Copper Induced DNA Damage in the Gills of the Mussel Mytilus trossulus and Reversibility after Depuration
Abstract
1. Introduction
2. Material and Methods
2.1. Description of the Experiment
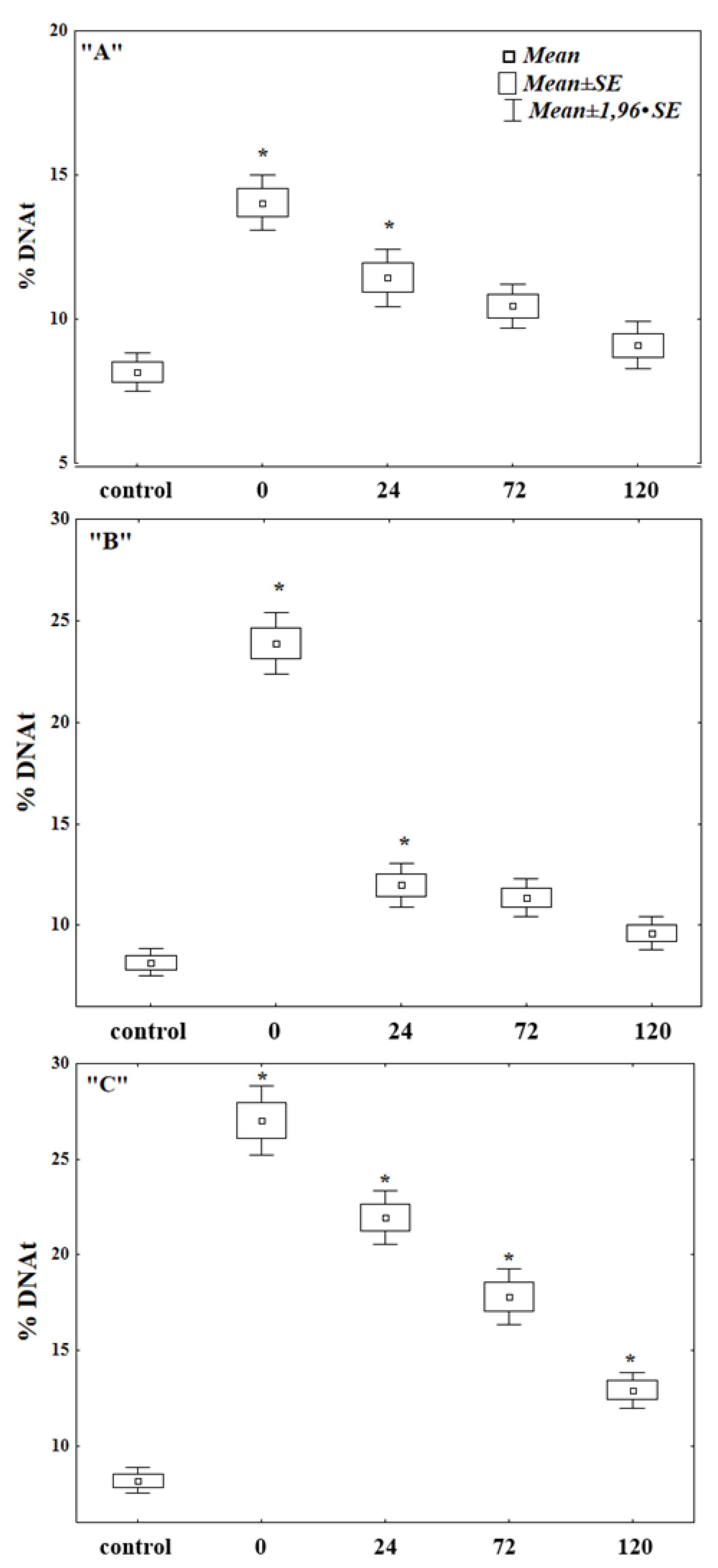
2.2. Comet Assay
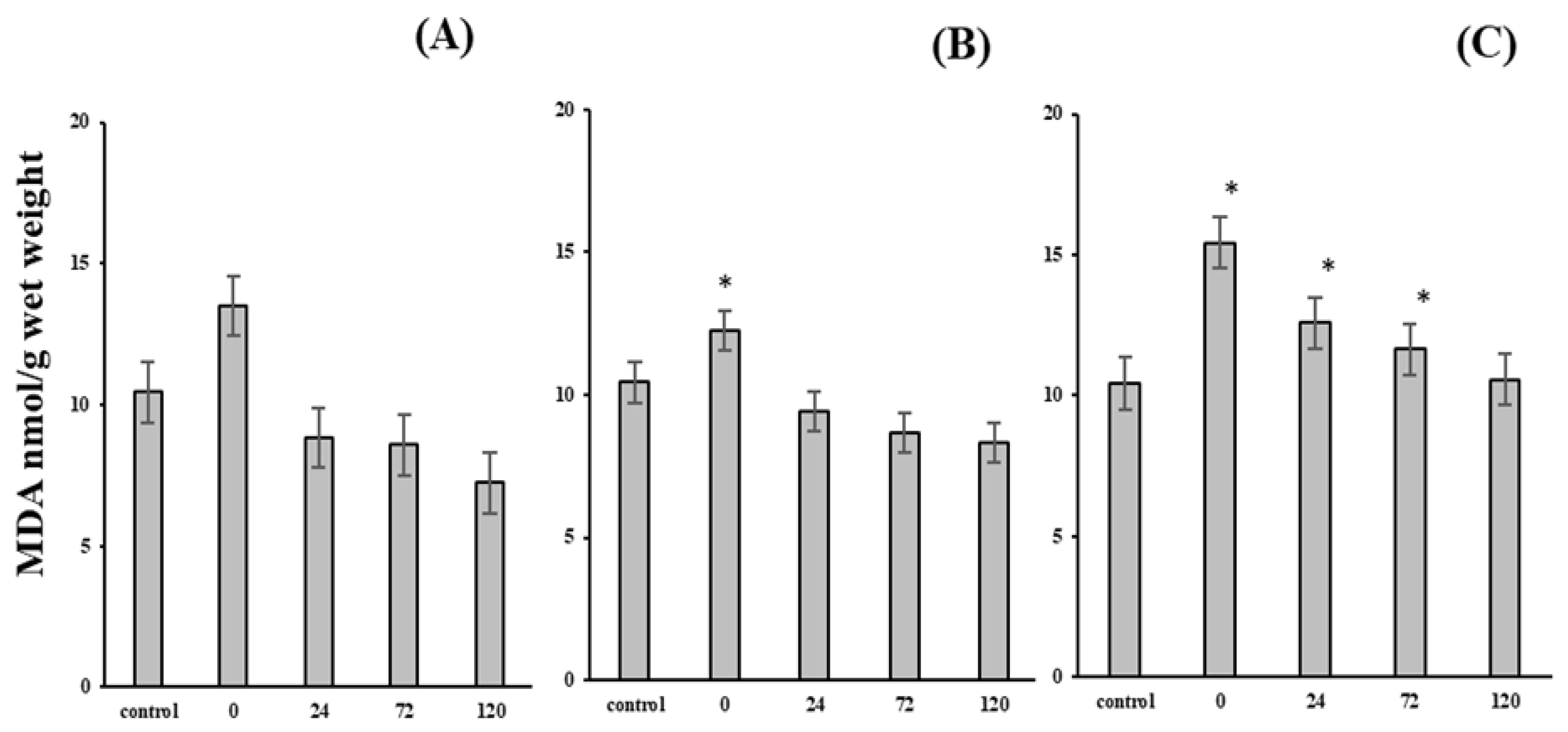
2.3. Malonic Dialdehyde
2.4. Copper Concentration Measurement
2.5. Statistical Analysis
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viarengo, A. Biochemical effects of trace metals. Mar. Pollut. Bull. 1985, 16, 153–158. [Google Scholar] [CrossRef]
- Viarengo, A. Heavy metals in marine invertebrates: Mechanisms of regulation and toxicity at the cellular level. Crit. Rev. Aquat. Sci. 1989, 1, 295–317. [Google Scholar]
- Han, B.C.; Jeng, W.L.; Tsai, Y.N.; Jeng, M.S. Depuration of copper and zinc by green oysters and blue mussels of Taiwan. Environ. Pollut. 1993, 82, 93–97. [Google Scholar] [CrossRef]
- Grout, J.A.; Levings, C.D. Effects of acid mine drainage from an abandoned copper mine, brilannia mines, howe sound, British Columbia, Canada, on transplanted blue mussels (Mytilus edulis). Mar. Environ. Res. 2001, 51, 265–288. [Google Scholar] [CrossRef]
- Rosen, G.; Rivera-Duarte, I.; Bart Chadwick, D.; Ryan, A.; Santore, R.C.; Paquin, P.R. Critical tissue copper residues for marine bivalve (Mytilus galloprovincialis) and echinoderm (Strongylocentrotus purpuratus) embryonic development: Conceptual, regulatory and environmental implications. Mar. Environ. Res. 2008, 66, 327–336. [Google Scholar] [CrossRef]
- Nyberg, K.A.; Michelson, R.J.; Putnam, C.W.; Weinert, A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002, 36, 617–656. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Belcheva, N.N.; Zakhartsev, M. Biochemical mechanisms of adaptation to cadmium and copper ions in the mussel Mytilus trossulus. Russ. J. Mar. Bio. 1998, 24, 330–336. [Google Scholar]
- Gomes, T.; Pereiraa, C.G.; Cardosoa, C.; Pinheiro, J.P.; Cancio, I.; Bebianno, M.J. Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquat. Toxicol. 2012, 118–119, 72–79. [Google Scholar] [CrossRef]
- Watson, G.J.; Pini, J.M.; Richir, J. Chronic exposure to copper and zinc induces DNA damage in the polychaete Alitta virens and the implications for future toxicity of coastal sites. Environ. Pollut. 2018, 243, 1498–1508. [Google Scholar] [CrossRef]
