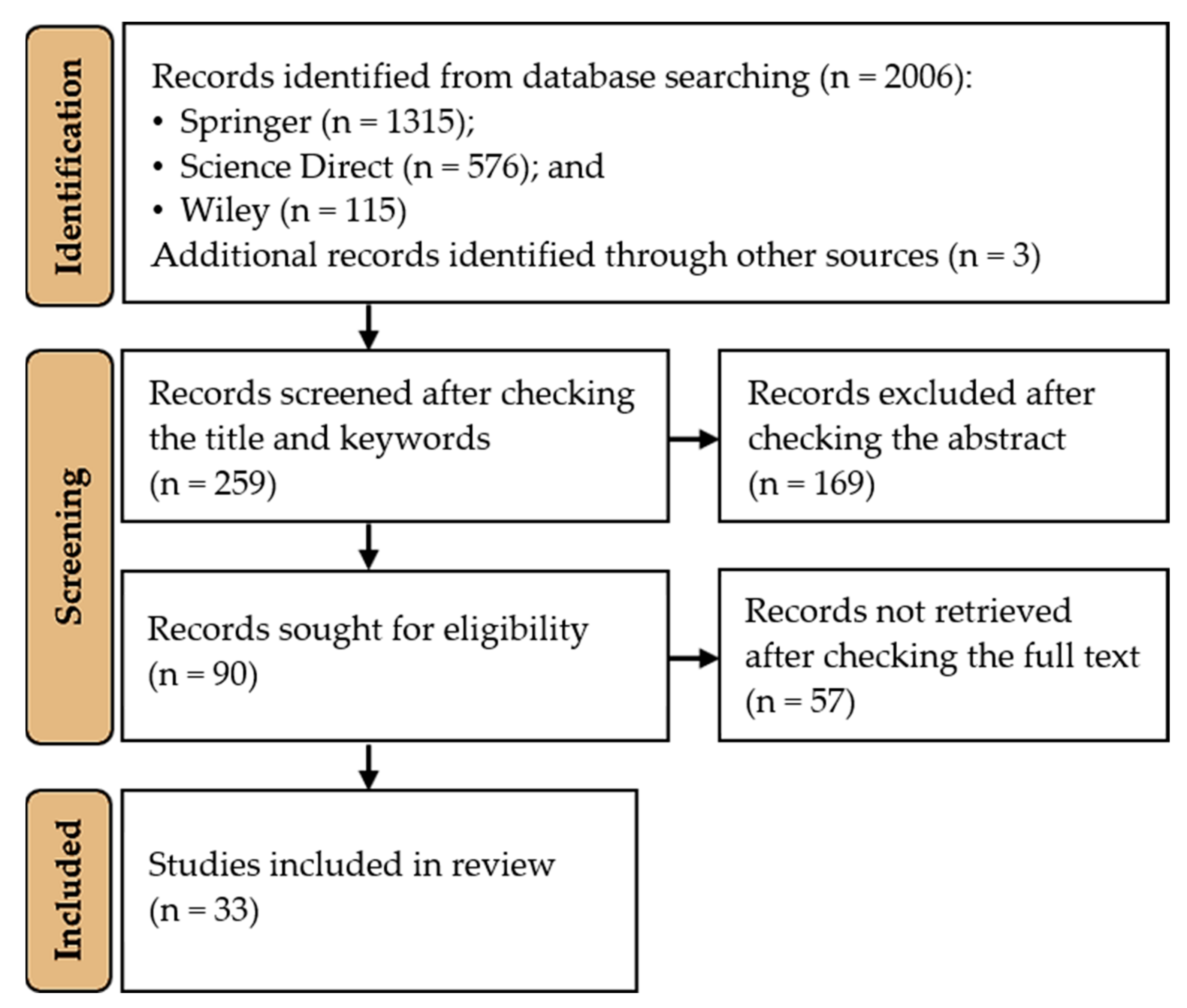
The Influence of Abiotic Factors on the Induction of Seaweed Callus
Abstract
1. Introduction
2. Materials and Methods
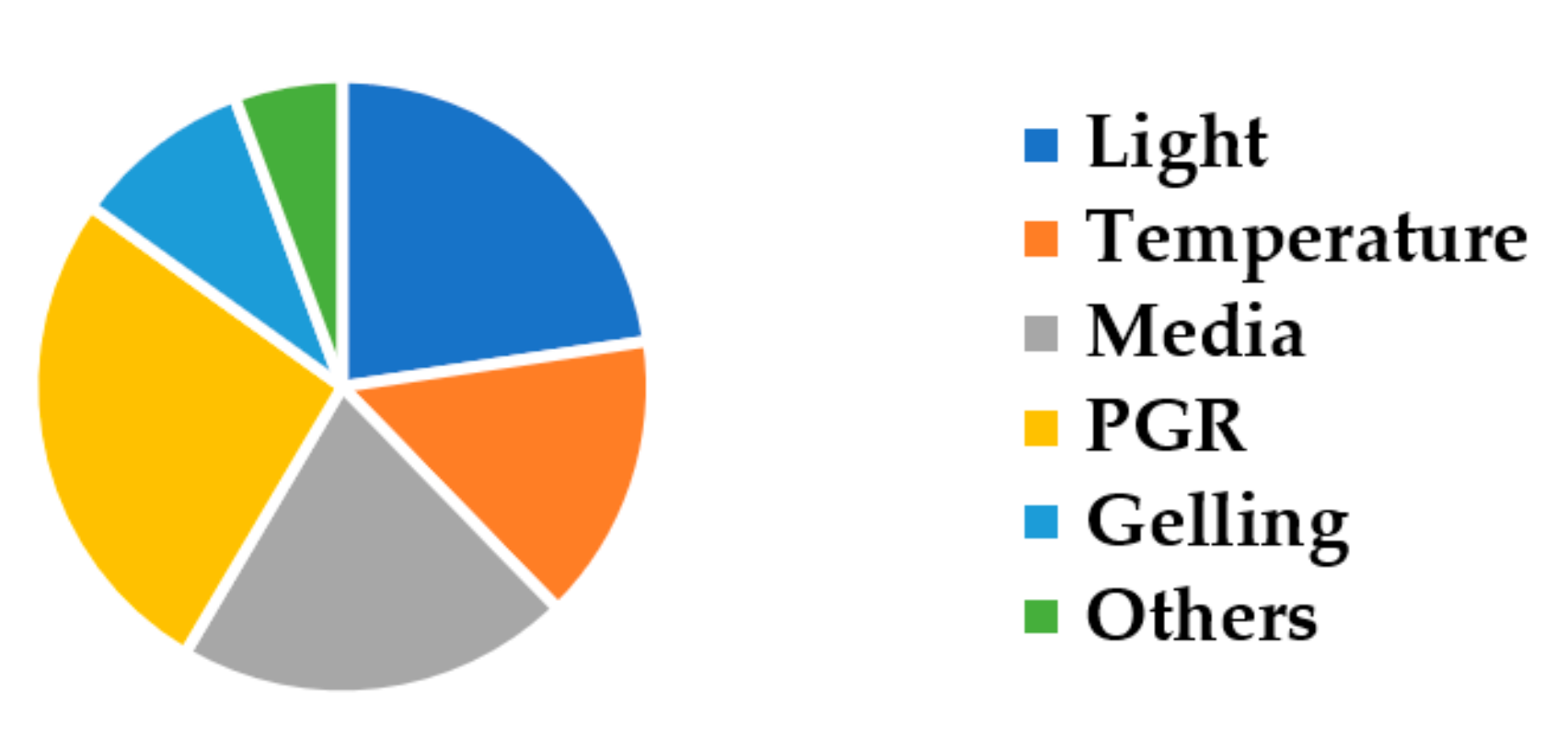
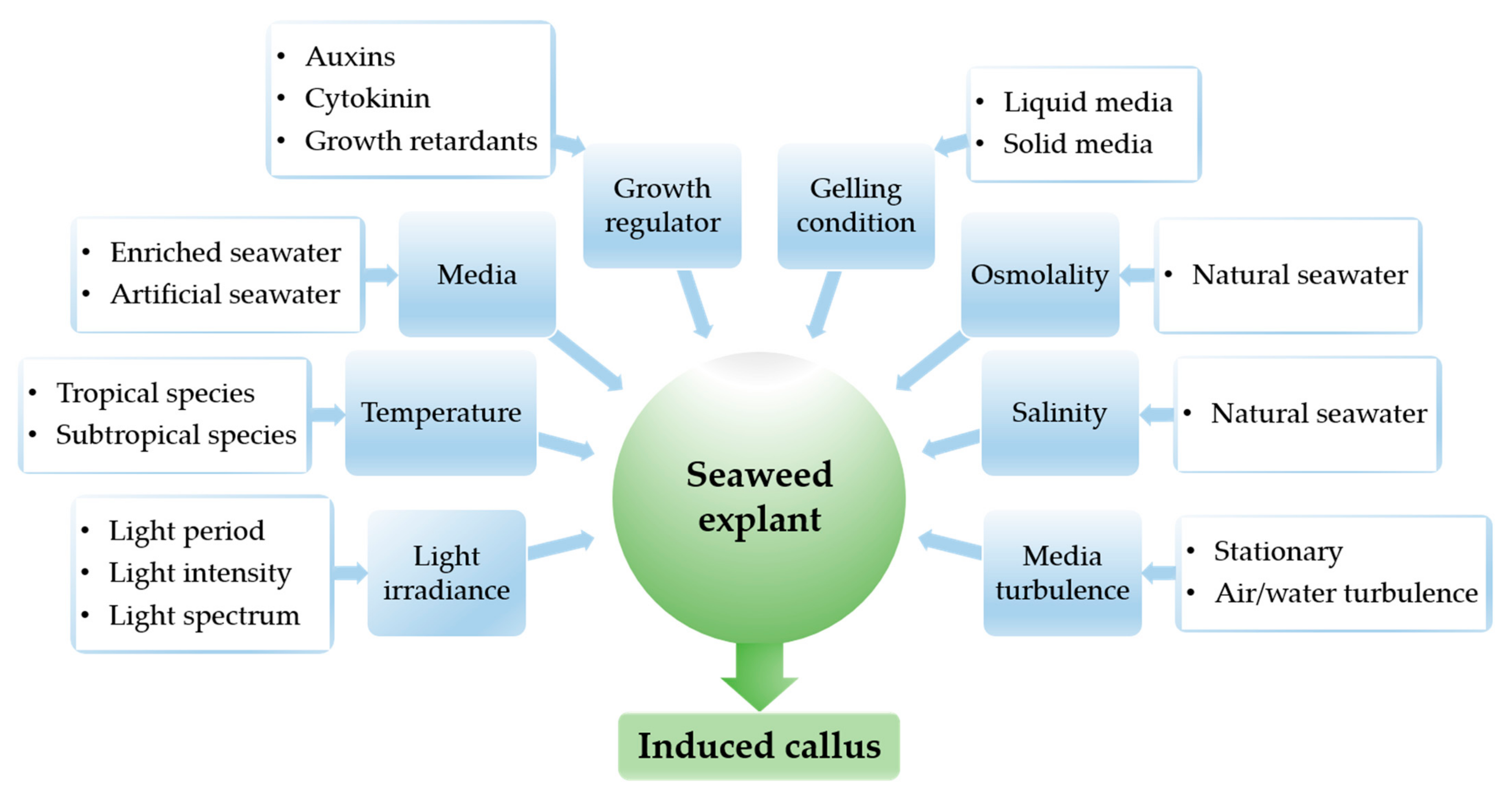
3. Results and Discussion
3.1. The Influence of Light
3.2. The Influence of Temperature
3.3. The Influence of Media Types
3.4. The Influence of Plant Growth Regulators
3.5. The Influence of Gelling Conditions
3.6. The Influence of Other Factors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stiger-Pouvreau, V.; Zubia, M. Macroalgal diversity for sustainable biotechnological development in French tropical overseas territories. Bot. Mar. 2020, 63, 17–41. [Google Scholar] [CrossRef]
- Sanches, P.F.; Pellizzari, F.; Horta, P.A. Multivariate analyses of Antarctic and sub-Antarctic seaweed distribution patterns: An evaluation of the role of the Antarctic Circumpolar Current. J. Sea Res. 2016, 110, 29–38. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Budzałek, G.; Śliwińska-Wilczewska, S.; Wiśniewska, K.; Wochna, A.; Bubak, I.; Latała, A.; Wiktor, J.M. Macroalgal defense against competitors and herbivores. Int. J. Mol. Sci. 2021, 22, 7865. [Google Scholar] [CrossRef] [PubMed]
- Bedoux, G.; Bourgougnon, N. Bioactivity of secondary metabolites from macroalgae. In The Algae World. Cellular Origin, Life in Extreme Habitats and Astrobiology; Sahoo, D., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 26, pp. 391–401. [Google Scholar]
- Hay, M. The ecology and evolution of seaweed-herbivore interactions on coral reefs. Coral Reefs 1997, 16, S67–S76. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae-A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, A.; Ali, H.; Mehdi, A. Seaweed proteins as a source of bioactive peptides. Curr. Pharm. Des. 2021, 27, 1342–1352. [Google Scholar]
- Lopes, D.; Rey, F.; Leal, M.C.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Bioactivities of lipid extracts and complex lipids from seaweeds: Current knowledge and future prospects. Mar. Drugs 2021, 19, 686. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Valado, A. The seaweed diet in prevention and treatment of the neurodegenerative diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.; Moon, I.S.; Hong, Y.K. The tropical carrageenophyte Kappaphycus alvarezii extract promotes axodendritic maturation of hippocampal neurons in primary culture. J. Appl. Phycol. 