The Effect of Schizochytrium sp. on Growth, Fatty Acid Profile and Gut Microbiota of Silver Pomfret (Pampus argenteus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diet and Sample
2.2. Determination of Fish Growth Performance and Fatty Acid Composition
2.2.1. Growth Performance
2.2.2. Fatty Acid Composition
Total Lipid (TL) Extraction
Fatty Acid Analysis
2.3. Gut Microbiota Analysis
2.3.1. DNA Extraction
2.3.2. 16S rRNA PCR and Pyrosequencing
2.3.3. Operational Taxonomic Units (OTUs) Cluster
2.3.4. Alpha and Beta Diversity
2.4. Statistical Analysis
3. Results
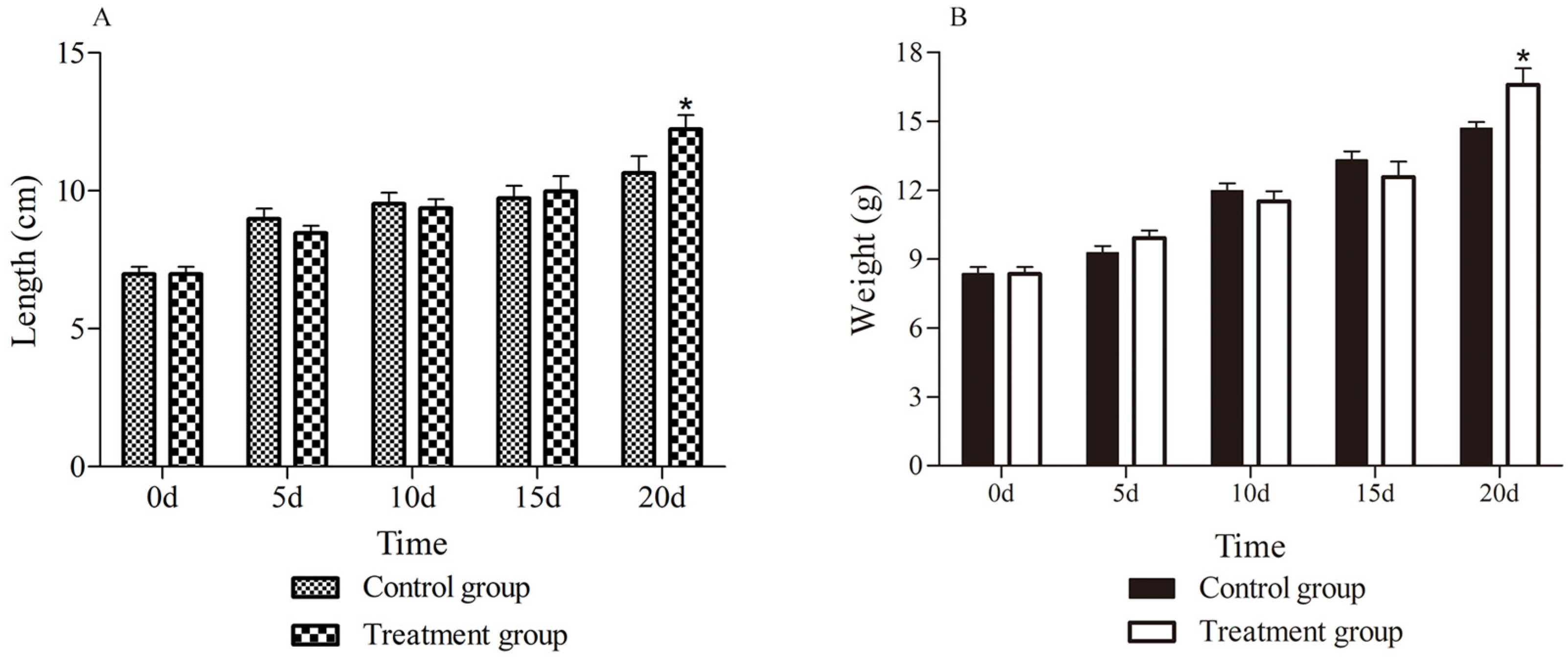
3.1. Growth Performance
3.2. FA Composition
FA Composition at Different Times
3.3. Effects of Schizochytrium sp. on the Gut Microbiota in Pampus argenteus
3.3.1. 16S rRNA Sequencing Results and Diversity Analysis
3.3.2. Difference in the Gut Microbiota between the Control and Treatment Groups
4. Discussion
4.1. Feeding Schizochytrium sp. for Better Growth Performance
4.2. Fatty Acid Composition
4.3. Changes and Functional of Dominant Phyla and Special in Different Period
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Huang, C.; Xu, Z.; Xu, H.; Wang, Z.; Yu, X. The strategies to reduce cost and improve productivity in DHA production by Aurantiochytrium sp.: From biochemical to genetic respects. Appl. Microbiol. Biotechnol. 2020, 104, 9433–9447. [Google Scholar] [CrossRef]
- Choi, S.A.; Jung, J.Y.; Kim, K.; Lee, J.S.; Kwon, J.H.; Kim, S.W.; Yang, J.W.; Park, J.Y. Acid-catalyzed hot-water extraction of docosahexaenoic acid (DHA)-rich lipids from Aurantiochytrium sp. KRS101. Bioresour. Technol. 2014, 161, 469–472. [Google Scholar] [CrossRef]
- dos Santos, S.K.A.; Schorer, M.; Moura, G.D.S.; Lanna, E.A.T.; Pedreira, M.M. Evaluation of growth and fatty acid profile of Nile tilapia (Oreochromis niloticus) fed with Schizochytrium sp. Aquac. Res. 2019, 50, 1068–1074. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, X.; Ji, L.; Song, X.; Kuang, C. Changes of lipid content and fatty acid composition of Schizochytrium limacinum in response to different temperatures and salinities. Process Biochem. 2007, 42, 210–214. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef]
- Chang, M.C.J.; Bell, J.M.; Purdon, A.D.; Chikhale, E.G.; Grange, E. Dynamics of Docosahexaenoic Acid Metabolism in the Central Nervous System: Lack of Effect of Chronic Lithium Treatment. Neurochem. Res. 1999, 24, 399–406. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, L.; Li, D.; Feng, Z.; Zhao, L.; Dong, T. Effects of fish oil on lymphocyte proliferation, cytokine production and intracellular signalling in weanling pigs. Arch. Tierernährung 2003, 57, 151. [Google Scholar] [CrossRef] [PubMed]
- Leifert, W.R.; Dorian, C.L.; Jahangiri, A.; Mcmurchie, E.J. Dietary fish oil prevents asynchronous contractility and alters Ca 2+ handling in adult rat cardiomyocytes. J. Nutr. Biochem. 2001, 12, 365–376. [Google Scholar] [CrossRef]
