Impacts of Mesoscale Eddies on Biogeochemical Variables in the Northwest Pacific
Abstract
:1. Introduction
2. Materials and Methods
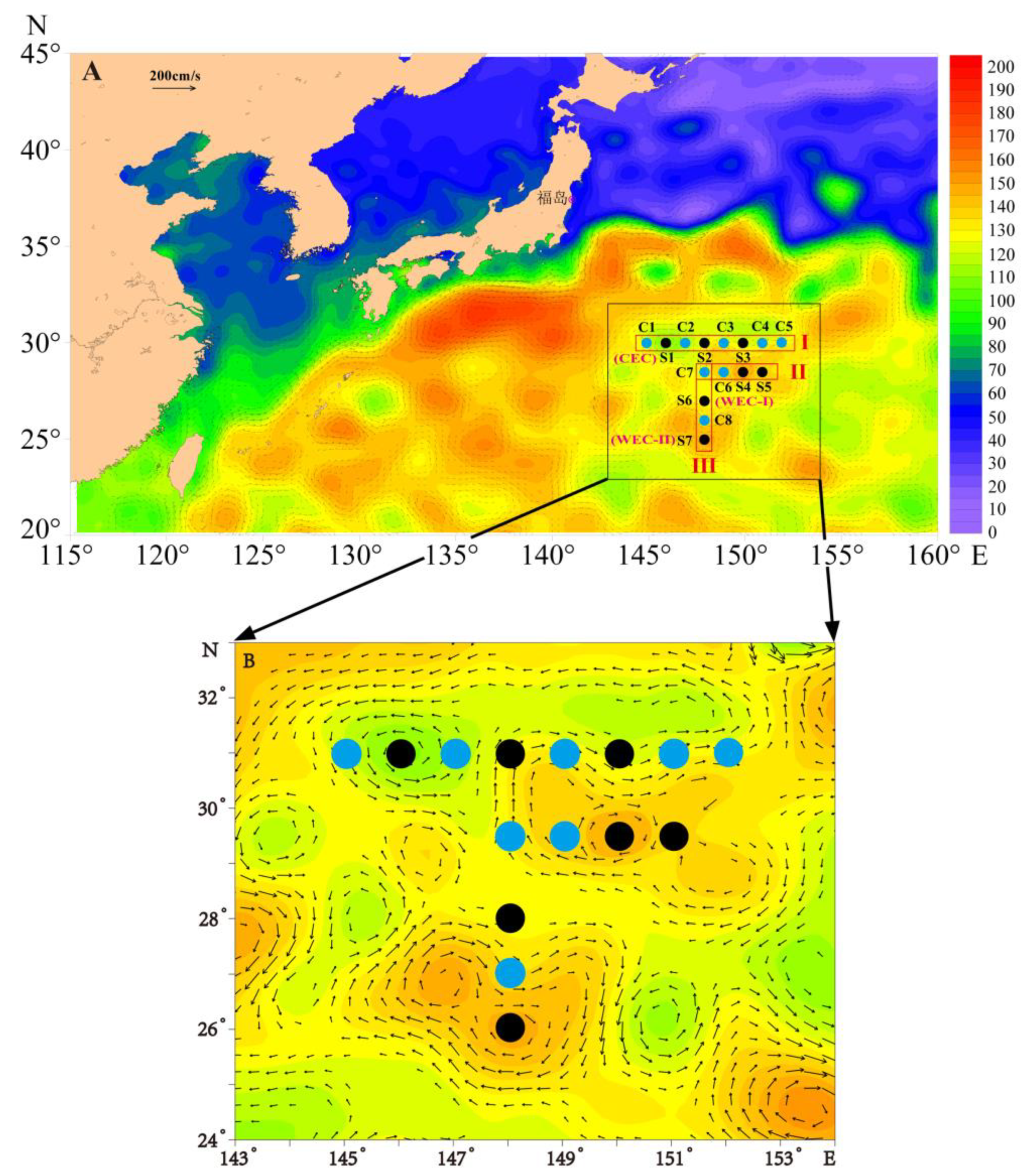
2.1. Sampling Dates and Stations
2.2. Sampling Procedures and Analytical Methods
3. Results
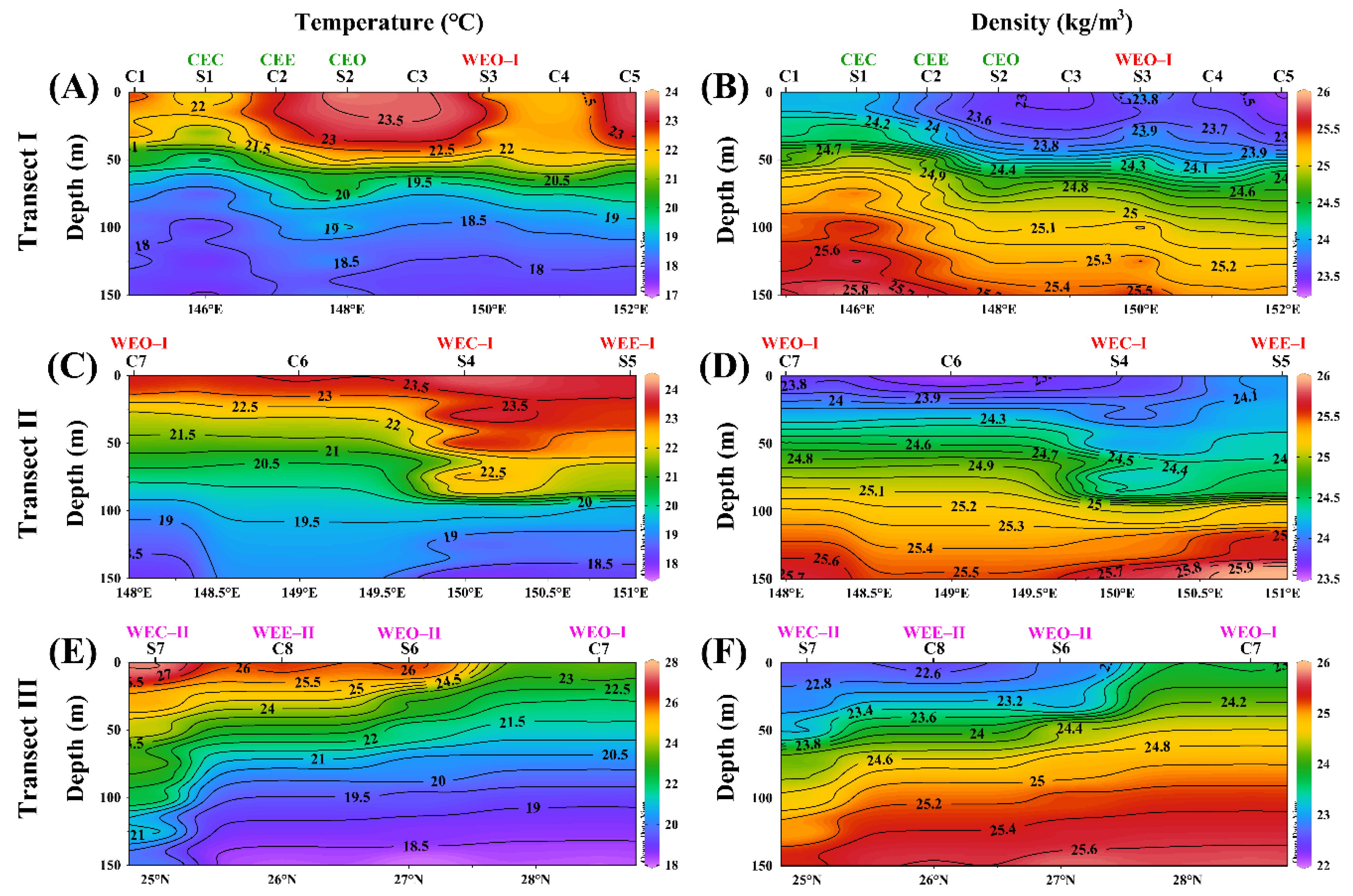
3.1. The Cold and Warm Eddy Characteristics
3.2. Biogeochemical Variables in Different Eddies
3.2.1. Comparisons of Stations
3.2.2. Cold Eddies
3.2.3. Warm Eddies
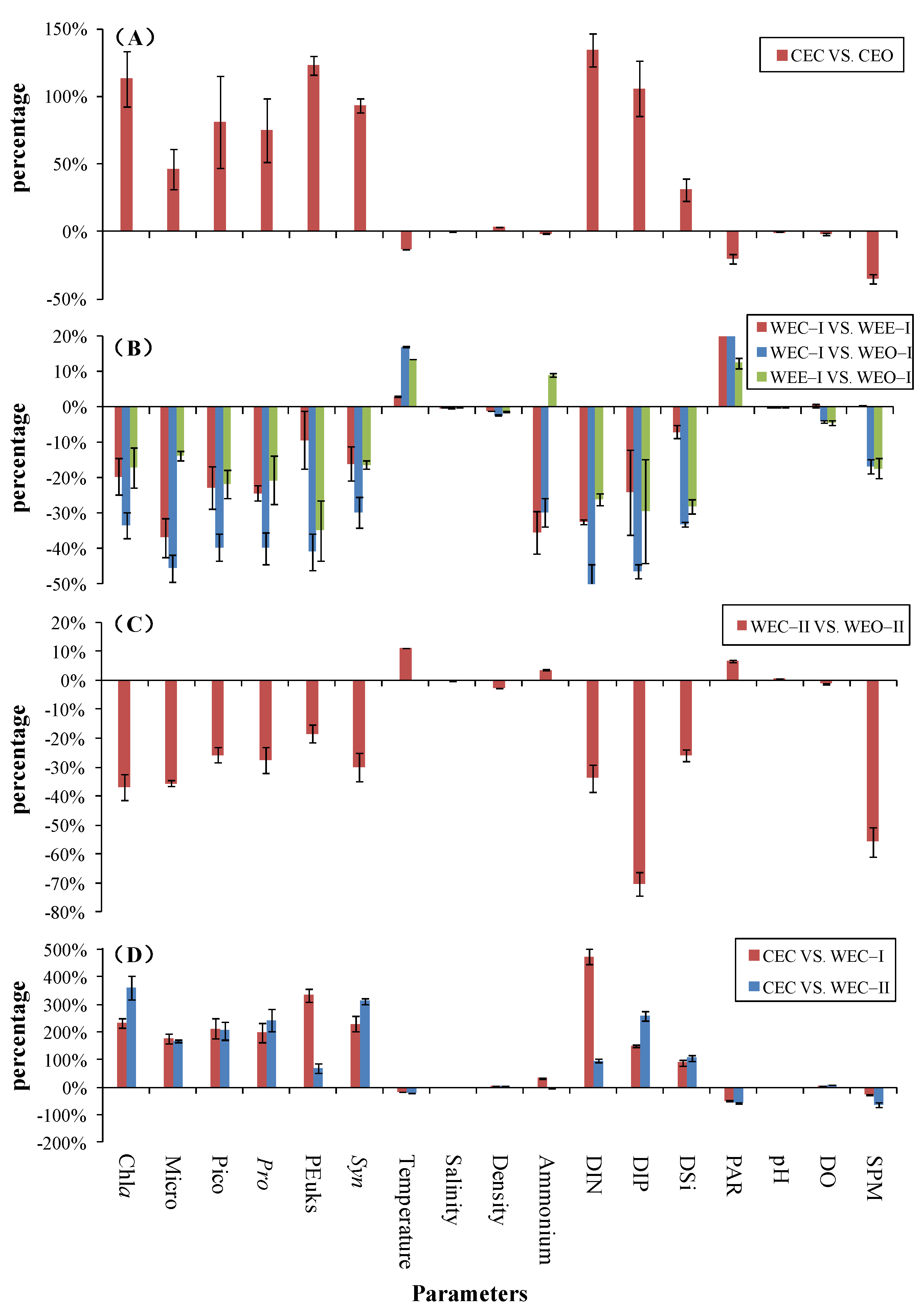
3.2.4. Comparisons between Cold and Warm Eddies
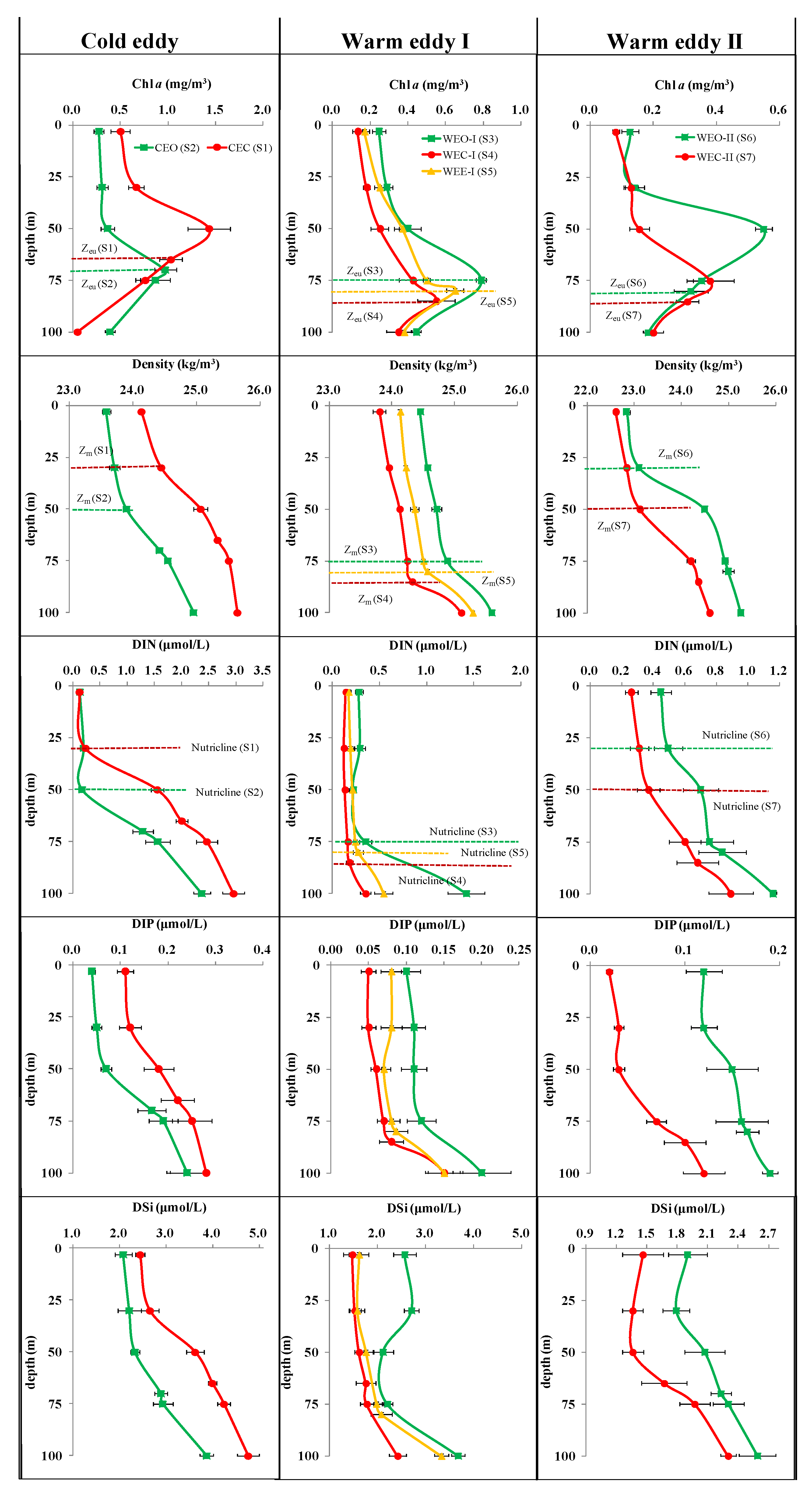
3.3. Vertical Distribution in Different Eddies
3.3.1. Biogeochemical Variables
3.3.2. Phytoplankton Community
3.4. Relationship between Biological and Physicochemical Factors
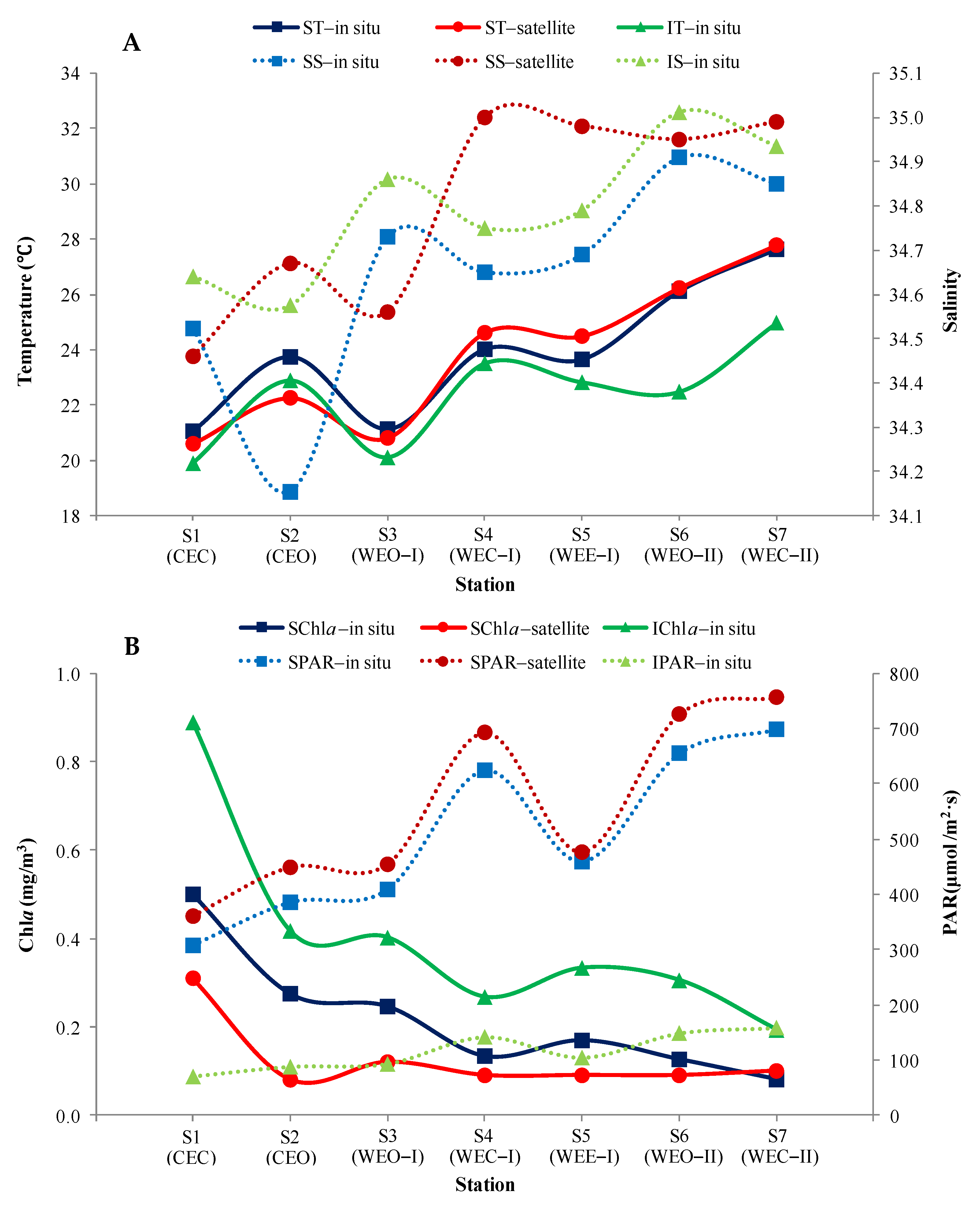
3.5. Comparison between Satellite and In Situ Observations
4. Discussion
4.1. Response of Geochemical Variables to Cold and Warm Eddies
4.2. Response of Biological Variables to Cold and Warm Eddies
4.2.1. Response of Chla to Eddies
4.2.2. Response of Phytoplankton to Eddies
4.3. Main Physicochemical Factors Affecting Biological Variables
4.3.1. Nutrient Concentrations
4.3.2. Nutrient Stoichiometry
4.3.3. Photosynthetically Active Radiation
5. Conclusions
- (1)
- Temperature, ammonium, DO, PAR and SPM decreased by 1–35%, while most of the biological variables (Chla, Micro, Pico, Pro, Syn, PEuks) and geochemical variables (salinity, density, DIN, DIP, DSi) increased by 0.2–134% in the CEC compared to the CEO. In contrast, WEC–I and WEC–II showed an increasing percentage of 7–53% in temperature and PAR and a decrease in percentage of 0.2–70% in biological variables (Chla, Micro, Pico, Pro, Syn, PEuks) and other geochemical variables (salinity, DIN, DIP, DSi, DO, SPM) compared to WEO–I and WEO–II. The magnitude of variation between WEC and WEO were not as pronounced as that between CEC and CEO.
- (2)
- The cold and warm eddies were able to force the DCM to rise or drop with pycnocline, nutricline and Zeu as a whole. In particular, cold eddies with a raised thermocline could lead to the DCM uplift by 20 m and enhance phytoplankton biomass when the nutricline and thermocline were coincident. Furthermore, warm eddies drove isopycnals downward, resulting in a 10–25 m drop in DCM and a decrease in nutrient and Chla concentrations at the WEC.
- (3)
- In terms of the depth of the maximum abundance of Micro and Pico, there were significant differences between the center and outside of the eddy, but in terms of the overall vertical structure, both in the cold and warm eddies, Pro and PEuks maintained a unimodal pattern basically consistent with Chla, Syn showed a surface or subsurface maximum type, while multiple types of Micro existed. The alteration of nutricline was usually deepened by downward movement of the warm eddy or was shoaled by upward movement of the cold eddy. The vertical shift of nutricline could enhance or reduce the dominance of Pico by making eutrophic water more oligotrophic or oligotrophic water more eutrophic.
- (4)