2018, 30, 3233–3241. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Choi, J.-S. Seaweed exhibits therapeutic properties against chronic disease: An overview. Appl. Sci. 2022, 12, 2638. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Antony, T.; Chakraborty, K. Pharmacological Properties of Seaweeds against Progressive Lifestyle Diseases. J. Aquat. Food Prod. Technol. 2019, 28, 1092–1104. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Fortune Business Insights. Commercial Seaweed Market Size, Share & COVID-19 Impact Analysis, by Type (Red Seaweed, Brown Seaweed, and Green Seaweed), form (Flakes, Powder, and Liquid), End-Uses (Food & Beverages, Agricultural Fertilizer, Animal Feed Additives, Pharmaceutical, and Cosmetics & Personal Care), and Regional Forecast. 2021–2028. Available online: https://www.fortunebusinessinsights.com/industry-reports/commercial-seaweed-market-100077 (accessed on 26 March 2022).
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant In Vitro Culture; Leva, A., Rinaldi, L., Eds.; Interchopen: London, UK, 2012; pp. 1–28. [Google Scholar]
- Zhang, Y.; Restall, J.; Crisp, P.; Godwin, I.; Liu, G. Current status and prospects of plant genome editing in Australia. Vitr. Cell. Dev. Biol. Plant 2021, 57, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Rorrer, G.L.; Modrell, J.; Zhi, C.; Yoo, H.-D.; Nagle, D.N.; Gerwick, W.H. Bioreactor seaweed cell culture for production of bioactive oxylipins. J. Appl. Phycol. 1995, 7, 187–198. [Google Scholar] [CrossRef]
- Shen, S.; Wu, X.; Yan, B.; He, L. Tissue culture of three species of Laurencia complex. Chin. J. Oceanol. Limnol. 2010, 28, 514–520. [Google Scholar] [CrossRef]
- Polne-Fuller, M.; Gibor, A. Calluses and callus-like growth in seaweeds: Induction and culture. In Twelfth International Seaweed Symposium. Developments in Hydrobiology; Ragan, M.A., Bird, C.J., Eds.; Springer: Dordrecht, The Netherlands, 1987; Volume 41, pp. 131–138. [Google Scholar]
