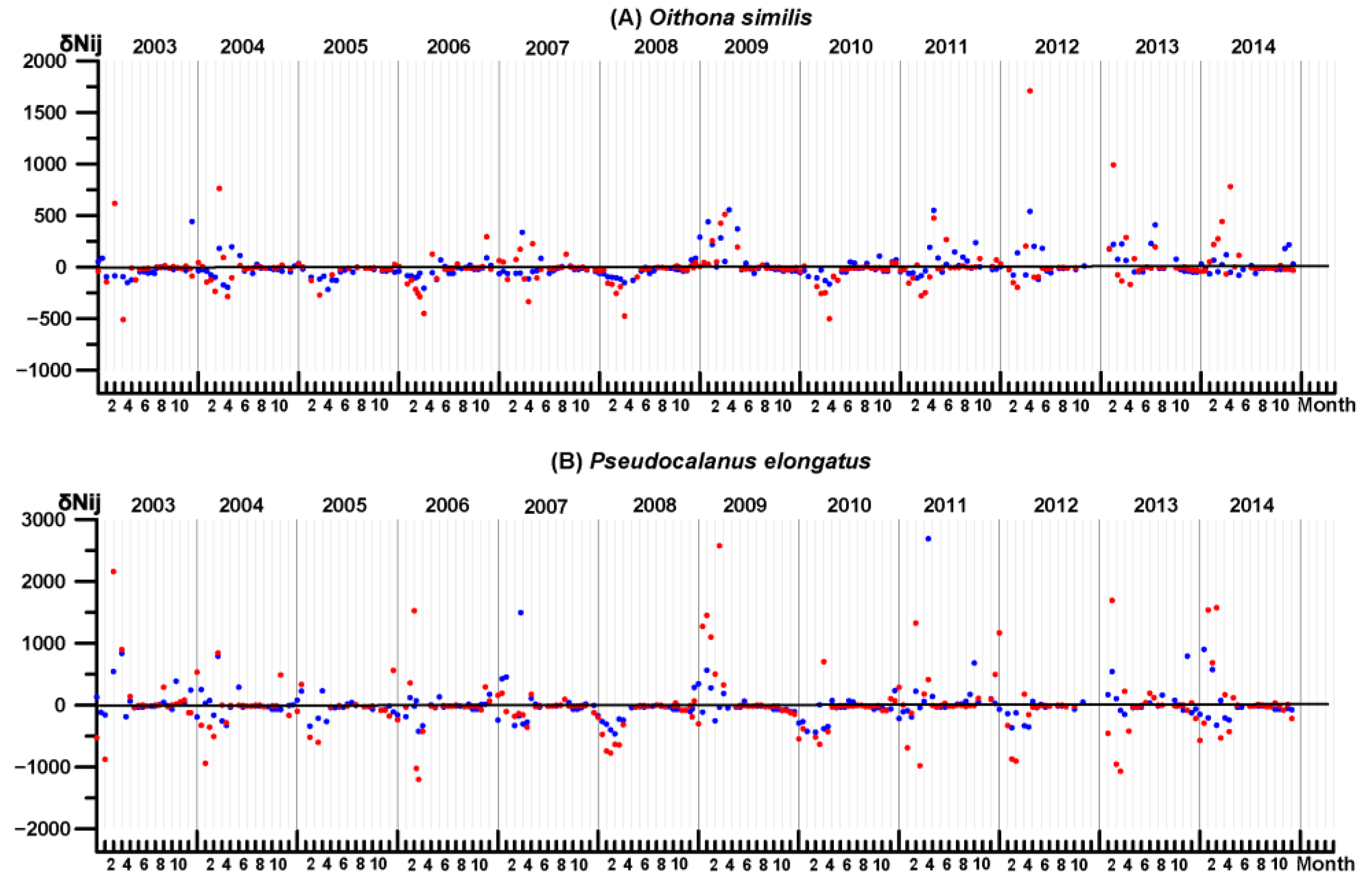
3.4. Key Species Variability
4. Discussion
4.1. Warm-Water Assemblages
4.2. Eurythermal Assemblages
4.3. Cold-Water Assemblage
4.4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Roberts, D.C.; Masson-Delmotte, V.; Zhai, P.; Tignor, M.; Poloczanska, E. Summary for policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Stanev, E.V.; Peneva, E.; Chtirkova, B. Climate Change and Regional Ocean Water Mass Disappearance: Case of the Black Sea. J. Geophys. Res. Ocean. 2019, 124, 4803–4819. [Google Scholar] [CrossRef]
- Mohamed, B.; Ibrahim, O.; Nagy, H. Sea Surface Temperature Variability and Marine Heatwaves in the Black Sea. Remote Sens. 2022, 14, 2383. [Google Scholar] [CrossRef]
- Barriopedro, D.; Fischer, E.M.; Luterbacher, J.; Trigo, R.M.; García-Herrera, R. The Hot Summer of 2010: Redrawing the Temperature Record Map of Europe. Science 2011, 332, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Soulié, T.; Vidussi, F.; Mas, S.; Mostajir, B. Functional Stability of a Coastal Mediterranean Plankton Community During an Experimental Marine Heatwave. Front. Mar. Sci. 2022, 9, 831496. [Google Scholar] [CrossRef]
- Richardson, A.J. In hot water: Zooplankton and climate change. ICES J. Mar. Sci. 2008, 65, 279–295. [Google Scholar] [CrossRef]
- Maskas, D.L.; Beaugrand, G. Comparisons of zooplankton time series. J. Mar. Syst. 2010, 79, 286–304. [Google Scholar] [CrossRef]
- Sazhina, L.T. Fertility of the mass pelagic copepoda in the Black Sea. Zool. Zhurnal 1971, 50, 586–588. (In Russian) [Google Scholar]
- Greze, V.N.; Baldina, E.P.; Bileva, O.K. Dynamics of the abundance and production of the principal components of Zooplankton in the Neritic Zone of the Black Sea. Mar. Biol. 1971, 24, 12–49. (In Russian) [Google Scholar]
- Evans, R.; Lea, M.-A.; Hindell, M.A.; Swadling, K.M. Significant shifts in coastal zooplankton populations through the 2015/16 Tasman Sea marine heatwave. Estuar. Coast. Shelf Sci. 2020, 235, 106538. [Google Scholar] [CrossRef]
