Review of the Status and Developments in Seaweed Farming Infrastructure
Abstract
:1. Introduction and Global Context
2. Traditional Farming Methods
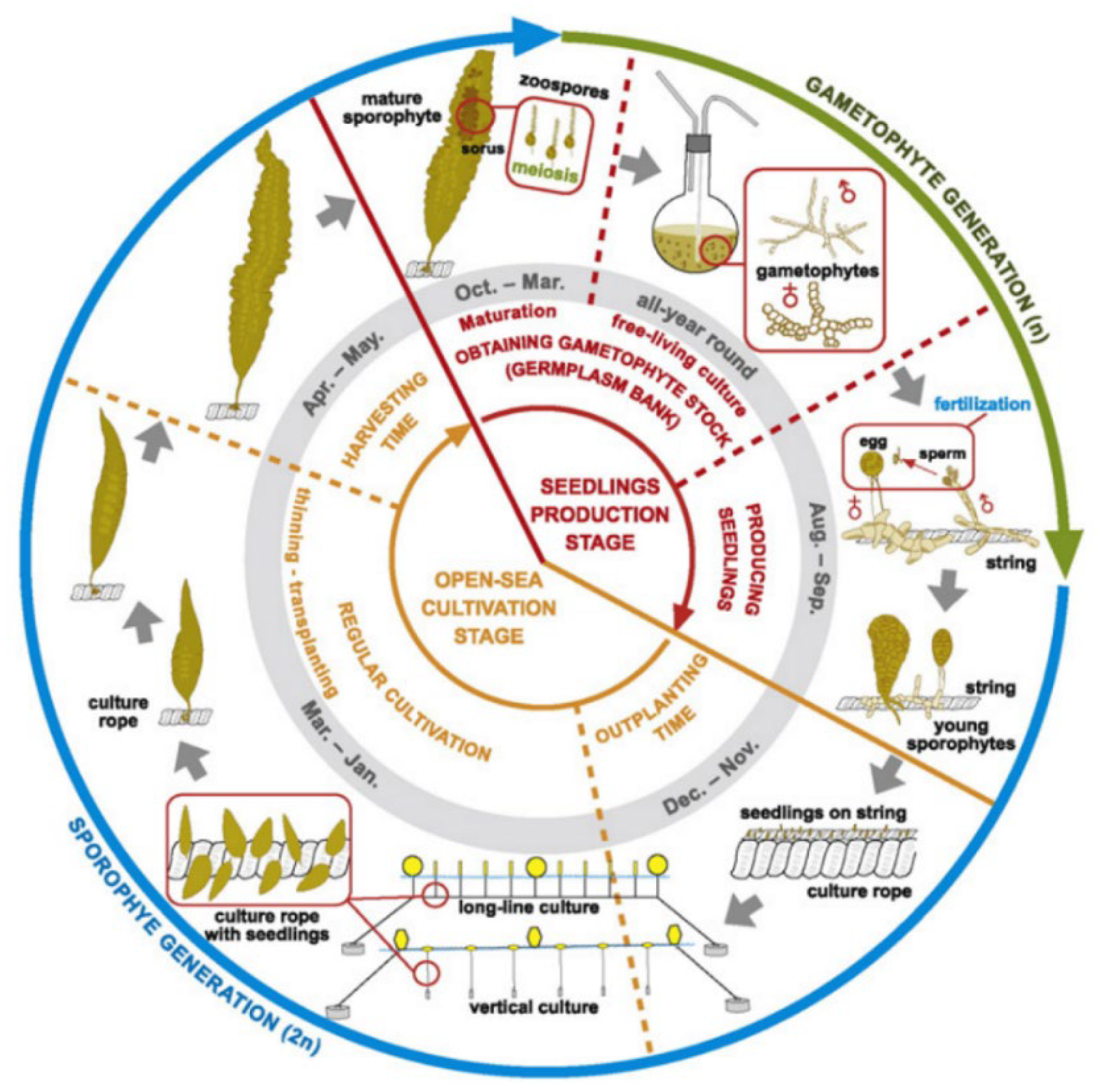
2.1. Background and Seaweed Species Classification
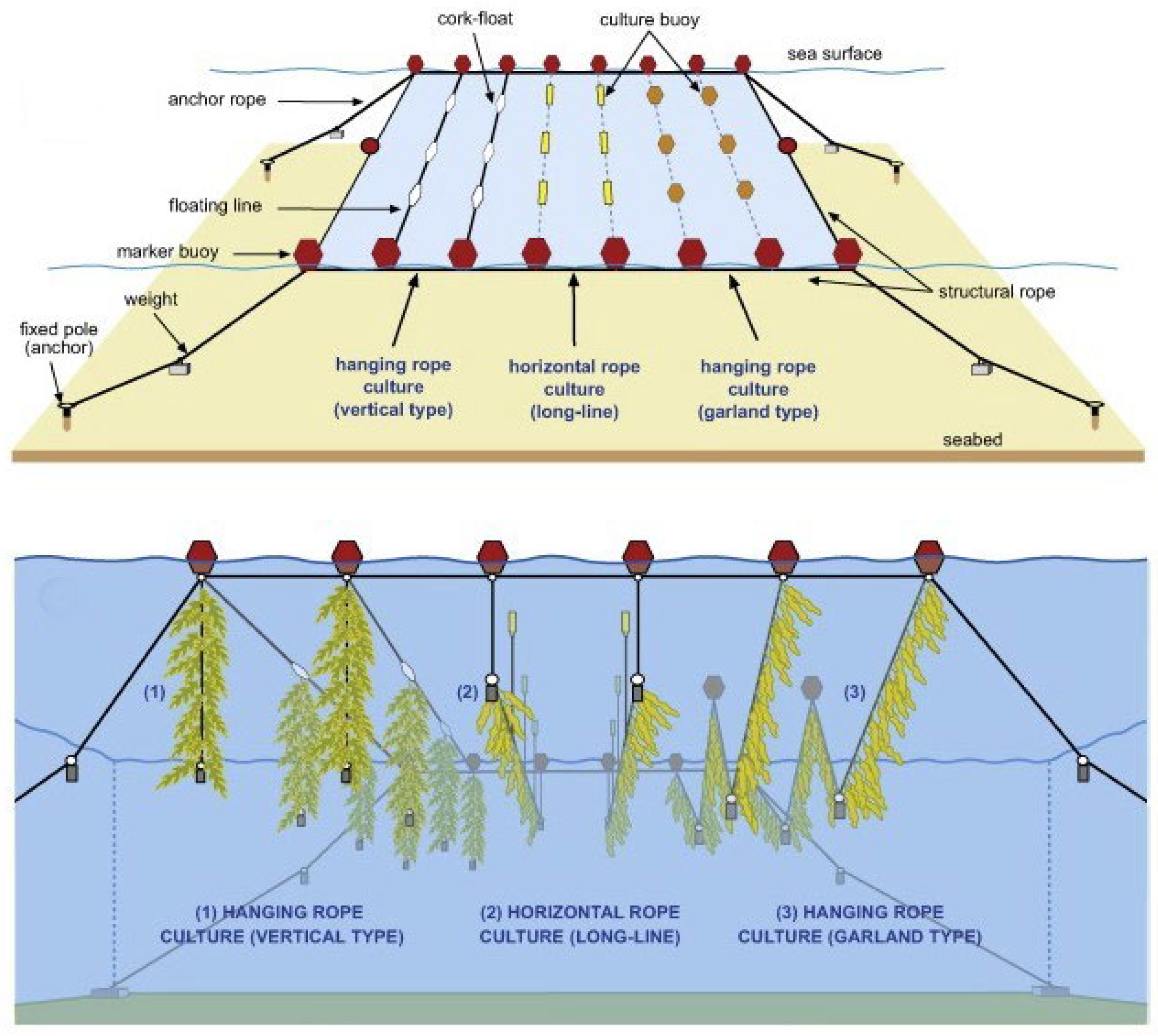
2.2. Cultivation Systems
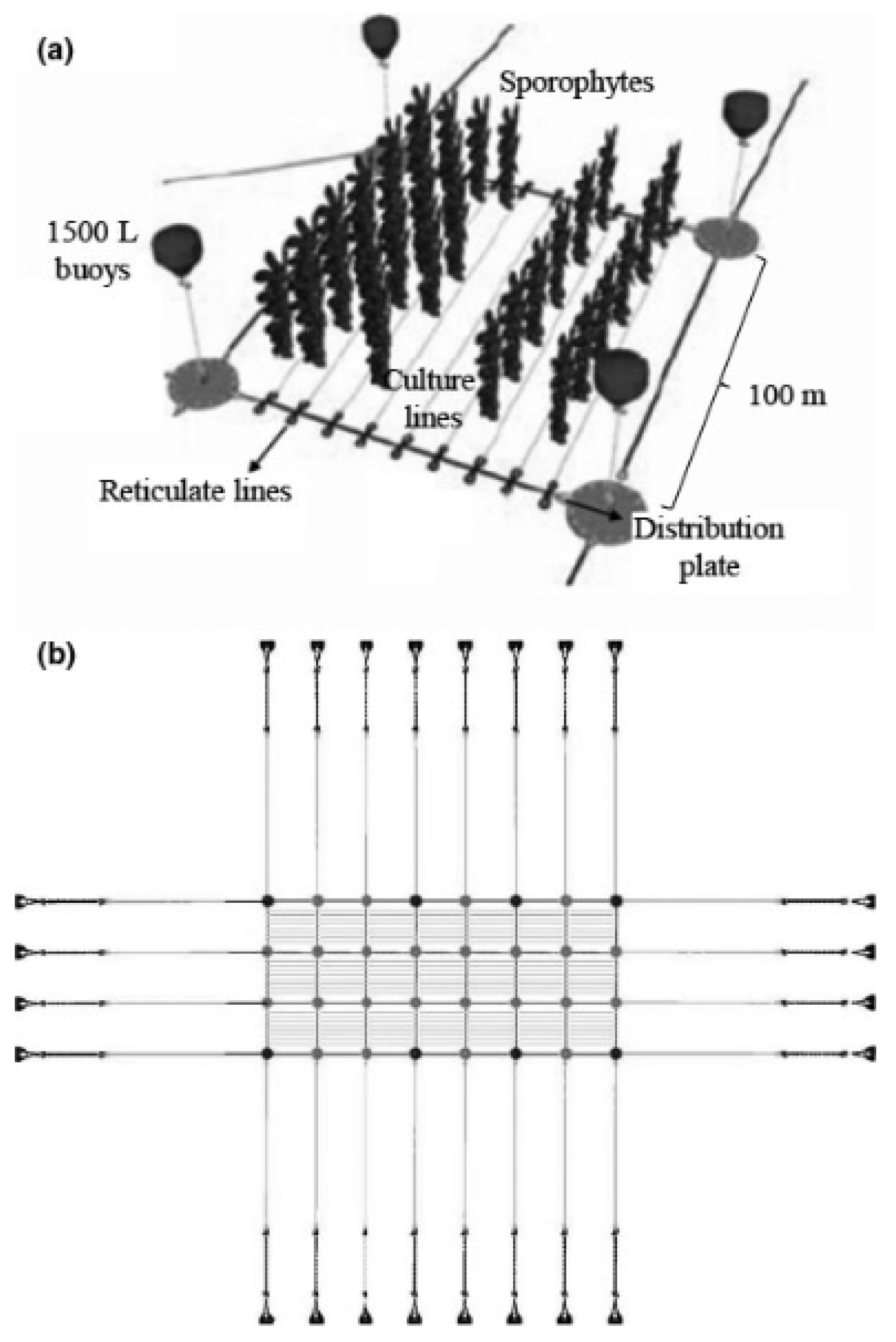
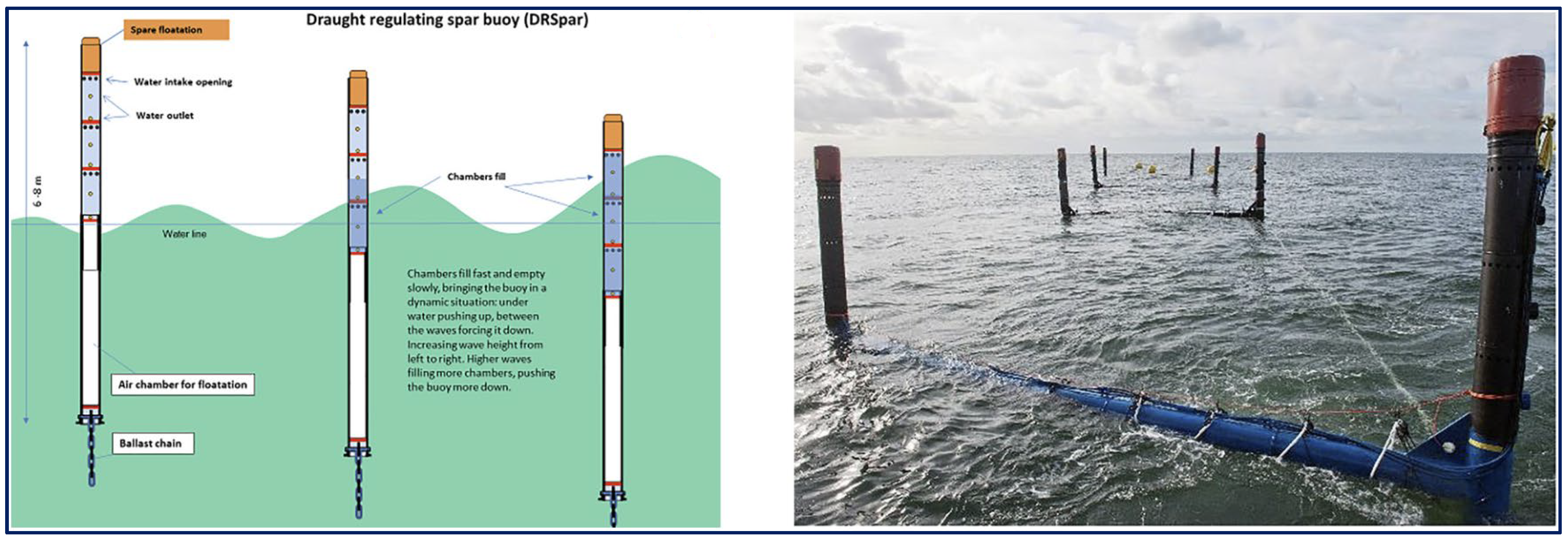
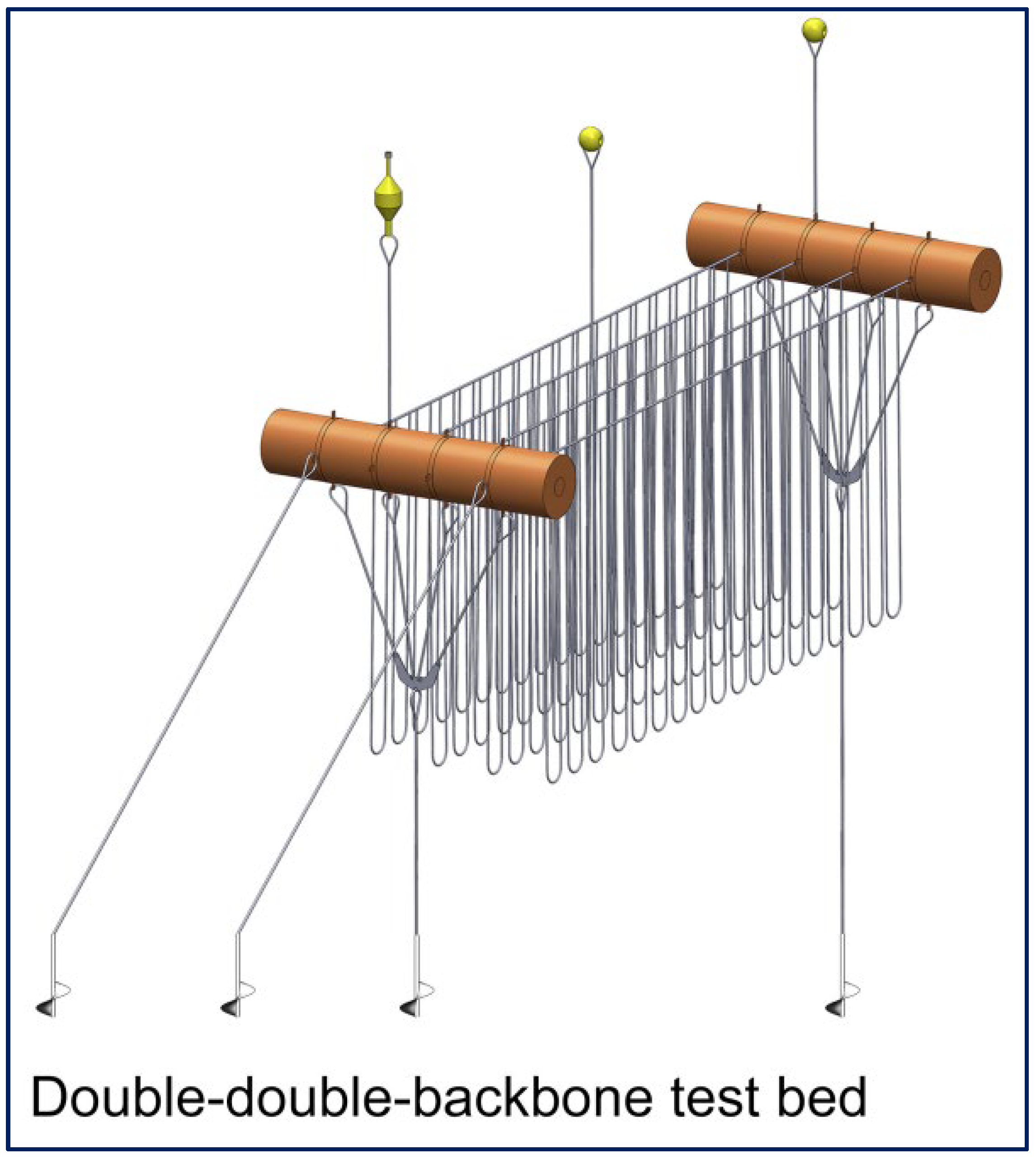
3. Evolution of Offshore and Semi-Automated Systems
- Cultivation structures must be able to withstand infrequent but intense weather events such as storms/cyclones and their associated high-energy waves, and strong currents depending on citing [3,13,28,29]. Whilst the occasional loss of a seaweed crop could be tolerated due to storm action the long-term integrity of structures must be ensured.
- Cultivation infrastructure systems need to be refined to support high productivity, and hence low overall cost harvesting and reseeding operations to be competitive with nearshore farming.
- Seaweed service vessels for harvesting, reseeding, and transport need to be further developed to suit the in-water infrastructure system and for sharing with other aquaculture and offshore renewables maintenance and repair.
- Linear—advances on the traditional longline systems.
- Circular—systems typically borrow technology from the seafood farming industry.
- Two-dimensional (2D)—based on substrates such as fabrics and 2D net structures.