- Villalta, M.; Estevez, A.; Bransden, M.P.; Bell, J.G. The effect of graded concentrations of dietary DHA on growth, survival and tissue fatty acid profile of Senegal sole (Solea senegalensis) larvae during the Artemia feeding period. Aquaculture 2005, 249, 353–365. [Google Scholar] [CrossRef]
- Trushenski, J.; Schwarz, M.; Bergman, A.; Rombenso, A.; Delbos, B. DHA is essential, EPA appears largely expendable, in meeting the n−3 long-chain polyunsaturated fatty acid requirements of juvenile cobia Rachycentron canadum. Aquaculture 2012, 326–329, 81–89. [Google Scholar] [CrossRef]
- Rombenso, A.N.; Trushenski, J.T.; Jirsa, D.; Drawbridge, M. Successful Fish Oil Sparing in white seabass Feeds Using Saturated Fatty Acid-Rich Soybean Oil and 22:6n-3 (DHA) Supplementation. Aquaculture 2015, 448, 176–185. [Google Scholar] [CrossRef]
- Desai, A.R.; Links, M.G.; Collins, S.A.; Mansfield, G.S.; Drew, M.D.; Kessel, A.G.V.; Hill, J.E. Effects of plant-based diets on the distal gut microbiome of rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 350–353, 134–142. [Google Scholar] [CrossRef]
- Ingerslev, H.C.; Strube, M.L.; Jørgensen, L.V.G.; Dalsgaard, I.; Boye, M.; Madsen, L. Diet type dictates the gut microbiota and the immune response against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2014, 40, 624. [Google Scholar] [CrossRef]
- Ingerslev, H.C.; Jørgensen, L.V.G.; Strube, M.L.; Larsen, N.; Dalsgaard, I.; Boye, M.; Madsen, L. The development of the gut microbiota in rainbow trout (Oncorhynchus mykiss) is affected by first feeding and diet type. Aquaculture 2014, 424–425, 24–34. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Shouche, Y.S.; Gade, W.N. Deep Sequencing Reveals Highly Variable Gut Microbial Composition of Invasive Fish Mossambicus Tilapia (Oreochromis mossambicus) Collected from Two Different Habitats. Indian J. Microbiol. 2017, 57, 235–240. [Google Scholar] [CrossRef]
- Sugita, H.; Shibuya, K.; Shimooka, H.; Deguchi, Y. Antibacterial abilities of intestinal bacteria in freshwater cultured fish. Aquaculture 1996, 145, 195–203. [Google Scholar] [CrossRef]
- Xiao, D.; Ren, W.; Peng, B.; Chen, S.; Yin, J.; Gao, W.; Liu, G.; Nan, Z.; Hu, X.; He, J. Chitosan lowers body weight through intestinal microbiota and reduces IL-17 expression via mTOR signalling. J. Funct. Foods 2016, 22, 166–176. [Google Scholar] [CrossRef]
- Lyons, P.P.; Turnbull, J.F.; Dawson, K.A.; Crumlish, M. Effects of low-level dietary microalgae supplementation on the distal intestinal microbiome of farmed rainbow trout Oncorhynchus mykiss (Walbaum). Aquac. Res. 2017, 48, 2438–2452. [Google Scholar] [CrossRef]
- AlMomin, S.; Kumar, V.; Al-Amad, S.; Al-Hussaini, M.; Dashti, T.; Al-Enezi, K.; Akbar, A. Draft genome sequence of the silver pomfret fish, Pampus argenteus. Genome 2016, 59, 51–58. [Google Scholar] [CrossRef]
