Effect of Predation on Fouling Communities in an Italian Hotspot of Non-Indigenous Species
Abstract
1. Introduction
2. Materials and Methods
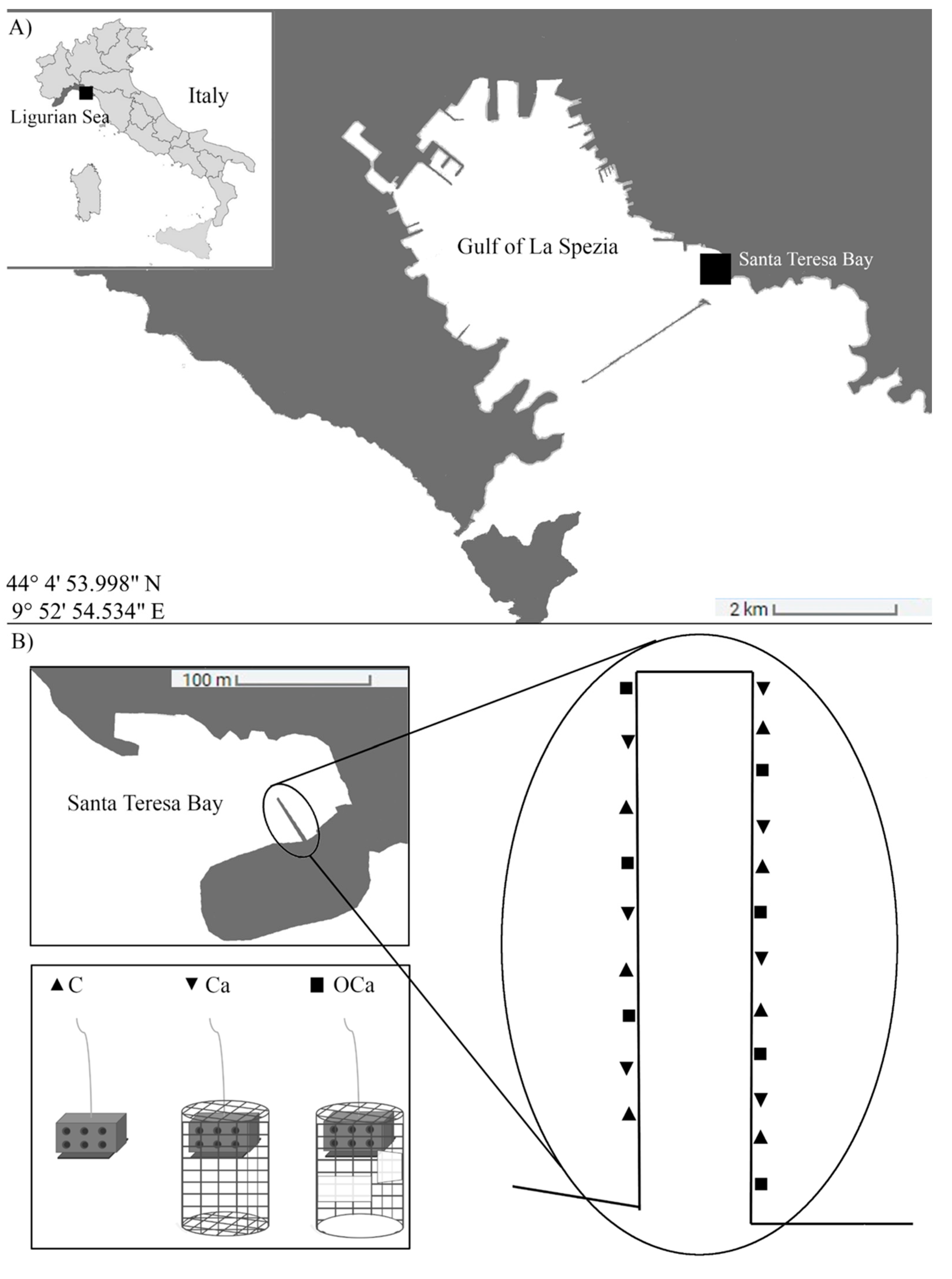
2.1. Study Area
2.2. Experimental Design
2.3. Field Measurements
2.4. Laboratory Analyses
2.5. Statistical Analyses
3. Results
3.1. Presence of Predators
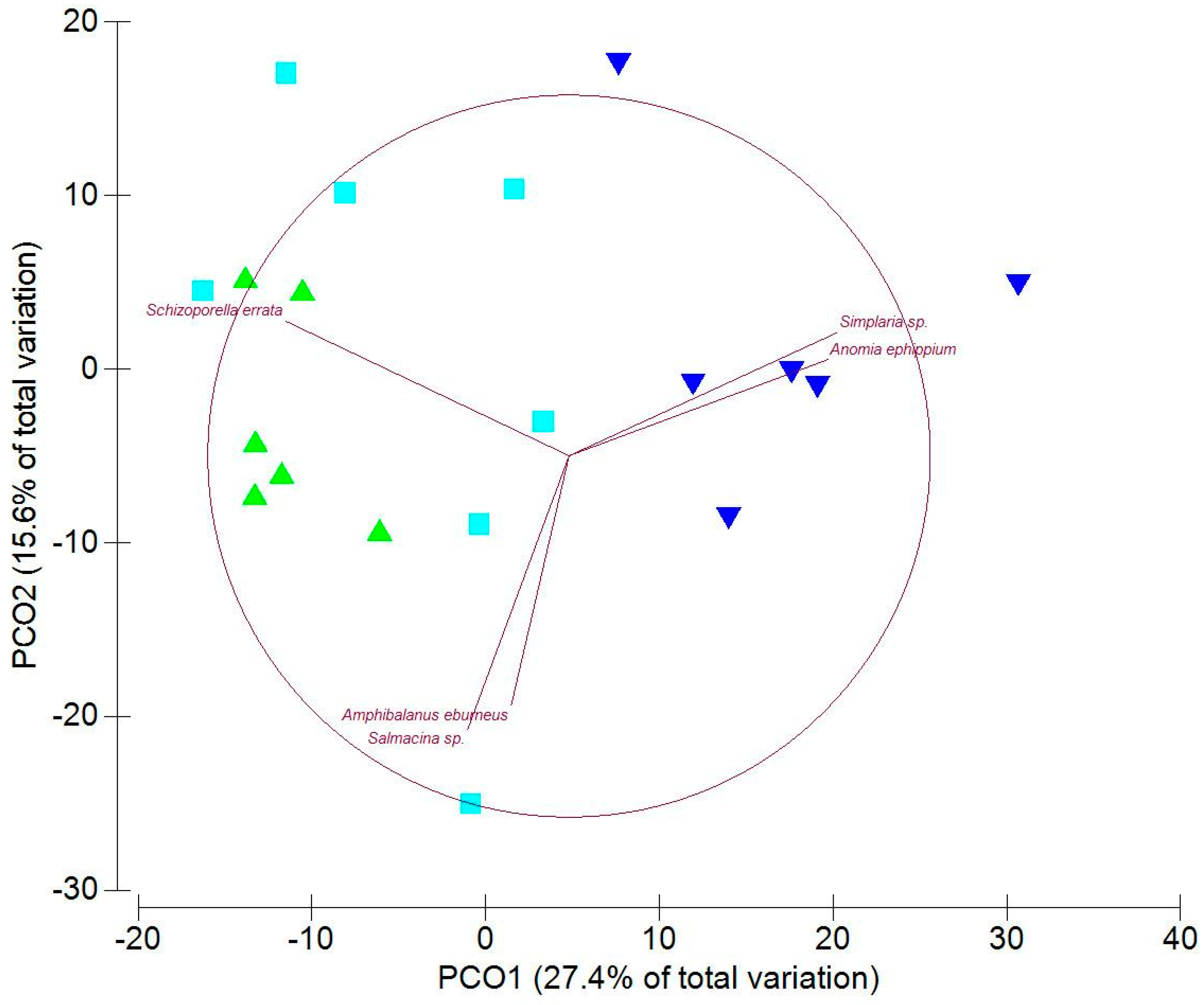
3.2. Sessile Fauna on Panels
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freestone, A.L.; Osman, R.W.; Ruiz, G.M.; Torchin, M.E. Stronger predation in the tropics shapes species richness patterns in marine communities. Ecology 2011, 92, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, L.J.; Freestone, A.L.; Ruiz, G.M.; Torchin, M.E. Prior predation alters community resistance to an extreme climate disturbance. Ecosphere 2017, 8, e01986. [Google Scholar] [CrossRef]
- Giachetti, C.B.; Battini, N.; Bortolus, A.; Tatián, M.; Schwindt, E. Macropredators as shapers of invaded fouling communities in a cold temperate port. J. Exp. Mar. Biol. Ecol. 2019, 518, 151177. [Google Scholar] [CrossRef]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuar. Coast. Shelf. Sci. 2018, 201, 7–16. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Chebaane, S.; Canning-Clode, J.; Ramalhosa, P.; Belz, J.; Castro, N.; Órfão, I.; Sempere-Valverde, J.; Hillebrand Engelen, A.; Pessanha Pais, M.; Monteiro, J.G. From plates to baits: Using a remote video foraging system to study the impact of foraging on fouling non-indigenous species. J. Mar. Sci. Eng. 2022, 10, 611. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef]
- Papacostas, K.J.; Freestone, A.L. Stronger predation in a subtropical community dampens an invasive species-induced trophic cascade. Biol. Invasions 2019, 21, 203–215. [Google Scholar] [CrossRef]
- Pereira, L.S.; Angulo-Valencia, M.A.; Occhi, T.V.; Padial, A.A.; Vitule, J.R.S.; Agostinho, A.A. Looking through the predator’s eyes: Another perspective in naïveté theory. Biol. Invasions 2019, 21, 2577–2588. [Google Scholar] [CrossRef]