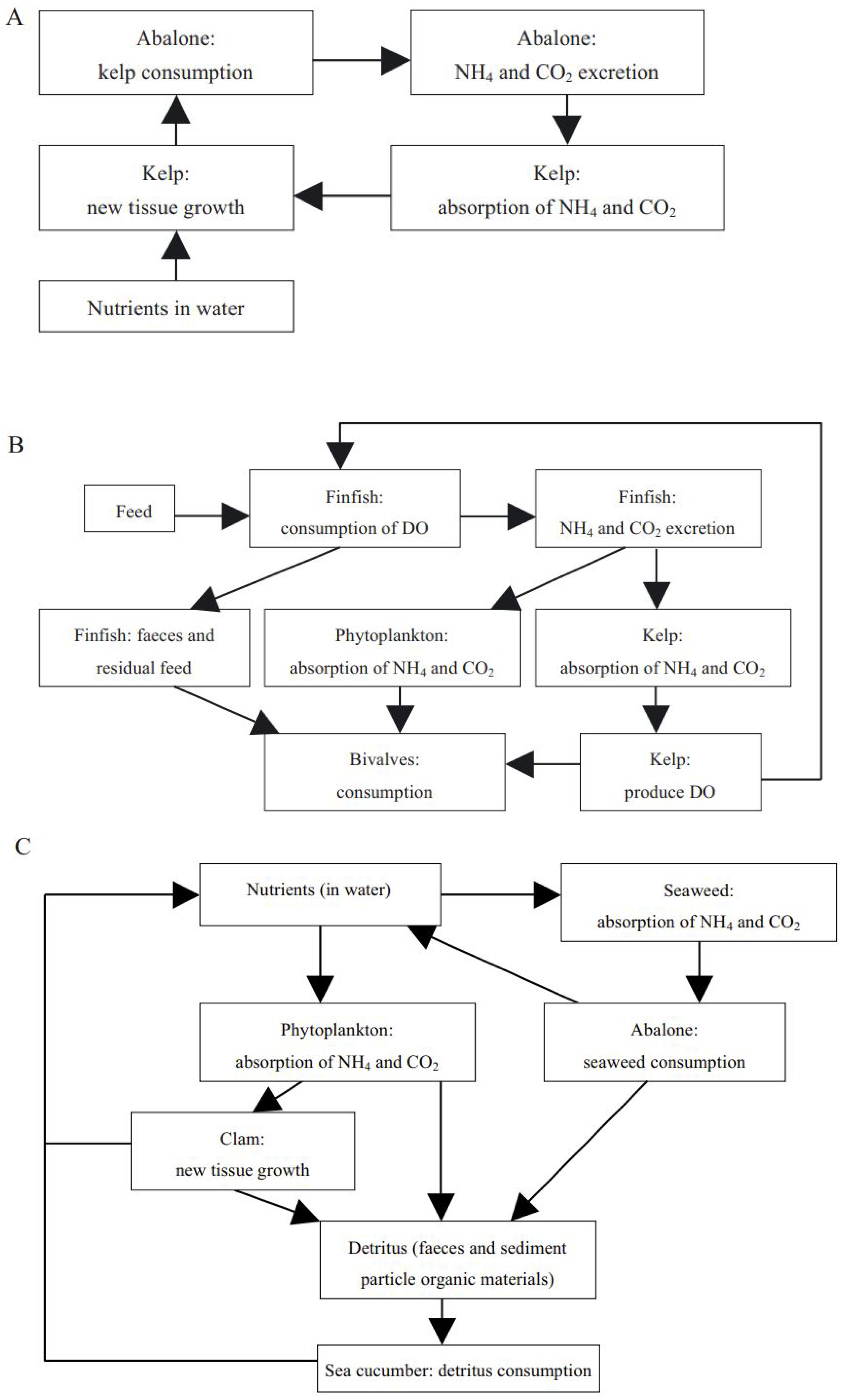
4. Co-Cultivation of Seaweed and Other Species
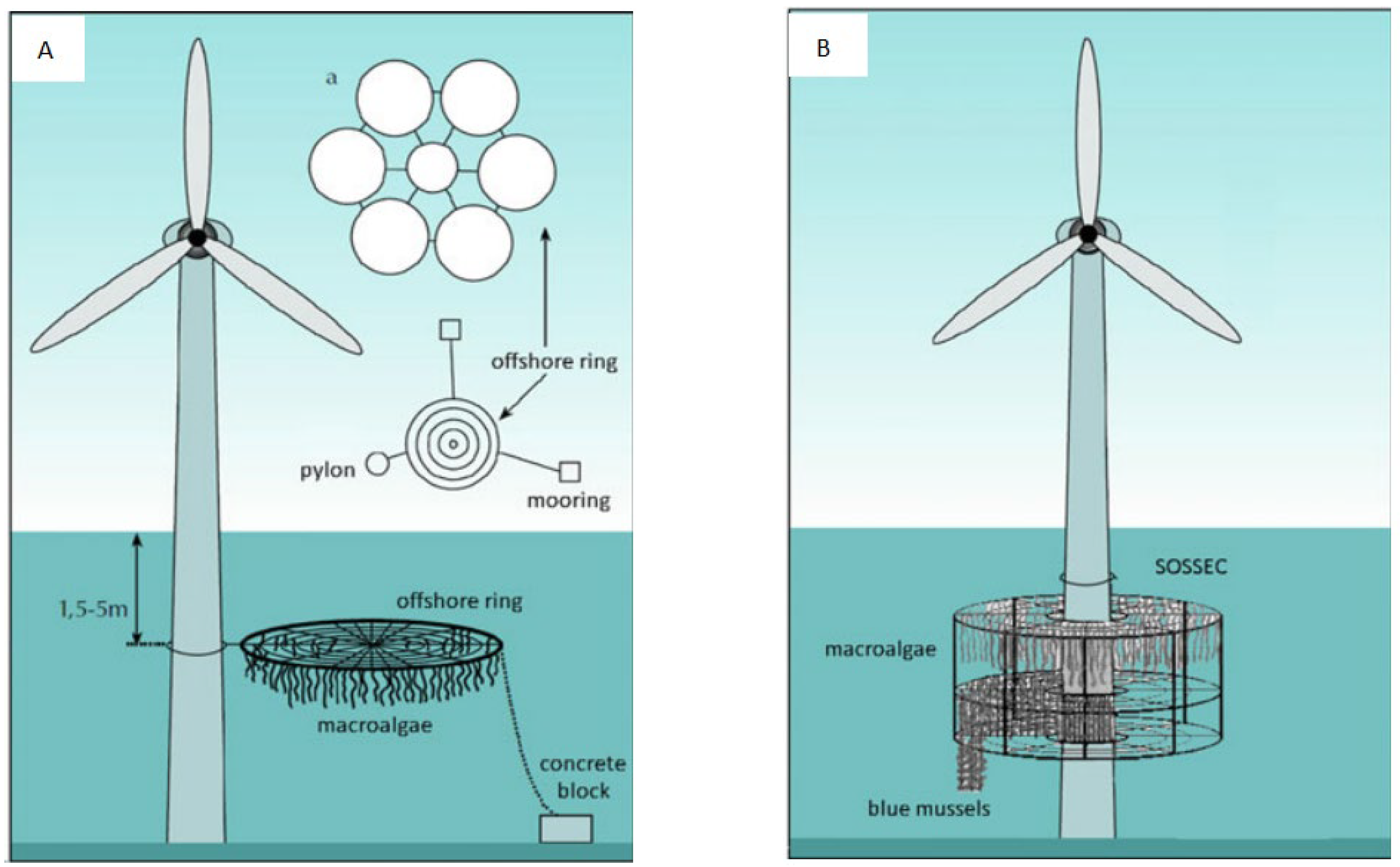
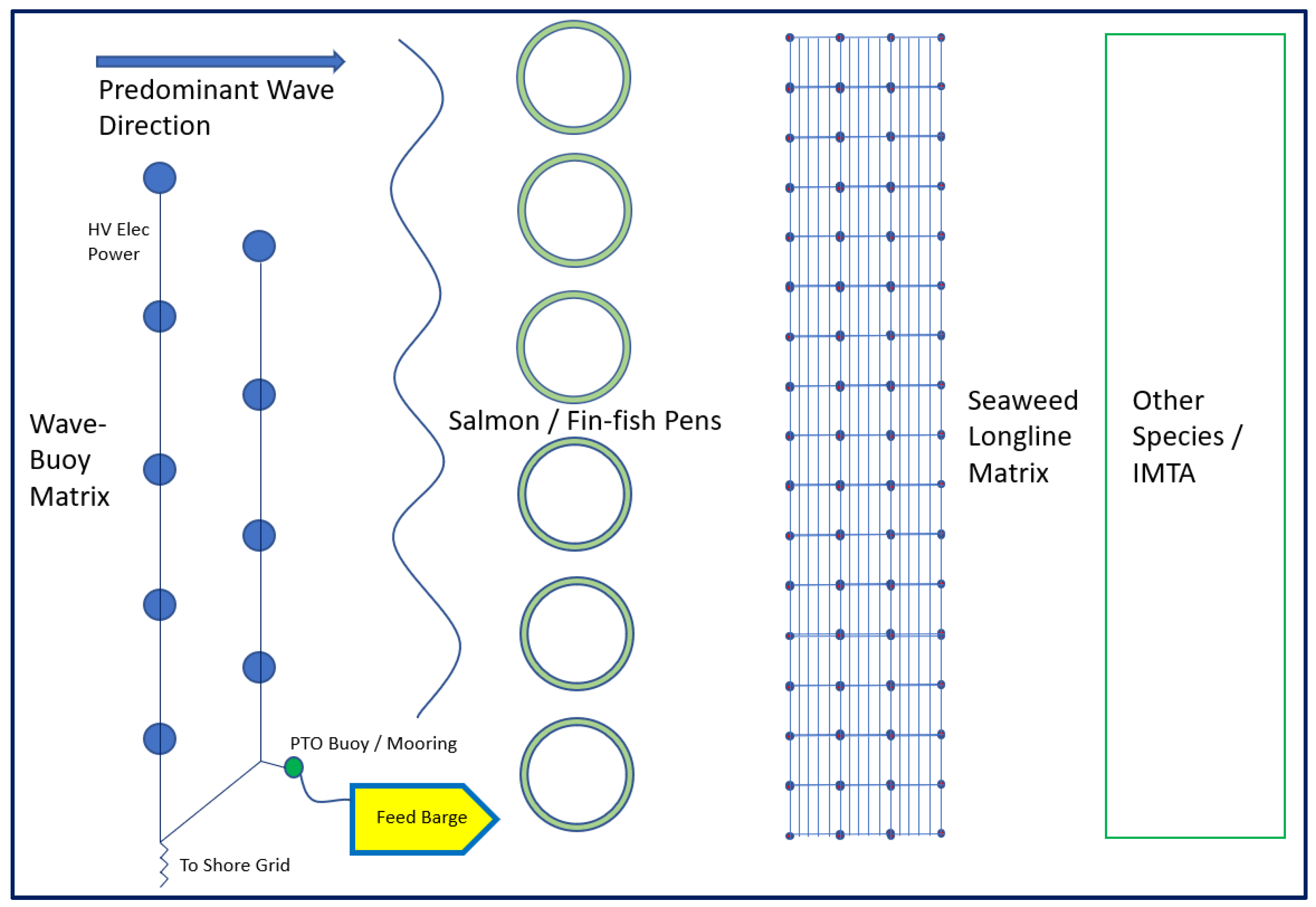
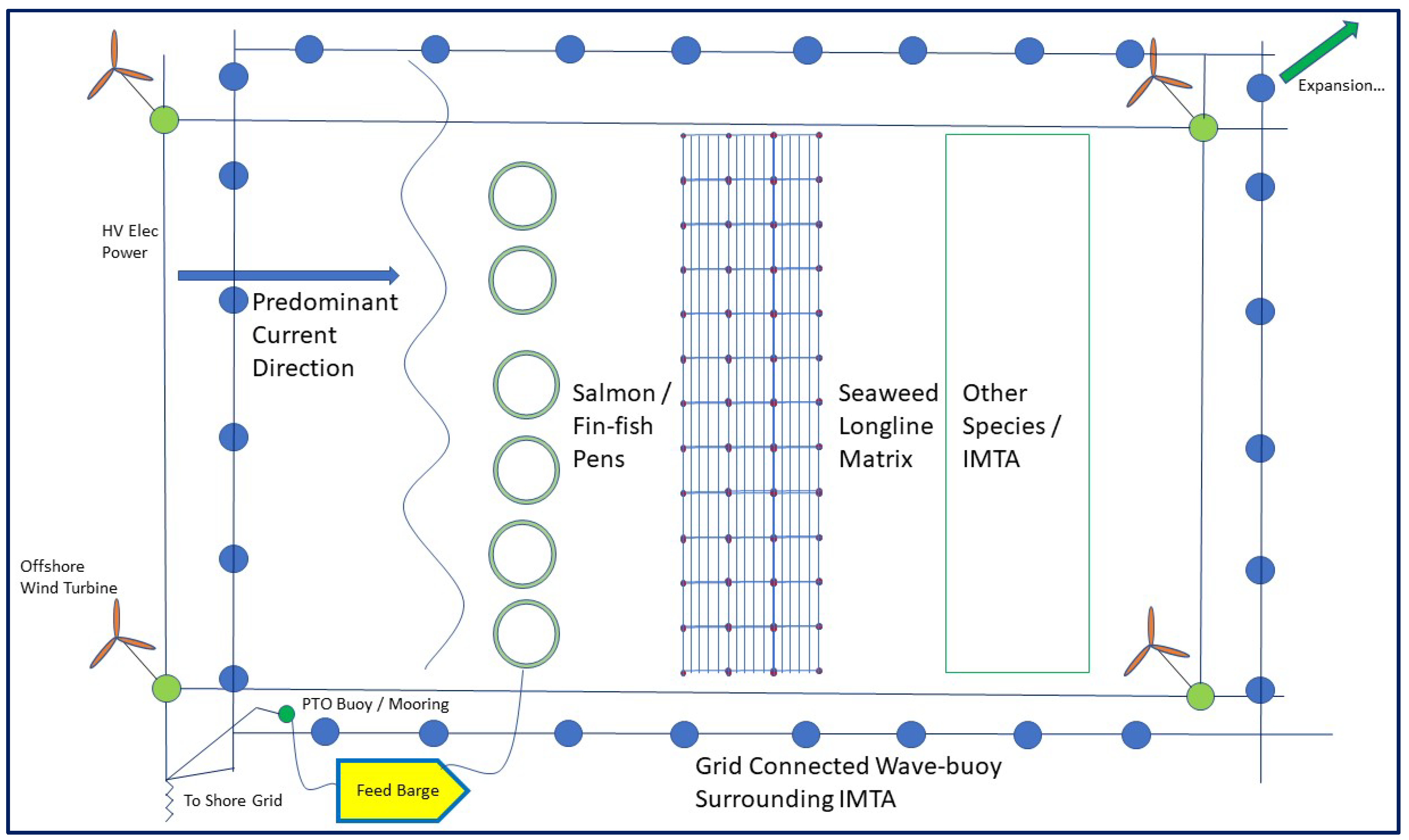
5. Colocation of Seaweed/Other Species and Offshore Renewable Energy Farms
6. Conclusions
Author Contributions
| Contributor | Contribution Type | Percentage (%) |
| R.M.T. | Conception, discussion, conclusions | 70 |
| Literature research | 60 | |
| Review, checking | 20 | |
| H.P.N. | Conception, discussion, conclusions | 20 |
| Literature research | 30 | |
| Review, checking | 30 | |
| C.M.W. | Conception, discussion, conclusions | 10 |
| Literature research | 10 | |
| Review, checking | 50 |
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bjerregaard, R.; Valderrama, D.; Radulovich, R.; Diana, J.; Capron, M.; Mckinnie, C.A.; Cedric, M.; Hopkins, K.; Yarish, C.; Goudey, C.; et al. Seaweed Aquaculture for Food Security, Income Generation and Environmental Health in Tropical Developing Countries; World Bank Group: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2022, 5, 185–193. [Google Scholar] [CrossRef]
- FAO. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Kelly, J. Australian Seaweed Industry Blueprint for Growth. 2020. Available online: www.agrifutures.com.au/wp-content/uploads/2020/09/20-072.pdf (accessed on 22 September 2022).
- Hasselström, L.; Thomas, J.B.; Nordström, J.; Cervin, G.; Nylund, G.M.; Pavia, H.; Gröndahl, F. Socioeconomic prospects of a seaweed bioeconomy in Sweden. Sci. Rep. 2020, 10, 1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, W.L.; Wilson, P. World seaweed utilization. In Seaweed Sustainability: Food and Non-Food Applications; Academic Press: Cambridge, MA, USA, 2015; pp. 7–25. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar] [CrossRef] [PubMed]
- FAO. The global status of seaweed production, trade and utilization. FAO Globefish Res. Program. 2018, 124, 120. [Google Scholar]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Vijn, S.; Compart, D.P.; Dutta, N.; Foukis, A.; Hess, M.; Hristov, A.N.; Kalscheur, K.F.; Kebreab, E.; Nuzhdin, S.V.; Price, N.N.; et al. Key Considerations for the Use of Seaweed to Reduce Enteric Methane Emissions From Cattle. Front. Vet. Sci. 2020, 7, 597430. [Google Scholar] [CrossRef]
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102. [Google Scholar] [CrossRef]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore Integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Ambo-Rappe, R.; Jones, B.L.; La Nafie, Y.A.; Irawan, A.; Hernawan, U.E.; Moore, A.M.; Cullen-Unsworth, L.C. Indonesia’s globally significant seagrass meadows are under widespread threat. Sci. Total Environ. 2018, 634, 279–286. [Google Scholar] [CrossRef]
- Bak, U.G.; Gregersen, Ó.; Infante, J. Technical challenges for offshore cultivation of kelp species: Lessons learned and future directions. Bot. Mar. 2020, 63, 341–353. [Google Scholar] [CrossRef]