- Almatar, S.; Chen, W. Deformities in silver pomfret Pampus argenteus caught from Kuwait waters. Chin. J. Oceanol. Limnol. 2010, 28, 1227–1229. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Y.; Shi, Z.; Li, W.; Zhou, H.; Lv, W. Lipid content and fatty acid composition in wild-caught silver pomfret (Pampus argenteus) broodstocks: Effects on gonad development. Aquaculture 2010, 310, 192–199. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yang, Y.; Zhang, Y.Y.; Hu, J.B.; Zhang, M.; Sun, J.C.; Tian, X.Y.; Jin, Y.X.; Zhang, D.Y.; Wang, Y.J.; et al. Expression and cellular localization of insulin-like growth factor 3 in gonads of the seasonal breeding teleost silver pomfret (Pampus argenteus). Fish Physiol. Biochem. 2022, 48, 1377–1387. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Hu, J.B.; Li, Y.Y.; Zhang, M.; Jacques, K.J.; Gu, W.W.; Sun, Y.B.; Sun, J.C.; Yang, Y.; Xu, S.L.; et al. Immune response of silver pomfret (Pampus argenteus) to Amyloodinium ocellatum infection. J. Fish Dis. 2021, 44, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.H.; Li, Y.Y.; Hu, J.B.; Wang, Y.J.; Tao, Z. Oocytes Skipped Spawning through Atresia Is Regulated by Somatic Cells Revealed by Transcriptome Analysis in Pampus argenteus. Front. Mar. Sci. 2022, 9, 1234. [Google Scholar] [CrossRef]
- Hu, J.B.; Le, Q.J.; Wang, Y.J.; Yu, N.; Cao, X.H.; Kuang, S.W.; Zhang, M.; Gu, W.W.; Sun, Y.B.; Yang, Y.; et al. Effects of formaldehyde on detoxification and immune responses in silver pomfret (Pampus argenteus). Fish Shellfish Immunol. 2019, 88, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Le, Q.; Zhang, M.; Kuang, S.; Gu, W.; Sun, Y.; Jean Jacques, K.; Zhang, Y.; Li, Y.; Sun, J.; et al. Effects of amino acids on olfactory-related receptors regulating appetite in silver pomfret. Aquac. Res. 2021, 52, 2528–2539. [Google Scholar] [CrossRef]
- Li, M.H.H.; Robinson, E.H.; Tucker, C.S.; Manning, B.B.; Khoo, L. Effects of dried algae Schizochytrium sp., a rich source of docosahexaenoic acid, on growth, fatty acid composition, and sensory quality of channel catfish Ictalurus punctatus. Aquaculture 2009, 292, 232–236. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Liu, J.; Jiang, Y.; Wu, Y.; Xu, Y.; Yang, C. The effects of bamboo leaf flavonoids on growth performance, immunity, antioxidant status, and intestinal microflora of Chinese mitten crabs (Eriocheir sinensis). Anim. Feed Sci. Technol. 2022, 288, 115297. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- An, Y.; Xu, W.; Li, H.; Lei, H.; Zhang, L.; Hao, F.; Duan, Y.; Yan, X.; Zhao, Y.; Wu, J.; et al. High-fat diet induces dynamic metabolic alterations in multiple biological matrices of rats. J. Proteome Res. 2013, 12, 3755–3768. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, X.; Qin, M.; Cheng, S.; Wang, Y.; Zhou, W. On-board saline black water treatment by bioaugmentation original marine bacteria with Pseudoalteromonas sp. SCSE709-6 and the associated microbial community. Bioresour. Technol. 2019, 273, 496–505. [Google Scholar] [CrossRef]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Wheat, W.; Regan, D.; Dow, S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl. Med. 2018, 7, 456–467. [Google Scholar] [CrossRef]
- Fang, H.; Xie, J.; Liao, S.; Guo, T.; Xie, S.; Liu, Y.; Tian, L.; Niu, J. Effects of Dietary Inclusion of Shrimp Paste on Growth Performance, Digestive Enzymes Activities, Antioxidant and Immunological Status and Intestinal Morphology of Hybrid Snakehead (Channa maculata ♀ × Channa argus ♂). Front Physiol. 2019, 10, 1027. [Google Scholar] [CrossRef]
