Control of Reactive Oxygen Species through Antioxidant Enzymes Plays a Pivotal Role during the Cultivation of Neopyropia yezoensis
Abstract
:1. Introduction
2. Materials and Methods
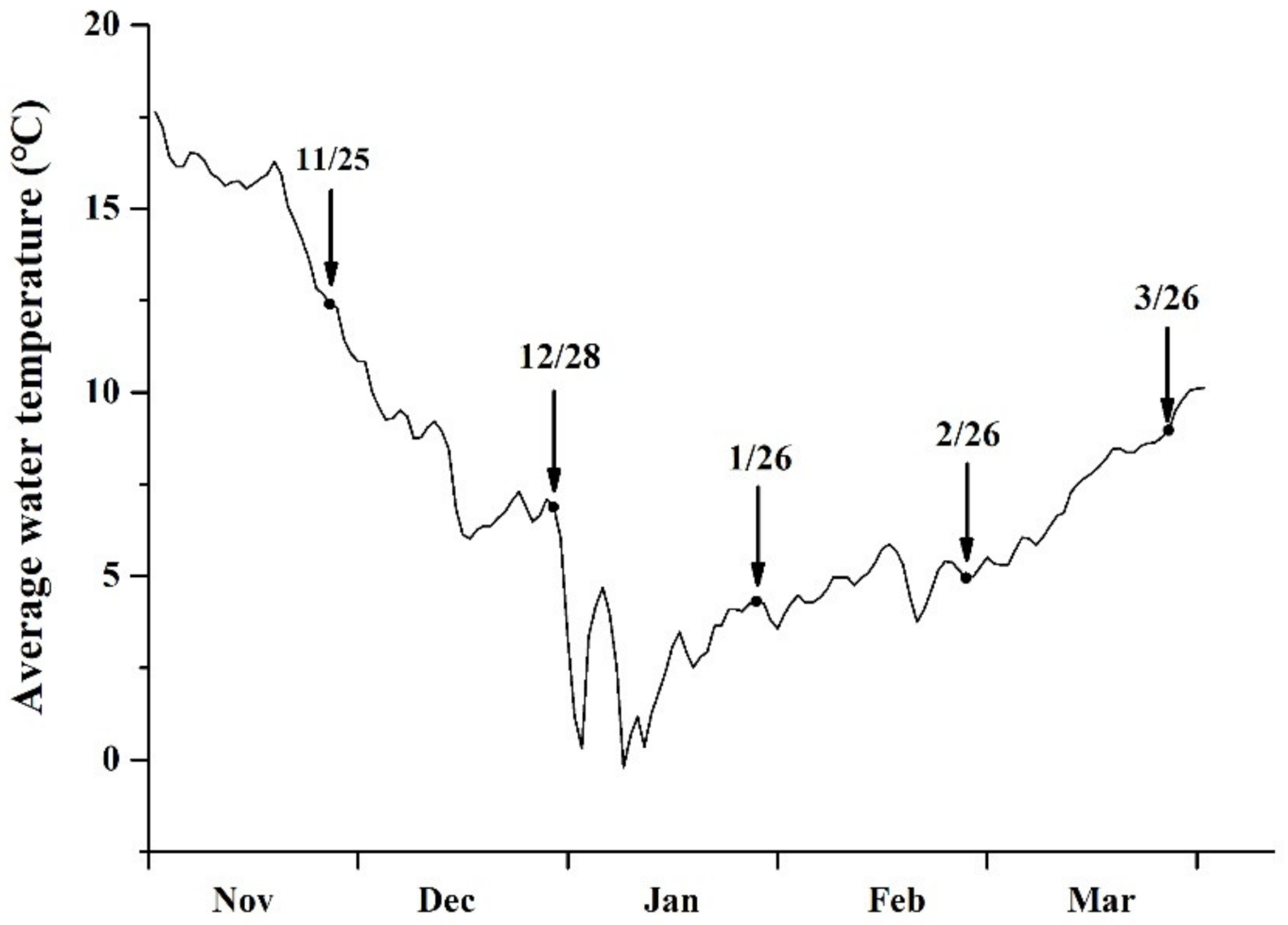
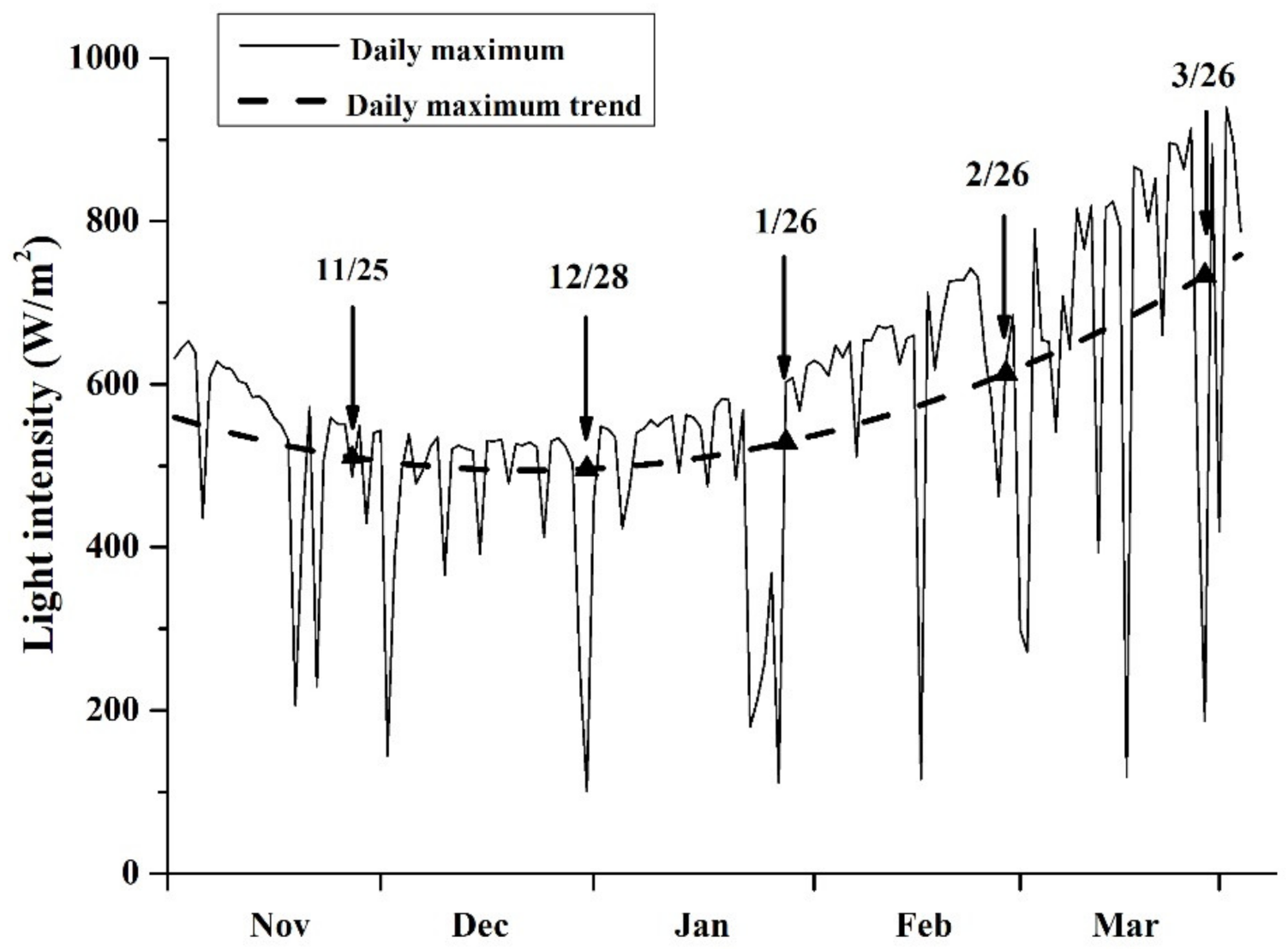
2.1. Observation of Sea Surface Temperature (SST) and Simulated Solar Light Intensity
2.2. Collection of Algae Materials
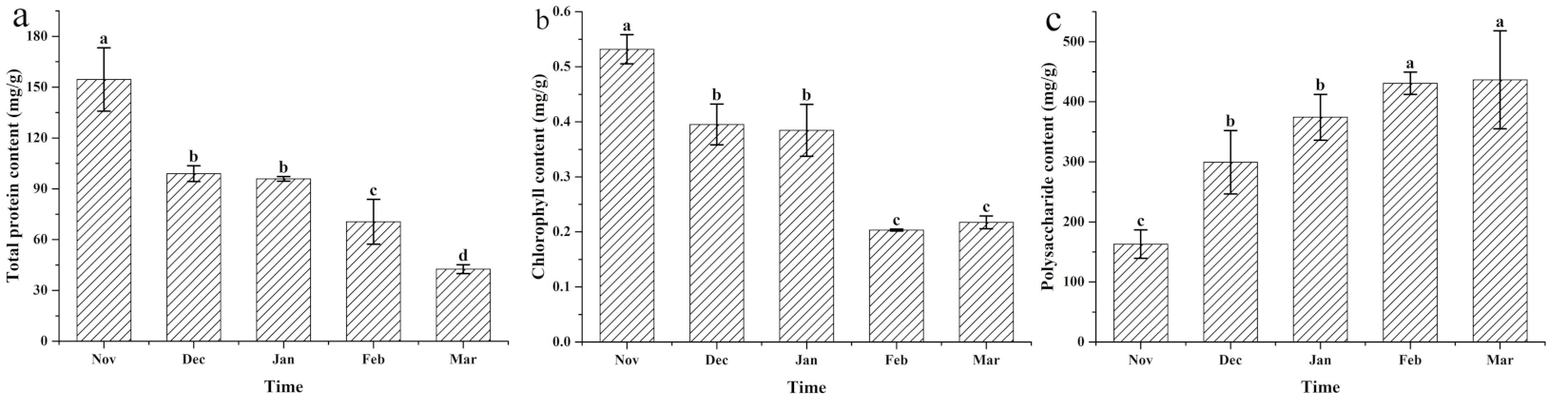
2.3. Determination of Soluble Protein, Chlorophyll, and Cell Wall Polysaccharides
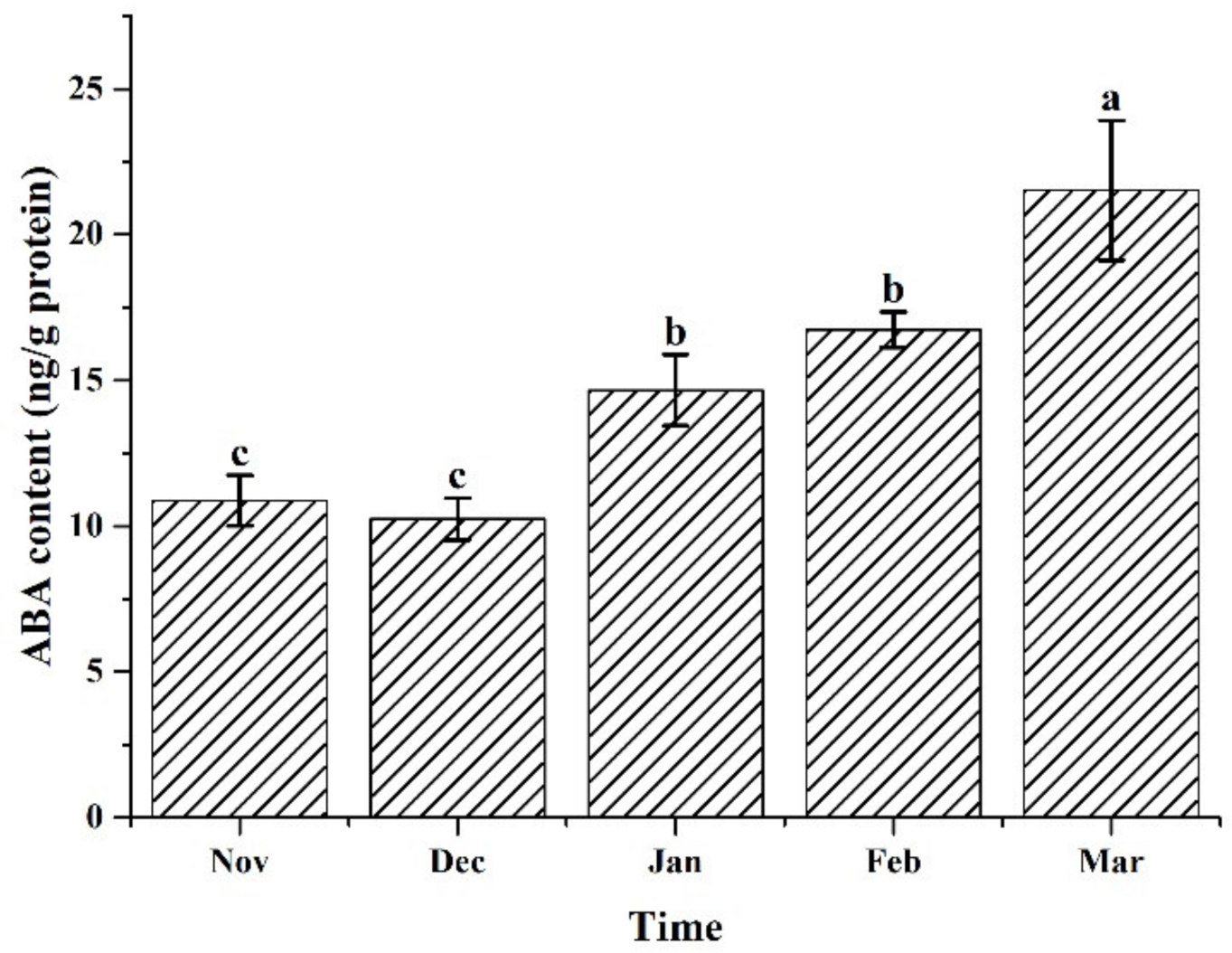
2.4. Determination of ABA Content
2.5. Assay of Antioxidant Enzymes
2.6. RNA Extraction, cDNA Library Construction, and Real-Time PCR Assay
2.7. Detection of H2O2, Malondialdehyde (MDA) and the Mycosporine-like Amino Acids Content
2.8. Statistical Analysis
3. Results
3.1. Variation in SST and Solar Light Intensity
3.2. Changes to the Protein, Chlorophyll, and Cell Wall Polysaccharides under Different Cultivation Periods
3.3. ABA Content of the Samples under the Different Cultivation Periods
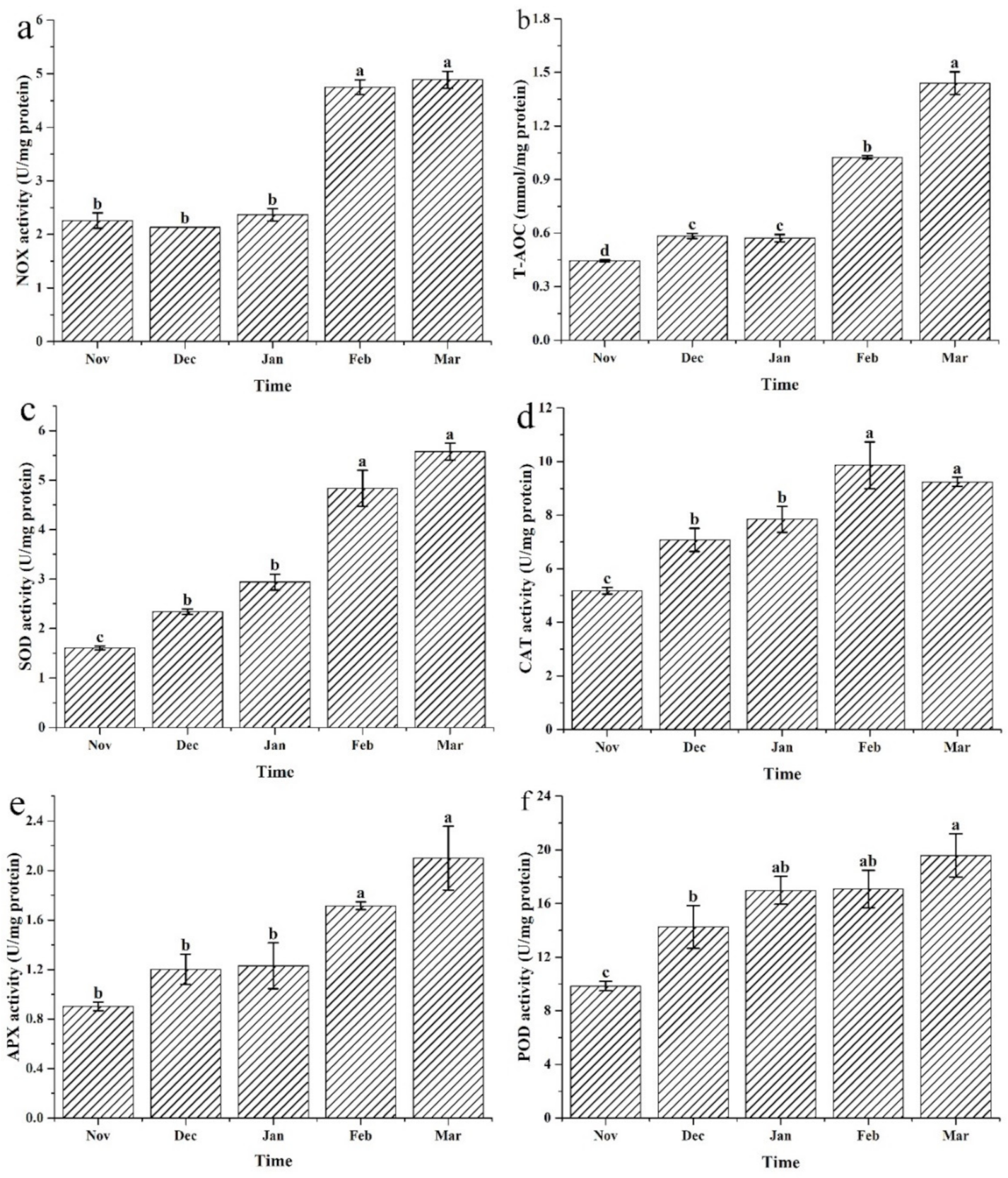
3.4. Changes to the Antioxidase Activities under Different Cultivation Periods
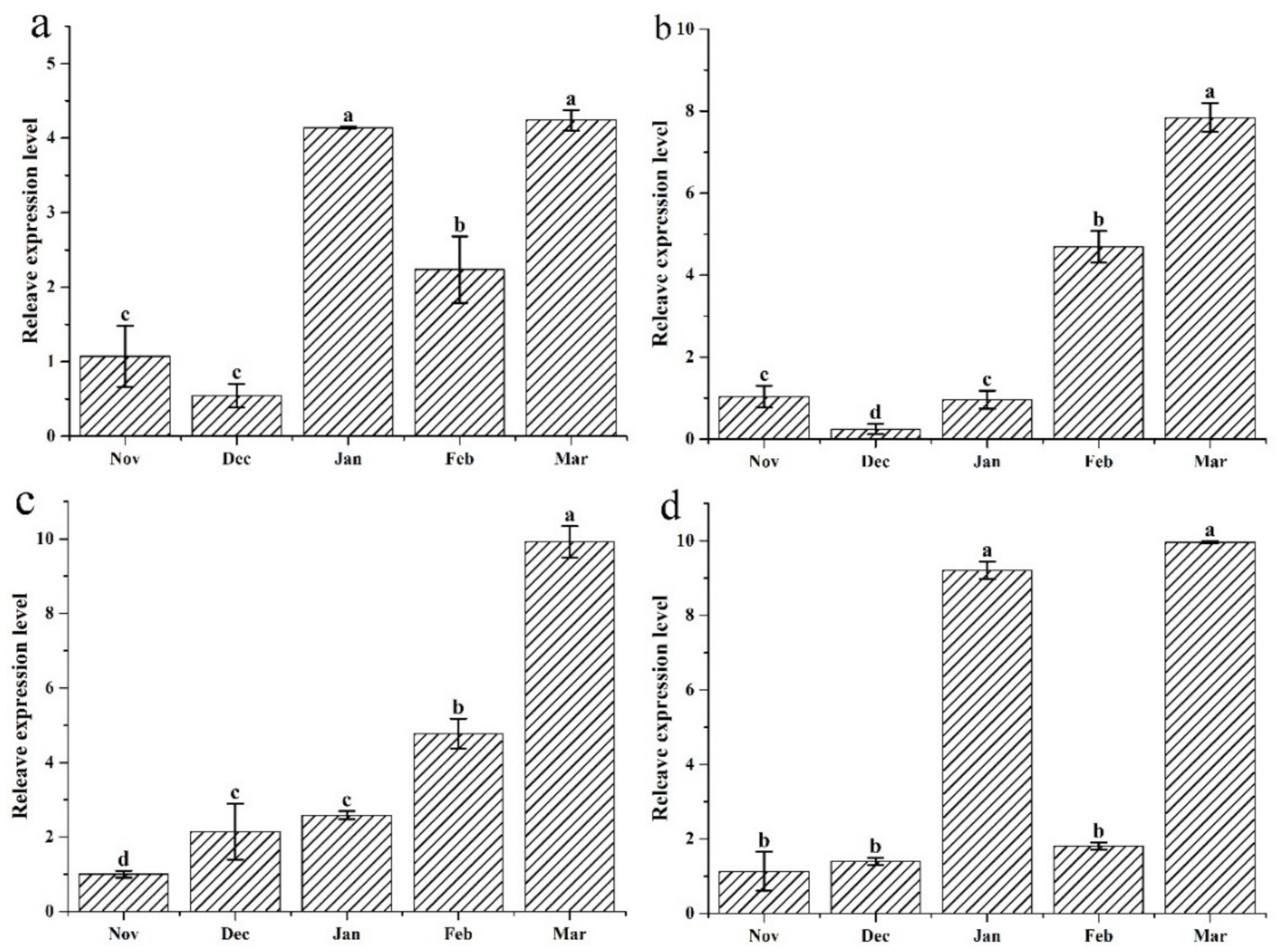
3.5. Antioxidase Genes Expression Profiles Determined by qRT-PCR
3.6. Variations in H2O2, MDA and MAAs Contents with the Cultivation Period
4. Discussion
4.1. Sampling at Different Cultivation Periods
4.2. Antioxidant Enzymes Play an Important Role during Cultivation of N. yezoensis in Sea Areas
4.3. The Up-Regulation of Antioxidant Enzymes May Be Regulated by ABA Signaling Pathway
4.4. Up-Regulation of Antioxidases Is Associated with the Accumulation of ROS Produced by NOX
4.5. Accumulation of ROS May Lead to Adjustment of Multiple Metabolic Pathways
4.6. ROS Accelerated Cell Aging
4.7. Accumulation of ROS May Also Result in the Synthesis of Stress-Related Molecules