- McKinstry, C.A.E.; Campbell, R.W.; Holderied, K. Influence of the 2014–2016 marine heatwave on seasonal zooplankton community structure and abundance in the lower Cook Inlet, Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2022, 195, 105012. [Google Scholar] [CrossRef]
- Zaika, V.E. Marine biodiversity of the Black Sea and Eastern Mediterranean. Ecol. Sea 2000, 51, 59–62. (in Russian). [Google Scholar]
- Benedetti, F.; Ayata, S.D.; Irisson, J.O.; Adloff, F.; Guilhaumon, F. Climate change may have minor impact on zooplankton functional diversity in the Mediterranean Sea. Divers. Distrib. 2019, 25, 568–581. [Google Scholar] [CrossRef]
- Gubanova, A.D.; Altukhov, D.A.; Stefanova, K.; Arashkevich, E.G.; Kamburska, L.; Prusova, I.Y.; Svetlichny, L.S.; Timofte, F.; Uysal, Z. Species composition of Black Sea marine planktonic copepods. J. Mar. Syst. 2014, 135, 44–52. [Google Scholar] [CrossRef]
- Gubanova, A.; Drapun, I.; Garbazey, O.; Krivenko, O.; Vodiasova, E. Pseudodiaptomus marinus Sato, 1913 in the Black Sea: Morphology, genetic analysis, and variability in seasonal and interannual abundance. PeerJ 2020, 8, e10153. [Google Scholar] [CrossRef]
- Greze, V.N. Zooplankton. In Basics of Biological Productivity of the Black Sea; Greze, V.N., Ed.; Naukova Dumka: Kiev, Ukraine, 1979; pp. 143–164. (In Russian) [Google Scholar]
- Finenko, Z. Biodiversity and Bioproductivity. In The Black Sea Environment. The Handbook of Environmental Chemistry; Water Pollution; Part, Q., Kostianoy, A.G., Kosarev, A.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5, pp. 351–374. [Google Scholar]
- Garmashov, A. Hydrological characteristics of Sevastopol Bay. Int. Multidiscip. Sci. Geo Conf. SGEM 2019, 19, 247–252. [Google Scholar] [CrossRef]
- Gubanov, V.I.; Gubanova, A.D.; Rodionova, N.Y. Diagnosis of water trophicity in the Sevastopol bay and its offshore. In Current issues in aquaculture: In Proceedings of the International Scientific Conference, Rostov-on-Don, Russia, 28 September–2 October 2015; pp. 64–67.