- The significant difference in the vertical structure of the phytoplankton community between the center and outside of the eddy might be explained by the direct influence of both nutrient concentrations and stoichiometry. The contribution of Micro to total biomass was much smaller than that of Pico in oligotrophic regions where the DIN:DIP and DSi:DIN ratios were exceedingly low. PAR was not the main factor controlling phytoplankton biomass and abundance in the eddies attributed to Zeu being consistently deeper than Zm, but it might be a key limiting factor affecting the vertical distribution of the phytoplankton community.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mcgillicuddy, D.J., Jr.; Robinson, A.R.; Siegel, D.A.; Jannasch, H.W.; Johnson, R.; Dickey, T.D. Influence of mesoscale eddies on new production in the sargasso sea. Nature 1998, 394, 263–266. [Google Scholar] [CrossRef]
- Aguilar–Islas, A.M.; Rember, R.; Nishino, S.; Kikuchi, T.; Itoh, M. Partitioning and lateral transport of iron to the canada basin. Polar. Sci. 2013, 7, 82–99. [Google Scholar] [CrossRef] [Green Version]
- Mcgillicuddy, D.J.; Johnson, R.; Siegel, D.A.; Michaels, A.F.; Bates, N.R.; Knap, A.H. Mesoscale variations of biogeochemical properties in the sargasso sea. J. Geophys. Res.-Oceans 1999, 104, 13381–13394. [Google Scholar] [CrossRef] [Green Version]
- Yun, M.S.; Kim, B.K.; Joo, H.T.; Yang, E.J.; Nishino, S.; Chung, K.H.; Kang, S.H.; Lee, S.H. Regional productivity of phytoplankton in the western Arctic Ocean during early summer in 2010. Deep-Sea Res. Pt. II 2015, 120, 61–71. [Google Scholar] [CrossRef]
- Mcgillicuddy, D.J.; Anderson, L.A.; Doney, S.C.; Maltrud, M.E. Eddy-driven sources and sinks of nutrients in the upper ocean: Results from a 0.1 resolution model of the north atlantic. Global Biogeochem. Cycles 2003, 17, 14–204. [Google Scholar] [CrossRef]
- Wang, J.X.; Tan, Y.H.; Huang, L.M.; Ke, Z.X.; Tan, J.Y.; Hu, Z.F.; Wang, Q. Response of picophytoplankton to a warm eddy in the northern south china sea. Oceanol. Hydrobiol. Stud. 2016, 45, 145–158. [Google Scholar] [CrossRef]
- Chelton, D.B.; Gaube, P.; Schlax, M.G.; Early, J.J.; Samelson, R.M. The influence of nonlinear mesoscale eddies on near—Surface oceanic chlorophyll. Science 2011, 334, 328–332. [Google Scholar] [CrossRef]
- Chang, Y.L.; Miyazawa, Y.; Oey, L.Y.; Kodaira, T.; Huang, S. The formation processes of phytoplankton growth and decline in mesoscale eddies in the western north pacific ocean. J. Geophys. Res. Oceans 2017, 122, 4444–4455. [Google Scholar] [CrossRef]
- Polovina, J.J.; Howell, E.A.; Kobayashi, D.R.; Seki, M.P. The transition zone chlorophyll front updated: Advances from a decade of research. Prog. Oceanogr. 2017, 150, 79–85. [Google Scholar] [CrossRef]
- Perruche, C.; Rivière, P.; Lapeyre, G.; Carton, X.; Pondaven, P. Effects of surface quasi-geostrophic turbulence on phytoplankton competition and coexistence. J. Mar. Res. 2011, 69, 105–135. [Google Scholar] [CrossRef]
- Clayton, S.; Dutkiewicz, S.; Jahn, O.; Follows, M.J. Dispersal, eddies, and the diversity of marine phytoplankton. Limnol. Oceanogr. 2013, 3, 182–197. [Google Scholar] [CrossRef]