4.8. Stress Conditions Enhanced the Synthesis of the Cell Wall
4.9. Potential Factors of ROS Induction and the Influences on N. yezoensis Cultivated in the Sea
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.L.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.K.; He, C.Z. Regulation of plant reactive oxygen species (ROS) in stress responses: Learning from AtRBOHD. Plant Cell Rep. 2016, 35, 995–1007. [Google Scholar] [CrossRef]
- Moon, E.Y.; Oh, J.M.; Kim, Y.H.; Ryoo, I.J.; Yoo, I.D. Clitocybins, Novel Isoindolinone Free Radical Scavengers, from Mushroom Clitocybe aurantiaca Inhibit Apoptotic Cell Death and Cellular Senescence. Biol. Pharm. Bull. 2009, 32, 1689–1694. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.H.; Yang, G.W. Signal Function Studies of ROS, Especially RBOH-Dependent ROS, in Plant Growth, Development and Environmental Stress. J. Plant Growth Regul. 2020, 39, 157–171. [Google Scholar] [CrossRef]
- Sutherland, J.E.; Lindstrom, S.C.; Nelson, W.A.; Brodie, J.; Lynch, M.D.J.; Hwang, M.S.; Choi, H.G.; Miyata, M.; Kikuchi, N.; Oliveira, M.C.; et al. A New Look at an Ancient Order: Generic Revision of the Bangiales (Rhodophyta). J. Phycol. 2011, 47, 1131–1151. [Google Scholar] [CrossRef] [PubMed]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2011, 16, 29–37. [Google Scholar] [CrossRef]
- Tijero, V.; Teribia, N.; Munoz, P.; Munne-Bosch, S. Implication of Abscisic Acid on Ripening and Quality in Sweet Cherries: Differential Effects during Pre- and Post-harvest. Front. Plant Sci. 2016, 7, 602. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.G.; Zhou, L.Y.; Chen, W.T.; Ye, N.H.; Xia, J.X.; Zhuang, C.X. Overexpression of a microRNA-targeted NAC transcription factor improves drought and salt tolerance in Rice via ABA-mediated pathways. Rice 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.T.S.; de Rosa, V.E.; Menossi, M.; Ulian, E.C.; Arruda, P. RNA expression profiles and data mining of sugarcane response to low temperature. Plant Physiol. 