Effects of Dietary Vegetable Oils Replacing Fish Oil on Fatty Acid Composition, Lipid Metabolism and Inflammatory Response in Adipose Tissue of Large Yellow Croaker (Larimichthys crocea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Diets and Fish Culture
2.2. Analysis of Fatty Acid Profiles
2.3. Triglyceride (TG) Content Quantification
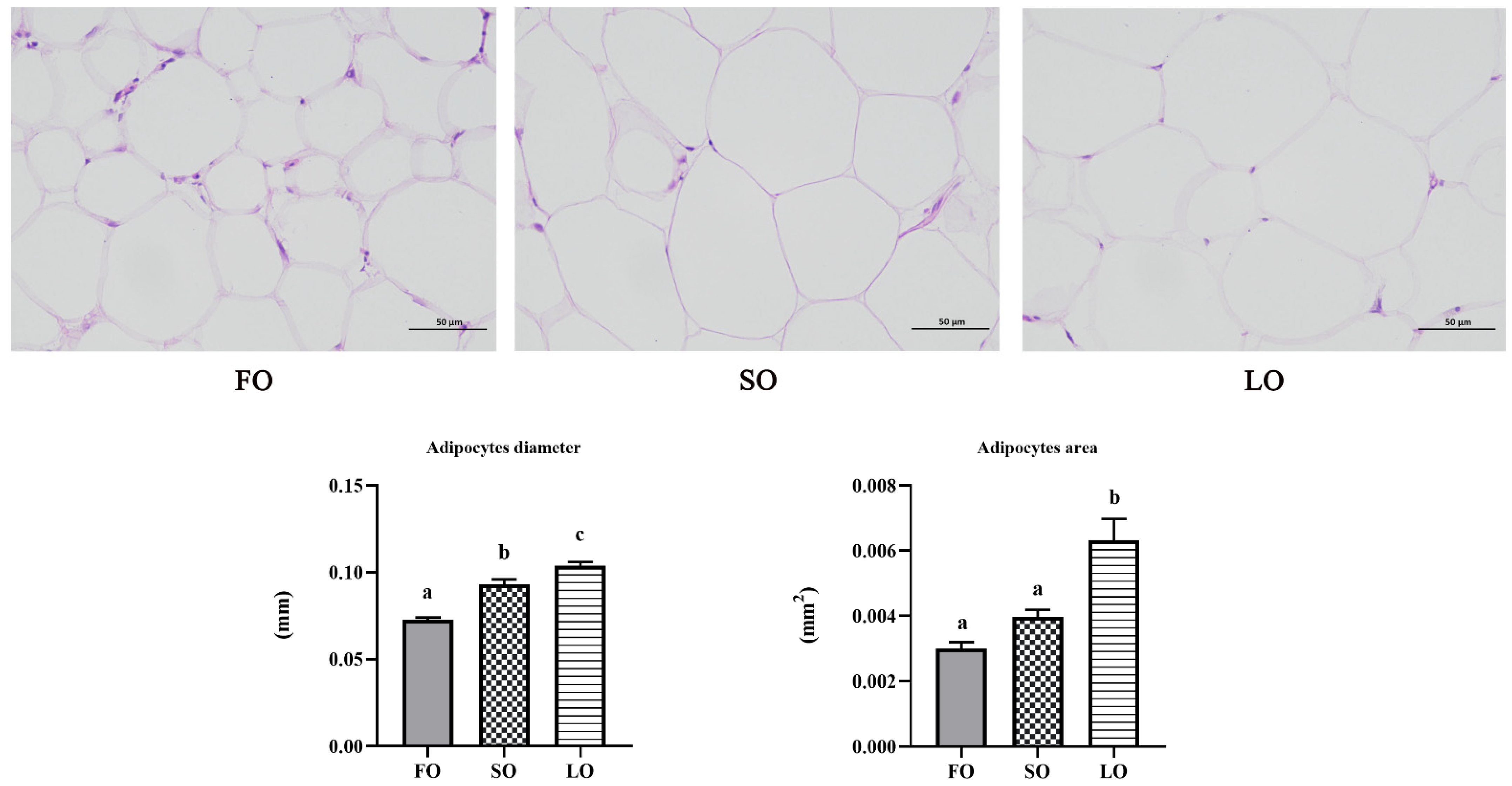
2.4. H&E Staining
2.5. RNA Extraction, Complementary DNA (cDNA) Synthesis and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
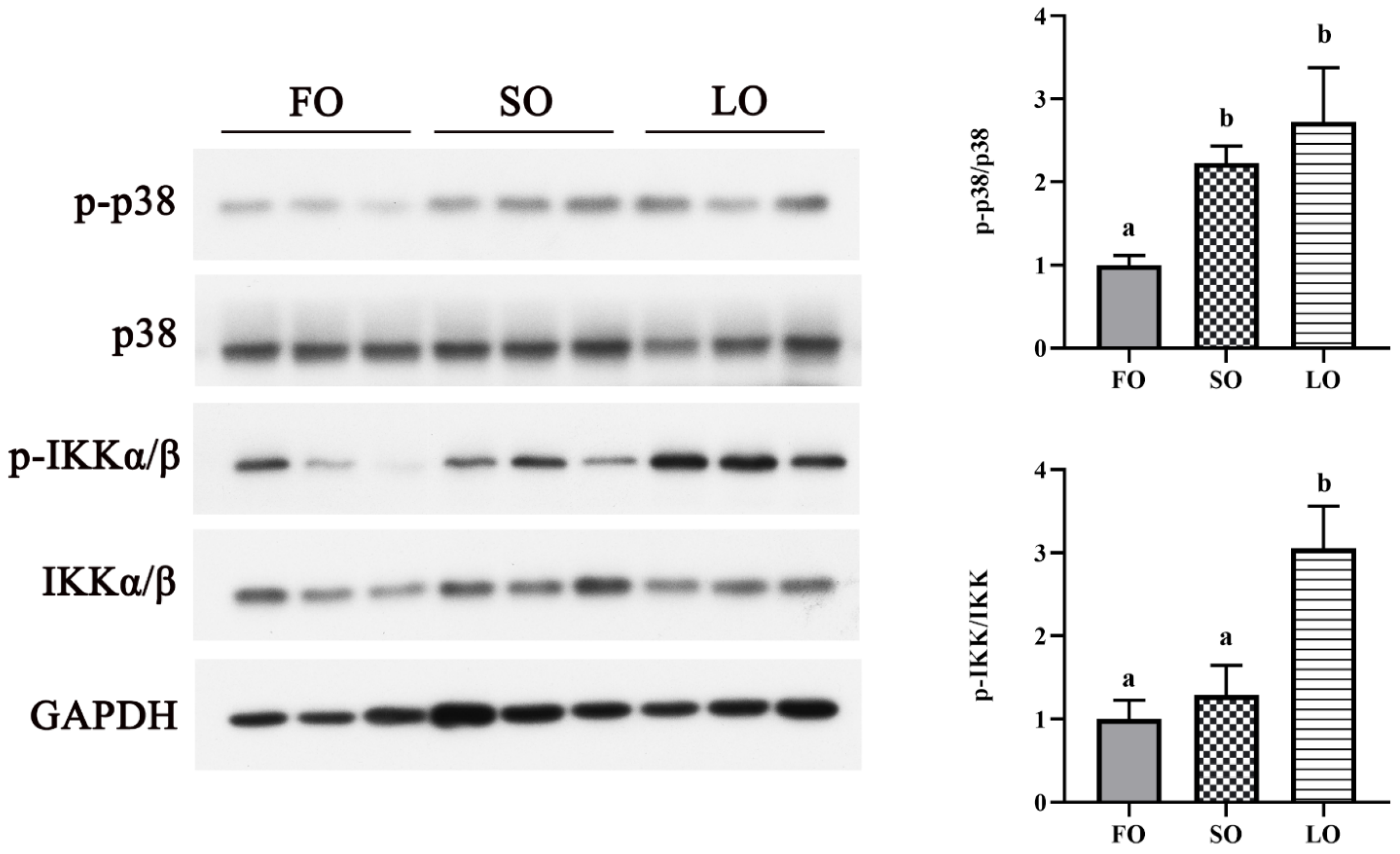
2.6. Western Blot Analysis
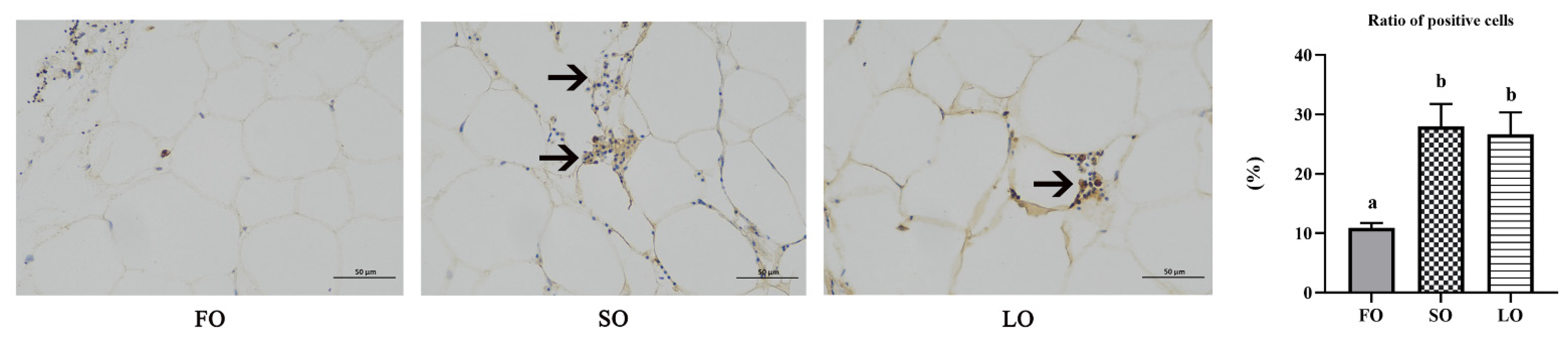
2.7. Immunohistochemical (IHC)
2.8. Statistical Analysis
3. Results
3.1. Dietary SO and LO Affected the Fatty Acid Profile of Adipose Tissue
3.2. Dietary SO and LO Induced Excess Fat Accumulation of Adipose Tissue
3.2.1. Dietary SO and LO Increased the TG Content in Adipose Tissue
3.2.2. Dietary SO and LO Induced Hypertrophy in Adipose Tissue
3.3. Effects of Dietary SO and LO on the Expression of Lipid-Metabolism-Related Genes in Adipose Tissue
3.4. Dietary SO and LO Induced the Inflammatory Response of Adipose Tissue
3.4.1. Dietary SO and LO Induced the Inflammation-Related Gene Expression
3.4.2. Dietary SO and LO Activated p38 MAPK and NF-κB Signaling Pathway
3.4.3. Dietary SO and LO Induced Infiltration and Polarization of Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacon, A.G.; Metian, M. Aquaculture feed and food safety: The role of the food and agriculture organization and the Codex Alimentarius. Ann. N. Y. Acad. Sci. 2008, 1140, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Menoyo, D.; Izquierdo, M.S.; Robaina, L.; Gines, R.; Lopez-Bote, C.J.; Bautista, J.M. Adaptation of lipid metabolism, tissue composition and flesh quality in gilthead sea bream (Sparus aurata) to the replacement of dietary fish oil by linseed and soyabean oils. Br. J. Nutr. 2004, 92, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Kaushik, S.; Larroquet, L.; Panserat, S.; Corraze, G. Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2006, 96, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Kutluyer, F.; Sirkecioğlu, A.N.; Aksakal, E.; Aksakal, F.İ.; Tunç, A.; Günaydin, E. Effect of dietary fish oil replacement with plant oils on growth performance and gene expression in Juvenile Rainbow Trout (Oncorhynchus mykiss). Ann. Anim. Sci. 2017, 17, 1135–1153. [Google Scholar] [CrossRef]