- Ma, K.; Chen, S.W.; Wu, Y.; Ma, Y.T.; Qiao, H.C.; Fan, J.H.; Wu, H.Z. Dietary supplementation with microalgae enhances the zebrafish growth performance by modulating immune status and gut microbiota. Appl. Microbiol. Biotechnol. 2022, 106, 773–788. [Google Scholar] [CrossRef]
- Garcia-Ortega, A.; Kissinger, K.R.; Trushenski, J.T. Evaluation of fish meal and fish oil replacement by soybean protein and algal meal from Schizochytrium limacinum in diets for giant grouper Epinephelus lanceolatus. Aquaculture 2016, 452, 1–8. [Google Scholar] [CrossRef]
- Hu, J.B.; Le, Q.J.; Wang, Y.J.; Kuang, S.W.; Zhang, M.; Gu, W.W.; Sun, Y.B.; Jacques, K.J.; Li, Y.Y.; Zhang, Y.Y.; et al. Comparative transcriptome analysis of olfactory epithelium in large yellow croaker: Evidence for olfactory adaptation to feed phagostimulant in fish. Aquaculture 2020, 519, 734920. [Google Scholar] [CrossRef]
- Olsen, K.H.; Lundh, T. Feeding stimulants in an omnivorous species, crucian carp Carassius carassius (Linnaeus 1758). Aquac. Rep. 2016, 4, 66–73. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.J.; Wang, L.N.; Cui, Q. Comprehensive analysis of metabolic alterations in Schizochytrium sp. strains with different DHA content. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2020, 1160, 122193. [Google Scholar] [CrossRef]
- Jobling, M.; Bendiksen, E.A. Dietary lipids and temperature interact to influence tissue fatty acid compositions of Atlantic salmon, Salmo salar L., parr. Aquac. Res. 2003, 34, 1423–1441. [Google Scholar] [CrossRef]
- SheikhEldin, M.; DeSilva, S.S.; Anderson, T.A.; Gooley, G. Comparison of fatty acid composition of muscle, liver, mature oocytes, and diets of wild and captive Macquarie perch, Macquaria australasica, broodfish. Aquaculture 1996, 144, 201–216. [Google Scholar] [CrossRef]
- Skuladottir, G.V.; Schith, H.B.; Gudmundsdottir, E.; Richards, B.; Jonsson, L.J. Fatty acid composition of muscle, heart and liver lipids in Atlantic salmon, Salmo salar, at extremely low environmental temperature. Aquaculture 1990, 84, 71–80. [Google Scholar] [CrossRef]
- Turchini, G.M.; Mentasti, T.; Frøyland, L.; Orban, E.; Caprino, F.; Moretti, V.M.; Valfré, F. Effects of alternative dietary lipid sources on performance, tissue chemical composition, mitochondrial fatty acid oxidation capabilities and sensory characteristics in brown trout (Salmo trutta L.). Aquaculture 2003, 225, 251–267. [Google Scholar] [CrossRef]
- Xu, R.; Hung SS, O.; German, J.B. Effects of dietary lipids on the fatty acid composition of triglycerides and phospholipids in tissues of white sturgeon. Aquac. Nutr. 1996, 2, 101–109. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Production of Biodiesel Fuel from the Microalga Schizochytrium limacinum by Direct Transesterification of Algal Biomass. Energy Fuels 2009, 23, 5179–5183. [Google Scholar] [CrossRef]