2003, 132, 1811–1824. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Phys. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.; Dietz, K.J. Alkyl hydroperoxide reductases: The way out of the oxidative breakdown of lipids in chloroplasts. Trends Plant Sci. 1999, 4, 166–168. [Google Scholar] [CrossRef]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Yu, B.; Yang, J.L.; Niu, J.F.; Wang, G. Antioxidant responses to hyperosmolarity stress in the intertidal Pyropia yezoensis (Bangiales, Rhodophyta). Algal Res. 2020, 48, 101930. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, C.; Lu, M.Q.; Xu, Q.G.; Yang, P.L.; Tian, N.C.; Deng, C.Y. Germplasm innovation and application of Porphyra yezoensis. J. Guangxi Acad. Sci. 2021, 37, 46–52. [Google Scholar]
- Yao, X.C.; Qiu, C.J.; Mu, C.L. Study on nutritional components and seasonal variation of Porphyra yezoensis. Aquaculture 2002, 5, 34–35. [Google Scholar]
- Zhong, M.; Zhang, Y. Changes of main nutrients in Porphyra yezoensis at different harvest stages. Chin. Feed. 2003, 23, 30–31. [Google Scholar]
- Qian, C.C.; Liu, A.C.; Huang, R.; Liu, Q.R.; Xu, W.K.; Zhong, S.; Yu, L. Quality control of marine big data-a case study of real-time observation station data in Qingdao. J. Oceanol. Limnol. 2019, 37, 1983–1993. [Google Scholar] [CrossRef]
- Liu, G.; Lian, Y.X.; Gao, H.S.; Gao, H.S.; Bai, T.; Dong, L.; Chen, L. Numerical study on the formation of atmospheric duct during a sea-land breeze process in the nearshore of Qingdao. Mar. Forecast. 2021, 38, 19–26. [Google Scholar]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts-Polyphenoloxidase in Beta-Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Zhang, H.H.; Xu, K.; Xu, Y.; Ji, D.H.; Wang, W.L.; Chen, C.S.; Xie, C.T. The Generation Difference of UVR Absoring Compounds Mycosporine-like Amino Acids (MAAs) of Pyropia haitanensis. J. Jimei Univ. 2018, 23, 161–170. [Google Scholar]