- Liu, H.; Stiling, P. Testing the enemy release hypothesis: A review and meta-analysis. Biol. Invasions 2006, 8, 1535–1545. [Google Scholar] [CrossRef]
- Blakeslee, A.M.; Keogh, C.L.; Byers, J.E.; Lafferty, A.M.K.K.D.; Torchin, M.E. Differential escape from parasites by two competing introduced crabs. Mar. Ecol. Prog. Ser. 2009, 393, 83–96. [Google Scholar] [CrossRef]
- Osman, R.W.; Whitlatch, R.B. Variation in the ability of Didemnum sp. to invade established communities. J. Exp. Mar. Biol. Ecol. 2007, 342, 40–53. [Google Scholar] [CrossRef]
- Cacabelos, E.; Olabarria, C.; Incera, M.; Troncoso, J.S. Do grazers prefer invasive seaweeds? J. Exp. Mar. Biol. Ecol. 2010, 393, 182–187. [Google Scholar] [CrossRef]
- Robinson, T.B.; Pope, H.R.; Hawken, L.; Binneman, C. Predation-driven biotic resistance fails to restrict the spread of a sessile rocky shore invader. Mar. Ecol. Prog. Ser. 2015, 522, 169–179. [Google Scholar] [CrossRef]
- Tomás, F.; Cebrian, E.; Ballesteros, E. Differential herbivory of invasive algae by native fish in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2011, 92, 27–34. [Google Scholar] [CrossRef]
- Rogers, T.L.; Byrnes, J.E.; Stachowicz, J.J. Native predators limit invasion of benthic invertebrate communities in Bodega Harbor, California, USA. Mar. Ecol. Prog. Ser. 2016, 545, 161–173. [Google Scholar] [CrossRef]
- Giakoumi, S.; Pey, A.; Thiriet, P.; Francour, P.; Guidetti, P. Patterns of predation on native and invasive alien fish in Mediterranean protected and unprotected areas. Mar. Environ. Res. 2019, 150, 104792. [Google Scholar] [CrossRef] [PubMed]
- Crocetta, F.; Shokouros-Oskarsson, M.; Doumpas, N.; Giovos, I.; Kalogirou, S.; Langeneck, J.; Tanduo, V.; Tiralongo, F.; Virgili, R.; Kleitou, P. Protect the natives to combat the aliens: Could Octopus vulgaris Cuvier, 1797 be a natural agent for the control of the lionfish invasion in the Mediterranean Sea? J. Mar. Sci. Eng. 2021, 9, 308. [Google Scholar] [CrossRef]
