Abstract
Green tides are a serious global ecological disaster; the largest occur in the Southern Yellow Sea (SYS). Early-stage green tides in the SYS are composed of four species (Ulva prolifera, Ulva flexuosa, Ulva linza, and Ulva compressa). We found that U. aragoensis is a constituent species of green tides in the SYS based on molecular data. Furthermore, this study re-evaluated the proportion of U. aragoensis in green tides and found that it was more prevalent in micro-propagules cultured from surface seawater during an early-stage green tide in 2021. The internal transcribed spacers, tufA, 18S, rbcL, large subunit, psbA, and rps2-trnL gene sequences were compared; the tufA and rbcL gene sequences were the most suitable DNA barcodes for distinguishing U. aragoensis. A haplotype analysis of the sequences of floating U. aragoensis and its micro-propagules was performed to study the correlation between green tide macroalgae and micro-propagules; close haplotype similarities occurred between them. This study further clarified the species composition of SYS green tides and provided a reference for assessing the relationship between micro-propagules and green tide macroalgae.
1. Introduction
With the acceleration of urbanization, green tides are occurring frequently globally [1,2,3,4]. They are an ecological disaster caused by green macroalgae. The main causative genera are Ulva, Chaetomorpha, Cladophora, Rhizoclonium, Percursaria, and Ulvaria [5]. The green tides in the Southern Yellow Sea (SYS) are the largest in the world. They accumulate in the waters around the Subei Shoal from April to May every year. Driven by wind currents, the green tides constantly propagate and drift northward. From June to August, they land on the shore of Shandong Province, causing huge economic losses and ecological threats [6,7].
Studying the species composition of green tides is a prerequisite for exploring the source of green tides and for making effective management decisions. In the early stage of green tides in the SYS, the macroalgae species include many Ulva species such as Ulva flexuosa, U. compressa, U. linza, and U. prolifera [8]. U. prolifera is the dominant species because of its strong ability to resist stress and its rapid growth and reproduction rates [9]. The initial species composition of the green tides in the SYS is closely related to the species composition of the green macroalgae attached to Neoporphyra cultivation rafts in the radial sandbanks of Jiangsu Province, and the internal transcribed spacers (ITS) sequences of floating U. flexuosa and U. compressa in the early period of the green tides have shown a 100% homology with the green macroalgae attached on the Neoporphyra cultivation rafts [10]. This indicates that the floating species of the green tides in the SYS may originate from Neoporphyra cultivation rafts. From 2009 to 2012, U. flexuosa and U. compressa were the main floating species in the early-stage green tides; U. prolifera was not present. However, since 2012, U. prolifera has become the first floating green tide macroalgae in the early stage, and it occurs throughout the whole process of the early floating green tide. This may be related to the annual increase in sea temperature [10,11].
Due to the morphological similarity among species, Ulva exhibits large intraspecies morphological and cytological changes under different growth stages and environmental conditions [12,13], which makes it difficult to distinguish green tide species based on morphology alone. A convenient and effective method for distinguishing morphologically indistinguishable green macroalgae is the use of molecular markers. The rbcL gene sequence is widely used for distinguishing Ulva; however, it cannot effectively distinguish between U. linza-prolifera-procera (LPP) clusters [6,12,14]. Similarly, ITS cannot distinguish between LPP clusters, and the tufA marker gene has a higher sensitivity than rbcL and ITS; however, the use of tufA for U. linza and U. prolifera distinction remains controversial [5,15]. The 5S ribosomal DNA (5S rDNA) spacer sequence is the most widely used method for distinguishing LPP clusters, and ITS+5S rDNA is an effective method for distinguishing green tide macroalgae [16]. Furthermore, rps2-trnL can also effectively distinguish U. prolifera, U. flexuosa, U. compressa, and U. linza, which are commonly found in SYS green tides [17]. While DNA-based approaches provide a better understanding of species boundaries, they are associated with issues such as conflicts between different methods or markers and species-level differences indicated by the level of uncertain sequence divergency [18,19]. In addition to morphology-based taxonomy, crossing experiments have been used to test biological species concepts and refine species boundaries, with considerable application in Ulva. Recent studies have combined DNA and morpho-anatomical data and performed crossing experiments to describe new species such as Ulva ohnoi and Ulva limnetica [20,21] or to elucidate species boundaries in taxonomically challenging complexes such as that of U. prolifera and U. linza [22].
Ulva flexuosa is a significant constituent of early green tides and micro-propagules [10,19]. Through hybridization, Hiraoka et al. [22] discovered another species, which was previously mistaken as U. flexuosa and U. californica, and named it Ulva mediterranea, the genetic information of which was updated in GenBank. Ulva mediterranea is based on the type specimen of Enteromorpha aragoensis Bliding (1960, p. 174, ‘Aragoensis’) from Banyuls, Mediterranean France; however, the name Ulva mediterranea is a superfluous name change [23,24,25]. It is widely distributed globally and is particularly concentrated along the eastern coast of the United States and the Gulf Coast [26]. Ulva aragoensis may be an early floating species and micro-propagule species of green tides in the SYS. Therefore, it is necessary to reassess the species composition of green tides in the SYS. Investigating the role of U. aragoensis in green tide outbreaks in the SYS is of great significance for assessing its potential to cause such ecological disasters.
At present, it is widely accepted that the green tides in the SYS originate from Neoporphyra cultivation rafts in the north of the Subei Shoal [7,27]; however, the stage from macroalgae detachment to floating is still unclear. A large number of micro-propagules have been found across a wide area of the SYS. Micro-propagules including gametes, spores, zygotes, micro-germlings, and vegetative fragments of macroalgae are part of the life history of opportunistic macroalgae [28,29]. The number of micro-propagules is related to the biomass of green tide macroalgae [28,30]. However, it remains difficult to determine the contribution of micro-propagules to green tides in the SYS. The micro-propagules germinate and grow on the Neoporphyra cultivation rafts and form green tide after detachment. The haplotypes of the micro-propagule U. aragoensis and the floating green tide U. aragoensis should be closely related in gene sequences.
Therefore, this study investigated the proportion of U. aragoensis in an area of a green tide outbreak in the SYS in 2021 and conducted multiple primer (ITS, tufA, 18S, large subunit [LSU], psbA, rbcL, and rps2-trnL) sequencing for U. aragoensis in order to improve understanding of the species composition of the green tides and micro-propagules. The sequences of U. aragoensis and other Ulva species in the National Center for Biotechnology Information (NCBI) database were compared and analyzed based on the molecular markers. The findings of this study can help clarify the classification of U. aragoensis in the Ulva genus and determine its role in green tides in the SYS, which is of great importance for the management and control of green tides.
2. Materials and Methods
2.1. Sampling Point Setting and Sampling Process
A total of three sample collection voyages (6–9 May 2021, 14–16 May 2021, and 15–18 July 2021) were conducted during a green tide outbreak in the SYS. The sampling site was located in the green tide outbreak area of the SYS (between 33°–37° N and 119°–122° E) (Figure 1). A WP2 zooplankton net was used to randomly collect large floating green macroalgae at the sampling site, and 1.5 L of surface seawater was collected using a water-sampler (HQM-1, Jiangsu, China). The surface seawater was filtered through a sieve (200 µm) and transported to the laboratory where it was stored at 4 °C away from light for further micro-propagule cultivation.