- Kaur, G.; Pati, P.K. Analysis of cis-acting regulatory elements of Respiratory burst oxidase homolog (Rboh) gene families in Arabidopsis and rice provides clues for their diverse functions. Comput. Biol. Chem. 2016, 62, 104–118. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, Y.X.; Ding, H.L.; Zhang, P.X.; Lu, C.Q.; Xu, Q.P. Color Atlas of Chinese Laver; China Agricultural Press: Beijing, China, 2016. [Google Scholar]
- Niu, J.F.; Feng, J.H.; Xie, X.J.; Gao, S.; Wang, C.G. Involvement of cyclic electron flow in irradiance stress responding and its potential regulation of the mechanisms in Pyropia yezoensis. Chin. J. Oceanol. Limn. 2016, 34, 730–739. [Google Scholar] [CrossRef]
- Mikami, K.; Mori, I.C.; Matsuura, T.; Ikeda, Y.; Kojima, M.; Sakakibara, H.; Hirayama, T. Comprehensive quantification and genome survey reveal the presence of novel phytohormone action modes in red seaweeds. J. Appl. Phycol. 2016, 28, 2539–2548. [Google Scholar] [CrossRef]
- Saradhi, P.P.; Suzuki, I.; Katoh, A.; Sakamoto, A.; Sharmila, P.; Shi, D.J.; Murata, N. Protection against the photo-induced inactivation of the photosystem II complex by abscisic acid. Plant Cell Environ. 2000, 23, 711–718. [Google Scholar] [CrossRef]
- Yoshida, K.; Igarashi, E.; Mukai, M.; Hirata, K.; Miyamoto, K. Induction of tolerance to oxidative stress in the green alga, Chlamydomonas reinhardtii, by abscisic acid. Plant Cell Environ. 2003, 26, 451–457. [Google Scholar] [CrossRef]
- Guajardo, E.; Correa, J.A.; Contreras-Porcia, L. Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 2016, 243, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.M.; Ji, J.; Yue, J.Y.; Shi, S.Q.; Ou, C.; Jiang, Z.P.; Chang, E.M. Exogenous Abscisic Acid Modulates Reactive Oxygen Metabolism and Related Gene Expression in Platycladus orientalis under H2O2-Induced Stress. Russ. J. Plant Physiology. 2020, 67, 85–93. [Google Scholar] [CrossRef]