- Clements, J.C.; Chopin, T. Ocean acidification and marine aquaculture in North America: Potential impacts and mitigation strategies. Rev. Aquac. 2017, 9, 326–341. [Google Scholar] [CrossRef]
- Radulovich, R. Massive freshwater gains from producing food at sea. Water Policy 2011, 13, 547–554. [Google Scholar] [CrossRef]
- Hisas, L. The Food Gap the Impact of Climate Change on Food Production: A 2020 Perspective. 2011. Available online: https://legacy-assets.eenews.net/open_files/assets/2011/01/19/document_cw_02.pdf (accessed on 22 September 2022).
- Clay, J. Freeze the footprint of food. Nature 2011, 475, 287–289. [Google Scholar] [CrossRef]
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N.; Neish, I.C.; Hurtado, A.Q.; Msuya, F.E.; Krishnan, M.; Narayanakumar, R.; Kronen, M.; et al. The Economics of Kappaphycus Seaweed Cultivation in Developing Countries: A Comparative Analysis of Farming Systems. Aquac. Econ. Manag. 2015, 19, 251–277. [Google Scholar] [CrossRef] [Green Version]
- FAO. Social and Economic Dimensions of Carrageenan Seaweed Farming. 2013. Available online: https://www.fao.org/in-action/globefish/publications/details-publication/en/c/338356/ (accessed on 22 September 2022).
- Peteiro, C.; Sánchez, N.; Martínez, B. Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: An overview. Algal Res. 2016, 15, 9–23. [Google Scholar] [CrossRef]
- Neushul, M.; Benson, J.; Harger, B.W.W.; Charters, A.C. Macroalgal farming in the sea: Water motion and nitrate uptake. J. Appl. Phycol. 1992, 4, 255–265. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; pp. 1–551. [Google Scholar] [CrossRef]
- van den Burg, S.W.K.; van Duijn, A.P.; Bartelings, H.; van Krimpen, M.M.; Poelman, M. The economic feasibility of seaweed production in the North Sea. Aquac. Econ. Manag. 2016, 20, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Available online: www.minimum-wage.org/international/Netherlands (accessed on 13 September 2022).
- Camus, C.; Infante, J.; Buschmann, A.H. Overview of 3 year precommercial seafarming of Macrocystis pyrifera along the Chilean coast. Rev. Aquac. 2018, 10, 543–559. [Google Scholar] [CrossRef]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Roesijadi, G.; Copping, A.E.E.; Huesemann, M.H.H.; Forster, J.; Benemann, J.R.; Thom, R.M. Techno-Economic Feasibility Analysis of Offshore Seaweed Farming for Bioenergy and Biobased Products. 2008. Available online: https://arpa-e.energy.gov/sites/default/files/Techno-Economic%20Feasibility%20Analysis%20of%20Offshore%20Seaweed%20Farming%20for%20Bioenergy%20and%20Biobased%20Products-2008.pdf (accessed on 22 September 2022).
- Navarrete, I.A.; Kim, D.Y.; Wilcox, C.; Reed, D.C.; Ginsburg, D.W.; Dutton, J.M.; Heidelberg, J.; Raut, Y.; Wilcox, B.H. Effects of depth-cycling on nutrient uptake and biomass production in the giant kelp Macrocystis pyrifera. Renew. Sustain. Energy Rev. 2021, 141, 110747. [Google Scholar] [CrossRef]
- Climate Foundation Global Warming. Available online: https://www.climatefoundation.org/global-warming.html (accessed on 22 September 2022).
