Characteristic and Relative Environmental Risk of Disinfection by Products Associated with Simple Glucose or Naturally Occurring Algal Organic Matter as Tested in Ballast Water Treatment System
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Test Water and the Sampling Period
2.2. Water-Quality Parameters
2.3. Relevant Chemicals
2.4. Environment Risk Assessment
2.5. Whole Effluent Toxicity Testing
3. Results and Discussion
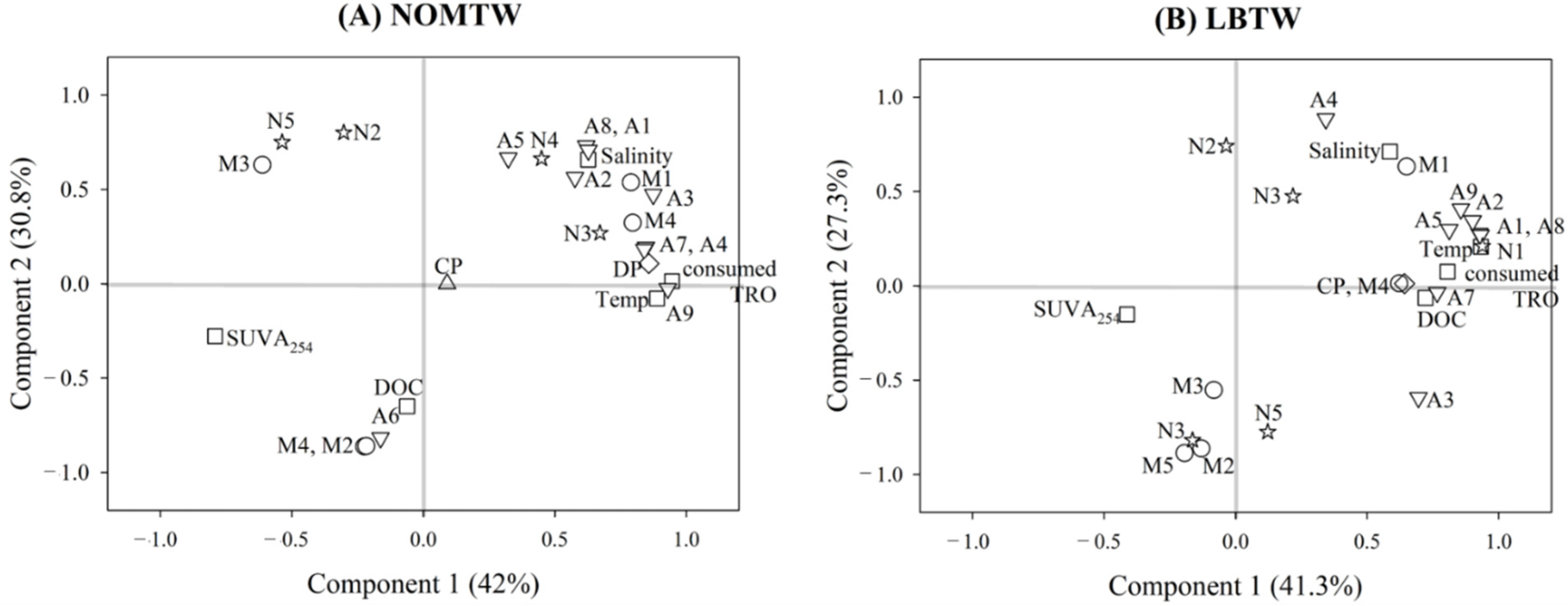
3.1. Comparison of Environmental Parameters
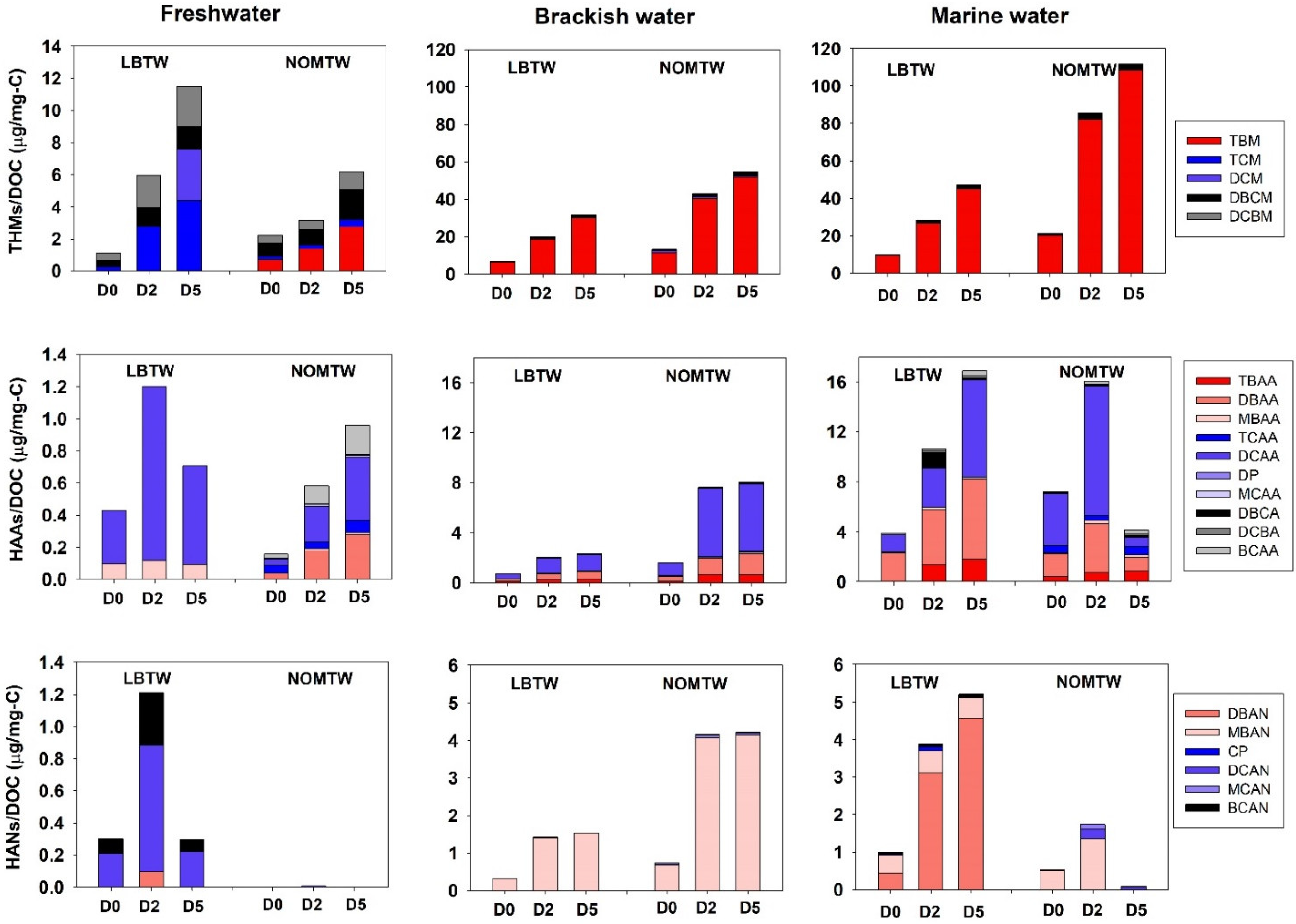
3.2. Comparison of the Disinfection By-Products
3.3. Comparison of MAMPEC Results
3.4. Comparison of the WET Test Results
4. Conclusions
- A greater number of DBP species in freshwater and higher concentrations of DBPs in brackish and marine water could be generated in real aquatic environments.
- Br-DBPs can be formed in relatively high concentration when ballast water is treated using active substances in a real freshwater environment.
- DBAN and CP exceeded a PEC/PNEC ratio of 1 only in the LBTW test, but the WET results showed that the chronic toxicity of phytoplankton was highest in the AOMTW, indicating that the total concentration of DBPs may be more important than individual concentrations.
- The WET testing showed that the concentrations of HAAs and HANs play an important role in environmental risk. Therefore, it is necessary to monitor the concentration of DBPs, especially HAAs and HANs, in major international ports where AOM concentrations may increase due to frequent phytoplankton outbreaks.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Maritime Organization (IMO). International Convention for the Control and Management of Ship’s Ballast Water and Sediment, 2004. Available online: http://www.imo.org/en/About/Conventions/ListOfConventions/Pages/International-Convention-for-the-Control-and-Management-of-Ships%27-Ballast-Water-and-Sediments-(BWM).aspx (accessed on 23 September 2022).