- Yang, J.; Gu, L.W.; Feng, H.Z.; Yu, Z.B. Synthesis of Abscisic Acid in Neopyropia yezoensis and Its Regulation of Antioxidase Genes Expressions Under Hypersaline Stress. Front. Microbiol. 2022, 12, 775710. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Phys. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Tan, J.L.; Guo, Z.F.; Lu, S.Y.; He, S.J.; Shu, W.; Zhou, B.Y. Increased abscisic acid levels in transgenic tobacco over-expressing 9 cis-epoxycarotenoid dioxygenase influence H2O2 and NO production and antioxidant defences. Plant Cell Environ. 2009, 32, 509–519. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Zhang, J.H. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagi, M.; Davydov, O.; Orazova, S.; Yesbergenova, Z.; Ophir, R.; Stratmann, J.W.; Fluhr, R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 2004, 16, 616–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Proels, R.K.; Oberhollenzer, K.; Pathuri, I.P.; Hensel, G.; Kumlehn, J.; Huckelhoven, R. RBOHF2 of Barley Is Required for Normal Development of Penetration Resistance to the Parasitic Fungus Blumeria graminis f. sp hordei. Mol. Plant-Microbe Interact. 2010, 23, 1143–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, S.M.B.; Brownlee, C.; Bothwell, J.H.F. A tip-high, Ca2+-interdependent, reactive oxygen species gradient is associated with polarized growth in Fucus serratus zygotes. Planta 2008, 227, 1037–1046. [Google Scholar] [CrossRef]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, R.; Mackerness, S.A.H.; Hancock, J.T.; Neill, S.J. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001, 127, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco-Cardenas, M.L.; Narvaez-Vasquez, J.; Ryan, C.A. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 2001, 13, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; van Remmen, H. Trends in oxidative aging theories. Free Radic. Bio. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef]
