Seasonal Variability in Present-Day Coccolithophore Fluxes in Deep Eastern Mediterranean Sea: A Multi-Year Study (2015–2017) of Coccolithophore Export in SE Ionian Sea at 4300 m Depth
Abstract
1. Introduction
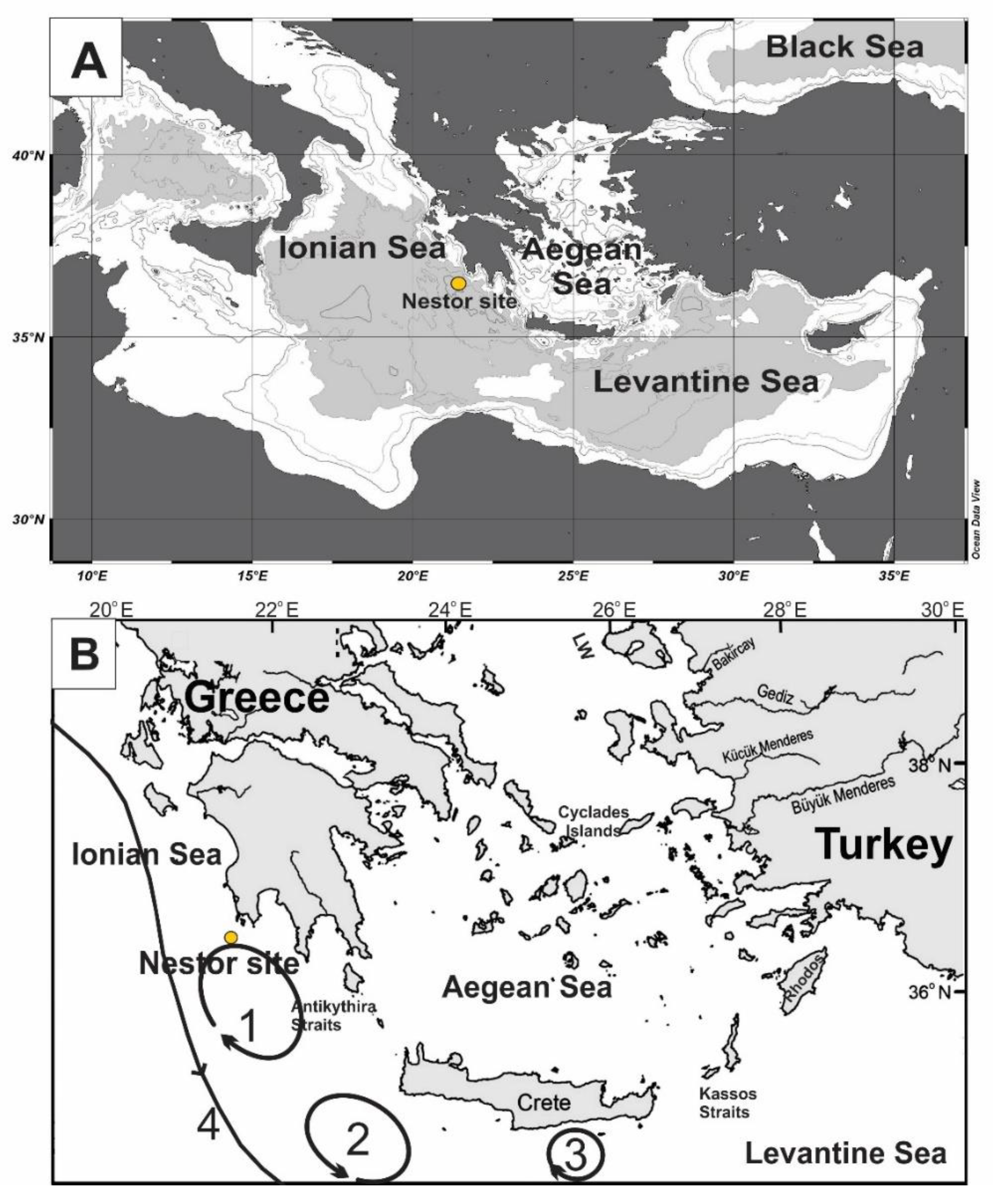
Oceanographic Setting
2. Materials and Methods
2.1. Satellite Environmental Data
2.2. Deployment and Recovery of the Sediment Trap Mooring
2.3. Sample Preparation
2.4. Quantitative Microscopic Analysis
3. Results
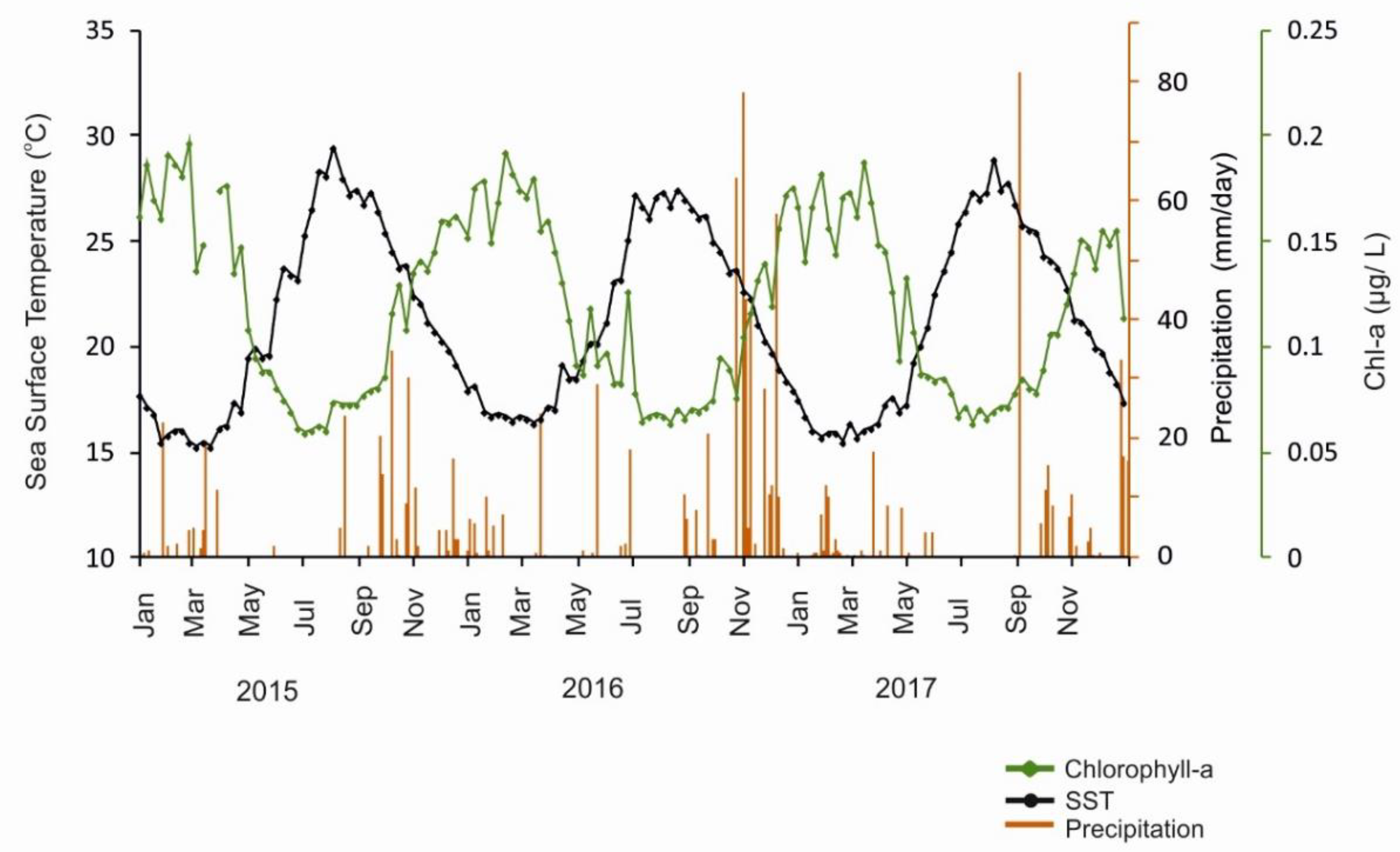
3.1. Satellite Measurements of Surface Chl-A Concentration, SST, and Precipitation
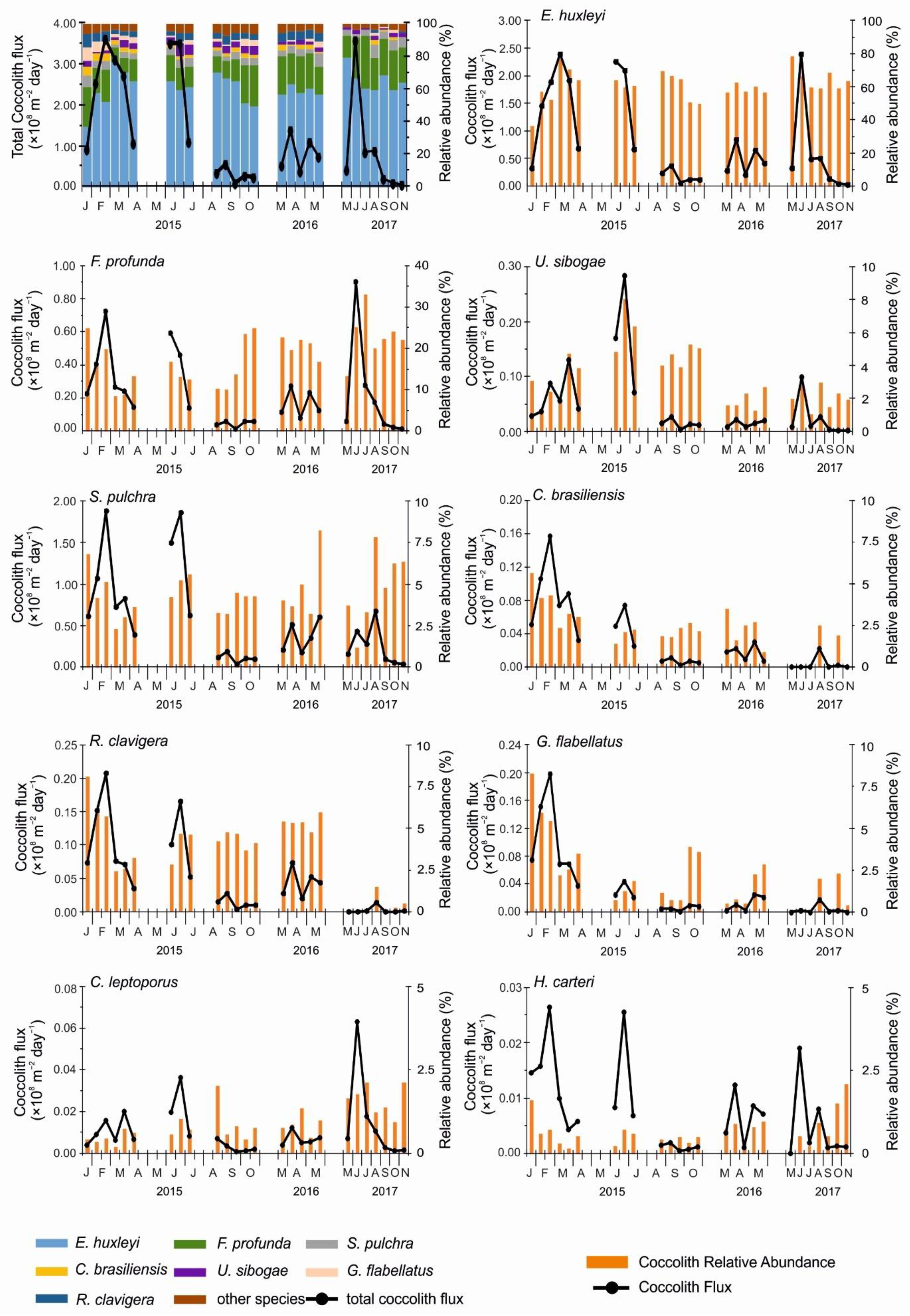
3.2. Total Coccolith Flux
3.3. Coccolithophore Assemblage Composition
4. Discussion
4.1. Total Coccolith Flux and Seasonal Trends of Major Coccolithophore Species
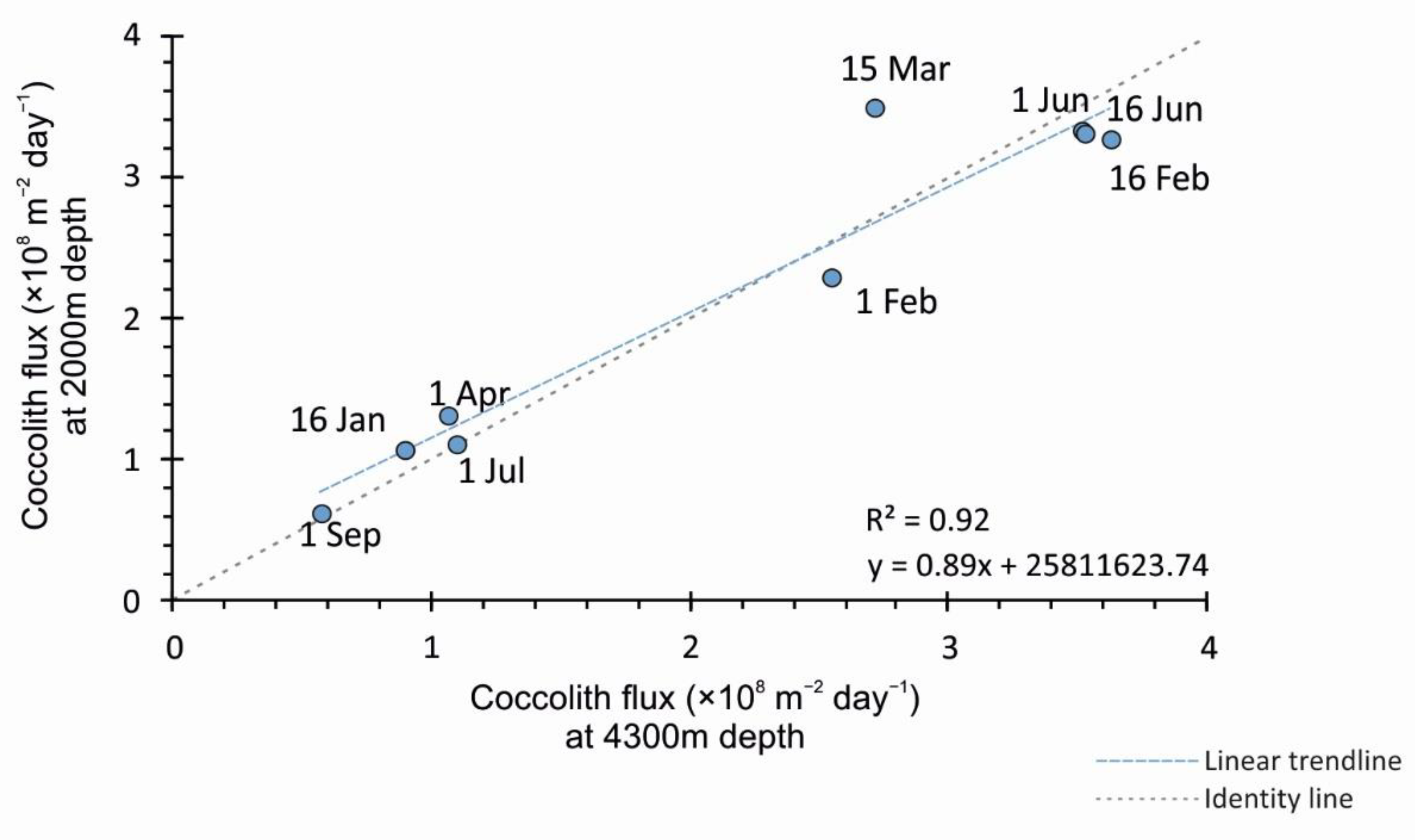
4.2. Comparison of Coccolith Fluxes at 2000 m and 4300 m for the 2015 Sampling Interval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pienaar, R.N. Ultrastructure and calcification of coccolithophores. In Coccolithophores; Winter, A., Siesser, W.G., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 13–38. [Google Scholar]
- Rost, B.; Riebesell, U. Coccolithophores and the biological pump: Responses to environmental changes. In Coccolithophores; Thierstein, H.R., Young, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 99–125. [Google Scholar] [CrossRef]
- Cermeño, P.; Dutkiewicz, S.; Harris, R.P.; Follows, M.; Schofield, O.; Falkowski, P.G. The role of nutricline depth in regulating the ocean carbon cycle. Proc. Natl. Acad. Sci. USA 2008, 105, 20344–20349. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Lee, C.; Hedges, J.I.; Honjo, S.; Wakeham, S.G. A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 49, 219–236. [Google Scholar] [CrossRef]