- Kissinger, K.R.; Garcia-Ortega, A.; Trushenski, J.T. Partial fish meal replacement by soy protein concentrate, squid and algal meals in low fish-oil diets containing Schizochytrium limacinum for longfin yellowtail Seriola rivoliana. Aquaculture 2016, 452, 37–44. [Google Scholar] [CrossRef]
- Rombenso, A.N.; Trushenski, J.T.; Jirsa, D.; Drawbridge, M. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) are essential to meet LC-PUFA requirements of juvenile California Yellowtail (Seriola dorsalis). Aquaculture 2016, 463, 123–134. [Google Scholar] [CrossRef]
- Keogh, J.B.; Grieger, J.A.; Noakes, M.; Clifton, P.M. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1274–1279. [Google Scholar] [CrossRef]
- Spence, J.D.; Srichaikul, K.K.; Jenkins, D.J.A. Cardiovascular Harm From Egg Yolk and Meat: More than Just Cholesterol and Saturated Fat. J. Am. Heart Assoc. 2021, 10, e017066. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, J.B.; Zhu, J.J.; Wang, Y.J.; Zhang, Y.Y.; Li, Y.Y.; Xu, S.L.; Yan, X.J.; Zhang, D.Y. Transcriptome, antioxidant enzymes and histological analysis reveal molecular mechanisms responsive to long-term cold stress in silver pomfret (Pampus argenteus). Fish Shellfish Immunol. 2022, 121, 351–361. [Google Scholar] [CrossRef]
- Kanazawa, A. Effects of docosahexaenoic acid and phospholipids on stress tolerance of fish. Aquaculture 1997, 155, 129–134. [Google Scholar] [CrossRef]
- Mourente, G.; Rodriguez, A.; Tocher, D.R.; Sargent, J.R. Effects of dietary docosahexaenoic acid (DHA; 22:6n-3) on lipid and fatty acid compositions and growth in gilthead sea bream (Sparus aurata L.) larvae during first feeding. Aquaculture 1993, 112, 79–98. [Google Scholar] [CrossRef]
- Li, T.; Long, M.; Ji, C.; Shen, Z.; Gatesoupe, F.J.; Zhang, X.; Zhang, Q.; Zhang, L.; Zhao, Y.; Liu, X.; et al. Alterations of the gut microbiome of largemouth bronze gudgeon (Coreius guichenoti) suffering from furunculosis. Sci. Rep. 2016, 6, 30606. [Google Scholar] [CrossRef]
- Romalde, J.L. Photobacterium damselae subsp. piscicida: An integrated view of a bacterial fish pathogen. Int. Microbiol. 2002, 5, 3–9. [Google Scholar] [CrossRef]
- Villasante, A.; Ramirez, C.; Catalan, N.; Opazo, R.; Dantagnan, P.; Romero, J. Effect of Dietary Carbohydrate-to-Protein Ratio on Gut Microbiota in Atlantic Salmon (Salmo salar). Animals 2019, 9, 89. [Google Scholar] [CrossRef]
- Souza, F.P.; Lima, E.C.S.; Urrea-Rojas, A.M.; Suphoronski, S.A.; Facimoto, C.T.; Bezerra Junior, J.D.S.; Oliveira, T.E.S.; Pereira, U.P.; Santis, G.W.D.; Oliveira, C.A.L.; et al. Effects of dietary supplementation with a microalga (Schizochytrium sp.) on the hemato-immunological, and intestinal histological parameters and gut microbiota of Nile tilapia in net cages. PLoS ONE 2020, 15, e0226977. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Defoirdt, T.; Halet, D.; Vervaeren, H.; Boon, N.; Van de Wiele, T.; Sorgeloos, P.; Bossier, P.; Verstraete, W. The bacterial storage compound poly-beta-hydroxybutyrate protects Artemia franciscana from pathogenic Vibrio campbellii. Environ. Microbiol. 2007, 9, 445–452. [Google Scholar] [CrossRef]