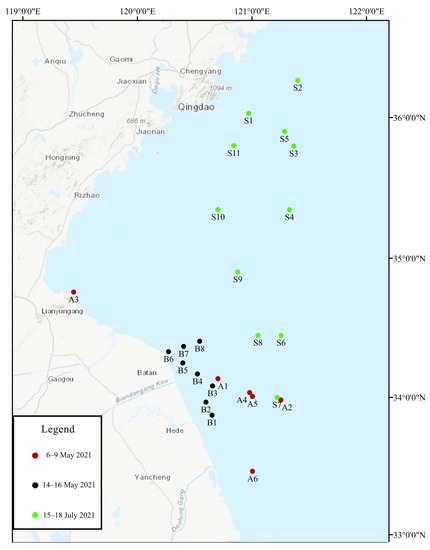
Figure 1.
Distribution of green tide outbreak sampling sites in the Southern Yellow Sea in 2021. Different colors represent different sample collection times. Red represents samples collected from 6 May 2021 to 9 May 2021, black represents samples collected from 14 May 2021 to 16 May 2021, and green represents samples collected from 15 July 2021 to 18 July 2021.
2.2. Micro-Propagule Cultivation and Pretreatment of Green Macroalgae Samples
The surface seawater samples from each site were divided into three 500 mL beakers, used as parallel samples (n = 3). The concentrations of dissolved inorganic N and PO4-P in the water were 500 μmol/L and 30 μmol/L, respectively, after being enriched with von Stosch’s enriched medium. At the same time, 500 μL of saturated GeO2 was added to the water to inhibit microalgal growth. The enriched surface seawater samples were then incubated in a light incubator with a temperature of 18 ± 2 °C, a light intensity of 100 μmol/(m2·s), and a light cycle of 12 h light (L):12 h dark (D). After 3–4 weeks of incubation, green macroalgae seedlings approximately 5–10 cm long on the walls and bottom of the beaker were visible to the naked eye. The seedlings were carefully scraped out of the beaker, and the number of individual seedlings was counted and regarded as the number of micro-propagules in the water. The seedlings were washed with sterilized seawater, dried with absorbent paper, and stored in a refrigerator (Haier, Qingdao, China) at −20 °C [11,31,32,33]. The abundance of micro-propagules (A, inds·L−1) was calculated as follows:
A = N/V (N: total number of germlings, V: volume of water sample cultured).
In addition, floating green macroalgae were collected at each sampling point (Figure 1) and transported back to the laboratory at 4 °C. After removing miscellaneous algae from the surface of the macroalgae with a brush in the laboratory, the samples were cleaned with sterilized seawater, dried with absorbent paper (Vinda, Jiangmen, China), and stored in a refrigerator at −80 °C.
2.3. Identification of U. aragoensis
The collected green tide floating macroalgae and surface seawater micro-propagules were roughly classified according to their morphological characteristics, and the morphology of the macroalgae was recorded under a microscope (Nikon, Tokyo, Japan) in the laboratory. Three single algae (n = 3) of each form from the green tide floating macroalgae and surface seawater micro-propagules at each site were then selected and cleaned with sterile seawater for DNA extraction.
The DNA of macroalgae samples was extracted using Dzup (Plant) Genomic DNA Isolation Reagent produced by Sangon Biotechnology (Shanghai) Co., Ltd. (Shanghai, China). The rbcL sequence primers used are shown in Table 1, and the amplification procedure is detailed in Table 2. The amplification products of the rbcL sequence were sent to Sangon Biotechnology Co., Ltd. for Sanger generation sequencing [32].
The rbcL gene sequences obtained by Sanger sequencing were manually proofread, and the Basic Local Alignment Search Tool of the NCBI was used to confirm the species of the macroalgae samples. The proportion of U. aragoensis in the floating green macroalgae and micro-propagule samples was calculated, and Origin 2022 (OriginLab, Northampton, MA, USA) was used to plot the histogram and for data analysis.
2.4. Sequencing of Multiple DNA Barcodes and Phylogenetic Tree Construction of U. aragoensis
Twenty-five U. aragoensis samples were identified by rbcL gene sequences, and then multiple pairs of DNA-barcoding polymerase chain reaction amplification of the tufA, 18S, LSU, psbA, and rps2-trnL gene sequences were performed on the detected U. aragoensis. At the same time, three U. flexuosa subsp. flexuosa (Uf1, Uf2, and Uf3) collected at 121°40′5.746″ E, 32°3′20.664″ N and U. californica (Uc1, Uc2, and Uc3) collected at 120°16′6.326″ E, 34°18′13.421″ N were identified by ITS sequences in the laboratory [33]. Their ITS, rbcL, tufA, rps2-trnL, 18S, and LSU sequences were amplified for further analysis. The primer sequences are shown in Table 1, and the polymerase chain reaction procedures are shown in Table 2. The sequences of the relevant species were downloaded from the NCBI. Clustal X [34] software was used for multiple sequence alignment analysis of the proofread U. aragoensis sequences. A Kimura 2-Paramete model with MEGA v.7 software was used to construct the phylogenetic tree with the adjacency method, and the reliability of each branch was verified with bootstrap values for 1000 replicates [35].

Table 1.
Polymerase chain reaction primers used in the study.
Table 1.
Polymerase chain reaction primers used in the study.
| Primers | Sequence | Direction | Reference |
|---|---|---|---|
| ITS-F | 5′-TCGTAACAAGGTTTCCGTAGG-3′ | Forward | [36] |
| ITS-R | 5′-TTCCTTCCGCTTATTGATATGC-3′ | Reverse | [36] |
| tufA-F | 5′-GGNGCNGCNCAAATGGAYGG-3′ | Forward | [37] |
| tufA-R | 5′-CCTTCNCGAATMGCRAAWCGC-3′ | Reverse | [37] |
| 18S-F | 5′-GGAGGATTAGGGTCCGATTCC-3′ | Forward | [38] |
| 18S-R | 5′-CTTCCGTCAATTCCTTTAAG-3′ | Reverse | [38] |
| LSU-F | 5′-AMAAGTACCRYGAGGGAAAG-3′ | Forward | [15] |
| LSU-R | 5′-GCACTAATCATTCGCTTTACC-3′ | Reverse | [15] |
| psbA-F | 5′-YTHTAYCCWATHTGGGAAGC-3′ | Forward | [39] |
| psbA-R | 5′-GGGAAGTTRTGNGCRTTRCG-3′ | Reverse | [39] |
| rbcL-F | 5′-ATGTCACCACAAACAGAAACTAAAGC-3′ | Forward | [40] |
| rbcL-R | 5′-AATTCAAATTTAATTTCTTTCC-3′ | Reverse | [40] |
| rps2-trnL-F | 5′-AAAATCAAAATCTAGTAAACCAGGC-3′ | Forward | [41] |
| rps2-trnL-R | 5′-GCTAGCGATTCTTAACGCGATTGGG-3′ | Reverse | [41] |

Table 2.
Polymerase chain reaction profiles for different gene.
Table 2.