- Rorrer, G.L.; Cheney, D.P. Bioprocess engineering of cell and tissue cultures for marine seaweeds. Aquac. Eng. 2004, 32, 11–41. [Google Scholar] [CrossRef]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2018, 31, 281–299. [Google Scholar] [CrossRef]
- Kumar, G.R.; Reddy, C.R.K.; Jha, B. Callus induction and thallus regeneration from callus of phycocolloid yielding seaweeds from the Indian coast. J. Appl. Phycol. 2007, 19, 15–25. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Yoneshigue-Valentin, Y. Micropropagation as a tool for sustainable utilization and conservation of populations of Rhodophyta. Rev. Bras. Farmacogn. 2011, 21, 334–339. [Google Scholar] [CrossRef]
- Baweja, P.; Sahoo, D.; García-Jiménez, P.; Robaina, R.R. Review: Seaweed tissue culture as applied to biotechnology: Problems, achievements and prospects. Phycol. Res. 2009, 57, 45–58. [Google Scholar] [CrossRef]
- Reddy, C.R.K.; Jha, B.; Fujita, Y.; Ohno, M. Seaweed micropropagation techniques and their potentials: An overview. J. Appl. Phycol. 2007, 20, 159–167. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Rudke, A.R.; de Andrade, C.J.; Salvador Ferreira, S.R. Kappaphycus alvarezii macroalgae: An unexplored and valuable biomass for green biorefinery conversion. Trends Food Sci. Technol. 2020, 103, 214–224. [Google Scholar] [CrossRef]
- Shirosaki, M.; Koyama, T. Laminaria japonica as a food for the prevention of obesity and diabetes. Adv. Food Nutr. Res. 2011, 64, 199–212. [Google Scholar] [PubMed]
- Hurtado, A.Q.; Critchley, A.T. A review of multiple biostimulant and bioeffector benefits of AMPEP, an extract of the brown alga Ascophyllum nodosum, as applied to the enhanced cultivation and micropropagation of the commercially important red algal carrageenophyte Kappaphycus alvarezii and its selected cultivars. J. Appl. Phycol. 2018, 30, 2859–2873. [Google Scholar]
- Notoya, M.; Nagashima, M.; Aruga, Y. Influence of light intensity and temperature on callus development in young sporophytes of four species of Laminariales (Phaeophyta). Algae 1992, 7, 101–107. [Google Scholar]
- Kawashima, Y.; Tokuda, H. Callus formation in Ecklonia cava Kjellman (Laminariales, Phaeophyta). Hydrobiologia 1990, 204, 375–380. [Google Scholar] [CrossRef]