- Al-Subiai, S.N.; Moody, A.J.; Mustafa, S.A.; Jha, A.N. A multiple biomarker approach to investigate the effects of copper on the marine bivalve mollusc, Mytilus edulis. Ecotoxicol. Environ. Saf. 2011, 74, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Istomina, A.; Chelomin, V.; Kukla, S.; Zvyagintsev, A.; Karpenko, A.; Slinko, E.; Dovzhenko, N.; Slobodskova, V.; Kolosova, L. Copper effect on the biomarker state of the Mizuhopecten yessoensis tissues in the prespawning period. Environ. Toxicol. Pharmacol. 2019, 70, 103189. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.A.; Mitchelmore, C.L.; Jones, R.; O’Dea, A.; Seymour, S. Exposure to copper induces oxidative and stress responses and DNA damage in the coral Montastraea franksi. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Gabbianelli, R.; Lupidi, G.; Villarini, M.; Falcioni, G. DNA Damage induced by copper on erythrocytes of gilthead sea bream Sparus aurata and mollusk Scapharca inaequivalvis. Arch. Environ. Contam. Toxicol. 2003, 45, 350–356. [Google Scholar] [CrossRef]
- Gomes, T.; Araújo, O.; Pereira, R.; Almeida, A.C.; Cravo, A.; Bebianno, M.J. Genotoxicity of copper oxide and silver nanoparticles in the mussel Mytilus galloprovincialis. Mar. Environ. Res. 2013, 84, 51–59. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Zakhartsev, M.K.; Kukla, S.P. Genotoxic potential of copper oxide nanoparticles in the bivalve mollusk Mytilus trossulus. J. Ocean Univ. China 2017, 16, 339–345. [Google Scholar] [CrossRef]
- Amiard-Triquet, C.; Berthet, B.; Metayer, C.; Amiard, J.C. Contribution to the ecotoxicological study of cadmium, copper and zinc in the mussel Mytilus edulis. Mar. Biol. 1986, 92, 7–13. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; Birmelin, C.; Livingstone, D.R.; Chipman, J.K. Detection of DNA strand breaks in isolated mussels (Mytilus edulis) digestive gland cells using the “comet” assay. Ecotoxicol. Environ. Saf. 1998, 41, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Slobodskova, V.V.; Kukla, S.P.; Chelomin, V.P. An analysis of the quality of the marine environment based on determina-tion of the genotoxicity of DNA in the gill cells of the Yesso Scallop Mizuhopecten yessoensis (Jay, 1856). Russ. J. Mar. Biol. 2015, 41, 495–498. [Google Scholar] [CrossRef]
- Mazur, A.A.; Chelomin, V.P.; Zhuravel, E.V.; Kukla, S.P.; Slobodskova, V.V.; Dovzhenko, N.V. Genotoxicity of Polystyrene (PS) Microspheres in Short-Term Exposure to Gametes of the Sand Dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea). J. Mar. Sci. Eng. 2021, 9, 1088. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic Properties of Polystyrene (PS) Microspheres in the Filter-Feeder Mollusk Mytilus trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- Julshamn, K.; Andersen, K.J. Subcellular distribution of major and minor elements in unexposed molluscs in western norway-I. The distribution and binding of cadmium, zinc and copper in the liver and the digestive system of the oyster Ostrea edulis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1983, 75, 9–12. [Google Scholar] [CrossRef]