Polymerase chain reaction profiles for different gene.
| Target | PCR Reaction Profile |
|---|---|
| ITS | 94 °C for 5 min, followed by 30 cycles of 94 °C for 40 s, 55 °C for 40 s, and 65 °C for 70 s, with the final step at 65 °C for 10 min |
| tufA | 94 °C for 2 min, followed by 35 cycles of 94 °C for 1 min, 50 °C for 30 s, and 72 °C for 1 min, with the final step at 72 °C for 10 min |
| 18S | 95 °C for 2 min, followed by 35 cycles of 95 °C for 1 min, 50 °C for 1 min, and 72 °C for 4 min, with the final step at 72 °C for 6 min |
| LSU | 94 °C for 5 min, followed by 38 cycles of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min, with the final step at 72 °C for 7 min |
| psbA | 94 °C for 10 min, followed by 30 cycles of 94 °C for 1 min, 57 °C for 1 min, and 72 °C for 50 s, with the final step at 72 °C for 10 min |
| rbcL | 94 °C for 3 min, followed by 35 cycles of 94 °C for 1 min, 45 °C for 2 min, and 65 °C for 3 min, with the final step at 72 °C for 10 min |
| rps2-trnL | 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 52 °C for 40 s, and 72 °C for 40 s, with the final step at 72 °C for 5 min |
2.5. Haplotype Analysis of U. aragoensis
The sequences amplified by the various primers (rbcL, tufA, psbA, 18S, ITS, LSU, and rps2-trnL) were manually corrected. The DNASP5 software [42] was then used to calculate and analyze the haplotypes of the samples that were identified as U. aragoensis. The number of haplotypes, haplotype diversity, and nucleotide diversity were calculated. The PopART software [43] was next used to construct a median joining network for the amplified and spliced sequences to assess the genetic relationship between U. aragoensis samples (Table 3) from the SYS.

Table 3.
Description of the different macroalgae and micro-propagules.
3. Results
3.1. Morphological Characteristics of U. aragoensis
Ulva aragoensis in the floating green tide macroalgae mostly comprised broken algae without pseudorhizes (Figure 2a). It was dark green and singular, or with a few branches at the base. The macroalgae thickened from the base to top, the lower part was tubular or approximately cylindrical, and the upper part was sometimes slightly flattened, irregularly rotated and twisted, and contained folds. The morphology of the micro-propagules was quite complete, with pseudorhizes, obviously long main branches, and small branches at the base (Figure 2b). The cells were arranged irregularly and were nearly rectangular, square, or polygonal, with two to six pyrenoids (Figure 2d,e). Ulva aragoensis is morphologically similar to U. flexuosa (Figure 2c).
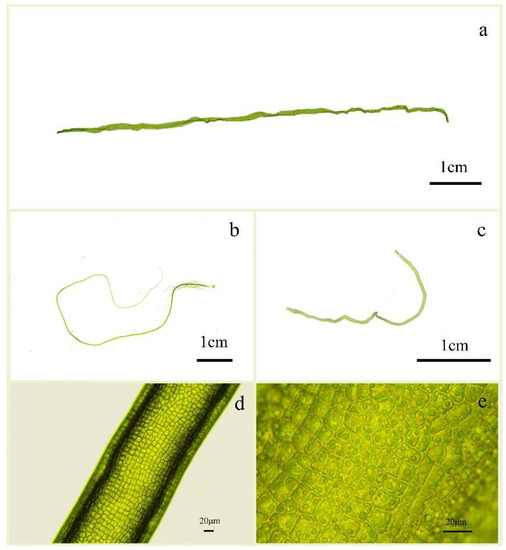
Figure 2.
Morphology and cell diagram of U. aragoensis and U. flexuosa subp. flexuosa; (a) Morphology of U. aragoensis floating in the early-stage green tide of the Southern Yellow Sea. (b) Micro-propagules of U. aragoensis cultured from the surface seawater in the area of green tide outbreak in the Southern Yellow Sea. (c) Morphology of U. flexuosa subp. flexuosa. cultured from the surface seawater in the Southern Yellow Sea. (d) The middle part of U. aragoensis under a microscope at 40 × 10 magnification. (e) The middle part of U. aragoensis under a microscope at 10 × 10 magnification.
3.2. Proportion of U. aragoensis at Each Site
During the three surveys, a total of 25 sites were investigated, among which U. aragoensis was found in the floating green macroalgae at six sites (Figure 3) and in the seawater micro-propagules at 16 sites (Figure 4). The proportion of U. aragoensis in the micro-propagules at each site was greater than that in the floating green macroalgae, and the maximum proportion of U. aragoensis in the floating macroalgae was only 9% (Figure 3). However, U. aragoensis accounted for 33.3% of the micro-propagule samples (Figure 4). In two early-stage green tide surveys conducted from 6 May 2021 to 9 May 2021, and 14 May 2021 to 16 May 2021, the average proportion of U. aragoensis was much larger than that in the third survey conducted from 15 July 2021 to 18 July 2021, which was during the dissipation period of the green tide (Figure 5). This indicated that the proportion of U. aragoensis decreased gradually as the green tide moved northward.
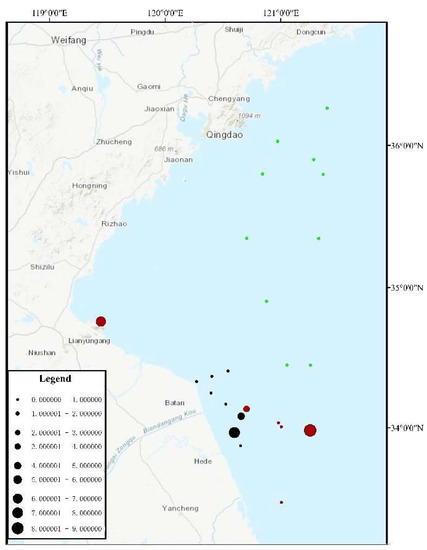
Figure 3.
Percrntage of U. aragoensis in the floating green macroalgae. Circle size represents the proportion of U. aragoensis. Different colors represent different sample collection times. Red represents samples collected from 6 May 2021 to 9 May 2021, black represents samples collected from 14 May 2021 to 16 May 2021, and green represents samples collected from 15 July 2021 to 18 July 2021.
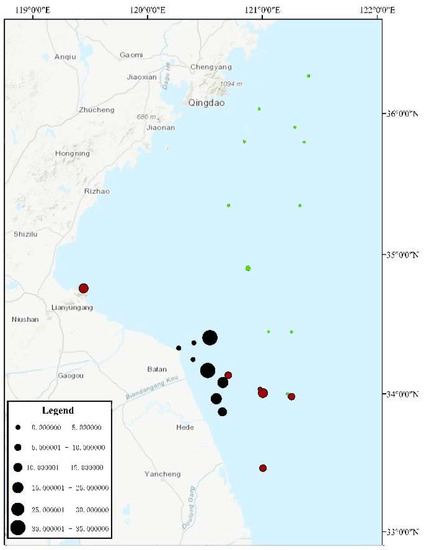
Figure 4.
Percrntage of U. aragoensis in the micro-propagules. Circle size represents the proportion of U. aragoensis. Different colors represent different sample collection times. Red represents samples collected from 6 May 2021 to 9 May 2021, black represents samples collected from 14 May 2021 to 16 May 2021, and green represents samples collected from 15 July 2021 to 18 July 2021.
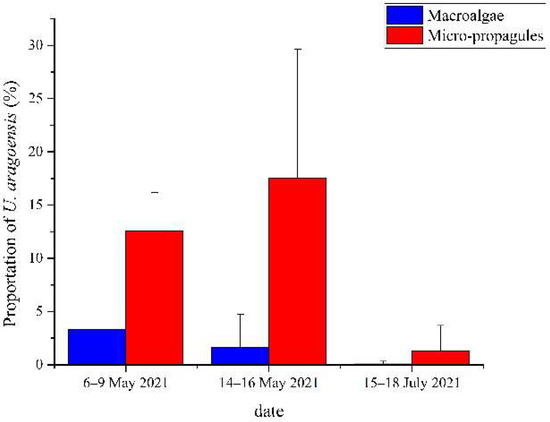
Figure 5.