- Li, X.; Ji, R.; Cui, K.; Chen, Q.; Chen, Q.; Fang, W.; Mai, K.; Zhang, Y.; Xu, W.; Ai, Q. High percentage of dietary palm oil suppressed growth and antioxidant capacity and induced the inflammation by activation of TLR-NF-kappaB signaling pathway in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2019, 87, 600–608. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Li, Q.; Li, J.; Cui, K.; Zhang, Y.; Kong, A.; Zhang, Y.; Wan, M.; Mai, K.; et al. Effects of High Levels of Dietary Linseed Oil on the Growth Performance, Antioxidant Capacity, Hepatic Lipid Metabolism, and Expression of Inflammatory Genes in Large Yellow Croaker (Larimichthys crocea). Front. Physiol. 2021, 12, 631850. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Liang, Z.; Xie, Y.; Su, J.; Luo, Q.; Zhu, J.; Liu, Q.; Han, T.; Wang, A. Effects of dietary fish oil replacement by soybean oil and l-carnitine supplementation on growth performance, fatty acid composition, lipid metabolism and liver health of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2020, 516, 734596. [Google Scholar] [CrossRef]
- Wu, J.-L.; Zhang, J.-L.; Du, X.-X.; Shen, Y.-J.; Lao, X.; Zhang, M.-L.; Chen, L.-Q.; Du, Z.-Y. Evaluation of the distribution of adipose tissues in fish using magnetic resonance imaging (MRI). Aquaculture 2015, 448, 112–122. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.W.; Dixit, V.D. Adipose tissue as an immunological organ. Obesity 2015, 23, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Zeyda, M.; Stulnig, T.M. Adipose tissue macrophages. Immunol. Lett. 2007, 112, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, C. Adipogenesis in fish. J. Exp. Biol. 2018, 221, jeb161588. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, K.A.; Wangkahart, E.; Wang, T.; Tubbs, L.; Ben Arous, J.; Secombes, C.J. Rainbow trout (Oncorhynchus mykiss) adipose tissue undergoes major changes in immune gene expression following bacterial infection or stimulation with pro-inflammatory molecules. Dev. Comp. Immunol. 