- Gaube, P.; Chelton, D.B.; Strutton, P.G.; Behrenfeld, M.J. Satellite observations of chlorophyll, phytoplankton biomass, and ekman pumping in nonlinear mesoscale eddies. J. Geophys. Res.-Oceans. 2013, 118, 6349–6370. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.Q.; Hu, J.; Xu, H.Z.; Cao, Z.; Wang, D.X. Phytoplankton community at warm eddies in the northern South China Sea in winter 2003/2004. Deep-Sea Res. II 2010, 57, 1792–1798. [Google Scholar] [CrossRef]
- Sweeney, E.N.; McGillicuddy, D.J.; Buesseler, K.O. Biogeochemical impacts due to mesoscale eddy activity in the Sargasso Sea as measured at the Bermuda Atlantic Time-Series Study (BATS). Deep-Sea Res. II 2003, 50, 3017–3039. [Google Scholar] [CrossRef]
- Chen, Y.L.L.; Chen, H.Y.; Lin, I.I.; Lee, M.A.; Chang, J. Effects of cold eddy on phytoplankton production and assemblages in luzon strait bordering the south china sea. J. Oceanogr. 2007, 63, 671–683. [Google Scholar] [CrossRef]
- Brown, S.L.; Landry, M.R.; Selph, K.E.; Yang, E.J.; Rii, Y.M.; Bidigare, R.R. Diatoms in the desert: Plankton community response to a mesoscale eddy in the subtropical north pacific. Deep-Sea Res. Pt. II 2008, 55, 1321–1333. [Google Scholar] [CrossRef]
- Mcgillicuddy, D.J.; Anderson, L.A.; Bates, N.R.; Bibby, T.; Buesseler, K.O.; Carlson, C.A. Eddy/wind interactions stimulate extraordinary mid-ocean plankton blooms. Science 2007, 316, 1021–1026. [Google Scholar] [CrossRef]
- Kouketsu, S.; Kaneko, H.; Okunishi, T.; Sasaoka, K.; Itoh, S.; Inoue, R. Mesoscale eddy effects on temporal variability of surface chlorophyll a in the kuroshio extension. J. Oceanog. 2016, 72, 439–451. [Google Scholar] [CrossRef]
- Yasuda, I.; Watanabe, T. Chlorophyll a variation in the kuroshio extension revealed with a mixed-layer tracking float: Implication on the long-term change of pacific saury (cololabis saira). Fish. Oceanogr. 2010, 6, 482–488. [Google Scholar] [CrossRef]
- Itoh, S.; Yasuda, I.; Saito, H.; Tsuda, A.; Komastu, K. Mixed layer depth and chlorophyll a: Profiling float observations in the Kuroshio–Oyashio Extension region. J. Mar. Syst. 2015, 151, 1–14. [Google Scholar] [CrossRef]
- Longhurst, A.R. Ecological Geography of the Sea, 2nd ed.; Academic Press: Oxford, UK, 2007; pp. 1–527. [Google Scholar] [CrossRef]
- Lin, P.; Chai, F.; Xue, H.; Xiu, P. Modulation of decadal oscillation on surface chlorophyll in the kuroshio extension. J. Geophys. Res.-Oceans 2014, 119, 187–199. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, X.H.; Chen, Y.; Chu, Q.; Yang, Y.; Shi, J.H.; Gao, H.W. Variations in the phytoplankton community due to dust additions in eutrophication, LNLC and HNLC oceanic zones. Sci. Total Environ. 2019, 669, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.J.; Dong, C.M.; Liu, Y.; Gaube, P.; Yang, J.S. Chlorophyll rings around ocean eddies in the North Pacific. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryther, J.H.; Menzel, D.W. Light Adaptation by Marine Phytoplankton. Limnol. Oceanogr. 1959, 4, 492–497. [Google Scholar] [CrossRef]