- Marić, M.; de Troch, M.; Occhipinti-Ambrogi, A.; Olenin, S. Trophic interactions between indigenous and non-indigenous species in Lampedusa Island, Mediterranean Sea. Mar. Environ. Res. 2016, 120, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Glamuzina, B.; Petrić, M.; Carrozzo, L.; Glamuzina, L.; Zotti, M.; Raho, D.; Vizzini, S. The trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): A first assessment using stable isotopes. Mediterr. Mar. Sci. 2016, 17, 634–643. [Google Scholar] [CrossRef]
- Locke, A.; Hanson, J.M.; Ellis, K.M.; Thompson, J.; Rochette, R. Invasion of the southern Gulf of St. Lawrence by the clubbed tunicate (Styela clava Herdman): Potential mechanisms for invasions of Prince Edward Island estuaries. J. Exp. Mar. Bio. Ecol. 2007, 342, 69–77. [Google Scholar] [CrossRef]
- Dias, G.M.; Vieira, E.A.; Pestana, L.; Marques, A.C.; Karythis, S.; Jenkins, S.R.; Griffith, K. Calcareous defence structures of prey mediate the effects of predation and biotic resistance towards the tropics. Divers. Distrib. 2020, 26, 1198–1210. [Google Scholar] [CrossRef]
- Ashton, G.V.; Freestone, A.L.; Duffy, J.E.; Torchin, M.E.; Sewall, B.J.; Tracy, B.; Albano, M.; Altieri, A.H.; Altvater, L.; Ruiz, G.M.; et al. Predator control of marine communities increases with temperature across 115 degrees of latitude. Science 2022, 376, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Freestone, A.L.; Ruiz, G.M.; Torchin, M.E. Stronger biotic resistance in tropics relative to temperate zone: Effects of predation on marine invasion dynamics. Ecology 2013, 94, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Freestone, A.L.; Torchin, M.E.; Jurgens, L.J.; Bonfim, M.; López, D.P.; Repetto, M.F.; Schlöder, C.; Sewall, B.J.; Ruiz, G.M. Stronger predation intensity and impact on prey communities in the tropics. Ecology 2021, 102, e03428. [Google Scholar] [CrossRef] [PubMed]