- Lawlor, H.J.; Mc Comb, J.A.; Borowitzka, M.A. Tissue culture of Ecklonia radiata (Phaeophyceae, Laminariales): Effects on growth of light, organic carbon source, and vitamins. J. Appl. Phycol. 1989, 1, 105–112. [Google Scholar] [CrossRef]
- Zayed, A.; Kovacheva, M.; Muffler, K.; Breiner, H.-W.; Stoeck, T.; Ulber, R. Induction and genetic identification of a callus-like growth developed in the brown alga Fucus vesiculosus. Eng. Life Sci. 2019, 19, 363–369. [Google Scholar] [CrossRef]
- Ramlov, F.; Plastino, E.M.; Yokoya, N.S. Growth, callus formation and plant regeneration in color morphs of Gracilaria domingensis (Gracilariales, Rhodophyta) cultured under different irradiance and plant growth regulators. Phycologia 2013, 52, 508–516. [Google Scholar] [CrossRef]
- Hwang, E.K.; Kim, C.H.; Sohn, C.H. Callus-like formation and differentiation in Hizikia fusiformis (Harvey) Okamura. Algae 1994, 9, 77–83. [Google Scholar]
- Reddy, C.R.K.; Kumar, G.R.K.; Siddhanta, A.K.; Tewari, A.; Eswaran, K. In vitro somatic embryogenesis and regeneration of somatic embryos from pigmented callus of Kappaphycus alvarezii (Doty) Doty (Rhodophyta, Gigartinales). J. Phycol. 2003, 39, 610–616. [Google Scholar] [CrossRef]
- Mo, V.T.; Cuong, L.K.; Tung, H.T.; Van Huynh, T.; Nghia, L.T.; Khanh, C.M.; Lam, N.N.; Nhut, D.T. Somatic embryogenesis and plantlet regeneration from the seaweed Kappaphycus striatus. Acta Physiol. Plant. 2020, 42, 104–114. [Google Scholar]
- Notoya, M.; Kim, H.G. Influence of light intensity and temperature on callus cell propagation and differentiation to bladelets from the explants of young sporophyte of Kjellmaniella crassifolia Miyabe (Phaeophyta, Laminariales). Algae 1996, 11, 179–182. [Google Scholar]
- Mizuta, H.; Kai, T.; Tabuchi, K.; Yasui, H. Effects of light quality on the reproduction and morphology of sporophytes of Laminaria japonica (Phaeophyceae). Aquac. Res. 2007, 38, 1323–1329. [Google Scholar]
- Tabuchi, K.; Mizuta, H.; Yasui, H. Promotion of callus propagation by 5-aminolevulinic acid in a Laminaria japonica sporophyte. Aquac. Res. 2009, 41, 1–10. [Google Scholar]
- Uji, T.; Nanaumi, D.; Kawagoe, C.; Saga, N.; Miyashita, K. Factors influencing the induction of adventitious bud and callus in the brown alga Sargassum horneri (Turner) C. Agardh. J. Appl. Phycol. 2015, 28, 2435–2443. [Google Scholar] [CrossRef]