- Collins, N.; Kumar Mediboyina, M.; Cerca, M.; Vance, C.; Murphy, F. Economic and environmental sustainability analysis of seaweed farming: Monetizing carbon offsets of a brown algae cultivation system in Ireland. Bioresour. Technol. 2022, 346, 126637. [Google Scholar] [CrossRef]
- Hurd, C.L.; Law, C.S.; Bach, L.T.; Britton, D.; Hovenden, M.; Paine, E.R.; Raven, J.A.; Tamsitt, V.; Boyd, P.W. Forensic carbon accounting: Assessing the role of seaweeds for carbon sequestration. J. Phycol. 2022, 58, 347–363. [Google Scholar] [CrossRef]
- Bach, L.T.; Tamsitt, V.; Gower, J.; Hurd, C.L.; Raven, J.A.; Boyd, P.W. Testing the climate intervention potential of ocean afforestation using the Great Atlantic Sargassum Belt. Nat. Commun. 2021, 12, 2556. [Google Scholar] [CrossRef]
- Hieronymus Buck, B.; Maria Buchholz, C. The offshore-ring: A new system design for the open ocean aquaculture of macroalgae. J. Appl. Phycol. 2004, 16, 355–368. [Google Scholar] [CrossRef]
- Solvang, T.; Bale, E.S.; Broch, O.J.; Handå, A.; Alver, M.O. Automation Concepts for Industrial-Scale Production of Seaweed. Front. Mar. Sci. 2021, 8, 613093. [Google Scholar] [CrossRef]
- Bak, U.G.; Mols-Mortensen, A.; Gregersen, O. Production method and cost of commercial-scale offshore cultivation of kelp in the Faroe Islands using multiple partial harvesting. Algal Res. 2018, 33, 36–47. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Water motion. In Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014; Available online: https://www.cambridge.org/core/books/abs/seaweed-ecology-and-physiology/water-motion/CBF4DF59A03DBE4EF5E0BCE370FEF373 (accessed on 22 September 2022).
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.; Zhou, Y.; Mao, X.Z. Eutrophication control strategies for highly anthropogenic influenced coastal waters. Sci. Total Environ. 2020, 705, 135760. [Google Scholar] [CrossRef] [PubMed]
- OECD; Chopin, T. Integrated Multi-Trophic Aquaculture. 2010. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/advancing-the-aquaculture-agenda/integrated-multi-trophic-aquaculture_9789264088726-15-en (accessed on 22 September 2022).
- Camus, C.; Infante, J.; Buschmann, A.H. Revisiting the economic profitability of giant kelp Macrocystis pyrifera (Ochrophyta) cultivation in Chile. Aquaculture 2019, 502, 80–86. [Google Scholar] [CrossRef]
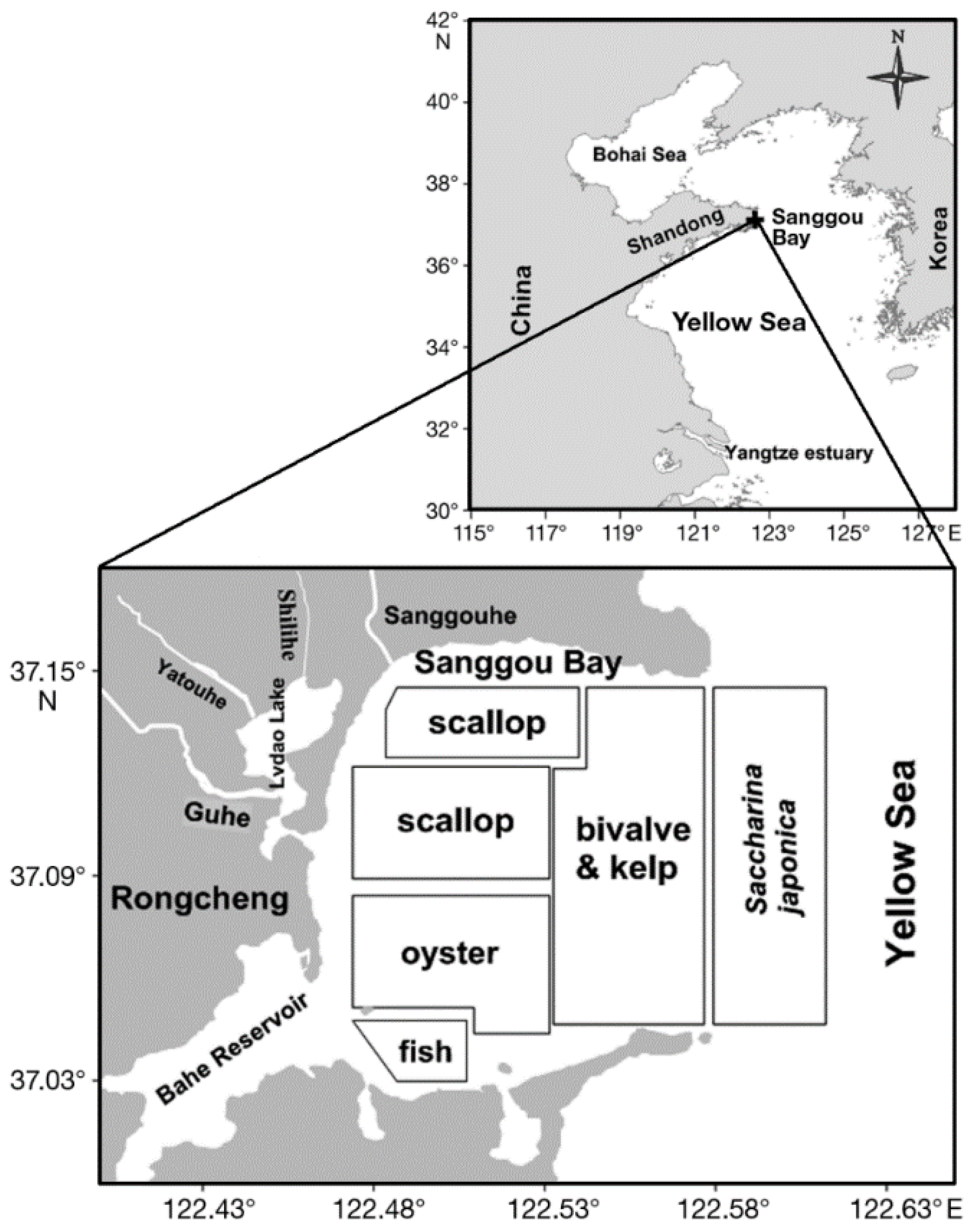
- Li, R.; Liu, S.; Zhang, J.; Jiang, Z.; Fang, J. Sources and export of nutrients associated with integrated multi-trophic aquaculture in Sanggou Bay, China. Aquac. Environ. Interact. 2016, 8, 285–309. [Google Scholar] [CrossRef] [Green Version]
- Yong, W.T.L.; Thien, V.Y.; Rupert, R.; Rodrigues, K.F. Seaweed: A potential climate change solution. Renew. Sustain. Energy Rev. 2022, 159, 112222. [Google Scholar] [CrossRef]
- Sanderson, J.C.; Dring, M.J.; Davidson, K.; Kelly, M.S. Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders adjacent to fish farm cages in northwest Scotland. Aquaculture 2012, 354–355, 128–135. [Google Scholar] [CrossRef]
- Nordvarg, L.; Johansson, T. The effects of fish farm effluents on the water quality in the Åland archipelago, Baltic Sea. Aquac. Eng. 2002, 25, 253–279. [Google Scholar] [CrossRef]
- Karakassis, I.; Pitta, P.; Krom, M.D. Contribution of fish farming to the nutrient loading of the Mediterranean. Sci. Mar. 2005, 69, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Gunn, K.; Stock-Williams, C. Quantifying the global wave power resource. Renew. Energy 2012, 44, 296–304. [Google Scholar] [CrossRef]
- IEA Electricity Consumption. Available online: https://www.iea.org/data-and-statistics?country=WORLD=Energyconsumption=Electricityconsumption (accessed on 22 September 2022).
- World Bank. World Bank: Technical Potential for Offshore Wind Worldwide Tops 15TW. Available online: https://ieefa.org/world-bank-technical-potential-for-offshore-wind-worldwide-tops-15tw/ (accessed on 22 September 2022).
- Graham, P.; Hayward, J.; Foster, J.; Havas, L. GenCost 2021-22: Final Report; CSIRO: Canberra, Australia, 2022.
- GWEC. Global Offshore Wind Report 2021. 2022. Available online: https://gwec.net/wp-content/uploads/2021/03/GWEC-Global-Wind-Report-2021.pdf (accessed on 22 September 2022).
- UNITED. 2020. Available online: https://www.h2020united.eu/ (accessed on 13 September 2022).
- Wier&Wind Wier and Wind. 2022. Available online: https://www.northseafarmers.org/projects/wier-en-wind (accessed on 31 May 2022).