2018, 81, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Dong, X.; Mai, K.; Xu, W.; Ai, Q. Vegetable oil induced inflammatory response by altering TLR-NF-kappaB signalling, macrophages infiltration and polarization in adipose tissue of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2016, 59, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Jin, M.; Li, Y.; Lu, Y.; Hou, Y.; Zhou, Q. Dietary lipid sources influence fatty acid composition in tissue of large yellow croaker (Larmichthys crocea) by regulating triacylglycerol synthesis and catabolism at the transcriptional level. PLoS ONE 2017, 12, e0169985. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Mu, H.; Shen, H.; Deng, K.; Liu, D.; Yang, M.; Zhang, Y.; Zhang, W.; Mai, K. High level of dietary soybean oil affects the glucose and lipid metabolism in large yellow croaker Larimichthys crocea through the insulin-mediated PI3K/AKT signaling pathway. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 231, 34–41. [Google Scholar] [CrossRef]
- Ding, T.; Xu, N.; Liu, Y.; Li, X.; Xiang, X.; Xu, D.; Yao, C.; Liu, Q.; Yin, Z.; Mai, K.; et al. Optimal amounts of coconut oil in diets improve the growth, antioxidant capacity and lipid metabolism of large yellow croaker (Larimichthys crocea). Mar. Life Sci. Technol. 2020, 2, 376–385. [Google Scholar] [CrossRef]
- Li, Y.; Pang, Y.; Xiang, X.; Du, J.; Mai, K.; Ai, Q. Molecular Cloning, Characterization, and Nutritional Regulation of Elovl6 in Large Yellow Croaker (Larimichthys crocea). Int. J. Mol. Sci. 2019, 20, 1801. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Fang, W.; Chen, Q.; Xu, D.; Mai, K.; Zhang, Y.; Ai, Q. High level of dietary olive oil decreased growth, increased liver lipid deposition and induced inflammation by activating the p38 MAPK and JNK pathways in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2019, 94, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Cui, K.; Li, Q.; Zhu, S.; Zhang, J.; Gao, S.; Hao, T.; Mai, K.; Ai, Q. Docosahexaenoic acid alleviates palmitic acid-induced inflammation of macrophages via TLR22-MAPK-PPARgamma/Nrf2 pathway in large yellow croaker (Larimichthys crocea). Antioxidants 2022, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, Q.; Zhou, Y.; Shen, Y.; Lai, W.; Hao, T.; Ding, Y.; Mai, K.; Ai, Q. Functional analysis and regulation mechanism of interferon gamma in macrophages of large yellow croaker (Larimichthys crocea). Int. J. Biol. Macromol. 2022, 194, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. Aquac. 2003, 11, 107. [Google Scholar] [CrossRef]