- Mohammad, S.; Khan, M.A.; Ali, A.; Khan, L.; Khan, M.S.; Mashwani, Z.-R. Feasible production of biomass and natural antioxidants through callus cultures in response to varying light intensities in olive (Olea europaea L.) cult. Arbosana. J. Photochem. Photobiol. B Biol. 2019, 193, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Abbasi, B.H.; Khan, M.A.; Azeem, M. Production of biomass and useful compounds through elicitation in adventitious root cultures of Fagonia indica. Ind. Crops Prod. 2017, 108, 451–457. [Google Scholar] [CrossRef]
- Staehr, P.A.; Wernberg, T. Physiological responses of Ecklonia radiata (Laminariales) to and latitudinal gradient in ocean temperature. J. Phycol. 2009, 45, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Miki, O.; Okumura, C.; Marzuki, M.; Tujimura, Y.; Fujii, T.; Kosugi, C.; Kato, T. Contrasting effects of blue and red LED irradiations on the growth of Sargassum horneri during the germling and immature stages. J. Appl. Phycol. 2016, 29, 1461–1469. [Google Scholar] [CrossRef]
- Gusev, M.V.; Tambiev, A.H.; Kirikova, N.N.; Shelyastina, N.N.; Aslanyan, R.R. Callus formation in seven species of agarophyte marine algae. Mar. Biol. 1987, 95, 593–597. [Google Scholar] [CrossRef]
- Kawashima, Y.; Tokuda, H. Regeneration from callus of Undaria pinnatifida (Harvey) Suringar (Laminariales, Phaeophyta). Hydrobiologia 1993, 260, 385–389. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Reis, R.P.; Loureiro, R.R.; Critchley, A.T. Kappaphycus (Rhodophyta) cultivation: Problems and the impacts of Acadian marine plant extract powder. In Marine Algae: Biodiversity Taxonomy, Environmental Assessment and Biotechnology; Pereira, L., Neto, J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 251–291. [Google Scholar]
- Borlongan, I.A.; Gerung, G.S.; Kawaguchi, S.; Nishihara, G.N.; Terada, R. Thermal and PAR effects on the photosynthesis of Eucheuma denticulatum and Kappaphycus striatus (so-called Sacol strain) cultivated in shallow bottom of Bali, Indonesia. J. Appl. Phycol. 2016, 29, 395–404. [Google Scholar] [CrossRef]
- Borlongan, I.A.; Matsumoto, K.; Nakazaki, Y.; Shimada, N.; Kozono, J.; Nishihara, G.N.; Shimada, S.; Watanabe, Y.; Terada, R. Photosynthetic activity of two life history stages of Costaria costata (Laminariales, Phaeophyceae) in response to PAR and temperature gradient. Phycologia 2018, 57, 159–168. [Google Scholar] [CrossRef]