Average proportion of U. aragoensis in the floating green macroalgae and micro-propagules in the three surveys (6–9 May 2021, 14–16 May 2021, and 15–18 July 2021).
3.3. Phylogenetic Tree Results of Each DNA Barcode
The rbcL sequences of all samples were clustered with MZ582407, indicating that all of the samples were U. aragoensis (Figure 6). All samples of U. aragoensis had a high degree of consistency in the tufA, rbcL, and psbA sequences (Figure 6, Figure 7 and Figure 8). In addition, there were differences in the ITS, 18S, LSU, and rps2-trnL sequences (Figure 9, Figure 10, Figure 11 and Figure 12), indicating that U. aragoensis had a faster evolution rate in the ITS, 18S, LSU, and rps2-trnL sequences. The tufA sequences of U. aragoensis were clustered with MF614789 U. flexuosa, HE600177 U. flexuosa, and MT 859810 U. aragoensis (Figure 7), and were different from the standard U. flexuosa samples (Uf1, Uf2, and Uf3) and U. californica samples (Uc1, Uc2, and Uc3). The psbA sequences were clustered as one large branch, and there was no significant difference (Figure 8). The 18S sequences and ITS sequences of U. aragoensis were different from those of U. flexuosa and U. califonica (Figure 9 and Figure 10). The rbcL sequences of U. aragoensis were divided into two clades with HM447574 U. flexuosa and HM447566 U. flexuosa (standard sequence) and clustered with U. flexuosa annotated in KP233758 and KC411905 in the NCBI database. This indicated that KP233758 and KC411905 were U. aragoensis and not U. flexuosa (Figure 6). The LSU sequences of the FUm1-2, FUm1-3, and MUm3-1 samples were not completely clustered with other U. aragoensis samples (Figure 11), and the sequences of the other samples of U. aragoensis were clustered with MZ596067 in the NCBI database. In addition to the psbA sequences, the other sequences of U. aragoensis, U. flexuosa, and U. californica were obviously different. Furthermore, after comparing with the standard U. flexuosa sequences and U. californica sequences, there were a large number of sequences of U. aragoensis that were misidentified as U. flexuosa in the NCBI database (Figure 12).
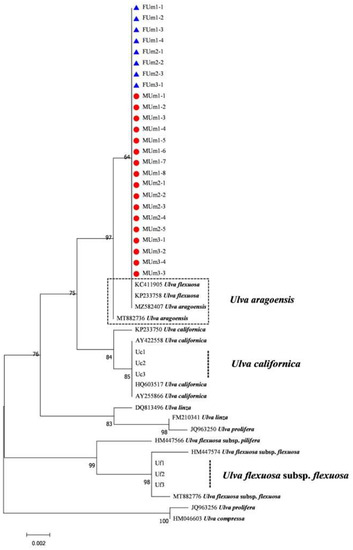
Figure 6.
Maximum likelihood tree constructed from the analysis of rbcL gene sequences of U. aragoensis samples and those downloaded from GenBank.
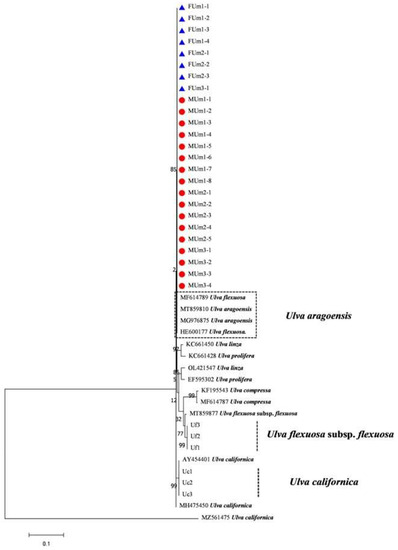
Figure 7.
Maximum likelihood tree constructed from the analysis of tufA gene sequences of U. aragoensis samples and those downloaded from GenBank.
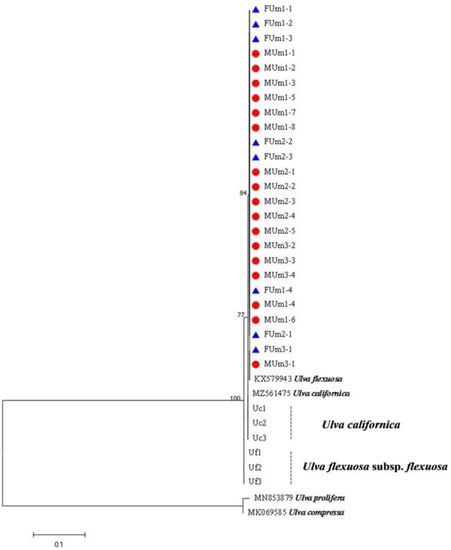
Figure 8.
Maximum likelihood tree constructed from the analysis of psbA gene sequences of U. aragoensis samples and those downloaded from GenBank.
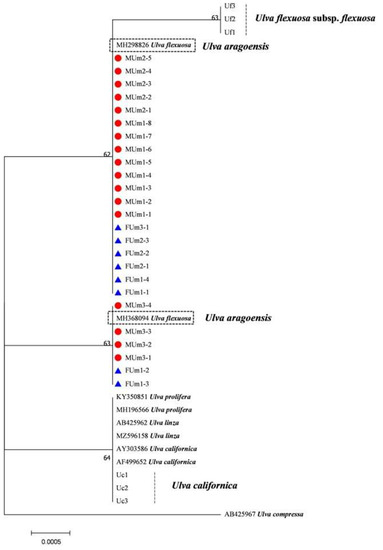
Figure 9.
Maximum likelihood tree constructed from the analysis of 18S gene sequences of U. aragoensis samples and those downloaded from GenBank.
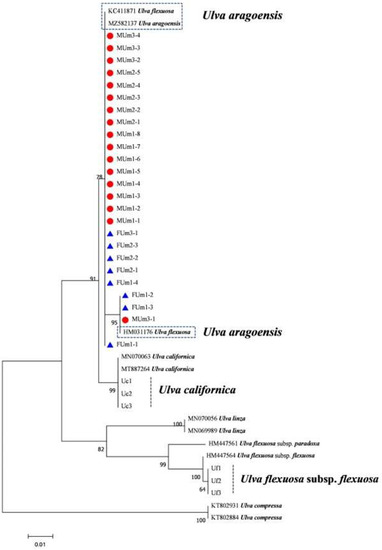
Figure 10.
Maximum likelihood tree constructed from the analysis of internal transcribed spacer gene sequences of U. aragoensis samples and those downloaded from GenBank.
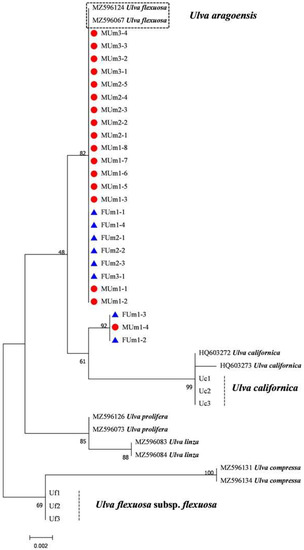
Figure 11.