- Code of Fedral Regulation (CFR). Title 46 Shipping, PART 162 Engineering Equipment, Subpart 162.060-Ballast Water Management Systems, 2016. Available online: https://www.ecfr.gov/current/title-46/chapter-l/subchapter-Q/part-162/subpart-162.060 (accessed on 23 September 2022).
- Marine Environmental Protection Committee (MEPC). Code for Approval of Ballast Water Management System (BWMS CODE) (MEPC 72/17/Add.1/Annex 5), 2018. Available online: https://docs.imo.org/Category.aspx?cid=47&session=72 (accessed on 28 September 2022).
- US Environmental Protection Agency (EPA). Generic Protocol for the Verification of Ballast Water Treatment Technology (EPA/600/R-10/146). 2010. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P10097A4.PDF?Dockey=P10097A4.PDF (accessed on 23 September 2022).
- Jang, P.G.; Cha, H.G. Long-term changes of disinfection byproducts in treatment of simulated ballast water. Ocean Sci. J. 2020, 55, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Gonsior, M.; Mitchelmore, C.; Heyes, A.; Harir, M.; Richardson, S.D.; Petty, W.T.; Wright, D.A.; Schmitt-Kopplin, P. Bromination of marine dissolved organic matter following full scale electrochemical ballast water disinfection. Environ. Sci. Technol. 2015, 49, 9048–9055. [Google Scholar] [CrossRef] [PubMed]
- International Maritime Organization (IMO). Methodology for Information Gathering and Conduct of Work of GESAMP-BWWG (BWM.2/Circ.13/Rev.4), 20 July 2017. Available online: www.Gesamp.org/site/assets/files/1708/bwm_2-circ_13-rev-4.pdf (accessed on 15 November 2022).