- Xu, H.; Turchini, G.M.; Francis, D.S.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef]
- Cui, K.; Li, X.; Chen, Q.; Li, Q.; Gao, S.; Tan, P.; Mai, K.; Ai, Q. Effect of replacement of dietary fish oil with four vegetable oils on prostaglandin E2 synthetic pathway and expression of inflammatory genes in marine fish Larimichthys crocea. Fish Shellfish Immunol. 2020, 107, 529–536. [Google Scholar] [CrossRef]
- Nayak, M.; Saha, A.; Pradhan, A.; Samanta, M.; Giri, S.S. Dietary fish oil replacement by linseed oil: Effect on growth, nutrient utilization, tissue fatty acid composition and desaturase gene expression in silver barb (Puntius gonionotus) fingerlings. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 205, 1–12. [Google Scholar] [CrossRef]
- Rondinone, C.M. Adipocyte-derived hormones, cytokines, and mediators. Endocrine 2006, 29, 81–90. [Google Scholar] [CrossRef]
- Teoh, C.-Y.; Turchini, G.M.; Ng, W.-K. Genetically improved farmed Nile tilapia and red hybrid tilapia showed differences in fatty acid metabolism when fed diets with added fish oil or a vegetable oil blend. Aquaculture 2011, 312, 126–136. [Google Scholar] [CrossRef]
- Ofori-Mensah, S.; Yıldız, M.; Arslan, M.; Eldem, V. Fish oil replacement with different vegetable oils in gilthead seabream, Sparus aurata diets: Effects on fatty acid metabolism based on whole-body fatty acid balance method and genes expression. Aquaculture 2020, 529, 735609. [Google Scholar] [CrossRef]
- Qin, G.; Xu, D.; Lou, B.; Chen, R.; Wang, L.; Tan, P. iTRAQ-based quantitative phosphoproteomics provides insights into the metabolic and physiological responses of a carnivorous marine fish (Nibea albiflora) fed a linseed oil-rich diet. J. Proteom. 2020, 228, 103917. [Google Scholar] [CrossRef]
- Cruz-Garcia, L.; Sanchez-Gurmaches, J.; Bouraoui, L.; Saera-Vila, A.; Perez-Sanchez, J.; Gutierrez, J.; Navarro, I. Changes in adipocyte cell size, gene expression of lipid metabolism markers, and lipolytic responses induced by dietary fish oil replacement in gilthead sea bream (Sparus aurata L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 391–399. [Google Scholar] [CrossRef]