- Ziveri, P.; de Bernardi, B.; Baumann, K.H.; Stoll, H.M.; Mortyn, P.G. Sinking of coccolith carbonate and potential contribution to organic carbon ballasting in the deep ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 659–675. [Google Scholar] [CrossRef]
- Šupraha, L.; Gerecht, A.C.; Probert, I.; Henderiks, J. Eco-physiological adaptation shapes the response of calcifying algae to nutrient limitation. Sci. Rep. 2015, 5, 16499. [Google Scholar] [CrossRef]
- Lazarus, D.; Barron, J.; Renaudie, J.; Diver, P.; Türke, A. Cenozoic planktonic marine diatom diversity and correlation to climate change. PLoS ONE 2014, 9, e84857. [Google Scholar] [CrossRef] [PubMed]
- Malinverno, E.; Maffioli, P.; Corselli, C.; De Lange, G.J. Present-day fluxes of coccolithophores and diatoms in the pelagic Ionian Sea. J. Mar. Syst. 2014, 132, 13–27. [Google Scholar] [CrossRef]
- Ziveri, P.; Grandi, C.; Stefanetti, A.; Cita, M.B. Biogenic fluxes in Bannock Basin: First results from a sediment trap study (November 1991–May 1992). Rend. Lincei 1995, 6, 131–145. [Google Scholar] [CrossRef]
- Malinverno, E.; Ziveri, P.; Corselli, C. Coccolithophorid distribution in the Ionian Sea and its relationship to eastern Mediterranean circulation during late fall to early winter 1997. J. Geophys. Res. Ocean. 2003, 108, 8115. [Google Scholar] [CrossRef]
- Stavrakakis, S.; Chronis, G.; Tselepides, A.; Heussner, S.; Monaco, A.; Abassi, A. Downward fluxes of settling particles in the deep Cretan Sea (NE Mediterranean). Prog. Oceanogr. 2000, 46, 217–240. [Google Scholar] [CrossRef]