- Wells, F.; Bieler, R. A low number of introduced marine species at low latitudes: A case study from southern Florida with a special focus on Mollusca. Manag. Biol. Invasions 2020, 11, 372–398. [Google Scholar] [CrossRef]
- Albano, M.J.; Obenat, S.M. Fouling assemblages of native, non-indigenous and cryptogenic species on artificial structures, depths and temporal variation. J. Sea. Res. 2019, 144, 1–15. [Google Scholar] [CrossRef]
- Brown, K.M.; Swearingen, D.C. Effects of seasonality, length of immersion, locality and predation on an intertidal fouling assemblage in the Northern Gulf of Mexico. J. Exp. Mar. Biol. Ecol. 1998, 225, 107–121. [Google Scholar] [CrossRef]
- Hiebert, L.S.; Vieira, E.A.; Dias, G.M.; Tiozzo, S.; Brown, F.D. Colonial ascidians strongly preyed upon, yet dominate the substrate in a subtropical fouling community. Proc. R. Soc. B. 2019, 286, 20190396. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, A.; Pearce, C.M.; Barker, D.J.; Paulson, A.; Therriault, T.W. Susceptibility of non-indigenous ascidian species in British Columbia (Canada) to invertebrate predation. Mar. Biol. 2009, 156, 1311–1320. [Google Scholar] [CrossRef]
- Giachetti, C.B.; Battini, N.; Castro, K.L.; Schwindt, E. Invasive ascidians: How predators reduce their dominance in artificial structures in cold temperate areas. J. Exp. Mar. Biol. Ecol. 2020, 533, 151459. [Google Scholar] [CrossRef]
- Leclerc, J.C.; Viard, F.; Brante, A. Experimental and survey-based evidences for effective biotic resistance by predators in ports. Biol. Invasions 2020, 22, 339–352. [Google Scholar] [CrossRef]
- Rodemann, J.; Brandl, S. Consumption pressure in coastal marine environments decreases with latitude and in artificial vs. natural habitats. Mar. Ecol. Prog. Ser. 2017, 574, 167–179. [Google Scholar] [CrossRef]