- Litvinyuk, D.; Mukhanov, V.; Evstigneev, V. The Black Sea Zooplankton Mortality, Decomposition, and Sedimentation Measurements Using Vital Dye and Short-Term Sediment Traps. J. Mar. Sci. Eng. 2022, 10, 1031. [Google Scholar] [CrossRef]
- Donlon, C.J.; Martin, M.; Stark, J.; Roberts-Jones, J.; Fielder, E.; Wimmer, W. The Operational Sea Surface Temperature and Sea Ice Analysis (OSTIA) system. Remote Sens. Environ. 2012, 116, 140–158. [Google Scholar] [CrossRef]
- Postel, L.; Fock, H.; Hagen, W. Biomass and abundance. In ICES Zooplankton Methodology Manual; Harris, R.P., Wiebe, P.H., Lenz, J., Skjoldal, H.R., Huntley, M., Eds.; Academic Press: London, UK, 2000; pp. 83–174. [Google Scholar]
- Aleksandrov, B.; Arashkevich, E.; Gubanova, A.; Korshenko, A. Black Sea monitoring guidelines mesozooplankton. Publ. EMBLAS Proj. BSC. 2014. Available online: http://www.blacksea-commission.org/Downloads/Mesozooplankton_Manual_2015_ISBN%20%20978-617-7953-33-2.pdf (accessed on 15 October 2020).
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Gubanova, A.; Altukhov, D. Establishment of Oithona brevicornis Giesbrecht, 1892 (Copepoda: Cyclopoida) in the Black Sea. Aquat. Invasions 2007, 2, 407–410. Available online: http://www.aquaticinvasions.net/2007/index4.html (accessed on 15 May 2007). [CrossRef]
- Temnykh, A.; Nishida, S. New record of copepod Oithona davisae Ferrari and Orsi in the Black Sea with notes on the identity of Oithona brevicornis. Aquat. Invasions 2012, 7, 425–431. [Google Scholar] [CrossRef]
- Altukhov, D.; Gubanova, A.; Mukhanov, V. New invasive copepod Oithona davisae Ferrari and Orsi, 1984: Seasonal dynamics in Sevastopol Bay and expansion along the Black Sea coasts. Mar. Ecol. 2014, 35, 28–34. [Google Scholar] [CrossRef]
- Odum, E. Fundamentals of Ecology; Saunders, W.B., Ed.; Saunders: Philadelphia, PA, USA; Moscow, Russia, 1975. [Google Scholar]
- Gubanova, A.D.; Garbazey, O.A.; Popova, E.V.; Altukhov, D.A.; Mukhanov, V.S. Oithona davisae: Naturalization in the Black Sea, interannual and Seasonal dynamics, and effect on the structure of the planktonic copepod community. Oceanology 2019, 59, 912–919. [Google Scholar] [CrossRef]
- Belmonte, G.; Mazzocchi, M.G.; Prusova, I.Y.; Shadrin, N.V. Acartia tonsa: A species new for the Black Sea fauna. Hydrobiologiya 1994, 292, 9–15. [Google Scholar] [CrossRef]
- Gubanova, A.D. Occurrence of Acartia tonsa Dana in the Black Sea. Was it introduced from the Metiterranean? Mediterr. Mar. Sci. 2000, 1, 105–109. [Google Scholar] [CrossRef]
- Itoh, H.; Nishida, S. Spatiotemporal distribution of planktonic copepod communities in Tokyo Bay where Oithona davisae ferrari and orsi dominated in mid-1980s. J. Nat. Hist. 2015, 49, 2759–2782. [Google Scholar] [CrossRef]