- Stavrakakis, S.; Gogou, A.; Krasakopoulou, E.; Karageorgis, A.P.; Kontoyiannis, H.; Rousakis, G.; Velaoras, D.; Perivoliotis, L.; Kambouri, G.; Stavrakaki, I.; et al. Downward fluxes of sinking particulate matter in the deep Ionian Sea (NESTOR site), eastern Mediterranean: Seasonal and interannual variability. Biogeosciences 2013, 10, 7235–7254. [Google Scholar] [CrossRef]
- Skampa, E.; Triantaphyllou, M.V.; Dimiza, M.D.; Gogou, A.; Malinverno, E.; Stavrakakis, S.; Parinos, C.; Panagiotopoulos, I.P.; Tselenti, D.; Archontikis, O.; et al. Coccolithophore export in three deep-sea sites of the Aegean and Ionian Seas (Eastern Mediterranean): Biogeographical patterns and biogenic carbonate fluxes. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 171, 104690. [Google Scholar] [CrossRef]
- Ziveri, P.; Rutten, A.; De Lange, G.J.; Thomson, J.; Corselli, C. Present-day coccolith fluxes recorded in central eastern Mediterranean sediment traps and surface sediments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 158, 175–195. [Google Scholar] [CrossRef]
- Triantaphyllou, M.V.; Ziveri, P.; Tselepides, A. Coccolithophore export production and response to seasonal surface water variability in the oligotrophic Cretan Sea (NE Mediterranean). Micropaleontology 2004, 50 (Suppl. S1), 127–144. [Google Scholar] [CrossRef]
- Malinverno, E.; Triantaphyllou, M.V.; Stavrakakis, S.; Ziveri, P.; Lykousis, V. Seasonal and spatial variability of coccolithophore export production at the South-Western margin of Crete (Eastern Mediterranean). Mar. Micropaleontol. 2009, 71, 131–147. [Google Scholar] [CrossRef]
- Dimiza, M.D.; Triantaphyllou, M.V.; Dermitzakis, M.D. Seasonality and ecology of living coccolithophores in Eastern Mediterranean coastal environment (Andros Island, Middle Aegean Sea). Micropaleontology 2008, 54, 159–175. [Google Scholar] [CrossRef]