- Sánchez-Moya, A.; García-Meilán, I.; Riera-Heredia, N.; Vélez, E.J.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2020, 519, 734881. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Espe, M.; Stubhaug, I.; Lie, O. Dietary plant proteins and vegetable oil blends increase adiposity and plasma lipids in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2011, 106, 633–647. [Google Scholar] [CrossRef]
- Schreurs, M.; Kuipers, F.; van der Leij, F.R. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, S.I.; Lee, H.R.; Hwang, I.S.; Lee, Y.J.; An, B.S.; Lee, S.H.; Kim, H.J.; Kang, B.C.; Hwang, D.Y. Selenium significantly inhibits adipocyte hypertrophy and abdominal fat accumulation in OLETF rats via induction of fatty acid beta-oxidation. Biol. Trace Elem. Res. 2012, 150, 360–370. [Google Scholar] [CrossRef]
- Noh, H.; Lee, H.; Kim, E.; Mu, L.; Rhee, Y.K.; Cho, C.W.; Chung, J. Inhibitory effect of a Cirsium setidens extract on hepatic fat accumulation in mice fed a high-fat diet via the induction of fatty acid beta-oxidation. Biosci. Biotechnol. Biochem. 2013, 77, 1424–1429. [Google Scholar] [CrossRef]
- Bouraoui, L.; SÁNchez-Gurmaches, J.; Cruz-Garcia, L.; Gutiérrez, J.; Benedito-Palos, L.; Pérez-Sánchez, J.; Navarro, I. Effect of dietary fish meal and fish oil replacement on lipogenic and lipoprotein lipase activities and plasma insulin in gilthead sea bream (Sparus aurata). Aquac. Nutr. 2011, 17, 54–63. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Nanton, D.A.; Olsvik, P.A.; Sundvold, H.; Stubhaug, I. Gene expression of fatty acid-binding proteins, fatty acid transport proteins (cd36 and FATP) and β-oxidation-related genes in Atlantic salmon (Salmo salar L.) fed fish oil or vegetable oil. Aquac. Nutr. 2009, 15, 440–451. [Google Scholar] [CrossRef]
- Richard, N.; Mourente, G.; Kaushik, S.; Corraze, G. Replacement of a large portion of fish oil by vegetable oils does not affect lipogenesis, lipid transport and tissue lipid uptake in European seabass (Dicentrarchus labrax L.). Aquaculture 2006, 261, 1077–1087. [Google Scholar] [CrossRef]
- Shi, X.C.; Jin, A.; Sun, J.; Tian, J.J.; Ji, H.; Chen, L.Q.; Du, Z.Y. The protein-sparing effect of alpha-lipoic acid in juvenile grass carp, Ctenopharyngodon idellus: Effects on lipolysis, fatty acid beta-oxidation and protein synthesis. Br. J. Nutr. 2018, 120, 977–987. [Google Scholar] [CrossRef]