- Tabakaeva, O.V.; Tabakaev, A.V.; Piekoszewski, W. Nutritional composition and total collagen content of two commercially important edible bivalve molluscs from the Sea of Japan coast. J. Food Sci. Technol. 2018, 55, 4877–4886. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Farmena, E.; Heier, L.S.; Blanco-Rayónd, E.; Izagirre, U. Differences in copper bioaccumulation and biological responses in three Mytilus species. Aquat. Toxicol. 2015, 160, 1–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Widdows, J.; Johnson, D. Physiological energetics of Mytilus edulis: Scope for growth. Mar. Ecol. 1988, 46, 113–121. [Google Scholar] [CrossRef]
- White, S.L.; Rainbow, P.S. On the metabolic requirements for copper and zinc in molluscs and crustaceans. Mar. Environ. Res. 1985, 16, 215–229. [Google Scholar] [CrossRef]
- Shukla, A.K.; Pragya, P.; Chowdhuri, K.D. A modified alkaline Comet assay for in vivo detection of oxidative DNA damage in Drosophila melanogaster. Mutat. Res. 2011, 726, 222–226. [Google Scholar] [CrossRef]
- Trevisan, R.; Mello, D.F.; Fisher, A.S.; Schuwerack, P.M.; Dafre, A.L.; Moody, A.J. Selenium in water enhances antioxidant defenses and protects against copper-induced DNA damage in the blue mussel Mytilus edulis. Aquat. Toxicol. 2011, 101, 64–71. [Google Scholar] [CrossRef]
- Sandrini, J.Z.; Bianchini, A.; Trindade, G.S.; Nery, L.E.M.; Marins, L.F.F. Reactive oxygen species generation and expression of DNA repair-related genes after copper exposure in zebrafish (Danio rerio) ZFL cells. Aquat. Toxicol. 2009, 95, 285–291. [Google Scholar] [CrossRef]
- Musgrave, M.E.; Gould, S.P.; Ablett, R.F. Enzymatic lipid peroxidation in the gonadal and hepatopancreatic microsomal fraction of cultivated mussels (Mytilus edulis L.). J. Food Sci. 1987, 52, 609–612. [Google Scholar] [CrossRef]
- Winston, G.W.; Livingston, D.R.; Lips, F. Oxygen reduction metabolism by the digestive gland of the common marine mussel, Mytilus edulis L. J. Exp. Zool. 1990, 255, 296–308. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Belcheva, N.N. The effect of heavy metals onprocesses of lipid peroxidation in microsomal membranes fromthe hepatopancreas of bivalve mollusks Mizuhopecten yessoensis. Comp. Biochem. Physiol. 1992, 103, 419–422. [Google Scholar] [CrossRef]