- Dimiza, M.D.; Triantaphyllou, M.V.; Malinverno, E.; Psarra, S.; Karatsolis, B.T.; Mara, P.; Lagaria, A.; Gogou, A. The composition and distribution of living coccolithophores in the Aegean Sea (NE Mediterranean). Micropaleontology 2015, 61, 521–540. [Google Scholar] [CrossRef]
- El Hourany, R.; Abboud-abi Saab, M.; Faour, G.; Mejia, C.; Crépon, M.; Thiria, S. Phytoplankton diversity in the Mediterranean Sea from satellite data using self-organizing maps. J. Geophys. Res. Ocean. 2019, 124, 5827–5843. [Google Scholar] [CrossRef]
- Trimonis, E.; Rudenko, M. Geomorphology and bottom sediments of the Pylos area. In Proceedings of the 2nd NESTOR International Workshop; Resvanis, L.K., Ed.; Fortress of Niokastro: Pylos, Greece, 1992; p. 321. [Google Scholar]
- D’Ortenzio, F.; Ragni, M.; Marullo, S.; Ribera d’Alcalà, M. Did biological activity in the Ionian Sea change after the Eastern Mediterranean Transient? Results from the analysis of remote sensing observations. J. Geophys. Res. Ocean. 2003, 108, 8113. [Google Scholar] [CrossRef]
- Crombet, Y.; Leblanc, K.; Queguiner, B.; Moutin, T.; Rimmelin, P.; Ras, J.; Claustre, H.; Leblond, N.; Oriol, L.; Pujo-Pay, M. Deep silicon maxima in the stratified oligotrophic Mediterranean Sea. Biogeosciences 2011, 8, 459–475. [Google Scholar] [CrossRef]
- Karageorgis, A.P.; Georgopoulos, D.; Kanellopoulos, T.D.; Mikkelsen, O.A.; Pagou, K.; Kontoyiannis, H.; Pavlidou, A.; Anagnostou, C. Spatial and seasonal variability of particulate matter optical and size properties in the Eastern Mediterranean Sea. J. Mar. Syst. 2012, 105, 123–134. [Google Scholar] [CrossRef]