- Shah, A.D.; Liu, Z.Q.; Salhi, E.; Hoefer, T.; Werschkun, B.; Von Gunten, U. Formation of disinfection by-products during ballast water treatment with ozone, chlorine, and peracetic acid: Influence of water quality parameters. Environ. Sci. Water Res. Technol. 2015, 1, 465–480. [Google Scholar] [CrossRef]
- Yu, H.W.; Oh, S.G.; Kim, I.S.; Pepper, I.; Snyder, S.; Jang, A. Formation and speciation of haloacetic acids in seawater desalination using chlorine dioxide as disinfectant. J. Ind. Eng. Chem. 2015, 26, 193–201. [Google Scholar] [CrossRef]
- Cha, H.G.; Seo, M.H.; Lee, H.Y.; Lee, J.H.; Lee, D.S.; Shin, K.; Choi, K.H. Enhancing the efficacy of electrolytic chlorination for ballast water treatment by adding carbon dioxide. Mar. Pollut. Bull. 2015, 95, 315–323. [Google Scholar] [CrossRef]
- Delacroix, S.; Vogelsang, C.; Tobiesen, A.; Liltved, H. Disinfection by-products and ecotoxicity of ballast water after oxidative treatment–Results and experiences from seven years of full-scale testing of ballast water management systems. Mar. Pollut. Bull. 2013, 73, 24–36. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, J.; Wang, Q.; Yuan, L.; Wu, H. Formation of halogenated disinfection by-products during ballast water chlorination. Environ. Sci. Water Res. Technol. 2022, 8, 648–656. [Google Scholar] [CrossRef]
- Lee, J.; Shon, M.B.; Cha, H.G.; Choi, K.H. The impact of adding organic carbon on the concentrations of total residual oxidants and disinfection by-products in approval tests for ballast water management systems. Sci. Total Environ. 2017, 605, 852–859. [Google Scholar] [CrossRef]
- Biddanda, B.; Benner, R. Oceanography. Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. Limnol. Oceanogr. 1997, 42, 506–518. [Google Scholar] [CrossRef]
- Chrost, R.H.; Faust, M.A. Organic carbon release by phytoplankton: Its composition and utilization by bacterioplankton. J. Plankton Res. 1983, 5, 477–493. [Google Scholar] [CrossRef]
- Zohdi, E.; Abbaspour, M. Technology. Harmful algal blooms (red tide): A review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar] [CrossRef]
- Myklestad, S.M. Dissolved organic carbon from phytoplankton. In Marine Chemistry; Springer: Berlin/Heidelberg, Germany, 2000; pp. 111–148. ISBN 978-3-540-48776-0. [Google Scholar]
- Liu, C.; Ersan, M.S.; Plewa, M.J.; Amy, G.; Karanfil, T. Formation of regulated and unregulated disinfection byproducts during chlorination of algal organic matter extracted from freshwater and marine algae. Water Res. 2018, 142, 313–324. [Google Scholar] [CrossRef]
- Huang, J.; Graham, N.; Templeton, M.; Zhang, Y.; Collins, C.; Nieuwenhuijsen, M. A comparison of the role of two blue–green algae in THM and HAA formation. Water Res. 2009, 43, 3009–3018. [Google Scholar] [CrossRef]
- Rostad, C.E.; Martin, B.S.; Barber, L.B.; Leenheer, J.A.; Daniel, S.R. Effect of a constructed wetland on disinfection byproducts: Removal processes and production of precursors. Environ. Sci. Technol. 2000, 34, 2703–2710. [Google Scholar] [CrossRef]
- Ziegler, G.; Gonsior, M.; Fisher, D.J.; Schmitt-Kopplin, P.; Tamburri, M.N. Formation of brominated organic compounds and molecular transformations in dissolved organic matter (DOM) after ballast water treatment with sodium dichloroisocyanurate dehydrate (DICD). Environ. Sci. Technol. 2019, 53, 8006–8016. [Google Scholar] [CrossRef]
- Hua, L.C.; Lin, J.L.; Syue, M.Y.; Huang, C.; Chen, P.C. Optical properties of algogenic organic matter within the growth period of Chlorella sp. and predicting their disinfection by-product formation. Sci. Total Environ. 2018, 621, 1467–1474. [Google Scholar] [CrossRef]
- Fang, J.; Yang, X.; Ma, J.; Shang, C.; Zhao, Q. Characterization of algal organic matter and formation of DBPs from chlor (am) ination. Water Res. 2010, 44, 5897–5906. [Google Scholar] [CrossRef]
- Li, L.; Gao, N.; Deng, Y.; Yao, J.; Zhang, K. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012, 46, 1233–1240. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Ismail, N.; Drouillard, K.G.; Maclasaac, H.J. Ship’s ballast water treatment by chlorination can generate toxic trihalomethanes. Bull. Environ. Contam. Toxicol. 2017, 99, 194–199. [Google Scholar] [CrossRef]
- Bai, M.; Tian, Y.; Yu, Y.; Zheng, Q.; Zhang, X.; Zhang, W.; Zhang, Z. Application of a hydroxyl-radical-based disinfection system for ballast water. Chemosphere 2018, 208, 541–549. [Google Scholar] [CrossRef]
- Marine Environmental Protection Committee, MEPC, Information on the GESAMP-BWWG Database of Chemicals Most Commonly Associated with Treated Ballast Water, 2014, MEPC 67/INF17. Available online: https://docs.imo.org/Documents/Detail.aspx?did=88063 (accessed on 26 February 2020).