- Uriarte, I.; Villate, F.; Iriarte, A. Zooplankton recolonization of the inner estuary of Bilbao: Influence of pollution abatement, climate and non-indigenous species. J. Plankton Res. 2016, 38, 718–731. [Google Scholar] [CrossRef][Green Version]
- Kimmel, D.G.; Roman, M.R. Long-term trends in mesozooplankton abundance in Chesapeake Bay, USA: Influence of freshwater input. Mar. Ecol. Prog. Ser. 2004, 267, 71–83. [Google Scholar] [CrossRef]
- Besiktepe, S.; Kurt, T.T.; Gubanova, A. Mesozooplankton composition and distribution in Izmir Bay, Aegean Sea: With special emphasis on copepods. Reg. Stud. Mar. Sci. 2022, 55, 102567. [Google Scholar] [CrossRef]
- Piontkovski, S.A.; Fonda-Umani, S.; De Olazabal, A.; Gubanova, A.D. Penilia avirostris: Regional and Global Patterns of Seasonal Cycles. Int. J. Ocean. Oceanogr. 2012, 6, 9–25. [Google Scholar]
- Sazhina, L.I.; Kovalev, A.V. About the synonymy of the Black Sea copepods. Zool. J. 1971, 50, 1099–1101. (In Russian) [Google Scholar]
- Papantoniou, G.; Danielidis, D.B.; Spyropoulou, A.; Fragopoulu, N. Spatial and temporal variability of small-sized copepod assemblages in a shallow semi-enclosed embayment (Kalloni Gulf, NE Mediterranean Sea). J. Mar. Biolog. Assoc. UK 2015, 95, 349–360. [Google Scholar] [CrossRef]
- Azeiteiro, U.M.; Marques, S.C.; Vieira, L.M.R.; Pastorinho, M.R.D.; Ré, P.A.B.; Pereira, M.J.; Morgado, F.M.R. Dynamics of the Acartia genus (Calanoida: Copepoda) in a temperate shallow estuary (the Mondego estuary) on the western coast of Portugal. Acta Adriat. 2005, 46, 7–20. [Google Scholar]
- Jeffries, H.P. Succession of two Acartia species in estuaries. Limnol. Oceanogr. 1962, 7, 354–364. [Google Scholar] [CrossRef]
- Lee, W.Y.; McAlice, B.J. Seasonal succession and breeding cycles of three species of Acartia (Copepoda: Calanoida) in a Marine estuary. Estuaries 1979, 2, 228–235. [Google Scholar] [CrossRef]
- Frost, B.W. A taxonomy of the marine calanoid copepod genus Pseudocalanus. Can. J. Zool. 1989, 67, 525–551. [Google Scholar] [CrossRef]
- Razouls, C.; de Bovée, F.; Kouwenberg, J.; Desreumaux, N. Diversity and Geographic Distribution of Marine Planktonic Copepods, 2005–2022. Available online: http://copepodes.obs-banyuls.fr/en (accessed on 18 November 2022).
- Nishida, S. Taxonomy and distribution of the family Oithonidae (Copepoda, Cyclopoida) in the Pacific and Indian oceans. Bull. Ocean Res. Inst. Univ. Tokyo 1985, 20, 1–167. [Google Scholar]
- Vijverberg, J. Effect of temperature in laboratory studies on development and growth of Cladocera and Copepoda from Tjeukemeer, The Netherlands. Freshw. Biol. 1980, 10, 317–340. [Google Scholar] [CrossRef]
- Huber, V.; Adrian, R.; Gerten, D. A matter of timing: Heat wave impact on crustacean zooplankton. Fresh Water Biol. 2010, 55, 1769–1779. [Google Scholar] [CrossRef]