- Gao, M.R.; Du, D.D.; Bo, Z.X.; Sui, L.Y. Poly-beta-hydroxybutyrate (PHB)-accumulating Halomonas improves the survival, growth, robustness and modifies the gut microbial composition of Litopenaeus vannamei postlarvae. Aquaculture 2019, 500, 607–612. [Google Scholar] [CrossRef]
- Gao, M.R.; Li, Y.; Xie, W.; Duan, H.; Du, D.D.; Sui, L.Y. Effect of Halomonas storage poly-beta-hydroxybutyrates on survival, growth and vibriosis resistance of half-smooth tongue sole Cynoglossus semilaevis juveniles. Aquac. Res. 2020, 51, 4631–4637. [Google Scholar] [CrossRef]
- Thai, T.Q.; Wille, M.; Garcia-Gonzalez, L.; Sorgeloos, P.; Bossier, P.; De Schryver, P. Poly--hydroxybutyrate content and dose of the bacterial carrier for Artemia enrichment determine the performance of giant freshwater prawn larvae. Appl. Microbiol. Biotechnol. 2014, 98, 5205–5215. [Google Scholar] [CrossRef]
- Alba, P.; Caprioli, A.; Cocumelli, C.; Ianzano, A.; Donati, V.; Scholl, F.; Sorbara, L.; Terracciano, G.; Fichi, G.; Di Nocera, F.; et al. A New Multilocus Sequence Typing Scheme and Its Application for the Characterization of Photobacterium damselae subsp. damselae Associated with Mortality in Cetaceans. Front. Microbiol. 2016, 7, 1656. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Miya, S.; Kimura, B.; Yamane, K.; Arakawa, Y.; Fujii, T. Difference of genotypic and phenotypic characteristics and pathogenicity potential of Photobacterium damselae subsp. damselae between clinical and environmental isolates from Japan. Microb. Pathog. 2008, 45, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Skall, H.F.; Lassen-Nielsen, A.M.; Bjerrum, L.; Olesen, N.J. Photobacterium damselae subsp. damselae, an emerging pathogen in Danish rainbow trout, Oncorhynchus mykiss (Walbaum), mariculture. J. Fish Dis. 2009, 32, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shen, C.; Zhou, S.M.; Yang, N.; Wang, G.L.; Wang, Y.J.; Xu, S.L. An outbreak of Photobacterium damselae subsp damselae infection in cultured silver pomfret Pampus argenteus in Eastern China. Aquaculture 2018, 492, 201–205. [Google Scholar] [CrossRef]
- Terceti, M.S.; Ogut, H.; Osorio, C.R. Photobacterium damselae subsp. damselae, an Emerging Fish Pathogen in the Black Sea: Evidence of a Multiclonal Origin. Appl. Environ. Microbiol. 2016, 82, 3736–3745. [Google Scholar] [CrossRef]
- Kankaanpaa, P.; Yang, B.; Kallio, H.; Isolauri, E.; Salminen, S. Effects of polyunsaturated fatty acids in growth medium on lipid composition and on physicochemical surface properties of lactobacilli. Appl. Environ. Microbiol. 2004, 70, 129–136. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]





| Growth Parameters | Control Group | Treat Group (Schi 2%) |
|---|---|---|
| WG | 6.42 ± 0.21 a | 8.08 ± 0.66 b |
| WGR (%) | 77.18 ± 7.04 a | 99.12 ± 8.79 b |
| SGR (%) | 2.86 ± 0.20 a | 3.39 ± 0.30 a |
| Fatty Acid | C1 | C2 | C3 | C4 | C5 | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|---|---|---|---|---|
| C14 | 3.93 ± 0.68 a/- | 2.61 ± 0.37 b/- | 3.05 ± 1.12 a/- | 3.83 ± 0.35 a/- | 1.92 ± 0.94 c/- | 3.93 ± 0.68 a/- | 4.01 ± 0.63 a/* | 3.89 ± 0.28 a/- | 2.65 ± 1.57 b/* | 1.15 ± 0.32 c/* |