Maximum likelihood tree constructed from the analysis of large subunit gene sequences of U. aragoensis samples and those downloaded from GenBank.
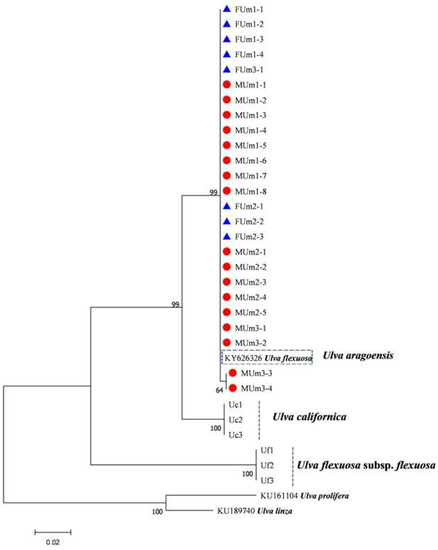
Figure 12.
Maximum likelihood tree constructed from the analysis of rps2-trnL gene sequences of U. aragoensis samples and those downloaded from GenBank.
3.4. Analysis of Haplotype Relationship between Macroalgae and Micro-Propagules
According to the sample source, the sequences were divided into floating macroalgae species or micro-propagule populations. Because the sequences of rbcL, tufA, and psbA of the 25 samples were completely consistent, haplotype analysis could not be carried out. Therefore, a haplotype analysis was performed on the sequences of the four different primers (18S, ITS, LSU, and rps2-trnL). The sequences of the four primers were divided into two haplotypes, and the differences between the two haplotypes were small (Figure 13a–d). The rps2-trnL sequences had the smallest difference and the LSU sequences had the largest. The two haplotypes of the 18S, ITS, and LSU sequences contained both macroalgae samples and micro-propagule samples, while the rps2-trnL sequences contained only two different sources of samples in Hap_1 and only micro-propagule samples in Hap_2, which may be the reason for the small number of samples of floating U. aragoensis.
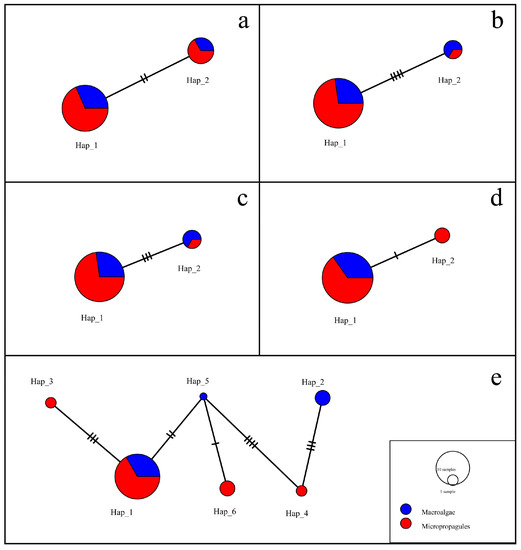
Figure 13.
Network diagram for the U. aragoensis haplotypes derived from the 18S (a), internal transcribed spacers (b), large subunit (c), rps2-trnL (d), and 18S-internal transcribed spacers-large subunit-rps2-trnL (e) gene sequences. The circle symbol represents one haplotype, and its size reflects the number of different haplotypes. Different colors represent different sources, from which the haplotypes were derived, and blank circles indicate undetected haplotypes.
The sequences of all four primers were spliced according to the sequence of 18S-ITS-LSU-rps2-trnL for haplotype analysis. There were four haplotypes, namely Hap_1, Hap_3, Hap_4, and Hap_6, in the micro-propagule samples, and three haplotypes in the floating macroalgae samples, namely Hap_1, Hap_2, and Hap_5. Hap_1 had the largest number of samples, including not only six floating samples but also twelve micro-propagule samples. Hap_2 and Hap_5 in only the macroalgae samples, and Hap_3, Hap_4, and Hap_6 in only the micro-propagule samples showed little difference (Figure 13e). Therefore, there was a close relationship between the micro-propagules and macroalgae samples.
4. Discussion
4.1. Ulva Aragoensis Is a Constituent Species of the Early Green Tide in the SYS
Ulva aragoensis is morphologically highly similar to U. flexuosa (Figure 2), with wide blades that are unbranched or present at the base. Previously, U. flexuosa comprised 68.70% of the green macroalgae on Neoporphyra cultivation rafts in December in the SYS [44] and also represented a certain proportion in the micro-propagules [31]. Ulva flexuosa was an important component of the early-stage green tide in a previous study [9], while in the present study, U. aragoensis comprised a large proportion in the early-stage green tide. It comprised up to 33.3% in the micro-propagules. The Ulva genus shows a high degree of morphological plasticity in different environments, and the combination of ITS sequences and 5S rDNA gene sequences is the most commonly used method for species identification of green tide macroalgae in the SYS [16]. Some U. aragoensis in the NCBI database have been mistakenly considered as U. flexuosa and U. californica. In the phylogenetic tree constructed from the ITS and rbcL sequences of U. aragoensis, the sample sequences were also clustered with U. flexuosa in the NCBI database. This indicated that a large number of U. aragoensis were also present in the early-stage floating green macroalgae and micro-propagules and were previously identified as U. flexuosa. Hiraoka et al. [22] corrected the ITS sequences of U. aragoensis in the NCBI database, and the present authors entered the sequences of rbcL, tufA, and rps2-trnL, three commonly used molecular markers of green tide macroalgae previously mistakenly considered U. flexuosa (Table 4) in order to provide a basis for species identification of green tide macroalgae in the SYS.

Table 4.
Sequences of U. aragoensis mistakenly identified as U. flexuosa in the NCBI database.
4.2. The Proportion of U. aragoensis Decreased as the Green Tide Moved Northward
As the green tide gradually moved northward, the temperature and light intensity increased, and the proportion of U. aragoensis in the floating green algae decreased. This may be because U. aragoensis is not suited to floating for a long time and cannot adapt to the high temperature and strong light in the coastal waters of Shandong Province [9,45,46]. Therefore, U. aragoensis did not contribute significantly to the green tide in the SYS. In addition, the proportion of U. aragoensis in the micro-propagules was much larger than that in the floating green macroalgae (Figure 5), and the large number of U. aragoensis attached on Neoporphyra cultivation rafts may be the main source of micro-propagules (unpublished data). In the process of green tide drift, a large number of pieces of bamboo used in the cultivation of Neoporphyra also drifted with the green tide. These floating pieces of bamboo provided a suitable attachment substrate for U. aragoensis, which is not suited for floating life. The U. aragoensis attached to the floating pieces of bamboo also contributed to the micro-propagules in the sea.