- Guidetti, P. Fish assemblages associated with coastal defence structures in south-western Italy (Mediterranean Sea). J. Mar. Biolog. Assoc. UK 2004, 84, 669–670. [Google Scholar] [CrossRef]
- Kincaid, E.S.; de Rivera, C.E. Predators associated with marinas consume indigenous over non-indigenous ascidians. Estuaries Coast. 2021, 44, 579–588. [Google Scholar] [CrossRef]
- Janiak, D.S.; Branson, D. Impacts of habitat and predation on epifaunal communities from seagrass beds and artificial structures. Mar. Environ. Res. 2021, 163, 105225. [Google Scholar] [CrossRef]
- Dumont, C.P.; Gaymer, C.F.; Thiel, M. Predation contributes to invasion resistance of benthic communities against the non-indigenous tunicate Ciona intestinalis. Biol. Invasions 2011, 13, 2023–2034. [Google Scholar] [CrossRef]
- Forrest, B.M.; Fletcher, L.M.; Atalah, J.; Piola, R.F.; Hopkins, G.A. Predation limits spread of Didemnum vexillum into natural habitats from refuges on anthropogenic structures. PLoS ONE 2013, 8, e82229. [Google Scholar] [CrossRef]
- Yorisue, T.; Ellrich, J.A.; Momota, K. Mechanisms underlying predator-driven biotic resistance against introduced barnacles on the Pacific coast of Hokkaido, Japan. Biol. Invasions 2019, 21, 2345–2356. [Google Scholar] [CrossRef]
- Gestoso, I.; Ramalhosa, P.; Canning-Clode, J. Biotic effects during the settlement process of non-indigenous species in marine benthic communities. Aquat. Invasions 2018, 13, 247–259. [Google Scholar] [CrossRef]
- Tamburini, M.; Keppel, E.; Marchini, A.; Repetto, M.F.; Ruiz, G.M.; Ferrario, J.; Occhipinti-Ambrogi, A. Monitoring non-indigenous species in port habitats: First application of a standardized North American protocol in the Mediterranean Sea. Front. Mar. Sci. 2021, 8, 700730. [Google Scholar] [CrossRef]
- Steele, M.A. Effects of predators on reef fishes: Separating cage artifacts from effects of predation. J. Exp. Mar. Biol. Ecol. 1996, 198, 249–267. [Google Scholar] [CrossRef]