- Theodosi, C.; Markaki, Z.; Pantazoglou, F.; Tselepides, A.; Mihalopoulos, N. Chemical composition of downward fluxes in the Cretan Sea (Eastern Mediterranean) and possible link to atmospheric deposition: A 7 year survey. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 164, 89–99. [Google Scholar] [CrossRef]
- Rutten, A.; De Lange, G.J.; Ziveri, P.; Thomson, J.; Van Santvoort, P.J.M.; Colley, S.; Corselli, C. Recent terrestrial and carbonate fluxes in the pelagic eastern Mediterranean; a comparison between sediment trap and surface sediment. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 158, 197–213. [Google Scholar] [CrossRef]
- Tanaka, T.; Zohary, T.; Krom, M.D.; Law, C.S.; Pitta, P.; Psarra, S.; Rassoulzadegan, F.; Thingstad, T.F.; Tselepides, A.; Woodward, E.M.S.; et al. Microbial community structure and function in the Levantine Basin of the eastern Mediterranean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 1721–1743. [Google Scholar] [CrossRef]
- Powley, H.R.; Van Cappellen, P.; Krom, M.D. Nutrient cycling in the Mediterranean Sea: The key to understanding how the unique marine ecosystem functions and responds to anthropogenic pressures. In Mediterranean Identities—Environment, Society, Culture; Soc Cult. InTech: London, UK, 2017; pp. 47–77. [Google Scholar] [CrossRef]
- Casotti, R.; Landolfi, A.; Brunet, C.; d’Ortenzio, F.; Mangoni, O.; Ribera d’Alcalà, M.; Denis, M. Composition and dynamics of the phytoplankton of the Ionian Sea (eastern Mediterranean). J. Geophys. Res. Oceans 2003, 108. [Google Scholar] [CrossRef]
- Nittis, K.; Pinardi, N.; Lascaratos, A. Characteristics of the summer 1987 flow field in the Ionian Sea. J. Geophys. Res. Ocean. 1993, 98, 10171–10184. [Google Scholar] [CrossRef]