- Mu, H.; Wei, C.; Zhang, Y.; Zhou, H.; Pan, Y.; Chen, J.; Zhang, W.; Mai, K. Impacts of replacement of dietary fish oil by vegetable oils on growth performance, anti-oxidative capacity, and inflammatory response in large yellow croaker Larimichthys crocea. Fish Physiol. Biochem. 2020, 46, 231–245. [Google Scholar] [CrossRef]
- Wong, S.W.; Kwon, M.-J.; Choi, A.M.; Kim, H.-P.; Nakahira, K.; Hwang, D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar] [CrossRef]
- De Boer, A.A.; Monk, J.M.; Robinson, L.E. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PLoS ONE 2014, 9, e85037. [Google Scholar] [CrossRef]
- Thompson, K.; Tatner, M.; Henderson, R. Effects of dietary (n-3) and (n-6) polyunsaturated fatty acid ratio on the immune response of Atlantic salmon, Salmo salar L. Aquac. Nutr. 1996, 2, 21–31. [Google Scholar] [CrossRef]
- Montero, D.; Mathlouthi, F.; Tort, L.; Afonso, J.M.; Torrecillas, S.; Fernandez-Vaquero, A.; Negrin, D.; Izquierdo, M.S. Replacement of dietary fish oil by vegetable oils affects humoral immunity and expression of pro-inflammatory cytokines genes in gilthead sea bream Sparus aurata. Fish Shellfish Immunol. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]



| Ingredient a | Dietary Treatments b | ||
|---|---|---|---|
| Fish Oil (FO) | Soybean Oil (SO) | Linseed Oil (LO) | |
| White fish meal b | 35 | 35 | 35 |
| Soybean meal b | 28 | 28 | 28 |
| Wheat meal b | 23.8 | 23.8 | 23.8 |
| Soybean lecithin | 1.5 | 1.5 | 1.5 |
| Vitamin premix c | 2 | 2 | 2 |
| Mineral premix c | 2 | 2 | 2 |
| Attractant mixture d | 0.1 | 0.1 | 0.1 |
| Mold inhibitor e | 0.1 | 0.1 | 0.1 |
| Fish oil | 7.5 | ||
| Soybean oil | 7.5 | ||
| Linseed oil | 7.5 | ||
| Total | 100.00 | 100.00 | 100.00 |
| Proximate analysis (%) | |||
| Crude protein | 42.23 | 41.98 | 42.42 |
| Crude lipid | 12.05 | 12.19 | 12.14 |
| Fatty Acid (% Total Fatty Acid) | FO | SO | LO |
|---|---|---|---|
| 14:0 | 8.77 | 0.08 | 0.07 |
| 16:0 | 19.15 | 10.32 | 5.39 |
| 18:0 | 4.26 | 3.95 | 3.76 |
| 20:0 | 1.18 | 0.27 | 0.17 |
| ∑SFA 1 | 33.26 | 14.62 | 9.39 |
| 16:1n-7 | 11.85 | 0.09 | 0.10 |
| 18:1n-9 | 9.75 | 26.10 | 20.45 |
| ∑MUFA 2 | 21.6 | 26.19 | 20.55 |