- Jin, X.; Zha, J.; Xu, Y.; Giesy, J.P.; Richardson, K.L.; Wang, Z. Derivation of predicted no effect concentrations (PNEC) for 2,4,6-trichlorophenol based on Chines resident species. Chemosphere 2012, 86, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, P.; Chang, H.S.; Vagliasindi, F.G.A.; Korshin, G.V. Differential absorbance study of effects of temperature on chlorine consumption and formation of disinfection by-products in chlorinated water. Water Res. 2008, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Biswas, H.; Jie, J.; Li, Y.; Zhang, G.; Zhu, Z.Y.; Wu, Y.; Zhang, G.-L.; Li, Y.-W.; Liu, S.M.; Zhang, J. Response of a natural Phytoplankton community from the Qingdao coast (Yellow Sea, China) to variable CO 2 levels over a short-term incubation experiment. Curr. Sci. 2015, 108, 1901–1909. [Google Scholar]
- Smith, W.O.; Barber, R.T.; Huntsman, S.A. Primary production off the coast of northwest Africa: Excretion of dissolved organic matter and its heterotrophic uptake. Deep Sea Res. 1977, 24, 35–47. [Google Scholar] [CrossRef]
- Kitis, M.; Karanfil, T.; Wigton, A.; Kilduff, J.E. Probing reactivity of dissolved organic matter for disinfection by-product formation using XAD-8 resin adsorption and ultrafiltration fractionation. Water Res. 2002, 36, 3834–3848. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A.; Abusallout, I. Correlation between SUVA and DBP formation during chlorination and chloramination of NOM fractions from different sources. Chemosphere 2015, 130, 82–89. [Google Scholar] [CrossRef]
- Hua, L.C.; Chao, S.J.; Huang, K.; Huang, C. Characteristics of low and high SUVA precursors: Relationships among molecular weight, fluorescence, and chemical composition with DBP formation. Sci. Total Environ. 2020, 727, 138638. [Google Scholar] [CrossRef]
- Ates, N.; Kitis, M.; Yetis, U. Formation of chlorination by-products in waters with low SUVA—Correlations with SUVA and differential UV spectroscopy. Water Res. 2007, 41, 4139–4148. [Google Scholar] [CrossRef]
- Chow, A.T.; Leech, D.M.; Boyer, T.H.; Singer, P.C. Impact of simulated solar irradiation on disinfection byproduct precursors. Environ. Sci. Technol. 2008, 42, 5586–5593. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, X.Y.; Xie, Y.F.; Tang, H. Characterization of soluble microbial products as precursors of disinfection byproducts in drinking water supply. Sci. Total Environ. 2014, 472, 818–824. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, X.Y. Biodegradation and biotransformation of wastewater organics as precursors of disinfection byproducts in water. Chemosphere 2010, 81, 1075–1083. [Google Scholar] [CrossRef]
- Kim, D.; Amy, G.L.; Karanfil, T. Disinfection by-product formation during seawater desalination: A review. Water Res. 2015, 81, 343–355. [Google Scholar] [CrossRef]