- Wever, L.; Krause, G.; Buck, B.H. Lessons from stakeholder dialogues on marine aquaculture in offshore wind farms: Perceived potentials, constraints and research gaps. Mar. Policy 2015, 51, 251–259. [Google Scholar] [CrossRef]
- Buck, B.H.; Langan, R. Aquaculture Perspective of Multi-Use Sites in the Open Ocean: The Untapped Potential for Marine Resources in the Anthropocene; Springer Nature: Berlin, Germany, 2017; ISBN 9783319511597. [Google Scholar] [CrossRef] [Green Version]
- Heasman, K.G.; Scott, N.; Ericson, J.A.; Taylor, D.I.; Buck, B.H. Extending New Zealand’s Marine Shellfish Aquaculture Into Exposed Environments—Adapting to Modern Anthropogenic Challenges. Front. Mar. Sci. 2020, 7, 565686. [Google Scholar] [CrossRef]



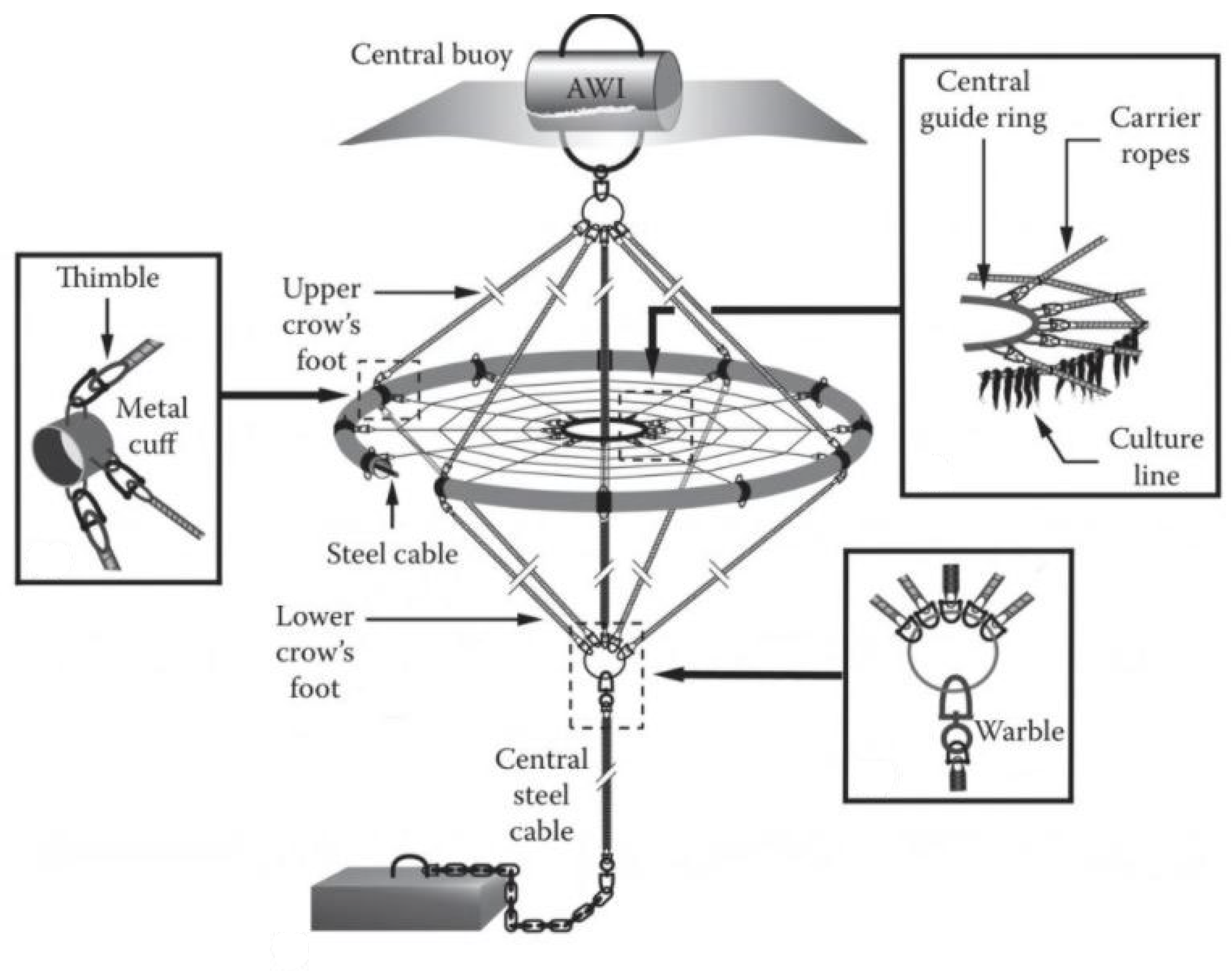
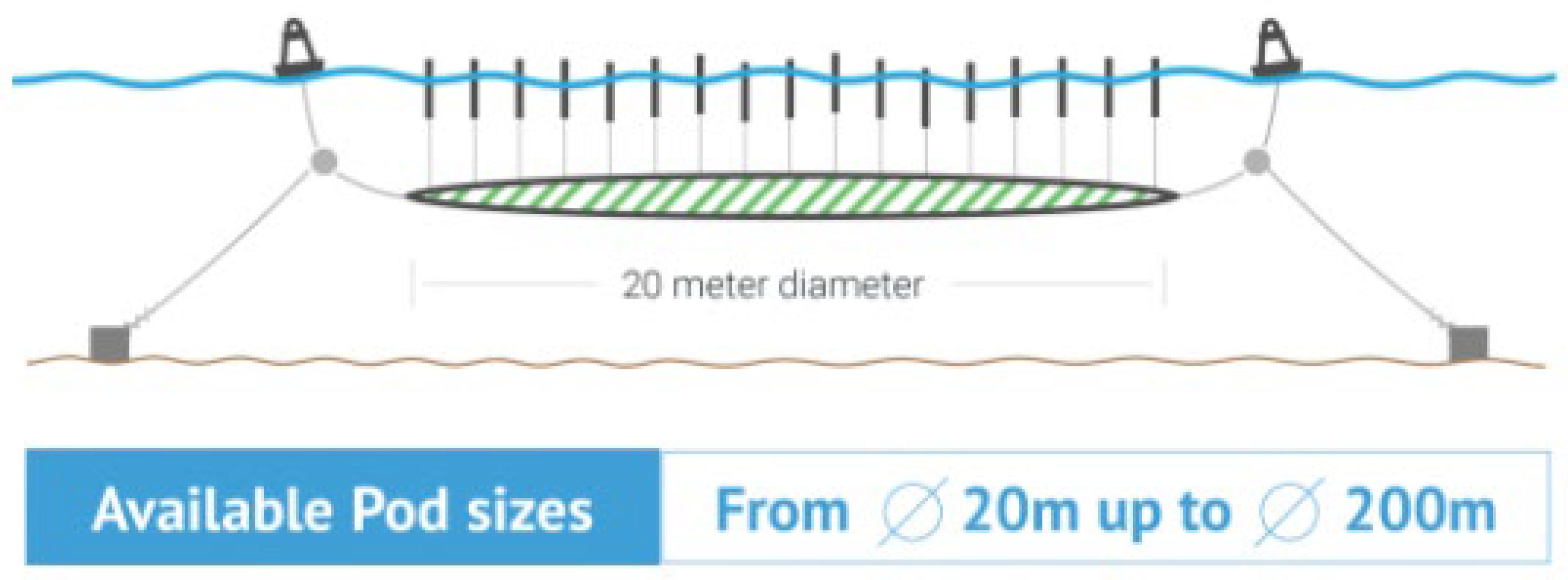
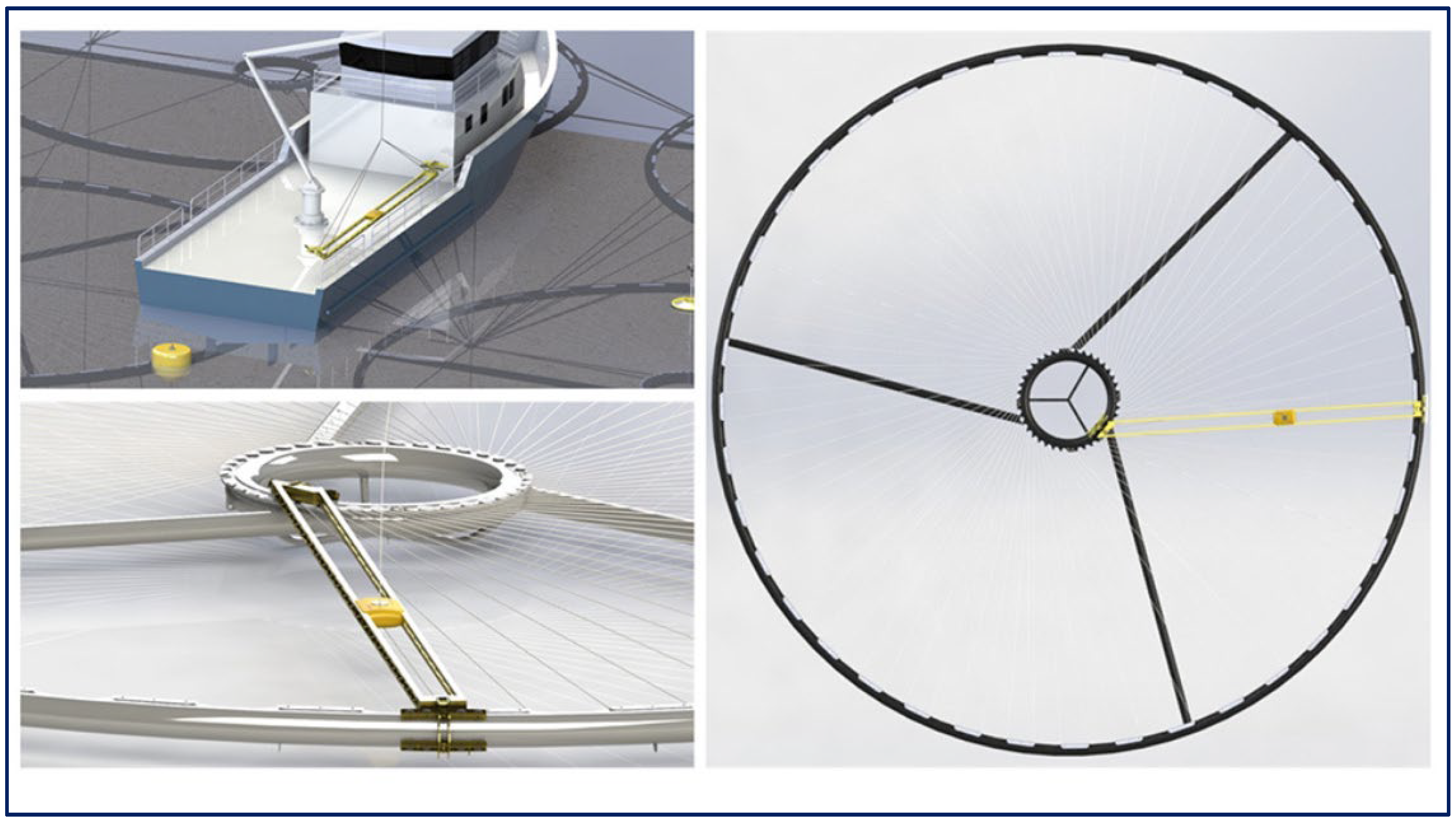
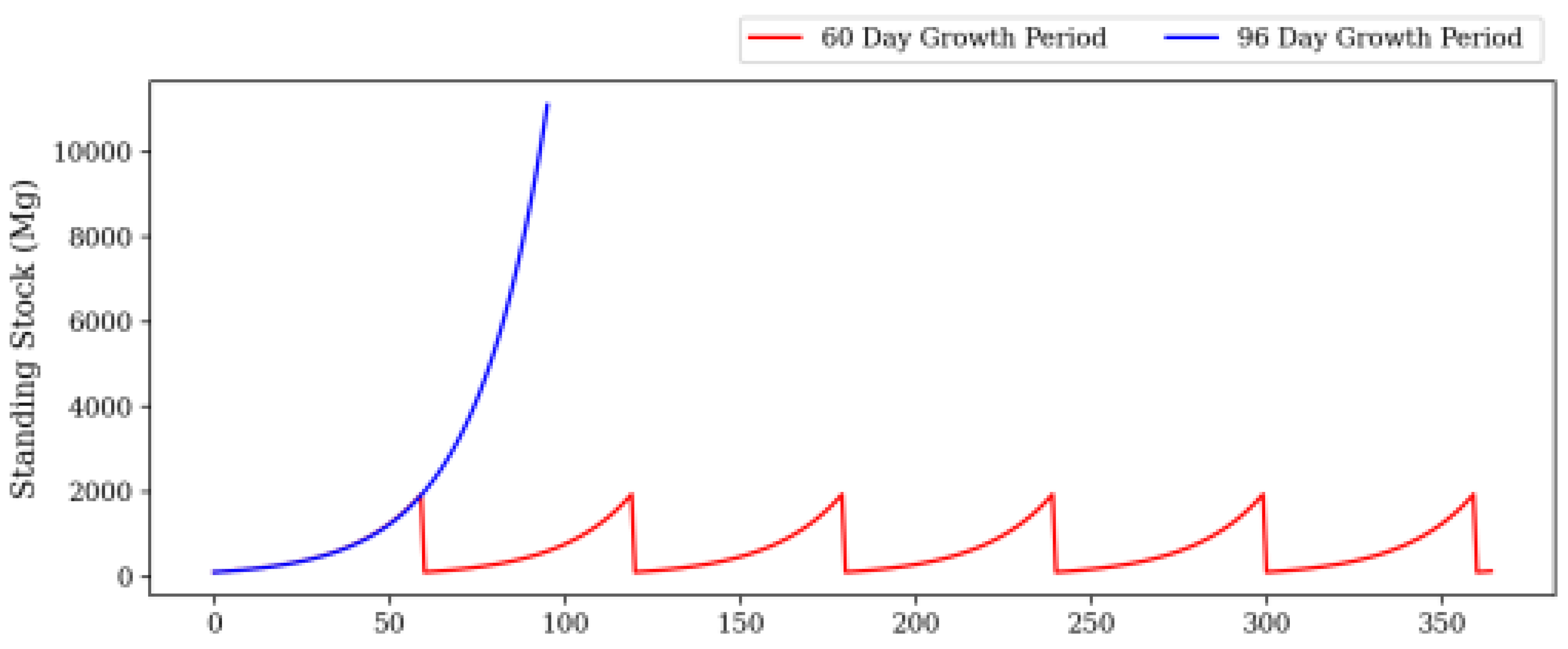

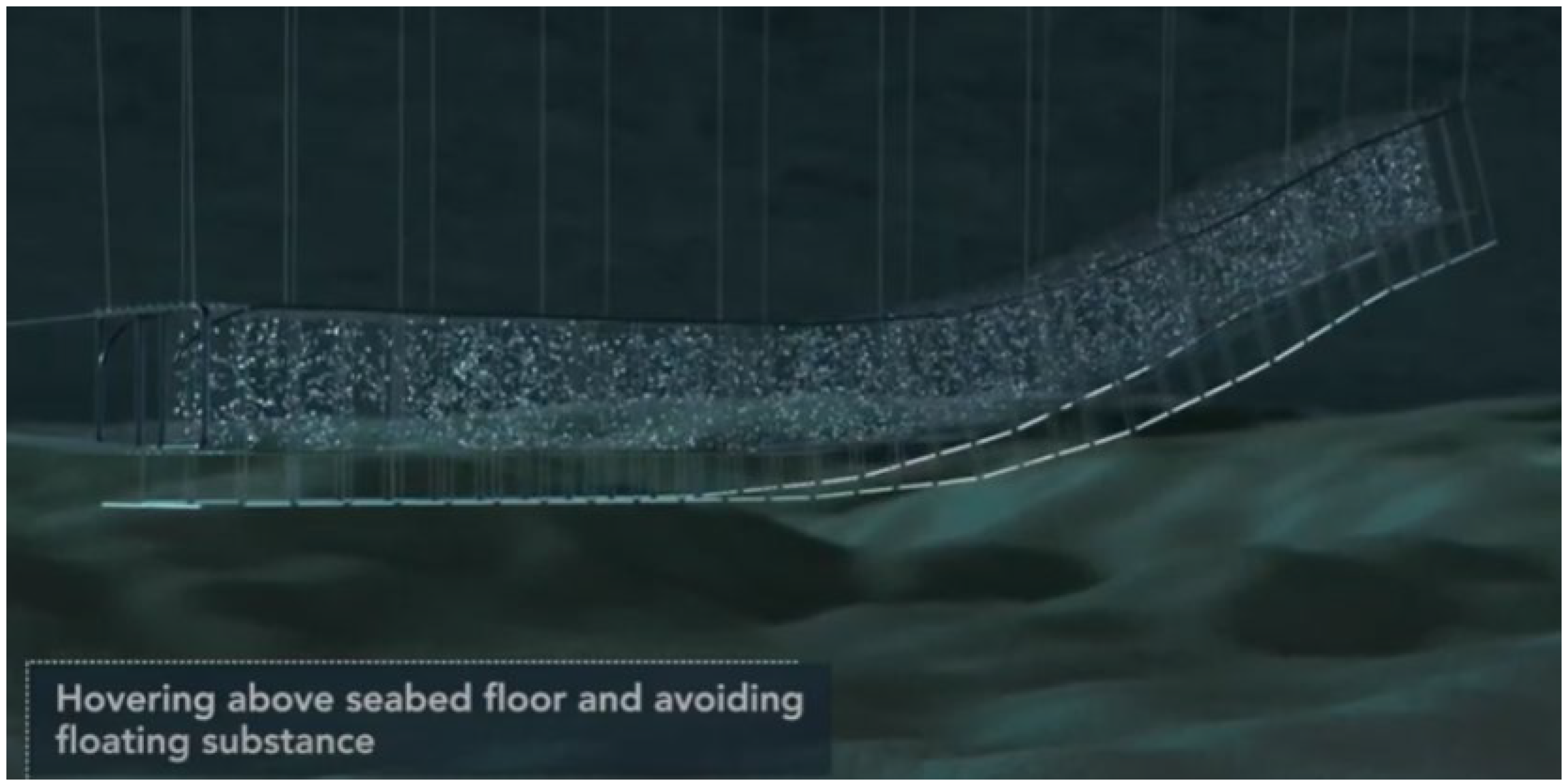

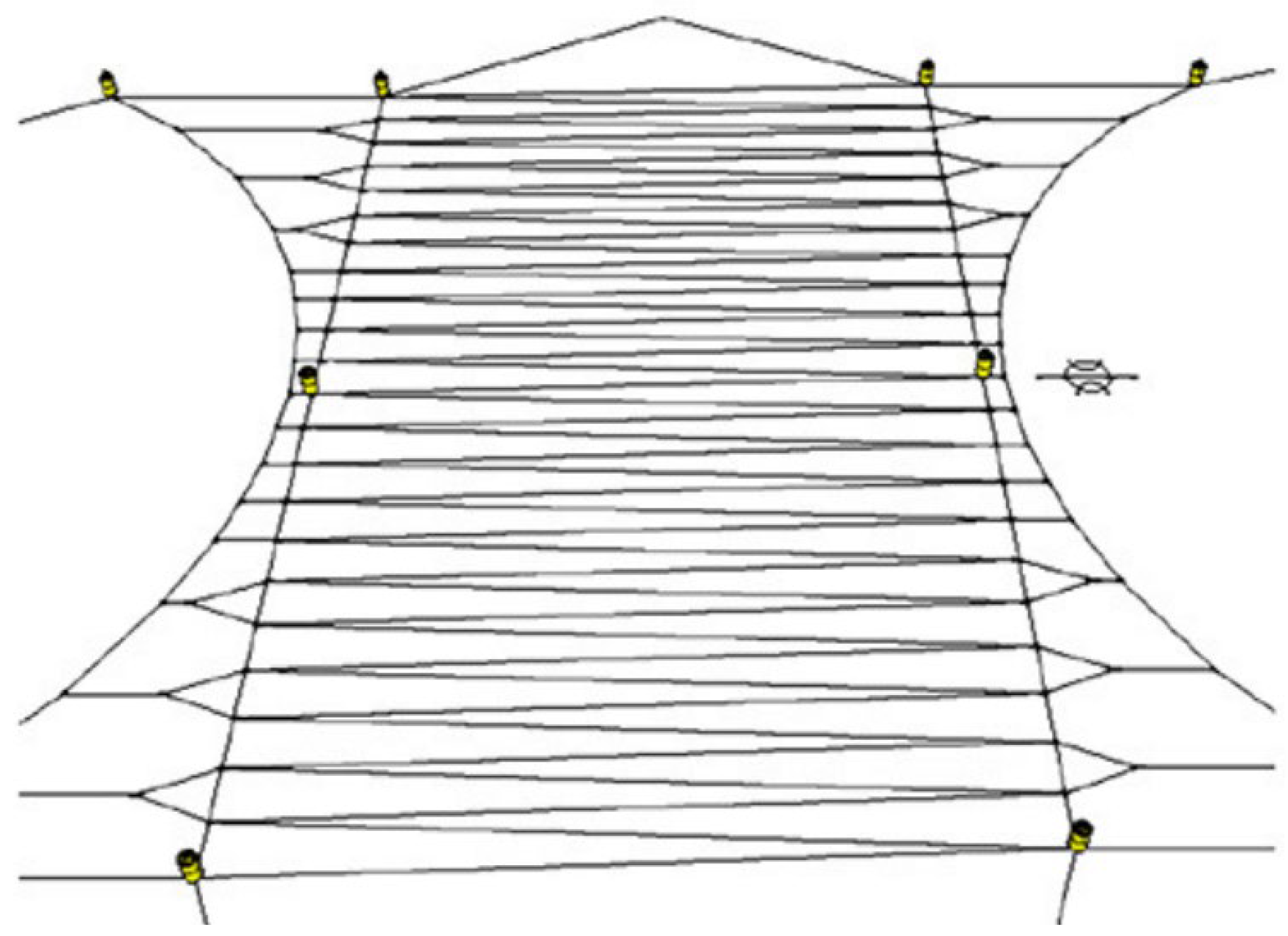




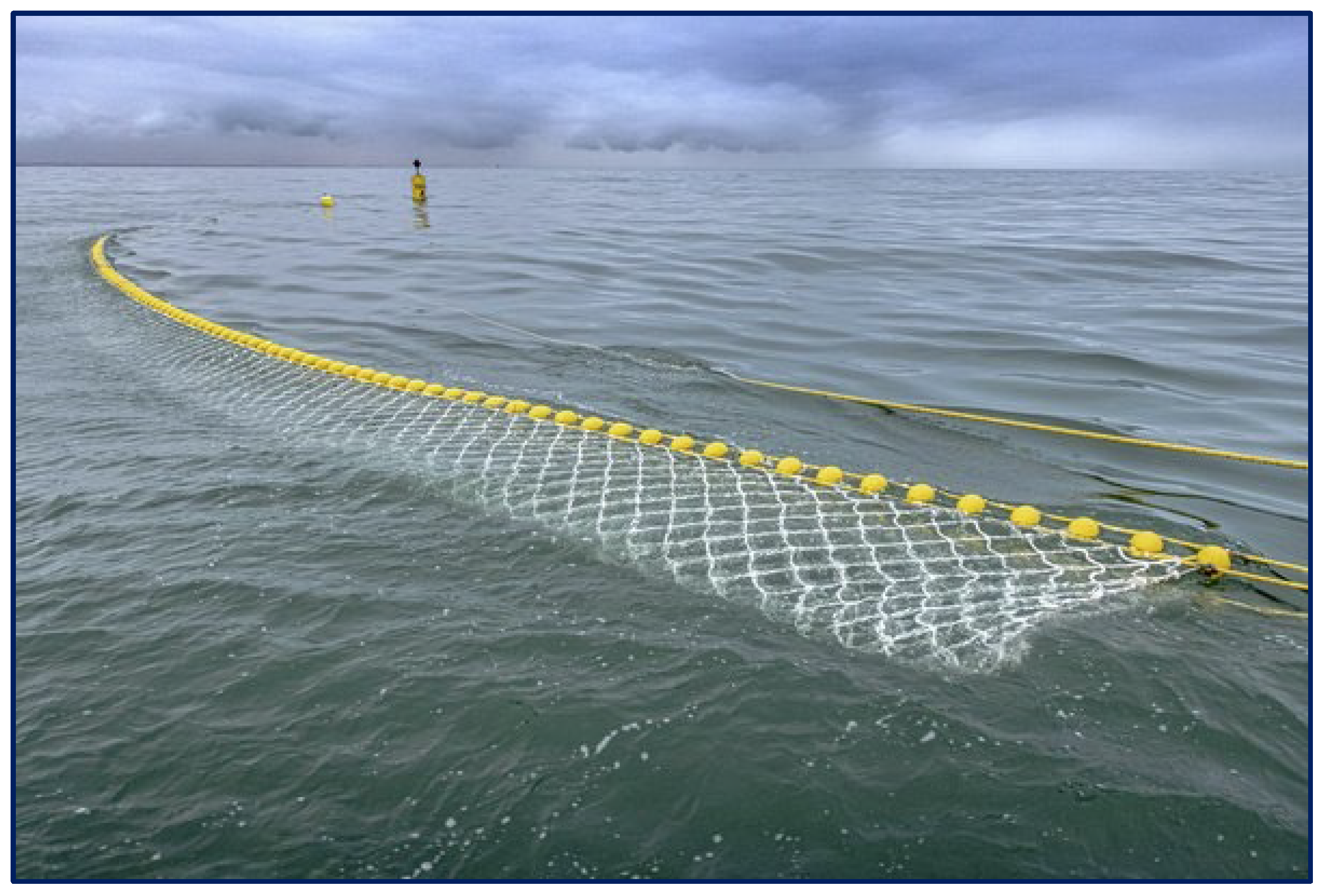




| Category | Water Depth (m) | Distance to Shore (NM) |
|---|---|---|
| Offshore | ≥50 m | >3 NM |
| <50 m | >3 NM | |
| Nearshore-exposed | ≥50 m | <3 NM |
| Nearshore-sheltered | <50 m | <3 NM |
| Reef Protected | <50 m | >3 NM |
| Species Group | Seaweed Colour | Biomass Load per Metre (Scale 1–3) | Tonnes (Wet) Annual Cultivation [3] | % World Market [3] | Buoyancy | Region | Applications | Cultivation Method(s) | Hydrodynamic Suitability (Scale 1–3) |
|---|---|---|---|---|---|---|---|---|---|
| Laminaria/Saccharina | Brown | 2—Moderate | 12,273,748 | 35.4% | Neutral/slightly negative | Temperate | Human consumption; Raw material for alginate, mannitol, iodine, Abalone feed | Longlines | 2—Moderate, grows in exposed water |
| Undaria (wakame) | Brown | 2—Moderate | 2,563,582 | 7.4% | Negative | Temperate | Sea mustard, Abalone Feed | Longlines | 1–2—Low–moderate, grows in exposed waters |
| Macrocystis pyrifera | Brown | 3—High | 2 | 0.0% | Positive | Temperate | Food and Cosmetic Products, Animal Feed | Longlines | 2—Moderate, grows in exposed waters |