- Yentsch, C.S.; Menzel, D.W. A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep Sea. Res. Oceanogr. Abstr. 1963, 10, 221–231. [Google Scholar] [CrossRef]
- Sukhanova, Z.N. Settling without inverted microscope, 97. In Phytoplankton Manual; Sourlna, A., Ed.; Unesco: Paris, France, 1978. [Google Scholar]
- Edler, L.; Elbrächter, M. The Utermöhl method for quantitative phytoplankton analysis, 13–20. In Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; Karlson, B., Cusack, C., Bresnan, E., Eds.; Unesco: Paris, France, 2010. [Google Scholar]
- Tomas, C.R. Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA, 1997; pp. 1–858. [Google Scholar]
- Hasle, G.R.; Syvertsen, E.E.; Steidinger, K.A.; Tangen, K.; Tomas, C.R. Identifying Marine Diatoms and Dinoflagellates; Academic Press: San Diego, CA, USA, 1996; pp. 5–385. [Google Scholar]
- Horner, R.A. A Taxonomic Guide to Some Common Marine Phytoplankton; Biopress: Bristol, UK, 2002. [Google Scholar]
- Jiao, N.Z.; Yang, Y.H.; Hong, N.; Ma, Y.; Harada, S.; Koshikawa, H.; Watanabe, M. Dynamics of autotrophic picoplankton and heterotrophic bacteria in the east china sea. Cont. Shelf. Res. 2005, 25, 1265–1279. [Google Scholar] [CrossRef]
- Bertilsson, S.; Berglund, O.; Karl, D.M.; Chisholm, S.W. Elemental composition of marine prochlorococcus and synechococcus: Implications for the ecological stoichiometry of the sea. Limnol. Oceanogr. 2003, 48, 1721–1731. [Google Scholar] [CrossRef]
- GB/T 12763.4–2007; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Specifications for Oceanographic Survey-Part 4: Survey of Chemical Parameters in Sea Water. China Standard Press: Beijing, China, 2008; pp. 1–65. (In Chinese)
- Pujol, M.I.; Faugère, Y.; Taburet, G.; Dupuy, S.; Pelloquin, C.; Ablain, M. DUACS DT2014: The new multi-mission altimeter data set reprocessed over 20 years. Ocean Sci. 2016, 12, 1067–1090. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.M.; McCarthy, J.J.; Joyce, T.M.; Ducklow, H.W. Enhanced near-surface nutrient availability and new production resulting from the frictional decay of a gulf stream warm-core ring. Deep-Sea Res. Pt. I 1989, 36, 705–714. [Google Scholar] [CrossRef]
- Mahaffey, C.; Benitez-Nelson, C.R.; Bidigare, R.R.; Rii, Y.; Karl, D.M. Nitrogen dynamics within a wind-driven eddy. Deep-Sea Res. Pt. II 2008, 55, 1398–1411. [Google Scholar] [CrossRef]
- Vaillancourt, R.D.; Marra, J.; Seki, M.P.; Parsons, M.L.; Bidigare, R.R. Impact of a cyclonic eddy on phytoplankton community structure and photosynthetic competency in the subtropical north pacific ocean. Deep-Sea Res. Pt. I 2003, 50, 829–847. [Google Scholar] [CrossRef]
- Thompson, P.A.; Pesant, S.; Waite, A.M. Contrasting the vertical differences in the phytoplankton biology of a dipole pair of eddies in the south-eastern indian ocean. Deep-Sea Res. Pt. II 2007, 54, 1003–1028. [Google Scholar] [CrossRef]
- Seki, M.P.; Polovina, J.J.; Brainard, R.E.; Bidigare, R.R.; Leonard, C.L.; Foley, D.G. Biological enhancement at cyclonic eddies tracked with goes thermal imagery in hawaiian waters. Geophys. Res. Lett. 2001, 28, 1583–1586. [Google Scholar] [CrossRef] [Green Version]