- Westerhoff, P.; Chao, P.; Mash, H. Reactivity of natural organic matter with aqueous chlorine and bromine. Water Res. 2004, 38, 1502–1513. [Google Scholar] [CrossRef]
- Muellner, M.G.; Wagner, E.D.; McCalla, K.; Richardson, S.D.; Woo, Y.T.; Plewa, M.J. Haloacetonitriles vs. regulated haloacetic acids: Are nitrogen-containing DBPs more toxic? Environ. Sci. Technol. 2007, 41, 645–651. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Pambudi, D.S.A.; Ahmad, M.M.; Alfanda, B.D.; Imron, M.F.; Abdullah, S.R.S. Ecological impacts of ballast water loading and discharge: Insight into the toxicity and accumulation of disinfection by-products. Heliyon 2022, 8, 1–8. [Google Scholar] [CrossRef]
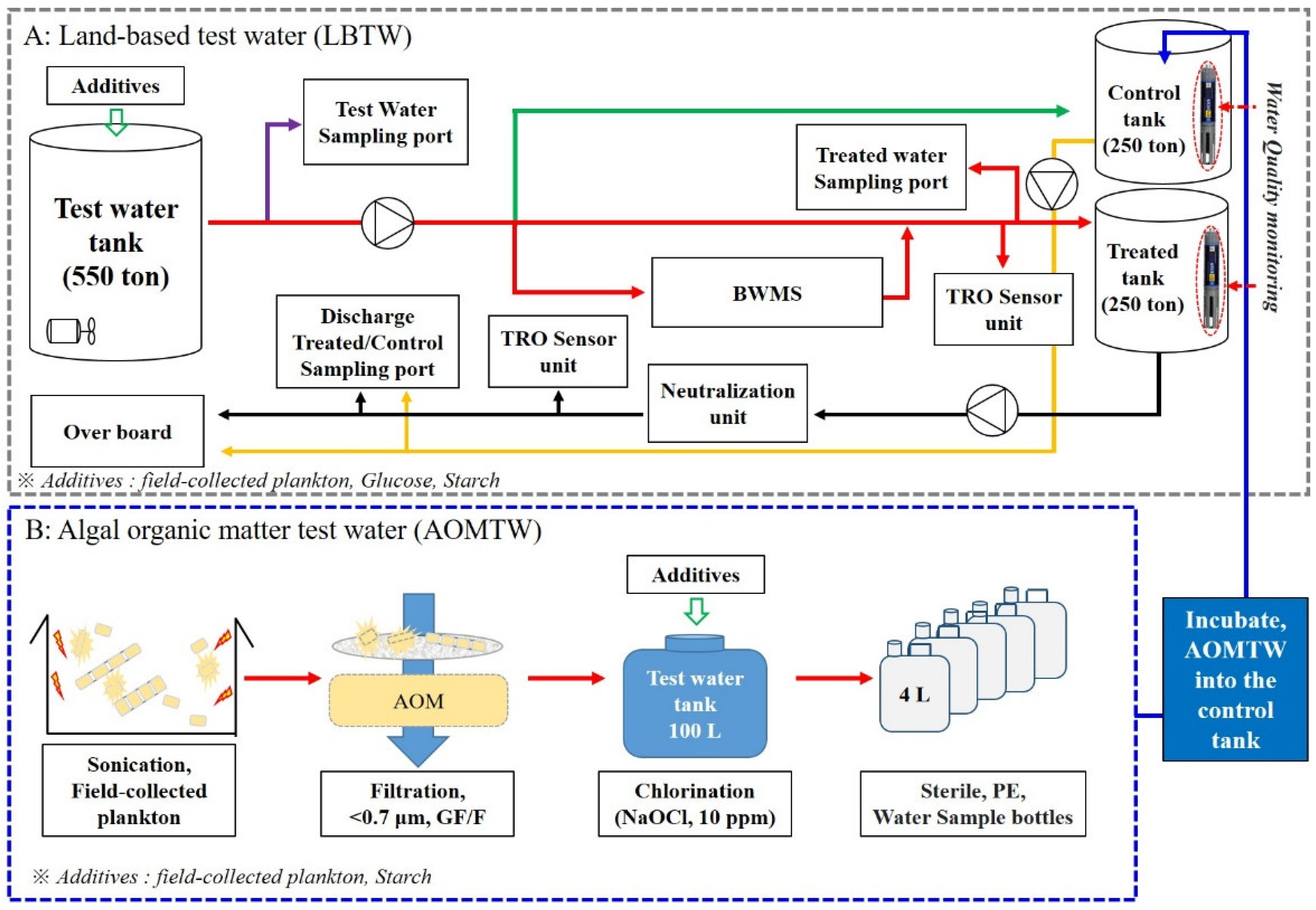
| Parameter | Test Water Condition | Discharge Water | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IMO | USCG | Control Water | Treated Water (D-2) | ||||||
| unit | F | B | M | F | B | M | |||
| Salinity | <1 | 10–20 | 28–34 | <1 | 10–20 | 28–36 | |||
| POC | mg/L | 1 | 5 | 4 | - | - | |||
| DOC | 1 | 5 | 6 | - | - | ||||
| TSS | 1 | 50 | 24 | - | - | ||||
| Escherichia coli | cfu/100 mL | Measured | Measured | - | <250 | ||||
| Intestinal Enterococci | Measured | Measured | - | <100 | |||||
| Vibrio cholera (O1&O129) | Measured | Measured | - | <1 | |||||
| Heterotrophic bacteria | cells/ml | 104 | 103 | - | Measured | ||||
| Organisms of ≥10 & <50 μm | organisms/mL | 103 | 103 | >100 | <10 | ||||
| Organisms of ≥50 μm | organisms/m3 | 105 | 105 | >100 | <10 | ||||
| Compounds | Acronyms | Mark * | Compound | Acronyms | Mark |
|---|---|---|---|---|---|
| Trihalomethanes | THMs | Trichloroacetic acid | TCAA | A4 | |
| Haloacetic acids | HAAs | Dichloroacetic acid | DCAA | A5 | |
| Haloacetonitriles | HANs | Monochloroacetic acid | MCAA | A6 | |
| Halogenated propionic acid | HPA | Dibromochloroacetic acid | DBCA | A7 | |
| Halogenated nitroalkane | HN | Dichlorobromoacetic acid | DCBA | A8 | |
| Bromoform | TBM | M1 | Bromochloroacetic acid | BCAA | A9 |
| Chloroform | TCM | M2 | Dibromoacetonitrile | DBAN | N1 |
| Dichloromethane | DCM | M3 | Monobromoacetonitrile | MBAN | N2 |
| Dibromochloromethane | DBCM | M4 | Dichloroacetonitrile | DCAN | N3 |
| Dichlorobromomethane | DCBM | M5 | Monochloroacetonitrile | MCAN | N4 |
| Tribromoacetic acid | TBAA | A1 | Bromochloroacetonitrile | BCAN | N5 |
| Dibromoacetic acid | DBAA | A2 | Chloropicrin | CP | CP |
| Monobromoacetic acid | MBAA | A3 | Dalapon | DP | DP |
| Taxon | Condition | Species | End-Point | Test Duration | References |
|---|---|---|---|---|---|
| Algae | Freshwater | Raphidocelis subcapitata | Population growth Inhibition | 72 h | OECD 201 USEPA 2002b EPA-821-R-02-013 (Method 1003.0) |
| Seawater/ Brackish water | Isochrysis galbana * | OECD 201 or ISO 10253; 2006 | |||