- Livingston, D.R.; Pipe, R.K. Mussels and environmental contaminants: Molecular and cellular aspects The Mussel Mytilus. Ecology, Physiology, Genetics and Culture. Dev. Aquac. Fish. Sci. 1992, 25, 425–464. [Google Scholar]
- Emmanouil, C.; Green, R.M.; Willey, F.R.; Chipman, J.K. Oxidative damage in gill of Mytilus edulis from Merseyside, UK, and reversibility after depuration. Environ. Pollut. 2008, 151, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, P.S. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. Pollut. 2002, 120, 497–507. [Google Scholar] [CrossRef]
- Mason, A.Z.; Jenkins, K.D. Metal detoxification in aquatic organisms. In Metal Speciation and Bioavailability in Aquatic Systems; Tessier, A., Turner, D.R., Eds.; John Wiley & Sons: Chichester, UK, 1995; pp. 479–608. [Google Scholar]
- Soto, M.; Ireland, M.P.; Marigomez, I. The contribution of metal/shell-weight index in target-tissues to metal body burden in sentinel marine molluscs. 2. Mytilus galloprovincialis. Sci. Total Environ. 1997, 198, 149–160. [Google Scholar] [CrossRef]
- Glei, M.; Hovhannisyan, G.; Pool-Zobel, B.L. Use of Comet-FISH in the study of DNA damage and repair: Review. Mutat. Res. 2009, 681, 33–43. [Google Scholar] [CrossRef]
- Kienzler, A.; Bony, S.; Devaux, A. DNA repair activity in fish and interest in ecotoxicology: A review. Aquat. Toxicol. 2013, 134–135, 47–56. [Google Scholar] [CrossRef]
- Pruski, A.M.; Dixon, D.R. Effects of cadmium on nuclear integrity and DNA repair efficiency in the gill cells of Mytilus edulis L. Aquat. Toxicol. 2002, 57, 127–137. [Google Scholar] [CrossRef]
- El-Bibany, A.H.; Bodnar, A.G.; Reinardy, H.C. Comparative DNA damage and repair in echinoderm coelomocytes exposed to genotoxicants. PLoS ONE 2014, 17, e107815. [Google Scholar] [CrossRef]
- Whiteside, J.R.; Box, C.L.; McMillan, T.J.; Allinson, S.L. Cadmium and copper inhibit both DNA repair activities of polynucleotide kinase. DNA Repair. 2010, 9, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ching, E.W.K.; Siu, W.H.L.; Lam, P.K.S.; Xu, L.; Zhang, Y.; Richardson, B.J.; Wu, R.S.S. DNA adduct formation and DNA strand breaks in green-lipped mussels (Perna viridis) exposed to Benzo[a]pyrene: Dose-and time-dependent relationships. Mar. Pollut. Bull. 2001, 42, 603–610. [Google Scholar] [CrossRef]
- Siu, W.H.L.; Hung, C.L.H.; Wong, H.L.; Richardson, B.J.; Lam, P.K.S. Exposure and time dependent DNA strand breakage in hepatopancreas of green-lipped mussels (Perna viridis) exposed to Aroclor 1254, and mixtures of B[a]P and Aroclor 1254. Mar. Pollut. Bull. 2003, 46, 1285–1293. [Google Scholar] [CrossRef]