- Taniguchi, K.; Agatsuma, Y. Marine afforestation of the kelp Eisenia bicyclis in coralline flats. Aquac. Sci. 2001, 49, 133–136. [Google Scholar]
- Kirihara, S.; Fujikawa, Y.; Notoya, M. Effect of the temperature and light intensity on the growth of zoospore germling of Kjellmaniella crassifolia Miyabe (Laminariales, Phaeophyceae) in culture. Aquac. Sci. 2003, 51, 281–286. [Google Scholar]
- Zhang, L.; Cui, C.; Li, X.; Zhang, Z.; Luo, S.; Liang, G.; Liu, Y.; Yang, G. Effect of temperature on the development of Saccharina japonica gametophytes. J. Appl. Phycol. 2013, 25, 261–267. [Google Scholar] [CrossRef]
- Morita, T.; Kurashima, A.; Maegawa, M. Temperature requirements for the growth and maturation of the gametophytes of Undaria pinnatifida and U. undarioides (Laminariales, Phaeophyceae). Phycol. Res. 2003, 51, 154–160. [Google Scholar] [CrossRef]
- Baweja, P.; Sahoo, D. Regeneration studies in Grateloupia filicina (J.V. Lamouroux) C. Agardh—An important carrageenophyte and edible seaweed. Algae 2009, 24, 163–168. [Google Scholar] [CrossRef][Green Version]
- Sulistiani, E.; Soelistyowati, D.T.; Alimuddin, Y.S. Callus induction and filaments regeneration from callus of cottonii seaweed Kappaphycus alvarezii (Doty) collected from Natuna Islands, Riau Islands Province. Biotropia 2012, 19, 103–114. [Google Scholar]
- Hayashi, L.; Yokoya, N.S.; Kikuchi, D.M.; Oliveira, E.C. Callus induction and micropropagation improved by colchicine and phytoregulators in Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J. Appl. Phycol. 2008, 20, 653–659. [Google Scholar] [CrossRef]
- Xi-hua, W.; Song, Q.; Xin-ping, L.; Peng, J.; Cheng-kui, Z.; Mei, Q. High efficiency induction of callus and regeneration of sporophytes of Laminaria japonica (Phaeophyta). Chin. J. Oceanol. Limnol. 1998, 16, 67–74. [Google Scholar] [CrossRef]
- Kanamori, M.; Mizuta, H.; Yasui, H. Effects of ambient calcium concentration on morphological form of callus-like cells in Saccharina japonica (Phaeophyceae) sporophyte. J. Appl. Phycol. 2011, 24, 701–706. [Google Scholar] [CrossRef]