- Qin, G.Z.; Meng, X.H.; Wang, Q.; Tian, S.P. Oxidative Damage of Mitochondrial Proteins Contributes to Fruit Senescence: A Redox Proteomics Analysis. J. Proteome Res. 2009, 8, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Hasty, P.; Campisi, J.; Hoeijmakers, J.; van Steeg, H.; Vijg, J. Aging and genome maintenance: Lessons from the mouse? Science 2003, 299, 1355–1359. [Google Scholar] [CrossRef]
- Fry, S.C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 1998, 332, 507–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, H.; Gutteridge, J.M.C. Free radicals in biology and medicine. Pergamon 1991, 10, 449–450. [Google Scholar] [CrossRef]
- Meng, X.Z.; Zhang, S.Q. MAPK Cascades in Plant Disease Resistance Signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Vos, D.L.; le Disquet, I.; Leprince, A.S.; Bordenave, M.; Maldiney, R.; Jdey, A.; Abdelly, C.; Savoure, A. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arab. Thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.M.; Berry, J.A. Recovery of Photosynthesis after Exposure of Intertidal Algae to Osmotic and Temperature Stresses - Comparative-Studies of Species with Differing Distributional Limits. Oecologia 1986, 70, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Portwich, A.; Garcia-Pichel, F. A novel prokaryotic UVB photoreceptor in the cyanobacterium Chlorogloeopsis PCC 6912. Photochem. Photobiol. 2000, 71, 493–498. [Google Scholar] [CrossRef]
- Liu, Z.W.; Hader, D.P.; Sommaruga, R. Occurrence of mycosporine-like amino acids (MAAs) in the bloom-forming cyanobacterium Microcystis aeruginosa. J. Plankton Res. 2004, 26, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.H. Preliminary Analysis on the Mechanism of UV Absorbing Substances of Porphyra haitanensis under Stress; Jimei University: Xiamen, China, 2016. [Google Scholar]