- Olaizola, M.; Ziemann, D.A.; Bienfang, P.K.; Walsh, W.A.; Conquest, L.D. Eddy-induced oscillations of the pycnocline affect the floristic composition and depth distribution of phytoplankton in the subtropical Pacific. Mar. Biol. 1993, 116, 533–542. [Google Scholar] [CrossRef]
- Landry, M.R.; Brown, S.L.; Rii, Y.M.; Selph, K.E.; Bidigare, R.R.; Yang, E.J. Depth-stratified phytoplankton dynamics in cyclone opal, a subtropical mesoscale eddy. Deep-Sea Res. Pt. II 2008, 55, 1348–1359. [Google Scholar] [CrossRef]
- Nishino, S.; Itoh, M.; Kawaguchi, Y.; Kikuchi, T.; Aoyama, M. Impact of an unusually large warm-core eddy on distributions of nutrients and phytoplankton in the southwestern canada basin during late summer/early fall 2010. Geophys. Res. Lett. 2011, 38, L16602. [Google Scholar] [CrossRef] [Green Version]
- Casey, J.R.; Lomas, M.W.; Mandecki, J.; Walker, D.E. Prochlorococcus contributes to new production in the sargasso sea deep chlorophyll maximum. Geophys. Res. Lett. 2007, 34, L10604. [Google Scholar] [CrossRef] [Green Version]
- Ning, X.R.; Chai, F.; Xue, H.; Cai, Y.; Liu, C.; Shi, J. Physical-biological oceanographic coupling influencing phytoplankton and primary production in the south china sea. J. Geophys. Res. 2004, 109, C10005. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chang, J.; Tseng, C.M.; Wen, L.S.; Liu, K.K. Seasonal variability of picoplankton in the northern south china sea at the seats station. Deep-Sea Res. Pt. II 2007, 54, 1602–1616. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Song, S.; Huang, B.; Sun, J.; Liu, H. Comparisons of picophytoplankton abundance, size, and fluorescence between summer and winter in northern south china sea. Cont. Shelf. Res. 2011, 31, 1527–1540. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Huang, D.Y.; Zhang, G.C.; Zhao, Y.Y.; Sun, J. Biogeographic variations of picophytoplankton in three contrasting seas: The Bay of Bengal, South China Sea and Western Pacific Ocean. Aquat. Microb. Ecol. 2020, 84, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.L.; Chen, H.Y.; Jan, S.; Lin, Y.H.; Kuo, T.H.; Hung, J.J. Biologically active warm- core anticyclonic eddies in the marginal seas of the western pacific ocean. Deep-Sea Res. Pt. I. 2015, 106, 68–84. [Google Scholar] [CrossRef]
- Oschlies, A. Can eddies make ocean deserts bloom? Global. Biogeochem. Cy. 2002, 16, 53-1–53-11. [Google Scholar] [CrossRef] [Green Version]
- Sarma, V.V.S.S.; Chopra, M.; Rao, D.N.; Priya, M.M.R.; Rao, V.D. Role of eddies on controlling total and size-fractionated primary production in the bay of bengal. Cont. Shelf. Res. 2020, 204, 104186. [Google Scholar] [CrossRef]
- Moore, L.R.; Goericke, R.; Chisholm, S.W. Comparative physiology of Synechococcus and Prochlorococcus: Influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar. Ecol-Prog. Ser. 1995, 116, 259–276. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Z.; Liu, Q.X.; Ye, F.; Tian, L.; Huang, X.P. Spatial and temporal variations of picoplankton in three contrasting periods in the Pearl River Estuary, South China. Cont. Shelf. Res. 2013, 56, 29–39. [Google Scholar] [CrossRef]