- Cornils, A.; Wend-Heckmann, B. First report of the planktonic copepod Oithona davisae in the northern Wadden Sea (North Sea): Evidence for recent invasion? Helgol. Mar. Res. 2015, 69, 243–248. [Google Scholar] [CrossRef][Green Version]
- Rice, E.; Dam, H.G.; Stewart, G. Impact of climate change on estuarine zooplankton: Surface water warming in long Island sound is associated with changes in copepod size and community structure. Estuaries Coasts 2015, 38, 13–23. [Google Scholar] [CrossRef]
- Uttieri, M.; Aguzzi, L.; Aiese Cigliano, R.; Amato, A.; Bojanić, N.; Brunetta, M.; Camatti, E.; Carotenuto, Y.; Damjanović, T.; Delpy, F.; et al. WGEUROBUS—Working Group ‘‘Towards a European Observatory of the non-indigenous calanoid copepod Pseudodiaptomus marinus”. Biol. Invasions 2020, 22, 885–906. [Google Scholar] [CrossRef]
- Bollens, S.M.; Breckenridge, J.; Cordell, J.R.; Simenstad, C.; Kalata, O. Zooplankton of tidal marsh channels in relation to environmental variables in the upper San Francisco Estuary). Aquat. Biol. 2014, 21, 205–219. [Google Scholar] [CrossRef][Green Version]
- Noss, R.F. Indicators for Monitoring Biodiversity: A Hierarchical Approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]


| Station 1. | ||||||||||||
| Year | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
| Samples Number | 21 | 21 | 19 | 24 | 21 | 24 | 23 | 23 | 22 | 22 | 21 | 22 |
| Penilia avirostris | 238 ± 117 | 433 ± 268 | 2391 ± 1596 | 792 ± 630 | 272 ± 166 | 334 ± 198 | 590 ± 370 | 1164 ± 597 | 825 ± 477 | 483 ± 284 | 518 ± 421 | 378 ± 241 |
| Pleopis polyphemoides | 227 ± 151 | 526 ± 246 | 741 ± 506 | 360 ± 114 | 383 ± 142 | 799 ± 513 | 372 ± 136 | 865 ± 374 | 795 ± 426 | 362 ± 219 | 376 ± 226 | 202 ± 97 |
| Acartia clausi | 582 ± 194 | 263 ± 56 | 232 ± 51 | 203 ± 52 | 142 ± 44 | 922 ± 408 | 198 ± 53 | 362 ± 162 | 389 ± 87 | 125 ± 35 | 573 ± 126 | 238 ± 42 |
| Acartia tonsa | 129 ± 47 | 480 ± 246 | 116 ± 53 | 99 ± 42 | 52 ± 26 | 62 ± 24 | 8 ± 7 | 72 ± 35 | 3 ± 1 | 13 ± 6 | 38 ± 18 | 13 ± 7 |
| Centropages ponticus | 62 ± 25 | 92 ± 54 | 37 ± 15 | 171 ± 50 | 346 ± 174 | 72 ± 31 | 321 ± 104 | 268 ± 109 | 177 ± 64 | 310 ± 174 | 471 ± 159 | 248 ± 57 |
| Oithona davisae | NA * | NA * | 22 ± 14 | 1892 ± 1056 | 2344 ± 1717 | 3256 ± 1349 | 5770 ± 1763 | 17,236 ± 5400 | 5043 ± 1384 | 4678 ± 2729 | 4211 ± 1159 | 7140 ± 3107 |
| Oithona similis | 30 ± 10 | 58 ± 20 | 23 ± 9 | 31 ± 7 | 63 ± 22 | 24 ± 8 | 160 ± 46 | 43 ± 10 | 122 ± 35 | 156 ± 57 | 127 ± 33 | 87 ± 21 |
| Paracalanus parvus | 173 ± 57 | 178 ± 41 | 377 ± 176 | 564 ± 169 | 524 ± 146 | 638 ± 207 | 1786 ± 354 | 1830 ± 567 | 1280 ± 249 | 474 ± 125 | 1261 ± 386 | 839 ± 165 |
| Pseudocalanus elongatus | 204 ± 74 | 193 ± 68 | 121 ± 44 | 120 ± 37 | 234 ± 102 | 55 ± 21 | 189 ± 61 | 64 ± 25 | 325 ± 141 | 118 ± 43 | 224 ± 67 | 180 ± 72 |
| Station 2. | ||||||||||||
| Year | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