| Sargassum | Brown | 3—High | 304,000 | 0.9% | Positive | Tropical | Food and Cosmetic Products, Animal Feed | Longlines | 2—Moderate, grows in exposed waters |
| Alaria esculenta | Brown | 105 | 0.0% | Temperate | Animal feed | Longlines | 2—Moderate, grows in exposed waters | ||
| Eckolonia, Lessonia | Brown | 2—Moderate | - | - | Negative | Temperate | Human consumption, fertiliser, animal feed (e.g., livestock, aquaculture), nutraceuticals, and biopolymers and bioplastics | Longlines | 2—Moderate, grows in exposed water |
| Durvillaea | Brown | 3—High | - | - | Negative | Temperate | Alginate industry, fertiliser | Longlines | 3—High, grows in very exposed waters |
| Kappaphycus/Eucheuma | Red | 2—Moderate | 11,622,213 | 33.5% | Negative | Tropical | For carrageenan extraction | Longlines/nets | 1–2—Low–moderate, grows in open water |
| Gracilaria | Red | 1—Low | 3,639,833 | 10.5% | Negative | Tropical | Feed for abalone; For agar extraction; Bioremediation | Longlines/nets | 1–2—Low–moderate, grows in exposed water |
| Porphyra | Red | 1—Low | 2,984,123 | 8.6% | - | Temperate | Food wrap | Nets | 1—Low–delicate |
| Asparagopsis | Red | 1—Low | - | - | - | Tropical/warm temperate | Animal feed | Longlines, net tubes | 1—Low–delicate, unsuitable for open waters |
| All Green | Green | 1—Low | 14,019 | 0.0% | Tropical/temperate | Human consumption | Land-based facilities | 1—Low–delicate | |
| TOTAL | 34,679,134 |
| Structure Name/Project Name | Origin Country, Location | Test Period | Site Category | Site Description | Size of Test Area (ha) | Aquaculture Output (Tonnes Ha−1 yr−1) | Yield (kg m−1 rope yr−1) | Species | Years Tested at Sea | Technically Viable | In Operation Today | Cost (US$) | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distance to Shore (km) | Location Depth (m) | Maximal Sign. Wave Height (m) | Maximal Current Speed (cm s−1) | |||||||||||||
| Marine Biomess Program | USA (California) | 1970–1983 | N.E. | ~1 | 0.48 | 300 # | MP | <1 | No | No | CAPEX: 570,000,000 OPEX: 61,400,000/yr. | Harger and Neushul (1983), Neushul (1987), Neushul et al. (1992) | ||||