| 18:2n-6 (LA) | 1.54 | 49.65 | 15.52 |
| 20:4n-6 | 1.30 | 0.00 | 0.00 |
| ∑n-6 PUFA 3 | 2.84 | 49.65 | 15.52 |
| 18:3n-3 (ALA) | 0.76 | 4.92 | 53.02 |
| 20:5n-3 (EPA) | 12.34 | 0.00 | 0.00 |
| 22:6n-3 (DHA) | 7.30 | 0.00 | 0.00 |
| ∑n-3 PUFA 4 | 20.40 | 4.92 | 53.02 |
| ∑n-3 LC-PUFA 5 | 19.64 | 0.00 | 0.00 |
| Target Gene | Forward (5’-3’) | Reverse (3’-5’) | Accession Number |
|---|---|---|---|
| β-actin | TTATGAAGGCTATGCCCTGCC | TGAAGGAGTAGCCACGCTCTGT | XM_010732326 |
| fas | CAGCCACAGTGAGGTCATCC | TGAGGACATTGAGCCAGACAC | XM_027289105 |
| dgat2 | TTCGGTGCTTTCTGCAACTTCG | AAGGATGGGGAAGCGGAAGT | XM_019255019 |
| fabp3 | CCAAACCCACCACTATCATCTCAG | GCACCATCTTTCCCTCCTCTATTG | XM_010731330 |
| fabp10 | CAATGGAACATGGCAGGTTTACG | TGATTGGCTTGATGTCCTTGGC | XM_010738161 |
| srebp1 | TCTCCTTGCAGTCTGAGCCAAC | TCAGCCCTTGGATATGAGCCT | XM_010730705 |
| cebpα | GAGGCGGGAAGCACAAGAAG | TTCGCCTTGTCGCGGCTCTTAC | XM_027281242 |
| pparγ | TGTCCGAGCTGGAAGACAAC | TGGGGTCATAGGGCATACCA | XM_010731330 |
| lpl | GAATTCAACGCGGAAACACAG | ACGCTCATAGAGGGCAGACAC | NM_001303397 |
| hsl | TCACAAAGTGCCTCAATGCC | CAAACGGGTTCCTGATAATGG | XM_019254673 |
| atgl | ATTTCCGACAACCTCCCTCA | CATCACTGCTCCCTCTTTCA | NM_001303332 |
| cpt1α | GCTGAGCCTGGTGAAGATGTTC | TCCATTTGGTTGAATTGTTTACTG | JX434612 |
| aco | AGTGCCCAGATGATCTTGAAGC | CTGCCAGAGGTAACCATTTCCT | XM_010748324 |
| il1β | CATAGGGATGGGGACAACGA | AGGGGACGGACACAAGGGTA | XM_010736551 |
| il6 | CGACACACCCACTATTTACAAC | TCCCATTTTCTGAACTGCCTC | XM_010734753 |
| tnfα | ACACCTCTCAGCCACAGGAT | CCGTGTCCCACTCCATAGTT | NM_001303385 |
| ifnγ | TCAGACCTCCGCACCATCA | GCAACCATTGTAACGCCACTTA | XM_010751697 |
| tgfβ | ATCTTCCGTCTTCCAAACCA | CCATTCACTCACTGCCTCTGT | XM_027280465 |
| il10 | AGTCGGTTACTTTCTGTGGTG | TGTATGACGCAATATGGTCTG | XM_010738826 |
| FO | SO | p-Value | LO | p-Value | |
|---|---|---|---|---|---|
| 12:0 | 0.325 ± 0.01 | 0.151 ± 0.022 | 0.006 | 0.09 ± 0.01 | <0.001 |
| 14:0 | 3.422 ± 0.11 | 1.149 ± 0.04 | <0.001 | 1.11 ± 0.06 | <0.001 |
| 16:0 | 19.544 ± 1.76 | 13.689 ± 0.43 | 0.035 | 12.82 ± 0.32 | 0.020 |
| 18:0 | 4.815 ± 0.39 | 4.39 ± 0.12 | 0.373 | 4.888 ± 0.38 | 0.899 |
| 20:0 | 0.143 ± 0.02 | 0.326 ± 0.06 | 0.003 | 0.323 ± 0.02 | 0.002 |
| ∑SFA 1 | 28.249 ± 0.92 | 19.705 ± 0.28 | 0.019 | 19.231 ± 0.26 | 0.013 |