4.3. Appropriate Molecular Markers for Green Tide Macroalgae Identification
The ITS, rbcL, and tufA sequences are molecular markers commonly used to identify macroalgae. The ITS sequence is a moderately conserved region from the process of evolution, with an interspecific difference of more than 14%. It is often used as a molecular marker to study the interspecific level of algae, but LPP clusters cannot be distinguished. In addition, the difference between the ITS sequences of U. aragoensis and U. flexuosa is small, only 2.4% [19]. Furthermore, the intraspecific difference in the ITS sequences of U. aragoensis is large, which increases the difficulty of its identification. Therefore, ITS is not suitable for distinguishing U. flexuosa from U. aragoensis [19]. The 5S rDNA spacer sequence is a highly variable non-transcribed (100–900 bp) conserved coding region with two to ten times more phylogenetic information than the ITS region, and it is a suitable fragment for identifying LPP clusters [16]. However, it does not seem to be able to amplify the 5S gene sequence of U. flexuosa. Three samples in the LSU sequence were isolated into a clade, which may be related to the higher evolutionary rate in the LSU fragment. Therefore, it was not suitable for the identification of U. aragoensis. The psbA sequences of U. aragoensis are difficult to distinguish due to the high similarity with U. flexuosa and U. californica. The rps2-trnL marker has played an effective role in the identification of four species of green tide macroalgae (U. prolifera, U. flexuosa, U. linza, and U. compressa) [17]. The rps2-trnL sequences of U. aragoensis were consistent with KY626326 U. flexuosa in the NCBI database, but not with the real U. flexuosa. This indicated that KY626326 U. flexuosa in the database was U. aragoensis, and there were significant differences between U. aragoensis and real U. flexuosa and U. californica. However, there are few reference rps2-trnL sequences in the NCBI database, and the identification of green tide macroalgae by rps2-trnL sequences still needs to be further improved.
Compared with LSU, rbcL, and UPA, the tufA gene sequences data of green macroalgae contained almost no introns, had a high amplification success rate, a higher evolutionary rate, an obvious discrimination effect, and a lower pollution level. It is a standard molecular marker for the classification of green algae. The rbcL enzyme (Rubis-Co) promotes the fixation of primary CO2 in photosynthesis and widely exists in algae. The sequence information of rbcL in Genbank is abundant and conserved. As a molecular marker, rbcL is universal, easy to amplify, and easy to contrast. The rbcL sequences of U. aragoensis in the 25 samples were consistent, thereby making it a relatively conserved molecular marker for distinguishing U. aragoensis from U. flexuosa. The tufA sequences of U. aragoensis in the present study were stable and specific and clearly distinguishable from U. californica and U. flexuosa; however, it is not clear whether tufA can distinguish U. linza from U. prolifera [5,15]. Therefore, it is proposed that tufA+5S rDNA sequences are a suitable approach for identifying green tide macroalgae. Furthermore, rbcL can also be used to identify green tide macroalgae outside LPP clusters. For the determination of U. aragoensis population geographic relationships, 18S, ITS, LSU, and rps2-trnL, with higher evolutionary rates, are more appropriate approaches.
4.4. The Micro-Propagule U. aragoensis and the Floating Green Tide U. aragoensis Are Related
Micro-propagules exist widely in the SYS in spring and summer, but the source of green tide outbreaks is particularly concentrated. Furthermore, green tide micro-propagules seem to have no ability to germinate directly in seawater [47] and must be attached to a certain substrate for germination; therefore, the micro-propagules cannot directly provide an initial biomass for green tide outbreaks. However, a large number of Neoporphyra cultivation rafts in the Subei Shoal provide suitable attachment substrates for micro-propagules [48]. The micro-propagules in the SYS attach to Neoporphyra cultivation rafts and then germinate in spring and summer every year. A large number of green macroalgae detach and fall into the water, providing the initial biomass for a green tide [49,50]. In autumn and winter, the micro-propagules are released into the seawater and sediments to survive the adverse environment to germinate again next year. This indicates that there should be close species overlap between floating green tide macroalgae, macroalgae attached to Neoporphyra cultivation rafts, and micro-propagules. This study revealed the existence of U. aragoensis in both green tide macroalgae and micro-propagules, in addition to the U. aragoensis from Neoporphyra cultivation rafts [51]. Furthermore, the haplotypes of the micro-propagule U. aragoensis and the floating green tide U. aragoensis were closely related in gene sequences, and the haplotypes were highly coincident in Hap_1, indicating that the micro-propagules indirectly contributed to the green tide.
5. Conclusions
In this study, we found that U. aragoensis was a constituent species of the early green tide in the SYS. The proportion of U. aragoensis decreased gradually as the green tide moved northward, and the proportion of U. aragoensis in the micro-propagules at each site was greater than that in the floating green macroalgae. This suggests that U. aragoensis is not suited to floating for a long time. Through amplification of the ITS, rbcL, rps2-trnL, tufA, 18S, LSU, and psbA gene sequences of the three confused species (U. aragoensis, U. flexuosa, and U. californica), there were obvious gene sequence differences between these three species except psbA. The sequences of tufA and rbcL were stable and suitable for the identification of U. aragoensis. Furthermore, through the analysis of the haplotypes of the floating U. aragoensis and the micro-propagules U. aragoensis, they were highly consistent, which also provided evidence for the indirect contribution of micro-propagules to the green tides.
Author Contributions