- Kang, J.H.; Liang, Q.Y.; Wang, J.J.; Lin, Y.L.; He, X.B.; Xia, Z. Size structure of biomass and primary production of phytoplankton: Environmental impact analysis in the Dongsha natural gas hydrate zone, northern South China Sea. Acta Oceanol. Sin. 2018, 37, 97–107. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.K.; Youn, S.; Roh, S. Influence of the physical forcing of different water masses on the spatial and temporal distributions of picophytoplankton in the northern east china sea. Cont. Shelf. Res. 2014, 88, 216–227. [Google Scholar] [CrossRef]
- Boyd, P.W.; Wong, C.S.; Merrill, J.; Whitney, F.; Snow, J.; Harrison, P.J.; Gower, J. Atmospheric iron supply and enhanced vertical carbon flux in the NE subarctic Pacific: Is there a connection? Glob. Biogeochem. Cycles 1998, 12, 429–441. [Google Scholar] [CrossRef]
- Coale, K.H.; Johnson, K.S.; Chavez, F.P.; Buesseler, K.O.; Barber, R.T.; Brzezinski, M.A.; Cochlan, W.P.; Millero, F.J.; Falkowski, P.G.; Bauer, J.E.; et al. Southern Ocean Iron Enrichment Experiment: Carbon Cycling in High- and Low-Si Waters. Science 2004, 304, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, A.; Takeda, S.; Saito, H.; Nishioka, J.; Nojiri, Y.; Kudo, I.; Kiyosawa, H.; Shiomoto, A.; Imai, K.; Ono, T.; et al. A Mesoscale Iron Enrichment in the Western Subarctic Pacific Induces a Large Centric Diatom Bloom. Science 2003, 300, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Sherry, N.D.; Kiyosawa, H.; Tsuda, A.; Harrison, P.J. Phytoplankton processes during a mesoscale iron enrichment in the NE subarctic Pacific: Part I—Biomass and assemblage. Deep-Sea Res. Pt. II 2006, 53, 2095–2113. [Google Scholar] [CrossRef]
- Dave, A.C.; Lozier, M.S. Local stratification control of marine productivity in the subtropical North Pacific. J. Geophys. Res. 2010, 115, C12032. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.H.; Gordon, R.M. Northeast Pacific iron distributions in relation to phytoplankton productivity. Deep Sea Res. Part I Oceanogr. Res. Pap. 1988, 35, 177–196. [Google Scholar] [CrossRef]
- Duce, R.A.; Tindale, N.W. Atmospheric transport of iron and its deposition in the ocean. Limnol. Oceanogr. 1991, 36, 1715–1726. [Google Scholar] [CrossRef]
- Fisher, T.R.; Peele, E.R.; Ammerman, J.W.; Harding, L.W.J. Nutrient limitation of phytoplankton in Chesapeake Bay. Mar. Ecol-Prog. Ser. 1992, 82, 51–63. [Google Scholar] [CrossRef]
- Justić, D.; Rabalais, N.N.; Turner, R.E.; Dortch, Q. Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences. Estuarine. Coast. Shelf. S. 1995, 40, 339–356. [Google Scholar] [CrossRef]
- Arrigo, K.R. Marine microorganisms and global nutrient cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef]
- Patel, R.S.; Llort, J.; Strutton, P.G.; Phillips, H.E.; Moreau, S.; Pardo, P.C. The biogeochemical structure of southern ocean mesoscale eddies. J. Geophys. Res.-Oceans 2020, 125, e2020JC016115. [Google Scholar] [CrossRef]