- Chapman, J.W.; Carlton, J.T. A test of criteria for introduced species: The global invasion by the isopod Synidotea laevidorsalis (Miers, 1881). J. Crustacean Biol. 1991, 11, 386–400. [Google Scholar] [CrossRef]
- Chang, A.L.; Brown, C.W.; Crooks, J.A.; Ruiz, G.M. Dry and wet periods drive rapid shifts in community assembly in an estuarine ecosystem. Glob. Chang. Biol. 2018, 24, e627–e642. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Gorley, R.N.; Clarke, K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Lafferty, K.D.; Suchanek, T.H. Revisiting Paine’s 1966 sea star removal experiment, the most-cited empirical article in the American Naturalist. Am. Nat. 2016, 188, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, J.M.; Heisey, D.M. The abuse of power: The pervasive fallacy of power calculations for data analysis. Am. Stat. 2001, 55, 19–24. [Google Scholar] [CrossRef]
- Grube, E. Beschreibungen neuer oder weniger bekannter von Hrn. Ehrenberg gesammelter anneliden des rothen meeres. Mon. Akad Wiss Berl. 1870, 1869, 484–521. [Google Scholar]
- De Barros, R.C.; da Rocha, R.M.; Pie, M.R. Human-mediated global dispersion of Styela plicata (Tunicata, Ascidiacea). Aquat. Invasions 2009, 4, 45–57. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Poursanidis, D.; Hoffmann, R.; Rizgalla, J.; Rothman, S.B.S.; Levitt-Barmats, Y.A.; Espinosa, T.F.; Zenetos, A. Unpublished Mediterranean records of marine alien and cryptogenic species. BioInvasions Rec. 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Lindquist, N.; Hay, M.E.; Fenical, W. Defense of ascidians and their conspicuous larvae: Adult vs. larval chemical defenses. Ecol. Monog. 1992, 62, 547–568. [Google Scholar] [CrossRef]
- Gauff, R.P.M.; Lejeusne, C.; Arsenieff, L.; Bohner, O.; Coudret, J.; Desbordes, F.; Jandard, A.; Loisel, S.; Schires, G.; Wafo, E.; et al. Alien vs. predator: Influence of environmental variability and predation on the survival of ascidian recruits of a native and alien species. Biol. Invasions 2022, 24, 1327–1344. [Google Scholar] [CrossRef]