- Jiang, H.X.; Gao, K.S.; Helbling, E.W. UV-absorbing compounds in Porphyra haitanensis (Rhodophyta) with special reference to effects of desiccation. J. Appl. Phycol. 2008, 20, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Lechat, H.; Amat, M.; Mazoyer, J.; Buleon, A.; Lahaye, M. Structure and distribution of glucomannan and sulfated glucan in the cell walls of the red alga Kappaphycus alvarezii (gigartinales, rhodophyta). J. Phycol. 2000, 36, 891–902. [Google Scholar] [CrossRef]
- Eppley, R.W.; Cyrus, C.C. Cation Regulation and Survival of the Red Alga, Porphyra-Perforata, in Diluted and Concentrated Sea Water. Biol. Bull. 1960, 118, 55–65. [Google Scholar] [CrossRef]
- Rees, D.A. Enzymic Synthesis of 3 6-Anhydro-L-Galactose within Porphyran from L-Galactose 6-Sulphate Units. Biochem. J. 1961, 81, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, D.A. Estimation of Relative Amounts of Isomeric Sulphate Esters in Some Sulphated Polysaccharides. J. Chem. Soc. 1961, 5168–5171. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; van-Moppes, D.; Grossman, S.; Arad, S. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Adda, M.; Merchuk, J.C.; Arad, S. Effect of Nitrate on Growth and Production of Cell-Wall Polysaccharide by the Unicellular Red Alga Porphyridium. Biomass 1986, 10, 131–140. [Google Scholar] [CrossRef]
- Gretz, M.R.; Mccandless, E.L.; Aronson, J.M.; Sommerfeld, M.R. The Galactan Sulfates of the Conchocelis Phases of Porphyra leucostricta and Bangia atropurpurea (Rhodophyta). J. Exp. Bot. 1983, 34, 705–711. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Burgstahler, R.; Buschmann, C.; Meier, D.; Prenzel, U.; Schönthal, A. Effect of High Light and High Light Stress on Composition, Function and Structure of the Photosynthetic Apparatus; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]