| 16:1n-7 | 5.686 ± 0.36 | 1.712 ± 0.06 | <0.001 | 1.599 ± 0.05 | <0.001 |
| 18:1n-9 | 15.444 ± 0.35 | 13.794 ± 1.85 | 0.566 | 10.678 ± 4.17 | 0.318 |
| ∑MUFA 2 | 21.13 ± 0.33 | 15.461 ± 1.82 | 0.110 | 12.277 ± 4.19 | 0.104 |
| 18:2n-6 (LA) | 10.679 ± 0.76 | 34.283 ± 1.27 | <0.001 | 20.502 ± 0.44 | <0.001 |
| 20:4n-6 | 0.174 ± 0.03 | 0.08 ± 0.02 | 0.109 | 0.127 ± 0.02 | 0.262 |
| ∑n-6 PUFA 3 | 10.853 ± 0.079 | 34.363 ± 1.19 | <0.001 | 20.629 ± 0.4 | <0.001 |
| 18:3n-3 (ALA) | 1.732 ± 0.11 | 4.285 ± 0.14 | <0.001 | 24.134 ± 0.18 | <0.001 |
| 20:5n-3 (EPA) | 0.712 ± 0.06 | 0.277 ± 0.03 | 0.004 | 0.375 ± 0.05 | 0.013 |
| 22:6n-3 (DHA) | 0.931 ± 0.09 | 0.366 ± 0.04 | 0.006 | 0.359 ± 0.02 | 0.003 |
| ∑n-3 PUFA 4 | 3.375 ± 0.13 | 4.928 ± 0,17 | 0.006 | 24.868 ± 0.19 | <0.001 |
| ∑n-3 LC-PUFA 5 | 1.643 ± 0.08 | 0.643 ± 0.03 | 0.005 | 0.734 ± 0.04 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Xiang, X.; Li, X.; Xu, N.; Zhang, W.; Mai, K.; Ai, Q. Effects of Dietary Vegetable Oils Replacing Fish Oil on Fatty Acid Composition, Lipid Metabolism and Inflammatory Response in Adipose Tissue of Large Yellow Croaker (Larimichthys crocea). J. Mar. Sci. Eng. 2022, 10, 1760. https://doi.org/10.3390/jmse10111760
Xu D, Xiang X, Li X, Xu N, Zhang W, Mai K, Ai Q. Effects of Dietary Vegetable Oils Replacing Fish Oil on Fatty Acid Composition, Lipid Metabolism and Inflammatory Response in Adipose Tissue of Large Yellow Croaker (Larimichthys crocea). Journal of Marine Science and Engineering. 2022; 10(11):1760. https://doi.org/10.3390/jmse10111760
Chicago/Turabian StyleXu, Dan, Xiaojun Xiang, Xueshan Li, Ning Xu, Wencong Zhang, Kangsen Mai, and Qinghui Ai. 2022. "Effects of Dietary Vegetable Oils Replacing Fish Oil on Fatty Acid Composition, Lipid Metabolism and Inflammatory Response in Adipose Tissue of Large Yellow Croaker (Larimichthys crocea)" Journal of Marine Science and Engineering 10, no. 11: 1760. https://doi.org/10.3390/jmse10111760
APA StyleXu, D., Xiang, X., Li, X., Xu, N., Zhang, W., Mai, K., & Ai, Q. (2022). Effects of Dietary Vegetable Oils Replacing Fish Oil on Fatty Acid Composition, Lipid Metabolism and Inflammatory Response in Adipose Tissue of Large Yellow Croaker (Larimichthys crocea). Journal of Marine Science and Engineering, 10(11), 1760. https://doi.org/10.3390/jmse10111760