- Wang, Z.H.; Song, S.H.; Qi, Y.Z. A comparative study of phytoneuston and the phytoplankton community structure in daya bay, South China Sea. J. Sea. Res. 2014, 85, 474–482. [Google Scholar] [CrossRef]
- Bibby, T.S.; Moore, C.M. Silicate: Nitrate ratios of upwelled waters control the phytoplankton community sustained by mesoscale eddies in sub-tropical north atlantic and pacific. Biogeosci. Discuss. 2010, 8, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Corredor-Acosta, A.; Morales, C.E.; Brewin, R.J.W.; Augur, P.A.; Pizarro, O.; Hormazabal, S.; Anabalón, V. Phytoplankton size structure in association with mesoscale eddies off central-aouthern Chile: The satellite application of a phytoplankton size-class model. Remote Sens. 2018, 10, 834. [Google Scholar] [CrossRef] [Green Version]
- Agusti, S.; Lubián, M.L.; Moreno-Ostos, E.; Estrada, M.; Duarte, C.M. Projected changes in photosynthetic picoplankton in a warmer subtropical ocean. Front. Mar. Sci. 2019, 5, 506. [Google Scholar] [CrossRef] [Green Version]
- Grébert, T.; Doré, H.; Partensky, F.; Farrant, G.K.; Garczarek, L. Light color acclimation is a key process in the global ocean distribution of Synechococcus cyanobacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 201717069. [Google Scholar] [CrossRef] [Green Version]
- Veldhuis, M.J.W.; Timmermans, K.R.; Croot, P.; Wagt, B.V.D. Picophytoplankton; a comparative study of their biochemical composition and photosynthetic properties. J. Sea Res. 2005, 53, 7–24. [Google Scholar] [CrossRef]


| Group | CEC vs. CEO | CEC vs. WEC–I | CEC vs. WEC–II | CEO vs. WEO–I | WEC–I vs. WEO–I | WEC–I vs. WEE–I | WEC–I vs. WEC–II | WEO–I vs. WEO–II | WEC–II vs. WEO–II |
|---|---|---|---|---|---|---|---|---|---|
| Temperature | −10.815 * (0.000) | −6.155 * (0.000) | −13.485 * (0.000) | 14.803 * (0.000) | 6.136 * (0.001) | 2.835 * (0.025) | −3.884 * (0.008) | −3.786 * (0.009) | 12.536 * (0.000) |
| Salinity | 0.184 (0.859) | −0.424 (0.685) | −8.230 * (0.000) | −3.344 * (0.016) | −4.279 * (0.005) | −4.661 * (0.002) | −8.419 * (0.000) | −2.716 * (0.035) | −5.692 * (0.001) |
| Density | 8.465 * (0.000) | 4.950 * (0.002) | −9.070 * (0.000) | −6.653 * (0.001) | −6.844 * (0.000) | −10.613 * (0.000) | 3.132 * (0.020) | 2.047 (0.087) | −4.894 * (0.002) |
| DIN | 4.106 * (0.005) | 4.200 * (0.004) | 3.970 * (0.005) | 1.136 (0.299) | −2.767 * (0.033) | −0.431 (0.679) | −2.801 * (0.031) | −0.506 (0.631) | −4.648 * (0.002) |
| DIP | 10.349 * (0.000) | 7.448 * (0.000) | 11.546 * (0.000) | −0.467 (0.657) | −10.392 * (0.000) | 3.976 * (0.005) | 2.322 (0.059) | −2.483 * (0.048) | −11.776 * (0.000) |
| DSi | 0.906 (0.395) | 1.814 (0.113) | 4.033 * (0.005) | 1.188 (0.280) | −1.523 (0.179) | −0.158 (0.879) | 1.203 (0.274) | 3.025 * (0.023) | −10.915 * (0.000) |
| pH | −1.895 (0.100) | −2.046 (0.080) | −3.908 * (0.006) | −0.509 (0.629) | 0.375 (0.721) | 1.146 (0.289) | −2.293 (0.062) | 0.057 (0.956) | 1.927 (0.095) |
| DO | −2.362 * (0.050) | 1.983 (0.088) | 1.730 (0.127) | 0.731 (0.492) | −0.513 (0.627) | −1.249 (0.252) | 1.302 (0.241) | 1.336 (0.230) | 0.158 (0.879) |