| Invertebrate | Freshwater | Daphnid (Daphnia magna) | Mortality | 48 h | USEPA 2002a EPA-821-R-02-012 (Method 2021.0) |
| Daphnid (Ceriodaphnia dubia) | Survival and reproduction | 8 days | USEPA 2002b EPA-821-R-02-013 (Method 1002.0) | ||
| Seawater/ Brackish water | Mysid (Neomysis awatschensis) | Mortality | 48 h | USEPA 2002a EPA-821-R-02-012 (Method 2007.0) | |
| Mysid (Neomysis awatschensis) | Survival and growth | 7 days | USEPA 2002c EPA-821-R-02-014 (Method 1007.0) | ||
| Vertebrate (Fish) | Freshwater | Oryzias latipes | Mortality | 96 h | USEPA 2002a EPA-821-R-02-012 (Method 2000.0) |
| Survival and growth | 7 days | USEPA 2000b EPA-821-R-02-013 (Method 1000.0) | |||
| Seawater/ Brackish water | Cyprindon variegates * | Mortality | 96 h | USEPA 2002a. EPA-821-R-02-012 (Method 2000.0 and 2004.0) | |
| Survival and growth | 7 days | USEPA 2002c EPA-821-R-02-014 (Method 1004.0) |
| Parameters | Time (Day) | LBTW | AOMTW | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Water | Brackish Water | Marine Water | Fresh Water | Brackish Water | Marine Water | ||||||||
| C | T | C | T | C | T | C | T | C | T | C | T | ||
| Temperature (°C) | 0 | 14.0 | 14.0 | 10.3 | 10.9 | 22.2 | 22.4 | ||||||
| 2 | 12.5 | 12.5 | 9.6 | 9.6 | 23.1 | 22.8 | |||||||
| 5 | 12.4 | 12.7 | 8.8 | 8.3 | 23.8 | 23.5 | |||||||
| Salinity (PSU) | 0 | 0.17 | 0.17 | 17.7 | 17.7 | 32.0 | 32.0 | ||||||
| TRO (mg L−1) n = 2 | 0 | - | 7.90(0.14) | - | 7.80(0.1) | - | 8.37(0.1) | - | 9.90(0.00) | - | 10.4(0.14) | - | 10.0(0.00) |
| 2 | - | 4.10(0.14) | - | 2.47(0.01) | - | 1.57(0.00) | - | 0.67(0.01) | - | 4.95(0.00) | - | 0.01(0.01) | |
| 5 | - | 2.30(0.00) | - | 1.03(0.01) | - | 0.67(0.14) | - | 0.09(0.00) | - | 3.14(0.00) | - | 0.01(0.00) | |
| DOC (mg L−1) n = 3 | 0 | 6.03 (0.12) | 6.63 (0.05) | 6.14 (0.14) | 6.57 (0.02) | 6.63 (0.11) | 6.98 (0.12) | 7.43 (0.18) | 11 (0.24) | 5.37 (0.08) | 6.18 (0.11) | 6.14 (0.06) | 9.39 (0.17) |
| 2 | 5.47 (0.07) | 7.00 (0.14) | 8.56 (0.13) | 7.53 (0.04) | 6.32 (0.09) | 9.01 (0.02) | 6.76 (0.02) | 14.78 (0.37) | 8.35 (0.24) | 7.55 (0.14) | 3.61 (0.07) | 9.99 (0.09) | |
| 5 | 4.03 (0.09) | 7.07 (0.11) | 3.73 (0.07) | 6.61 (0.10) | 7.11 (0.21) | 7.05 (0.05) | 4.67 (0.17) | 9.33 (0.19) | 5.19 (0.04) | 7.93 (0.01) | 3.16 (0.05) | 6.23 (0.01) | |
| SUVA254 (m−1 of absorbance per mg/L of DOC) | 0 | 0.437 | 1.176 | 0.342 | 2.261 | 0.196 | 1.526 | 1.306 | 1.109 | 0.87 | 1.401 | 0.741 | 1.128 |
| 2 | 0.548 | 2.372 | 0.206 | 0.416 | 1.316 | 0.880 | 0.425 | 1.106 | 0.499 | 0.095 | |||
| 5 | 0.678 | 1.278 | 0.870 | 2.459 | 1.350 | 1.248 | 1.886 | 1.826 | 0.694 | 1.192 | 0.680 | 0.385 | |
| Active chlorophyll-a (μg L−1) n = 3 | 0 | 33.6 (0.22) | 4.9 (0.11) | 21.9(0.85) | 1.1 (0.04) | 19.5 (0.24) | 2.3 (0.36) | 77.1 (1.70) | 7.0 (0.23) | 26.1 (0.85) | 0.4 (0.20) | 61.4 (1.94) | 1.9 (0.19) |