Data curation, Y.T., L.X., T.W. and J.L.; formal analysis, Y.T.; funding acquisition, J.Z.; methodology, J.L. and Y.T.; writing—original draft, Y.T.; writing—review and editing, J.L., J.Z., S.Z., Y.S., Z.X., S.L. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFC3106001, 2022YFC3106004), the Project of Prevention Strategies for Green Tides of Yellow Sea, M.N.R., the Natural Science Foundation of Shanghai (21ZR1427400), and the Project of Shanghai Municipal Bureau of Oceanography (2022-03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taylor, R.; Fletcher, R.L.; Raven, J.A. Preliminary Studies on the Growth of Selected ‘Green Tide’ Algae in Laboratory Culture: Effects of Irradiance, Temperature, Salinity and Nutrients on Growth Rate. Bot. Mar. 2001, 44, 327–336. [Google Scholar] [CrossRef]
- Ye, N.H.; Zhang, X.W.; Mao, Y.Z.; Liang, C.W.; Xu, D.; Zou, J.; Zhuang, Z.M.; Wang, Q.Y. ‘Green Tides’ are Overwhelming the Coastline of Our Blue Planet: Taking the World’s Largest Example. Ecol. Res. 2011, 26, 477–485. [Google Scholar] [CrossRef]
- Schreyers, L.; Van Emmerik, T.; Biermann, L.; Le Lay, Y.F. Spotting Green Tides over Brittany from Space: Three Decades of Monitoring with Landsat Imagery. Remote Sens. 2021, 13, 1308. [Google Scholar] [CrossRef]
- Lagourgue, L.; Gobin, S.; Brisset, M.; Vandenberghe, S.; Bonneville, C.; Jauffrais, T.; Van Wynsberge, S.; Payri, C.E. Ten New Species of Ulva (Ulvophyceae, Chlorophyta) Discovered in New Caledonia: Genetic and Morphological Diversity, and Bloom Potential. Eur. J. Phycol. 2022, 57, 458–478. [Google Scholar] [CrossRef]
- Song, W.; Han, H.B.; Wang, Z.L.; Li, Y. Molecular Identification of the Macroalgae That Cause Green Tides in the Bohai Sea, China. Aquat. Bot. 2019, 156, 38–46. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, N.; Jiang, P.; Boo, S.M.; Lee, W.J.; Cui, Y.L.; Lin, H.Z.; Zhao, J.; Liu, Z.Y.; Qin, S. Ulva and Enteromorpha (Ulvaceae, Chlorophyta) from Two Sides of the Yellow Sea: Analysis of Nuclear rDNA ITS and Plastid rbcL Sequence Data. Chin. J. Oceanol. Limnol. 2010, 28, 762–768. [Google Scholar] [CrossRef]
- Zhang, J.H.; Shi, J.T.; Gao, S.; Huo, Y.Z.; Cui, J.J.; Shen, H.; Liu, G.Y.; He, P.M. Annual Patterns of Macroalgal Blooms in the Yellow Sea during 2007–2017. PLoS ONE 2019, 14, e0210460. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, P.; Liu, Z.Y.; Wei, W.; Lin, H.Z.; Li, F.C.; Wang, J.F.; Qin, S. The Yellow Sea Green Tides Were Dominated by One Species, Ulva (Enteromorpha) prolifera, from 2007 to 2011. Chin. Sci. Bull. 2013, 58, 2298–2302. [Google Scholar] [CrossRef]
- Wang, S.Y.; Huo, Y.Z.; Zhang, J.H.; Cui, J.J.; Wang, Y.; Yang, L.L.; Zhou, Q.Y.; Lu, Y.W.; Yu, K.F.; He, P.M. Variations of Dominant Free-Floating Ulva Species in the Source Area for the World’s Largest Macroalgal Blooms, China: Differences of Ecological Tolerance. Harmful Algae 2018, 74, 58–66. [Google Scholar] [CrossRef]
- Han, W.; Chen, L.P.; Zhang, J.H.; Tian, X.L.; Hua, L.; He, Q.; Huo, Y.Z.; Yu, K.F.; Shi, D.J.; Ma, J.H.; et al. Seasonal Variation of Dominant Free-Floating and Attached Ulva Species in Rudong Coastal Area, China. Harmful Algae 2013, 28, 46–54. [Google Scholar] [CrossRef]
- Huo, Y.Z.; Zhang, J.H.; Chen, L.P.; Hu, M.; Yu, K.F.; Chen, Q.F.; He, Q.; He, P.M. Green Algae Blooms Caused by Ulva prolifera in the Southern Yellow Sea: Identification of the Original Bloom Location and Evaluation of Biological Processes Occurring during the Early Northward Floating Period. Limnol. Oceanogr. 2013, 58, 2206–2218. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.J.; Xu, N.; Shan, T.F.; Sun, S.; Hu, X.A.; Yang, J.Q. Ulva Diversity in the Yellow Sea during the Large-Scale Green Algal Blooms in 2008–2009. Phycol. Res. 2010, 58, 270–279. [Google Scholar] [CrossRef]
- Gao, G.; Zhong, Z.H.; Zhou, X.H.; Xu, J.T. Changes in Morphological Plasticity of Ulva prolifera under Different Environmental Conditions: A Laboratory Experiment. Harmful Algae 2016, 59, 51–58. [Google Scholar] [CrossRef]
- Leliaert, F.; Zhang, X.W.; Ye, N.H.; Malta, E.; Engelen, A.H.; Mineur, F.; Verbruggen, H.; De Clerck, O. Research Note: Identity of the Qingdao Algal Bloom. Phycol. Res. 2009, 57, 147–151. [Google Scholar] [CrossRef]
- Saunders, G.W.; Kucera, H. An Evaluation of rbcL, tufA, UPA, LSU and ITS as DNA Barcode Markers for the Marine Green Macroalgae. Cryptogam. Algol. 2010, 31, 487–528. [Google Scholar]
- Shimada, S.; Yokoyama, N.; Arai, S.; Hiraoka, M. Phylogeography of the Genus Ulva (Ulvophyceae, Chlorophyta), with Special Reference to the Japanese Freshwater and Brackish Taxa. J. Appl. Phycol. 2008, 20, 979–989. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhao, X.H.; Kang, X.Y.; Zhuang, M.M.; Ding, X.W.; Zhao, L.J.; Wen, Q.L.; Zhu, Y.; Gu, K.; Bao, Q.J.; et al. Good News: We Can Identify Ulva Species Erupted in the Yellow Sea More Easily and Cheaply Now. Conserv. Genet. Resour. 2020, 12, 447–449. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, J.E.; Kim, J.H.; Byeon, S.Y.; Kim, S.; Choi, S.K.; Kang, Y.H.; Park, S.R.; Lee, H.J. Species Composition, Diversity, and Distribution of the Genus Ulva along the Coast of Jeju Island, Korea Based on Molecular Phylogenetic Analysis. PLoS ONE 2019, 14, e0219958. [Google Scholar] [CrossRef]
- Hiraoka, M.; Ichihara, K.; Zhu, W.R.; Shimada, S.; Oka, N.; Cui, J.J.; Tsubaki, S.; He, P.M. Examination of Species Delimitation of Ambiguous DNA-Based Ulva (Ulvophyceae, Chlorophyta) Clades by Culturing and Hybridisation. Phycologia 2017, 56, 517–532. [Google Scholar] [CrossRef]
- Hiraoka, M.; Shimada, S.; Uenosono, M.; Masuda, M. A New Green-Tide-Forming Alga, Ulva ohnoi Hiraoka et Shimada sp. nov. (Ulvales, Ulvophyceae) from Japan. Phycol. Res. 2004, 52, 17–29. [Google Scholar] [CrossRef]
- Ichihara, K.; Arai, S.; Uchimura, M.; Fay, E.J.; Ebata, H.; Hiraoka, M.; Shimada, S. New Species of Freshwater Ulva, Ulva limnetica (Ulvales, Ulvophyceae) from the Ryukyu Islands, Japan. Phycol. Res. 2009, 57, 94–103. [Google Scholar] [CrossRef]
- Cui, J.J.; Monotilla, A.P.; Zhu, W.R.; Takano, Y.; Shimada, S.; Ichihara, K.; Matsui, T.; He, P.M.; Hiraoka, M. Taxonomic Reassessment of Ulva prolifera (Ulvophyceae, Chlorophyta) Based on Specimens from the Type Locality and Yellow Sea Green Tides. Phycologia 2018, 57, 692–704. [Google Scholar] [CrossRef]
- Krupnik, N.; Rinkevich, B.; Paz, G.; Douek, J.; Lewinsohn, E.; Israel, A.; Carmel, N.; Mineur, F.; Maggs, C.A. Native, Invasive and Cryptogenic Ulva Species from the Israeli Mediterranean Sea: Risk and Potential. Mediterr. Mar. Sci. 2018, 19, 132–146. [Google Scholar] [CrossRef]