| C15 | 0.31 ± 0.08 b/- | 0.59 ± 0.51 a/- | 0.28 ± 0.11 b/- | 0.27 ± 0.03 b/- | 0.15 ± 0.04 a/- | 0.31 ± 0.08 a/- | 0.28 ± 0.03 a/* | 0.31 ± 0.01 a/- | 0.43 ± 0.03 a/* | 0.2 ± 0.05 b/- |
| C16 | 29.81 ± 0.59 a/- | 30.42 ± 1.95 a/- | 30.08 ± 0.43 a/- | 29.17 ± 1.2 a/- | 30.46 ± 0.5 a/- | 29.81 ± 0.59 a/- | 29.11 ± 1.04 a/- | 27.61 ± 0.64 a/- | 28.37 ± 1.16 a/- | 30.52 ± 0.65 a/- |
| C16:1(n-9) | 3.53 ± 1.57 a/- | 2.12 ± 0.97 b/- | 3.26 ± 1.11 a/- | 3.47 ± 0.37 a/- | 0.41 ± 0.22 a/- | 3.53 ± 1.57 a/- | 3.5 ± 0.65 a/* | 3.79 ± 0.33 a/- | 3.34 ± 0.33 a/- | 0.55 ± 0.19 c/- |
| C17 | 0.8 ± 0.28 b/- | 1.85 ± 1.05 a/- | 0.79 ± 0.8 b/- | 0.55 ± 0.15 c/- | 1.67 ± 0.02 a/- | 0.8 ± 0.28 c/- | 0.75 ± 0.84 c/** | 0.22 ± 0.11 c/** | 4.2 ± 1.11 a/** | 1.71 ± 0.57 c/- |
| C18 | 6.82 ± 1.92 a/- | 7.65 ± 0.49 a/- | 6.94 ± 0.98 a/- | 5.96 ± 0.19 b/- | 9.37 ± 0.83 a/- | 6.82 ± 1.92 a/- | 6.17 ± 1.88 a/- | 4.87 ± 0.47 b/- | 6.31 ± 2.25 a/- | 9.96 ± 1.31 a/- |
| C18:1(n-9) | 22.8 ± 1.12 a/- | 16.85 ± 0.92 a/- | 19.31 ± 0.58 a/- | 21.7 ± 0.75 a/- | 20.36 ± 0.92 a/- | 22.8 ± 1.12 a/- | 21.46 ± 1 a/- | 20.47 ± 0.78 a/- | 13.95 ± 0.57 b/* | 10.87 ± 1.39 b/* |
| C18:1 | 3.68 ± 0.87 a/- | 2.79 ± 1.4 b/- | 2.84 ± 0.23 b/- | 4.27 ± 0.42 a/- | 1.55 ± 0.35 c/- | 3.68 ± 0.87 a/- | 3.42 ± 1.11 b/- | 4.51 ± 0.1 a/* | 2.96 ± 2.04 b/* | 1.67 ± 0.25 c/- |
| C18:2(n-9) | 1.71 ± 0.58 a/- | 2.25 ± 1.14 a/- | 2.23 ± 1.51 a/- | 2.37 ± 0.43 a/- | 1.5 ± 0.16 b/- | 1.71 ± 0.58 b/- | 2.01 ± 0.64 b/- | 3.24 ± 0.17 a/* | 1.95 ± 1.98 b/- | 1.39 ± 0.43 c/- |
| C20:1 | 0.79 ± 0.11 c/- | 1.79 ± 1.06 a/- | 1.21 ± 1.03 b/- | 1.19 ± 0.58 b/- | 1.02 ± 1.23 b/- | 0.79 ± 0.11 c/- | 1.09 ± 0.28 b/* | 1.57 ± 0.31 a/- | 1.65 ± 0.98 a/* | 1.29 ± 0.28 a/- |
| C20:4(n-6) | 0.5 ± 0.04 c/- | 1.57 ± 0.55 a/- | 1.06 ± 0.96 b/- | 0.54 ± 0.12 c/- | 0.95 ± 0.98 b/- | 0.5 ± 0.04 c/- | 0.44 ± 0.07 c/** | 0.41 ± 0.05 c/* | 1.15 ± 0.86 b/* | 1.62 ± 1.24 a/* |
| C20:5(n-3) | 4.9 ± 0.55 b/- | 9.27 ± 1.12 a/- | 7.02 ± 0.68 a/- | 5.95 ± 0.91 b/- | 10.11 ± 1.12 a/- | 4.9 ± 0.55 a/- | 6.5 ± 1.19 a/* | 7.18 ± 0.43 a/- | 8.96 ± 1.61 a/* | 9.27 ± 1.35 a/- |
| C22:1(n-9) | 0.48 ± 0.21 a/- | 0.34 ± 0.12 b/- | 0.66 ± 0.48 a/- | 0.45 ± 0.26 a/- | 0.36 ± 0.15 b/- | 0.48 ± 0.21 a/- | 0.34 ± 0.17 b/- | 0.51 ± 0.21 a/* | 0.61 ± 0.34 a/* | 0.26 ± 0.08 b/- |
| C22:6(n-3) | 19.94 ± 0.33 a/- | 20.14 ± 0.44 a/- | 21.28 ± 0.7 a/- | 20.3 ± 0.51 a/- | 21.72 ± 0.65 a/- | 19.94 ± 0.33 b/- | 21.08 ± 0.38 b/- | 21.42 ± 0.81 b/- | 24.15 ± 0.96 a/- | 29.65 ± 1.51 a/* |
| SFA | 41.67 ± 0.72 a/- | 43.11 ± 0.72 a/- | 41.14 ± 0.3 a/- | 39.77 ± 0.49 a/- | 43.57 ± 0.41 a/- | 41.67 ± 0.72 a/- | 40.32 ± 0.55 a/- | 36.9 ± 0.23 a/- | 41.95 ± 0.53 a/- | 43.53 ± 0.42 a/- |
| MUFA | 31.27 ± 0.62 a/- | 23.88 ± 0.47 b/- | 27.28 ± 0.37 a/- | 31.07 ± 0.19 a/- | 23.7 ± 0.47 b/- | 31.27 ± 0.62 a/- | 29.81 ± 0.42 a/- | 30.86 ± 0.26 a/- | 22.51 ± 0.72 b/* | 14.63 ± 0.54 c/* |