| 2 | 22.4 (1.10) | 0.7 (0.07) | 25.7 (0.40) | 0.0 | 0.3 (0.03) | 0.0 | 22.0 (0.12) | 2.0 (0.12) | 27.1 (1.27) | 0.0 | 3.1 (0.11) | 0.0 | |
| 5 | 18.7 (0.30) | 0.5 (0.10) | 22.7 (0.47) | 0.0 | 0.3 (0.02) | 0.0 (0.00) | 24.7 (0.25) | 0.1 (0.02) | 26.2 (1.37) | 0.0 | 0.7 (0.07) | 0.0 | |
| Heterotrophic bacteria (CFU/mL) n = 3 | 0 | 207,333 (3055) | 74,000 (2291) | 141,667 (7638) | - | - | - | - | - | - | |||
| 5 | 351,667 (10,408) | 3983 (126) | 281,667 (2887) | 1983 (126) | 403,333 (60,277) | 4500 (1000) | - | - | - | - | - | - | |
| LBTW | AOMTW | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Chemical Group | Asseseement Factor | PEC | PNEC | PEC/PNEC | PEC | PNEC | PEC/PNEC |
| TBM | THMs | 5.0 × 10 | 0.38 × 10 | 9.6 × 10 | 4.0 × 10−2 | 0.98 × 10 | 9.6 × 10 | 1.0 × 10−1 |
| TCM | 5.0 × 10 | 3.0 × 10−1 | 1.5 × 102 | 2.0 × 10−3 | 3.9 × 10−2 | 1.5 × 102 | 2.6 × 10−4 | |
| DCM | 5.0 × 10 | 2.0 × 10−2 | 1.2 × 102 | 1.7 × 10−4 | 4.4 × 10−3 | 1.2 × 102 | 3.7 × 10−5 | |
| DBCM | 1.0 × 10 | 1.6 × 10−1 | 0.63 × 10 | 2.5 x10−2 | 3.3 × 10−1 | 0.63 × 10 | 5.3 × 10−2 | |
| DCBM | 1.0 × 10 | 1.8 × 10−1 | 7.8 × 101 | 2.3 × 10−3 | 1.0 × 10−1 | 7.8 × 10 | 1.3 × 10−3 | |
| TBAA | HAAs | 1.0 × 10 | 2.7 × 10−1 | 1.4 × 104 | 2.0 × 10−5 | 1.3 × 10−1 | 1.4 × 104 | 8.9 × 10−6 |
| DBAA | 1.0 × 10 | 8.7 × 10−1 | 6.9 × 103 | 1.3 × 10−4 | 7.0 × 10−1 | 6.9 × 103 | 1.0 × 10−4 | |
| MBAA | 1.0 × 102 | 4.0 × 10−2 | 1.6 × 10 | 2.5 × 10−3 | 4.3 × 10−2 | 1.6 × 10 | 2.7 × 10−3 | |
| TCAA | 1.0 × 10 | 2.7 × 10−3 | 3.0 × 102 | 9.0 × 10−6 | 9.2 × 10−2 | 3.0 × 102 | 3.1 × 10−4 | |
| DCAA | 1.0 × 103 | 9.3 × 10−1 | 2.3 × 10 | 4.1 × 10−2 | 0.20 x10 | 2.3 × 10 | 8.8 × 10−2 | |
| MCAA | 1.0 × 10 | 2.0 × 10−3 | 5.8 × 10−1 | 3.5 × 10−3 | 3.6 × 10−3 | 5.8 × 10−1 | 6.1 × 10−3 | |
| DBCA | 1.0 × 10 | 3.2 × 10−1 | 3.0 × 102 | 1.1 × 10−3 | 1.6 × 10−2 | 3.0 × 102 | 5.2 × 10−5 | |
| DCBA | 5.0 × 10 | 3.3 × 10−2 | 6.0 × 10 | 5.4 × 10−4 | 1.3 × 10−2 | 6.0 × 10 | 2.1 × 10−4 | |
| BCAA | 1.0 × 102 | 4.9 × 10−2 | 1.6 × 10 | 3.0 × 10−3 | 5.1 × 10−2 | 1.6 × 10 | 3.2 × 10−3 | |
| DBAN | HANs | 1.0 × 104 | 0.11 × 10 | 5.5 × 10−2 | 1.9 × 10 | |||
| MBAN | 1.0 × 103 | 1.8 × 10−1 | 2.3 × 10 | 8.0 × 10−3 | 5.5 × 10−1 | 2.3 × 10 | 2.4 × 10−2 | |
| DCAN | 1.0 × 103 | 4.5 × 10−3 | 2.4 × 10 | 1.9 × 10−4 | 1.1 × 10−3 | 2.4 × 10 | 4.4 × 10−5 | |
| MCAN | 1.0 × 104 | 5.4 × 10−2 | 1.6 × 10−1 | 3.4 × 10−2 | 1.1 × 10−2 | 1.6 × 10−1 | 6.8 × 10−2 | |
| BCAN | 1.0 × 103 | 4.9 × 10−2 | 6.9 × 10−1 | 7.2 × 10−2 | 1.8 × 10−3 | 6.9 × 10−1 | 2.6 × 10−3 | |
| DP | HPA | 1.0 × 103 | 2.5 × 10−3 | 1.1 × 10 | 2.2 × 10−4 | |||
| CP | HN | 1.0 × 102 | 3.4 × 10−2 | 2.5 × 10−2 | 1.4 × 10 | 1.9 × 10−4 | 2.5 × 10−2 | 7.7 × 10−3 |
| Environmental Condition | Freshwater | Brackish Water | Marine Water | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | 0 | 2 | 5 | 0 | 2 | 5 | 0 | 2 | 5 | |