| BAL’s cultivation grid (BAL) | Chile (Quenac, Caldera & Ancud) | 2010–2013 | N.E. | 60 * | 3 | 115 | 21 | 124 * | Mean 12.4 * | MP | 3 | Yes | No | CAPEX: 6000/ha OPEX: 8000/ha | Buschmann et al. (2014), Camus et al. (2018b) | |
| Offshore Ring System | Germany (North Sea) | 1995–2002 | O.S. | <5 | 14 * | 6.4 | 152 | 109 # tonnes dw yr−1 | SL | Yes | No | Buck and Buchholz (2004, 2005), Buck et al. (2004) | ||||
| A culture raft | Spain (Matalena) | 2000–2008 | N.S. | ~1 | ~20 * | 3 | 92 | 0.12 | 45.6 # | Max. 16 * | SL, UP | <1 | No | Peteiro et al. (2014, 2016) | ||
| H-frame structure using SPAR | The Netherlands (Texel) | 2011–2013 | O.S. | 12 | 22 * | 8 | 0.04 | SL, LD | <1 | No | Pers. Comm. Hortimare 2018, The North Sea Farm Foundation (2018) | |||||
| Tension-Leg Platform (TLP) | Republic of Korea (Jeju Island) | 2010–2012 | O/N.E. | 4 | 300 # | Max. 80.6 | SJ | 2 | Yes | 0.5 million/ha | Chung et al. (2015) | |||||
| Seaweed Carrier | Norway Trondheim | 2009 | N.E./N.S. | SL | Yes | No | Seaweed Energy Solutions (2018) | |||||||||
| MacroAlgal Cultivation Rig (MACR) | The Faroe Islands (Funningsfjrdur) | 2010 | N.E. | 0.5 | 70 * 200 # | 4 * 6 # | 25 | 9 | 35 * | Mean 6 */58 | SL, AE, LD | 8 | Yes | Yes | CAPEX: 13,364/ha OPEX: 10,676/ha | Bak et al. (2018) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tullberg, R.M.; Nguyen, H.P.; Wang, C.M. Review of the Status and Developments in Seaweed Farming Infrastructure. J. Mar. Sci. Eng. 2022, 10, 1447. https://doi.org/10.3390/jmse10101447
Tullberg RM, Nguyen HP, Wang CM. Review of the Status and Developments in Seaweed Farming Infrastructure. Journal of Marine Science and Engineering. 2022; 10(10):1447. https://doi.org/10.3390/jmse10101447
Chicago/Turabian StyleTullberg, Robert Maxwell, Huu Phu Nguyen, and Chien Ming Wang. 2022. "Review of the Status and Developments in Seaweed Farming Infrastructure" Journal of Marine Science and Engineering 10, no. 10: 1447. https://doi.org/10.3390/jmse10101447
APA StyleTullberg, R. M., Nguyen, H. P., & Wang, C. M. (2022). Review of the Status and Developments in Seaweed Farming Infrastructure. Journal of Marine Science and Engineering, 10(10), 1447. https://doi.org/10.3390/jmse10101447