| PUFA | 27.05 ± 0.25 a/- | 33.23 ± 0.37 a/- | 31.59 ± 0.39 a/- | 29.16 ± 0.33 a/- | 34.28 ± 0.42 a/- | 27.05 ± 0.25 b/- | 30.02 ± 0.47 b/- | 32.24 ± 0.34 a/- | 36.21 ± 0.54 a/- | 41.92 ± 0.48 a/* |
| EPA + DHA | 24.84 ± 0.16 b/- | 29.42 ± 0.48 a/- | 28.3 ± 0.02 a/- | 26.25 ± 0.29 a/- | 31.83 ± 0.33 a/- | 24.84 ± 0.16 b/- | 27.58 ± 0.57 b/- | 28.6 ± 0.27 b/- | 33.11 ± 0.46 a/* | 38.92 ± 0.12 a/* |
| Sample | OTU | Simpson | Chao1 | Shannon | Coverage |
|---|---|---|---|---|---|
| C1-1 | 609 | 0.465 | 629.488 | 2.484 | 0.997 |
| C1-2 | 675 | 0.566 | 681.573 | 2.842 | 0.997 |
| C1-3 | 805 | 0.823 | 813.243 | 4.263 | 0.997 |
| C2-1 | 1173 | 0.943 | 1160.275 | 6.001 | 0.997 |
| C2-2 | 597 | 0.603 | 584.065 | 2.642 | 0.998 |
| C2-3 | 461 | 0.266 | 453.928 | 1.32 | 0.998 |
| C3-1 | 969 | 0.815 | 970.903 | 4.544 | 0.996 |
| C3-2 | 1477 | 0.973 | 1507.891 | 6.99 | 0.995 |
| C3-3 | 709 | 0.917 | 694.483 | 4.749 | 0.997 |
| C4-1 | 513 | 0.412 | 504.319 | 1.987 | 0.998 |
| C4-2 | 347 | 0.146 | 331.8 | 0.779 | 0.998 |
| C4-3 | 2193 | 0.85 | 2148.671 | 5.414 | 0.993 |
| C5-1 | 2504 | 0.98 | 2479.35 | 7.884 | 0.992 |
| C5-2 | 2014 | 0.657 | 1943.744 | 3.963 | 0.992 |
| C5-3 | 2299 | 0.936 | 2235.786 | 6.243 | 0.991 |
| S1-1 | 609 | 0.465 | 629.488 | 2.484 | 0.997 |
| S1-2 | 675 | 0.566 | 681.573 | 2.842 | 0.997 |
| S1-3 | 805 | 0.823 | 813.243 | 4.263 | 0.997 |
| S2-1 | 2292 | 0.898 | 2122.455 | 6.489 | 0.995 |
| S2-2 | 2071 | 0.869 | 1992.766 | 5.433 | 0.992 |
| S2-3 | 2463 | 0.759 | 2380.358 | 5.194 | 0.99 |
| S3-1 | 1146 | 0.839 | 1101.783 | 5.117 | 0.996 |
| S3-2 | 1358 | 0.969 | 1309.61 | 6.433 | 0.995 |
| S3-3 | 1126 | 0.938 | 1135.107 | 5.726 | 0.996 |
| S4-1 | 1072 | 0.948 | 1052.173 | 5.943 | 0.996 |
| S4-2 | 940 | 0.942 | 891.114 | 5.485 | 0.997 |
| S4-3 | 776 | 0.929 | 741.719 | 5.069 | 0.997 |
| S5-1 | 207 | 0.031 | 211.125 | 0.213 | 0.999 |
| S5-2 | 241 | 0.09 | 222.895 | 0.527 | 0.999 |
| S5-3 | 218 | 0.07 | 211.526 | 0.425 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Le, Q.; Zhang, M.; Xu, S.; He, S.; Yan, X.; Hu, J.; Wang, Y. The Effect of Schizochytrium sp. on Growth, Fatty Acid Profile and Gut Microbiota of Silver Pomfret (Pampus argenteus). J. Mar. Sci. Eng. 2023, 11, 414. https://doi.org/10.3390/jmse11020414
Li Y, Le Q, Zhang M, Xu S, He S, Yan X, Hu J, Wang Y. The Effect of Schizochytrium sp. on Growth, Fatty Acid Profile and Gut Microbiota of Silver Pomfret (Pampus argenteus). Journal of Marine Science and Engineering. 2023; 11(2):414. https://doi.org/10.3390/jmse11020414
Chicago/Turabian StyleLi, Yuanbo, Qijun Le, Man Zhang, Shanliang Xu, Shan He, Xiaojun Yan, Jiabao Hu, and Yajun Wang. 2023. "The Effect of Schizochytrium sp. on Growth, Fatty Acid Profile and Gut Microbiota of Silver Pomfret (Pampus argenteus)" Journal of Marine Science and Engineering 11, no. 2: 414. https://doi.org/10.3390/jmse11020414
APA StyleLi, Y., Le, Q., Zhang, M., Xu, S., He, S., Yan, X., Hu, J., & Wang, Y. (2023). The Effect of Schizochytrium sp. on Growth, Fatty Acid Profile and Gut Microbiota of Silver Pomfret (Pampus argenteus). Journal of Marine Science and Engineering, 11(2), 414. https://doi.org/10.3390/jmse11020414