| Samples number | 18 | 20 | 15 | 21 | 21 | 22 | 22 | 22 | 22 | 16 | 21 | 21 |
| Penilia avirostris | 284 ± 117 | 133 ± 100 | 1265 ± 1170 | 75 ± 29 | 73 ± 45 | 80 ± 62 | 664 ± 369 | 1202 ± 749 | 258 ± 221 | 348 ± 168 | 215 ± 144 | 2362 ± 2151 |
| Pleopis polyphemoides | 1051 ± 612 | 966 ± 475 | 959 ± 641 | 1636 ± 845 | 1401 ± 678 | 912 ± 501 | 688 ± 398 | 1056 ± 511 | 2027 ± 1086 | 1662 ± 820 | 1060 ± 777 | 617 ± 295 |
| Acartia clausi | 338 ± 162 | 308 ± 71 | 159 ± 50 | 372 ± 157 | 137 ± 45 | 472 ± 131 | 198 ± 43 | 232 ± 62 | 270 ± 63 | 83 ± 17 | 485 ± 117 | 375 ± 106 |
| Acartia tonsa | 1777 ± 1245 | 690 ± 361 | 599 ± 264 | 366 ± 215 | 292 ± 162 | 261 ± 152 | 30 ± 17 | 883 ± 609 | 73 ± 25 | 197 ± 110 | 483 ± 237 | 189 ± 130 |
| Centropages ponticus | 61 ± 36 | 40 ± 20 | 25 ± 10 | 36 ± 15 | 196 ± 98 | 32 ± 14 | 184 ± 63 | 79 ± 28 | 284 ± 130 | 183 ± 85 | 186 ± 53 | 122 ± 32 |
| Oithona davisae | NA * | NA * | 301 ± 168 | 4849 ± 2224 | 6867 ± 3128 | 16,312 ± 5456 | 22,069 ± 4345 | 41,754 ± 12,337 | 25,059 ± 6520 | 13,174 ± 6807 | 8946 ± 2017 | 18,131 ± 7869 |
| Oithona similis | 69 ± 50 | 120 ± 60 | 20 ± 7 | 49 ± 19 | 111 ± 36 | 22 ± 7 | 151 ± 60 | 36 ± 15 | 115 ± 37 | 247 ± 146 | 177 ± 67 | 193 ± 76 |
| Paracalanus parvus | 61 ± 17 | 85 ± 28 | 174 ± 60 | 364 ± 113 | 295 ± 65 | 484 ± 211 | 674 ± 160 | 731 ± 192 | 686 ± 137 | 214 ± 59 | 474 ± 91 | 548 ± 102 |
| Pseudocalanus elongatus | 324 ± 195 | 305 ± 118 | 224 ± 87 | 303 ± 145 | 262 ± 84 | 130 ± 40 | 598 ± 228 | 152 ± 64 | 370 ± 126 | 254 ± 115 | 241 ± 128 | 426 ± 184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubanova, A.; Goubanova, K.; Krivenko, O.; Stefanova, K.; Garbazey, O.; Belokopytov, V.; Liashko, T.; Stefanova, E. Response of the Black Sea Zooplankton to the Marine Heat Wave 2010: Case of the Sevastopol Bay. J. Mar. Sci. Eng. 2022, 10, 1933. https://doi.org/10.3390/jmse10121933
Gubanova A, Goubanova K, Krivenko O, Stefanova K, Garbazey O, Belokopytov V, Liashko T, Stefanova E. Response of the Black Sea Zooplankton to the Marine Heat Wave 2010: Case of the Sevastopol Bay. Journal of Marine Science and Engineering. 2022; 10(12):1933. https://doi.org/10.3390/jmse10121933
Chicago/Turabian StyleGubanova, Alexandra, Katerina Goubanova, Olga Krivenko, Kremena Stefanova, Oksana Garbazey, Vladimir Belokopytov, Tatiana Liashko, and Elitsa Stefanova. 2022. "Response of the Black Sea Zooplankton to the Marine Heat Wave 2010: Case of the Sevastopol Bay" Journal of Marine Science and Engineering 10, no. 12: 1933. https://doi.org/10.3390/jmse10121933
APA StyleGubanova, A., Goubanova, K., Krivenko, O., Stefanova, K., Garbazey, O., Belokopytov, V., Liashko, T., & Stefanova, E. (2022). Response of the Black Sea Zooplankton to the Marine Heat Wave 2010: Case of the Sevastopol Bay. Journal of Marine Science and Engineering, 10(12), 1933. https://doi.org/10.3390/jmse10121933