| SPM | −2.315 (0.054) | −0.768 (0.468) | −1.515 (0.173) | −0.836 (0.435) | −1.152 (0.293) | −0.916 (0.390) | −1.377 (0.218) | −1.910 (0.105) | −0.521 (0.619) |
| Chla | 1.098 (0.309) | 1.921 (0.096) | 2.511 * (0.014) | 0.865 (0.420) | −2.912 * (0.027) | −4.692 * (0.002) | 2.953 * (0.025) | 2.277 (0.063) | −0.727 (0.491) |
| Micro | 2.469 * (0.043) | 2.978 * (0.021) | 3.077 * (0.018) | −1.143 (0.297) | −1.838 (0.116) | −0.905 (0.396) | 0.121 (0.908) | 1.011 (0.351) | −3.504 * (0.010) |
| Pico | 1.003 (0.349) | 2.096 (0.074) | 2.485 * (0.042) | 0.393 (0.708) | −3.292 * (0.017) | −4.923 * (0.002) | 0.873 (0.416) | 2.102 (0.080) | −0.411 (0.694) |
| Pro | 0.759 (0.473) | 1.907 (0.098) | 2.404 * (0.047) | 0.256 (0.806) | −3.111 * (0.021) | −4.852 * (0.002) | 1.715 (0.137) | 2.488 (0.470) | −0.201 (0.847) |
| Syn | 2.967 * (0.021) | 3.026 * (0.019) | 1.755 (0.123) | 1.343 (0.228) | −3.684 * (0.010) | −11.504 * (0.000) | −3.536 * (0.012) | −3.715 (0.100) | −3.717 * (0.007) |
| PEuks | 1.144 (0.290) | 2.034 (0.081) | 2.628 * (0.034) | 2.172 (0.073) | −2.300 (0.061) | −4.361 * (0.003) | 2.644 * (0.038) | 1.758 (0.129) | −0.435 (0.677) |
| Variables | Chla | Micro | Pico | Pro | Syn | PEuks |
|---|---|---|---|---|---|---|
| Temperature | −0.777 * | −0.837 * | −0.780 * | −0.809 * | −0.397 | −0.736 |
| Salinity | −0.578 | −0.502 | −0.533 | −0.594 | −0.005 | −0.615 |
| Density | 0.672 | 0.683 | 0.625 | 0.687 | 0.090 | 0.617 |
| DIN | 0.688 | 0.686 | 0.741 | 0.661 | 0.991 ** | 0.697 |
| DIP | 0.694 | 0.766 * | 0.711 | 0.684 | 0.669 | 0.655 |
| DSi | 0.924 ** | 0.964 ** | 0.948 ** | 0.955 ** | 0.635 | 0.921 ** |
| PAR | −0.792 * | −0.859 * | −0.790 * | −0.850 * | −0.214 | −0.792 * |
| Zeu | −0.888 ** | −0.957 ** | −0.918 ** | −0.933 ** | −0.562 | −0.904 ** |
| Eddy | Station | DIN | DIP | DSi | DIN:DIP | DSi:DIP | DSi:DIN | Limitation |
|---|---|---|---|---|---|---|---|---|
| CEC | S1 | 0.79 | 0.15 | 3.04 | 5.40 | 20.70 | 3.84 | DIN |
| CEO | S2 | 0.34 | 0.07 | 2.32 | 4.75 | 32.57 | 6.86 | DIN and DIP |
| WEO–I | S3 | 0.28 | 0.11 | 2.41 | 2.52 | 21.96 | 8.72 | DIN |
| WEC–I | S4 | 0.14 | 0.06 | 1.61 | 2.38 | 27.38 | 11.51 | DIN and DIP |
| WEE–I | S5 | 0.20 | 0.08 | 1.73 | 2.65 | 22.44 | 8.47 | DIN and DIP |
| WEO–II | S6 | 0.61 | 0.14 | 1.99 | 4.41 | 14.44 | 3.28 | DIN |
| WEC–II | S7 | 0.40 | 0.04 | 1.48 | 9.78 | 35.95 | 3.67 | DIN and DIP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Wang, Y.; Huang, S.; Pei, L.; Luo, Z. Impacts of Mesoscale Eddies on Biogeochemical Variables in the Northwest Pacific. J. Mar. Sci. Eng. 2022, 10, 1451. https://doi.org/10.3390/jmse10101451
Kang J, Wang Y, Huang S, Pei L, Luo Z. Impacts of Mesoscale Eddies on Biogeochemical Variables in the Northwest Pacific. Journal of Marine Science and Engineering. 2022; 10(10):1451. https://doi.org/10.3390/jmse10101451
Chicago/Turabian StyleKang, Jianhua, Yu Wang, Shuhong Huang, Lulu Pei, and Zhaohe Luo. 2022. "Impacts of Mesoscale Eddies on Biogeochemical Variables in the Northwest Pacific" Journal of Marine Science and Engineering 10, no. 10: 1451. https://doi.org/10.3390/jmse10101451