- Siu, W.H.L.; Cao, J.; Jack, R.W.; Wu, R.S.S.; Richardson, B.J.; Xu, L.; Lam, P.K.S. Application of the comet and micronucleus assays to the detection of B[a]P genotoxicity in haemocytes of the green-lipped mussel (Perna viridis). Aquat. Toxicol. 2004, 66, 381–392. [Google Scholar] [CrossRef]
- Hook, S.E.; Lee, R.F. Interactive effects of UV, benzo[α] pyrene, and cadmium on DNA damage and repair in embryos of the grass shrimp Paleomonetes pugio. Mar. Environ. Res. 2004, 58, 735–739. [Google Scholar] [CrossRef]
- Michel, C.; Vincent-Hubert, F. DNA oxidation and DNA repair in gills of zebra mussels exposed to cadmium and benzo(a)pyrene. Ecotoxicology 2015, 24, 2009–2016. [Google Scholar] [CrossRef]
- Tang, S.; Wu, Y.; Ryan, C.N.; Yu, S.; Qin, G.; Edwards, D.S.; Mayer, G.D. Distinct expression profiles of stress defense and DNA repair genes in Daphnia pulex exposed to cadmium, zinc, and quantum dots. Chemosphere 2015, 120, 92–99. [Google Scholar] [CrossRef]
- Roesijadi, G.; Vestling, M.M.; Murphy, C.M.; Klerks, P.L.; Fenselau, C.C. Structure and time-dependent behavior of acetylated and non-acetylated forms of a molluscan metallothionein. Biochim. Biophys. Acta 1991, 1074, 230–236. [Google Scholar] [CrossRef]
- Carpene, E. Metallothionein in marine molluscs. In Ecotoxicology of Metals in Invertebrates; Dallinger, R., Rainbow, P.S.L., Eds.; Lewis Publishers: Devon, UK, 1993; pp. 55–72. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slobodskova, V.V.; Chelomin, V.P.; Kukla, S.P.; Mazur, A.A. Copper Induced DNA Damage in the Gills of the Mussel Mytilus trossulus and Reversibility after Depuration. J. Mar. Sci. Eng. 2022, 10, 1570. https://doi.org/10.3390/jmse10111570
Slobodskova VV, Chelomin VP, Kukla SP, Mazur AA. Copper Induced DNA Damage in the Gills of the Mussel Mytilus trossulus and Reversibility after Depuration. Journal of Marine Science and Engineering. 2022; 10(11):1570. https://doi.org/10.3390/jmse10111570
Chicago/Turabian StyleSlobodskova, Valentina Vladimirovna, Victor Pavlovich Chelomin, Sergey Petrovich Kukla, and Andrey Alexandrovich Mazur. 2022. "Copper Induced DNA Damage in the Gills of the Mussel Mytilus trossulus and Reversibility after Depuration" Journal of Marine Science and Engineering 10, no. 11: 1570. https://doi.org/10.3390/jmse10111570
APA StyleSlobodskova, V. V., Chelomin, V. P., Kukla, S. P., & Mazur, A. A. (2022). Copper Induced DNA Damage in the Gills of the Mussel Mytilus trossulus and Reversibility after Depuration. Journal of Marine Science and Engineering, 10(11), 1570. https://doi.org/10.3390/jmse10111570