- Shimada, S.; Hiraoka, M.; Nabata, S.; Iima, M.; Masuda, M. Molecular Phylogenetic Analyses of the Japanese Ulva and Enteromorpha (Ulvales, Ulvophyceae), with Special Reference to the Free-Floating Ulva. Phycol. Res. 2003, 51, 99–108. [Google Scholar] [CrossRef]
- Hayden, H.S.; Blomster, J.; Maggs, C.A.; Silva, P.C.; Stanhope, M.J.; Waaland, J.R. Linnaeus Was Right All along: Ulva and Enteromorpha Are Not Distinct Genera. Eur. J. Phycol. 2003, 38, 277–294. [Google Scholar] [CrossRef]
- Melton, J.T.; Lopez-Bautista, J.M. Diversity of the Green Macroalgal Genus Ulva (Ulvophyceae, Chlorophyta) from the East and Gulf Coast of the United States Based on Molecular Data. J. Phycol. 2021, 57, 551–568. [Google Scholar] [CrossRef]
- Liu, D.Y.; Keesing, J.K.; Xing, Q.U.; Shi, P. World’s Largest Macroalgal Bloom Caused by Expansion of Seaweed Aquaculture in China. Mar. Pollut. Bull. 2009, 58, 888–895. [Google Scholar] [CrossRef]
- Huo, Y.Z.; Han, H.B.; Hua, L.; Wei, Z.L.; Yu, K.F.; Shi, H.H.; Kim, J.K.; Yarish, C.; He, P.M. Tracing the Origin of Green Macroalgal Blooms Based on the Large Scale Spatio-Temporal Distribution of Ulva Microscopic Propagules and Settled Mature Ulva Vegetative Thalli in Coastal Regions of the Yellow Sea, China. Harmful Algae 2016, 59, 91–99. [Google Scholar] [CrossRef]
- Miao, X.X.; Xiao, J.; Xu, Q.Z.; Fan, S.L.; Wang, Z.L.; Wang, X.; Zhang, X.L. Distribution and Species Diversity of the Floating Green Macroalgae and Micro-Propagules in the Subei Shoal Southwestern Yellow Sea. PeerJ 2020, 8, e10538. [Google Scholar] [CrossRef]
- Song, W.; Li, Y.; Fang, S.; Wang, Z.L.; Xiao, J.; Li, R.X.; Fu, M.Z.; Zhu, M.Y.; Zhang, X.L. Temporal and Spatial Distributions of Green Algae Micro-Propagules in the Coastal Waters of the Subei Shoal, China. Estuar. Coast. Shelf Sci. 2015, 163, 29–35. [Google Scholar] [CrossRef]
- Huo, Y.Z.; Hua, L.; Wu, H.L.; Zhang, J.H.; Cui, J.J.; Huang, X.W.; Yu, K.F.; Shi, H.H.; He, P.M.; Ding, D.W. Abundance and Distribution of Ulva Microscopic Propagules Associated with a Green Tide in the Southern Coast of the Yellow Sea. Harmful Algae 2014, 39, 357–364. [Google Scholar] [CrossRef]
- Coat, G.; Dion, P.; Noailles, M.C.; De-Reviers, B.; Fontaine, J.M.; Berger-Perrot, Y.; Loiseaux-De, G.S. Ulva armoricana (Ulvales, Chlorophyta) from the Coasts of Brittany (France). I. Morphological Identification. Eur. J. Phycol. 1998, 33, 81–86. [Google Scholar] [CrossRef]
- Tian, X.L.; Huo, Y.Z.; Chen, L.P.; He, J.H.; Zhang, J.H.; Jia, R.; Liu, H.; Wang, J.H.; Xu, R.; Yang, J.Q.; et al. Molecular Detection and Analysis of Green Seaweeds from Rudong Coasts in Jiangsu Province. Chin. Sci. Bull. 2011, 56, 309–317. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Leskinen, E.; Pamilo, P. Evolution of the ITS Sequences of Ribosomal DNA in Enteromorpha (Chlorophyceae). Hereditas 1997, 126, 17–23. [Google Scholar] [CrossRef]
- Fama, P.; Wysor, B.; Kooistra, W.; Zuccarello, G.C. Molecular Phylogeny of the Genus Caulerpa (Caulerpales, Chlorophyta) Inferred from Chloroplast tufA Gene. J. Phycol. 2002, 38, 1040–1050. [Google Scholar] [CrossRef]
- Lin, Z.H.; Shen, S.D.; Chen, W.Z.; Li, H.H. Phylogenetic Analyses of Four Species of Ulva and Monostroma grevillei Using ITS, rbcL and 18s rDNA Sequence Data. Chin. J. Oceanol. Limnol. 2013, 31, 97–105. [Google Scholar] [CrossRef]
- Wang, J.F.; Jiang, P.; Cui, Y.L.; Li, N.; Wang, M.Q.; Lin, H.Z.; He, P.M.; Qin, S. Molecular Analysis of Green-Tide-Forming Macroalgae in the Yellow Sea. Aquat. Bot. 2010, 93, 25–31. [Google Scholar] [CrossRef]
- Manhart, J.R. Phylogenetic Analysis of Green Plant rbcL Sequences. Mol. Phylogenet. Evol. 1994, 3, 114–127. [Google Scholar] [CrossRef]
- Liu, J.L.; Tong, Y.C.; Xia, J.; Sun, Y.Q.; Zhao, X.H.; Sun, J.Y.; Zhao, S.; Zhuang, M.M.; Zhang, J.H.; He, P.M. Ulva Macroalgae within Local Aquaculture Ponds along the Estuary of Dagu River, Jiaozhou Bay, Qingdao. Mar. Pollut. Bull. 2021, 174, 113243. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. Popart: Full-Feature Software for Haplotype Network Construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Huo, Y.Z.; Han, H.B.; Shi, H.H.; Wu, H.L.; Zhang, J.H.; Yu, K.F.; Xu, R.; Liu, C.C.; Zhang, Z.L.; Liu, K.F.; et al. Changes to the Biomass and Species Composition of Ulva sp. on Porphyra Aquaculture Rafts, along the Coastal Radial Sandbank of the Southern Yellow Sea. Mar. Pollut. Bull. 2015, 93, 210–216. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Yao, L.L.; Liu, J.L.; Tong, Y.C.; Xia, J.; Zhao, X.H.; Zhao, S.; Fu, M.L.; Zhuang, M.M.; He, P.M.; et al. Prevention Strategies for Green Tides at Source in the Southern Yellow Sea. Mar. Pollut. Bull. 2022, 178, 113646. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Yuan, H.Q.; Liu, J.L.; Sun, Y.Q.; Tong, Y.C.; Zhao, S.; Xia, J.; Li, S.; Hu, M.J.; Cao, J.X.; et al. A Review of Physical, Chemical, and Biological Green Tide Prevention Methods in the Southern Yellow Sea. Mar. Pollut. Bull. 2022, 180, 113772. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xiao, J.; Fan, S.L.; Li, Y.; Liu, X.Q.; Liu, D.Y. Who Made the World’s Largest Green Tide in China?—An Integrated Study on the Initiation and Early Development of the Green Tide in Yellow Sea. Limnol. Oceanogr. 2015, 60, 1105–1117. [Google Scholar] [CrossRef]
- Geng, H.X.; Yan, T.; Zhou, M.J.; Liu, Q. Comparative Study of the Germination of Ulva prolifera Gametes on Various Substrates. Estuar. Coast. Shelf Sci. 2015, 163, 89–95. [Google Scholar] [CrossRef]
- Ma, Y.F.; Wong, K.P.; Tsou, J.Y.; Zhang, Y.Z. Investigating Spatial Distribution of Green-Tide in the Yellow Sea in 2021 Using Combined Optical and SAR Images. J. Mar. Sci. Eng. 2022, 10, 127. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Zhao, N.; Liu, T.R.; Hirose, N. Summer Wind Effects on Coastal Upwelling in the Southwestern Yellow Sea. J. Mar. Sci. Eng. 2021, 9, 1021. [Google Scholar] [CrossRef]
- Song, M.J.; Kong, F.Z.; Li, Y.F.; Zhao, J.; Yu, R.C.; Zhou, M.J.; Jiang, P.; Yan, T. A Massive Green Tide in the Yellow Sea in 2021: Field Investigation and Analysis. Int. J. Environ. Res. Public Health 2022, 19, 11753. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).