- Malanotte-Rizzoli, P.; Manca, B.B.; d’Alcalà, M.R.; Theocharis, A.; Bergamasco, A.; Bregant, D.; Budillon, G.; Civitarese, G.; Georgopoulos, D.; Michelato, A.; et al. A synthesis of the Ionian Sea hydrography, circulation and water mass pathways during POEM-Phase I. Prog. Oceanogr. 1997, 39, 153–204. [Google Scholar] [CrossRef]
- Estournel, C.; Marsaleix, P.; Ulses, C. A new assessment of the circulation of Atlantic and Intermediate Waters in the Eastern Mediterranean. Prog. Oceanogr. 2021, 198, 102673. [Google Scholar] [CrossRef]
- Larnicol, G.; Ayoub, N.; Le Traon, P.Y. Major changes in Mediterranean Sea level variability from 7 years of TOPEX/Poseidon and ERS-1/2 data. J Mar Syst. 2002, 33, 63–89. [Google Scholar] [CrossRef]
- Acker, J.G.; Leptoukh, G. Online analysis enhances use of NASA earth science data. Eos Trans. AGU 2007, 88, 14–17. [Google Scholar] [CrossRef]
- Ziveri, P.; Young, J.R.; Van Hinte, J.E. Coccolithophore export production and accumulation rates. GeoResearch Forum 1999, 5, 41–56. [Google Scholar]
- D’Ortenzio, F.; Ribera d’Alcalà, M. On the trophic regimes of the Mediterranean Sea: A satellite analysis. Biogeosciences 2009, 6, 139–148. [Google Scholar] [CrossRef]
- Patara, L.; Pinardi, N.; Corselli, C.; Malinverno, E.; Tonani, M.; Santoleri, R.; Masina, S. Particle fluxes in the deep Eastern Mediterranean basins: The role of ocean vertical velocities. Biogeosciences 2009, 6, 333–348. [Google Scholar] [CrossRef]
- Gogou, A.; Stavrakakis, S.; Triantaphyllou, M.; Paraskos, F.; Parinos, C.; Dimiza, M.; Kambouri, G.; Lykousis, V. Seasonal and interannual variability of sinking particulate matter in the deep Ionian Sea: Ecological and biogeochemical perspectives. Rapp. Comm. Int. Mer Medit. 2016, 41. Available online: https://ciesm.org/online/archives/abstracts/pdf/41/CIESM_Congress_2016_Kiel_article_0083.pdf (accessed on 11 October 2022).