- Needles, L.A.; Gosnell, J.S.; Waltz, G.T.; Wendt, D.E.; Gaines, S.D. Trophic cascades in an invaded ecosystem: Native keystone predators facilitate a dominant invader in an estuarine community. Oikos 2015, 124, 1282–1292. [Google Scholar] [CrossRef]
- Simkanin, C.; Dower, J.F.; Filip, N.; Jamieson, G.; Therriault, T.W. Biotic resistance to the infiltration of natural benthic habitats: Examining the role of predation in the distribution of the invasive ascidian Botrylloides violaceus. J. Exp. Mar. Biol. Ecol. 2013, 439, 76–83. [Google Scholar] [CrossRef]
- Fernandez-Jover, D.; Sanchez-Jerez, P. Comparison of diet and otolith growth of juvenile wild fish communities at fish farms and natural habitats. ICES J. Mar. Sci. 2015, 72, 916–929. [Google Scholar] [CrossRef]
- Pallaoro, A.; Santic, M.; Jardas, I. Feeding habits of the saddled bream, Oblada melanura (Sparidae), in the Adriatic Sea. Cybium 2003, 27, 261–268. [Google Scholar] [CrossRef]
- Havelange, S.; Lepoint, G.; Dauby, P.; Bouquegneau, J.-M. Feeding of the sparid fish Sarpa salpa in a seagrass ecosystem: Diet and carbon flux. Mar. Ecol. 1997, 18, 289–297. [Google Scholar] [CrossRef]
- Dobroslavic, T.; Zlatovic, A.; Bartulovic, V.; Lucic, D.; Glamuzina, B. Diet overlap of juvenile salema (Sarpa salpa), bogue (Boops boops) and common twobanded sea bream (Diplodus vulgaris) in the southeastern Adriatic. J. Appl. Ichthyol. 2012, 29, 181–185. [Google Scholar] [CrossRef]
- Quignard, J.-P.; Pras, A. Pomacentridae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 2, pp. 916–918. [Google Scholar]
- Aronson, R.B.; Heck, K.L., Jr. Tethering experiments and hypothesis testing in ecology. Mar. Ecol. Prog. Ser. 1995, 121, 307–309. [Google Scholar] [CrossRef]
- Astudillo, J.C.; Leung, K.M.Y.; Bonebrake, T.C. Seasonal heterogeneity provides a niche opportunity for ascidian invasion in subtropical marine communities. Mar. Environ. Res. 2016, 122, 1–10. [Google Scholar] [CrossRef]