| LBTW | Temp. (°C) | 5.4 | 4.8 | 5.3 | 6.7 | 5.6 | 5.2 | 7.9 | 8.3 | 6.5 |
| Sal. (PSU) | 0.2 | 0.2 | 0.3 | 17.9 | 17.6 | 17.7 | 32.3 | 32.4 | 31.7 | |
| pH | 7.02 | 7.20 | 7.22 | 8.07 | 8.10 | 7.76 | 8.01 | 7.98 | 7.90 | |
| DO (mg/L) | 7.49 | 7.29 | 7.29 | 8.14 | 8.20 | 8.57 | 6.58 | 6.43 | 6.74 | |
| AOMTW | Temp. (°C) | 4.8 | 2.2 | 2.8 | 4.5 | 3.3 | 2.4 | 5.9 | 6.4 | 4.3 |
| Sal. (PSU) | 2.0 | 1.9 | 1.9 | 23.1 | 23.1 | 23.2 | 33.2 | 33.1 | 33.2 | |
| pH | 7.59 | 7.60 | 7.59 | 8.32 | 8.21 | 8.33 | 7.98 | 7.96 | 7.94 | |
| DO (mg/L) | 5.92 | 5.54 | 5.68 | 8.98 | 8.68 | 8.60 | 7.69 | 7.65 | 7.53 | |
| LBTW | AOMTW | |||||||||
| Water Type | Test Species | End point | Day | NOEC (%) | LC50 or EC50 (%) | NOEC (%) | LC50 or EC50 (%) | |||
| Fresh Water | Raphidocelis subcapitata | Algal growth inhibition (72 h) | 0 | 50 | >100 | 100 | >100 | |||
| 2 | 25 | >100 | <6.25 | >100 | ||||||
| 5 | 0 | >100 | <6.25 | >100 | ||||||
| Brackish Water | Isochrysis galbana | Algal growth inhibition (72 h) | 0 | 100 | >100 | 62.5 | >100 | |||
| 2 | 25 | >100 | 25 | 57.6 | ||||||
| 5 | 25 | 76.7 | 25 | 60.9 | ||||||
| Marine Water | Isochrysis galbana | Algal growth inhibition (72 h) | 0 | 100 | >100 | 25 | 96.0 | |||
| 2 | 62.5 | >100 | 12.5 | 33.4 | ||||||
| 5 | 50 | >100 | 25 | 86.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, P.-G.; Cha, H.-G.; Jang, M.-C.; Hyun, B.; Choi, T.S.; Kang, Y.; Shin, K. Characteristic and Relative Environmental Risk of Disinfection by Products Associated with Simple Glucose or Naturally Occurring Algal Organic Matter as Tested in Ballast Water Treatment System. J. Mar. Sci. Eng. 2022, 10, 1928. https://doi.org/10.3390/jmse10121928
Jang P-G, Cha H-G, Jang M-C, Hyun B, Choi TS, Kang Y, Shin K. Characteristic and Relative Environmental Risk of Disinfection by Products Associated with Simple Glucose or Naturally Occurring Algal Organic Matter as Tested in Ballast Water Treatment System. Journal of Marine Science and Engineering. 2022; 10(12):1928. https://doi.org/10.3390/jmse10121928
Chicago/Turabian StyleJang, Pung-Guk, Hyung-Gon Cha, Min-Chul Jang, Bonggil Hyun, Tae Seob Choi, Younseok Kang, and Kyoungsoon Shin. 2022. "Characteristic and Relative Environmental Risk of Disinfection by Products Associated with Simple Glucose or Naturally Occurring Algal Organic Matter as Tested in Ballast Water Treatment System" Journal of Marine Science and Engineering 10, no. 12: 1928. https://doi.org/10.3390/jmse10121928
APA StyleJang, P.-G., Cha, H.-G., Jang, M.-C., Hyun, B., Choi, T. S., Kang, Y., & Shin, K. (2022). Characteristic and Relative Environmental Risk of Disinfection by Products Associated with Simple Glucose or Naturally Occurring Algal Organic Matter as Tested in Ballast Water Treatment System. Journal of Marine Science and Engineering, 10(12), 1928. https://doi.org/10.3390/jmse10121928