- Turner, J.T. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat. Microb. Ecol. 2002, 27, 57–102. [Google Scholar] [CrossRef]
- Schulz, M.; Prospero, J.M.; Baker, A.R.; Dentener, F.; Ickes, L.; Liss, P.S.; Mahowald, N.M.; Nickovic, S.; García-Pando, C.P.; Rodríguez, S.; et al. Atmospheric transport and deposition of mineral dust to the ocean: Implications for research needs. Environ. Sci. Technol. 2012, 46, 10390–10404. [Google Scholar] [CrossRef]
- Cascella, A.; Bonomo, S.; Jalali, B.; Sicre, M.A.; Pelosi, N.; Schmidt, S.; Lirer, F. Climate variability of the last ~2700 years in the Southern Adriatic Sea: Coccolithophore evidence. Holocene 2020, 30, 53–64. [Google Scholar] [CrossRef]
- Molinaroli, E.; Guerzoni, S.; Rampazzo, G. Contribution of Saharan dust to the Central Mediterranean Basin. Geol. Soc. Am. Spec. Pap. 1993, 284, 303–312. [Google Scholar] [CrossRef]
- Klaas, C.; Archer, D.E. Association of sinking organic matter with various types of mineral ballast in the deep sea: Implications for the rain ratio. Glob. Biogeochem. Cycles 2002, 16, 63-1. [Google Scholar] [CrossRef]
- Young, J.R. Variation in Emiliania huxleyi coccolith morphology in samples from the Norwegian EHUX experiment, 1992. Sarsia 1994, 79, 417–425. [Google Scholar] [CrossRef]
- Molfino, B.; McIntyre, A. Precessional forcing of nutricline dynamics in the equatorial Atlantic. Science 1990, 249, 766–769. [Google Scholar] [CrossRef]
- Triantaphyllou, M.V.; Ziveri, P.; Gogou, A.; Marino, G.; Lykousis, V.; Bouloubassi, I.; Emeis, K.-V.; Kouli, K.; Dimiza, M.; Rosell-Melé, A.; et al. Late Glacial–Holocene climate variability at the south-eastern margin of the Aegean Sea. Mar. Geol. 2009, 266, 182–197. [Google Scholar] [CrossRef]
- Bonomo, S.; Schroeder, K.; Cascella, A.; Alberico, I.; Lirer, F. Living coccolithophore communities in the central Mediterranean Sea (Summer 2016): Relations between ecology and oceanography. Mar. Micropaleontol. 2021, 165, 101995. [Google Scholar] [CrossRef]
- Kleijne, A. Morphology, taxonomy and distribution of extant coccolithophorids (calcareous nannoplankiton). Neth. J. Sea Res. 1993. Available online: https://www.amazon.co.uk/Morphology-Distribution-Coccolithophorids-Calcareous-Nannoplankton/dp/9090061614 (accessed on 11 October 2022).
- Knappertsbusch, M. Geographic distribution of living and Holocene coccolithophores in the Mediterranean Sea. Mar. Micropaleontol. 1993, 21, 219–247. [Google Scholar] [CrossRef]
- Flores, J.A.; Sierro, F.J.; Francés, G.; Vázquez, A.; Zamarren, I. The last 100,000 years in the western Mediterranean: Sea surface water and frontal dynamics as revealed by coccolithophores. Mar. Micropaleontol. 1997, 29, 351–366. [Google Scholar] [CrossRef]
- Colmenero-Hidalgo, E.; Flores, J.A.; Sierro, F.J.; Bárcena, M.Á.; Löwemark, L.; Schönfeld, J.; Grimalt, J.O. Ocean surface water response to short-term climate changes revealed by coccolithophores from the Gulf of Cadiz (NE Atlantic) and Alboran Sea (W Mediterranean). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 205, 317–336. [Google Scholar] [CrossRef]
- Dimiza, M.D.; Triantaphyllou, M.V.; Malinverno, E. New Evidence for The Ecology of Helicosphaera Carteri In Polluted Coastal Environments (Elefsis Bay, Saronikos Gulf, Greece). JNR 2014, 34, 37–43. [Google Scholar]
- Boeckel, B.; Baumann, K.-H. Vertical and lateral variations in coccolithophore community structure across the subtropical frontal zone in the South Atlantic Ocean. Mar. Micropaleontol. 2008, 67, 255–273. [Google Scholar] [CrossRef]

| 2015 | 2016 | 2017 | |||
|---|---|---|---|---|---|
| Sample Code | Sampling Date | Sample Code | Sampling Date | Sample Code | Sampling Date |