- Kremer, L.P.; da Rocha, R.M. The biotic resistance role of fish predation in fouling communities. Biol. Invasions 2016, 18, 3223–3237. [Google Scholar] [CrossRef]
- Atalah, J.; Newcombe, E.M.; Hopkins, G.A.; Forrest, B.M. Potential biocontrol agents for biofouling on artificial structures. Biofouling 2014, 30, 999–1010. [Google Scholar] [CrossRef] [PubMed]

| NIS (Mean Coverage > 1%) | Mean Coverage | F Value | p |
|---|---|---|---|
| Branchiomma sp. | 2.8% | 0.4316 | 0.6568 |
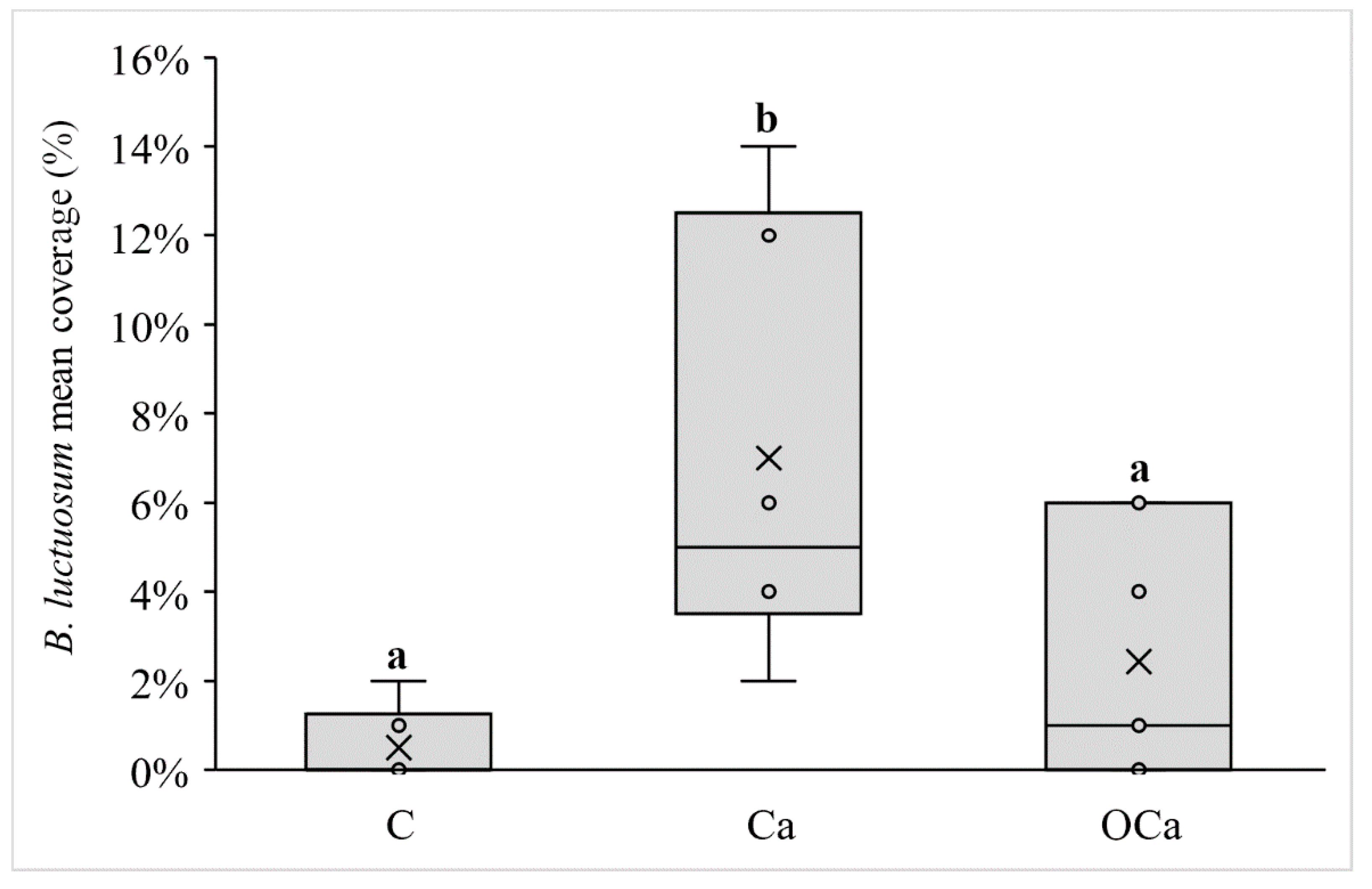
| Branchiomma luctuosum (Grube, 1870)° | 3.2% | 7.7513 | 0.0044 |
| Hydroides elegans (Haswell, 1883) | 9.7% | 2.1297 | 0.1513 |
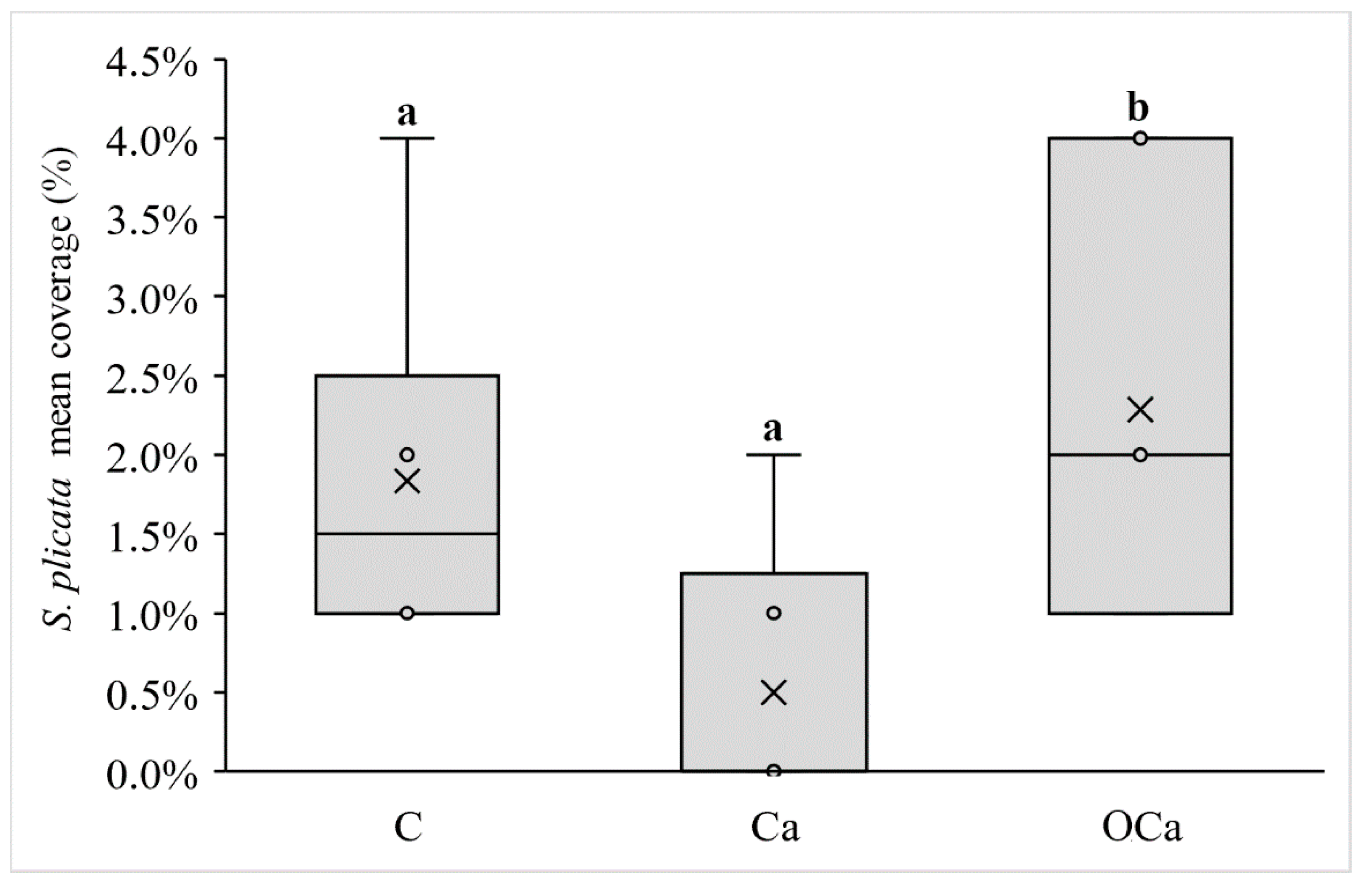
| Styela plicata (Lesueur, 1823) | 1.5% | 4.4003 | 0.0300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburini, M.; Ferrario, J.; Piazzese, L.; Occhipinti-Ambrogi, A. Effect of Predation on Fouling Communities in an Italian Hotspot of Non-Indigenous Species. J. Mar. Sci. Eng. 2022, 10, 1496. https://doi.org/10.3390/jmse10101496
Tamburini M, Ferrario J, Piazzese L, Occhipinti-Ambrogi A. Effect of Predation on Fouling Communities in an Italian Hotspot of Non-Indigenous Species. Journal of Marine Science and Engineering. 2022; 10(10):1496. https://doi.org/10.3390/jmse10101496
Chicago/Turabian StyleTamburini, Marco, Jasmine Ferrario, Laura Piazzese, and Anna Occhipinti-Ambrogi. 2022. "Effect of Predation on Fouling Communities in an Italian Hotspot of Non-Indigenous Species" Journal of Marine Science and Engineering 10, no. 10: 1496. https://doi.org/10.3390/jmse10101496
APA StyleTamburini, M., Ferrario, J., Piazzese, L., & Occhipinti-Ambrogi, A. (2022). Effect of Predation on Fouling Communities in an Italian Hotspot of Non-Indigenous Species. Journal of Marine Science and Engineering, 10(10), 1496. https://doi.org/10.3390/jmse10101496