| Gene Name | Gene Introduction | Primer Sequence 5′-3′ | Product Length |
|---|---|---|---|
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | CCAACAAGTGGGAGTAAGCG GGACAGAACCGAACAGCGTA | 104 |
| SOD | Superoxide dismutase | GGCTGCCTGCCTTTGACA CTCGTAGGTGCTCCGTTG | 438 |
| APX | Ascorbate peroxidase | AGATTATGAAGGAGACGC AAAAGAATGGACAGGAAG | 372 |
| NOX | NADPH oxidase | GAGCACCCCCCTGTCCATC GGCGAGCACAAATCCCGTC | 167 |
| CAT | Catalase | CCCTCTCTGCCCTTCACA CTTCCCGTAGCCGTCCTC | 192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Wu, L.; Sun, Z.; Yang, J.; Liu, G.; Niu, J.; Wang, G. Control of Reactive Oxygen Species through Antioxidant Enzymes Plays a Pivotal Role during the Cultivation of Neopyropia yezoensis. J. Mar. Sci. Eng. 2022, 10, 109. https://doi.org/10.3390/jmse10010109
Feng Z, Wu L, Sun Z, Yang J, Liu G, Niu J, Wang G. Control of Reactive Oxygen Species through Antioxidant Enzymes Plays a Pivotal Role during the Cultivation of Neopyropia yezoensis. Journal of Marine Science and Engineering. 2022; 10(1):109. https://doi.org/10.3390/jmse10010109
Chicago/Turabian StyleFeng, Zezhong, Lingjuan Wu, Zhenjie Sun, Jiali Yang, Guiyan Liu, Jianfeng Niu, and Guangce Wang. 2022. "Control of Reactive Oxygen Species through Antioxidant Enzymes Plays a Pivotal Role during the Cultivation of Neopyropia yezoensis" Journal of Marine Science and Engineering 10, no. 1: 109. https://doi.org/10.3390/jmse10010109
APA StyleFeng, Z., Wu, L., Sun, Z., Yang, J., Liu, G., Niu, J., & Wang, G. (2022). Control of Reactive Oxygen Species through Antioxidant Enzymes Plays a Pivotal Role during the Cultivation of Neopyropia yezoensis. Journal of Marine Science and Engineering, 10(1), 109. https://doi.org/10.3390/jmse10010109