| KMS XV 7 | 16–31 January 2015 | KMS XVII 8 | 16–31 March 2016 | KMS XVIII 2 | 1–31 May 2017 |
| KMS XV 8 | 1–15 February 2015 | KMS XVII 9 | 1–15 April 2016 | KMS XVIII 3 | 1–30 June 2017 |
| KMS XV 9 | 16–28 February 2015 | KMS XVII 10 | 16–30 April 2016 | KMS XVIII 4 | 1–31 July 2017 |
| KMS XV 10 | 1–15 March 2015 | KMSXVII 11 | 1–15 May 2016 | KMS XVIII 5 | 1–31 August 2017 |
| KMS XV 11 | 16–31 March 2015 | KMS XVII 12 | 16–31 May 2016 | KMSXVIII 6 | 1–30 September 2017 |
| KMS XV 12 | 1–15 April 2015 | No data (1 June 2016 to 30 April 2017) | KMSXVIII 7 | 1–31 October 2017 | |
| No data (16 April to 31 May 2015) | KMS XVIII 8 | 1–30 November 2017 | |||
| KMS XVIII 8 | 1–30 November 2017 | ||||
| KMS XVI 1 | 1–15 June 2015 | ||||
| KMS XVI 2 | 16–30 June 2015 | ||||
| KMS XVI 3 | 1–15 July 2015 | ||||
| No data (16 July to 15 August 2015) | |||||
| KMS XVI 6 | 16–31 August 2015 | ||||
| KMS XVI 7 | 1–15 September 2015 | ||||
| KMS XVI 8 | 16–30 September 2015 | ||||
| KMS XVI 9 | 1–15 October 2015 | ||||
| KMS XVI 10 | 16–31 October 2015 | ||||
| No data (1 November 2015 to 15 March 2016) | |||||
| Algirosphaera robusta (Lohmann, 1902) Norris, 1984 |
| Calcidiscus leptoporus (Murray and Blackman, 1898) Loeblich and Tappan, 1978 |
| Calciosolenia brasiliensis (Lohmann, 1919) Young, 2003 |
| Ceratolithus cristatus Kamptner, 1950 |
| Coccolithus pelagicus (Wallich, 1877) Schiller, 1930 subsp. pelagicus |
| Discosphaera tubifera (Murray and Blackman, 1898) Ostenfeld 1900 |
| Emiliania huxleyi (Lohmann, 1902) Hay and Mohler in Hay et al. 1967 |
| Florisphaera profunda Okada and Honjo, 1973 |
| Gephyrocapsa oceanica Kamptner, 1943 |
| Gladiolithus flabellatus (Halldal and Markali, 1955) Jordan and Chamberlain, 1993 |
| Helicosphaera carteri (Wallich, 1877) Kamptner, 1954 |
| Pontosphaera spp. |
| Rhabdosphaera clavigera Murray and Blackman, 1898 |
| Scyphosphaera spp. |
| Syracosphaera mediterranea Lohmann 1902 |
| Syracosphaera pulchra Lohmann, 1902 |
| Umbellosphaera tenuis (Kamptner, 1954) Paasche, 1955 |
| Umbilicosphaera sibogae (Weber-van Bosse, 1901) Gaarder, 1970 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, S.; Skampa, E.; Gogou, A.; Stavrakakis, S.; Parinos, C.; Triantaphyllou, M. Seasonal Variability in Present-Day Coccolithophore Fluxes in Deep Eastern Mediterranean Sea: A Multi-Year Study (2015–2017) of Coccolithophore Export in SE Ionian Sea at 4300 m Depth. J. Mar. Sci. Eng. 2022, 10, 1761. https://doi.org/10.3390/jmse10111761
Hayat S, Skampa E, Gogou A, Stavrakakis S, Parinos C, Triantaphyllou M. Seasonal Variability in Present-Day Coccolithophore Fluxes in Deep Eastern Mediterranean Sea: A Multi-Year Study (2015–2017) of Coccolithophore Export in SE Ionian Sea at 4300 m Depth. Journal of Marine Science and Engineering. 2022; 10(11):1761. https://doi.org/10.3390/jmse10111761
Chicago/Turabian StyleHayat, Sikandar, Elisavet Skampa, Alexandra Gogou, Spyros Stavrakakis, Constantine Parinos, and Maria Triantaphyllou. 2022. "Seasonal Variability in Present-Day Coccolithophore Fluxes in Deep Eastern Mediterranean Sea: A Multi-Year Study (2015–2017) of Coccolithophore Export in SE Ionian Sea at 4300 m Depth" Journal of Marine Science and Engineering 10, no. 11: 1761. https://doi.org/10.3390/jmse10111761
APA StyleHayat, S., Skampa, E., Gogou, A., Stavrakakis, S., Parinos, C., & Triantaphyllou, M. (2022). Seasonal Variability in Present-Day Coccolithophore Fluxes in Deep Eastern Mediterranean Sea: A Multi-Year Study (2015–2017) of Coccolithophore Export in SE Ionian Sea at 4300 m Depth. Journal of Marine Science and Engineering, 10(11), 1761. https://doi.org/10.3390/jmse10111761