- Kaczyna, F.; Megnet, R. The effects of glycerol and plant growth regulators on Gracilaria verrucosa (Gigartinales, Rhodophyceae). Hydrobiologia 1993, 268, 57–64. [Google Scholar] [CrossRef]
- Tatewaki, M. Formation of a crustaceous sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 1966, 6, 62–66. [Google Scholar] [CrossRef]
- Fries, L. Growth regulating effects of phenylacetic acid and phydroxyphenilacetic acid on Fucus spiralis in axenic culture. Phycologia 1977, 16, 451–455. [Google Scholar] [CrossRef]
- Saga, N.; Motomura, T.; Sakai, Y. Induction of callus from the marine brown alga Dictyosiphon foeniculaceus. Plant Cell Physiol. 1982, 23, 727–730. [Google Scholar]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Jamwal, K.; Bhattacharya, S.; Puri, S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants 2018, 9, 26–38. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Ávila, M.; Piel, M.I.; Villanueva, F.; Alcapan, A. Effects of plant growth regulators on growth and morphogenesis in tissue culture of Chondracanthus chamissoi (Gigartinales, Rhodophyta). J. Appl. Phycol. 2013, 26, 819–823. [Google Scholar] [CrossRef]
- Dawes, C.J.; Koch, E.W. Branch, micropropagule and tissue culture of the red algae Eucheuma denticulatum and Kappaphycus alvarezii farmed in the Philippines. J. Appl. Phycol. 1991, 3, 247–257. [Google Scholar] [CrossRef]
- Yokoya, N.S.; West, J.A.; Luchi, A.E. Effects of plant growth regulators on callus formation, growth and regeneration in axenic tissue cultures of Gracilaria tenuistipitata and Gracilaria perplexa (Gracilariales, Rhodophyta). Phycol. Res. 2004, 52, 244–254. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Kakita, H.; Obika, H.; Kitamura, T. Effects of environmental factors and plant growth regulators on growth of the red alga Gracilaria vermiculophylla from Shikoku Island, Japan. Hydrobiologia 1999, 398, 339–347. [Google Scholar] [CrossRef]
- Yokoya, N.S. Apical callus formation and plant regeneration controlled by plant growth regulators on axenic culture of the red alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Phycol. Res. 2000, 48, 133–142. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Handro, W. Effects of auxins and cytokinins on tissue culture of Grateloupia dichotoma (Gigartinales, Rhodophyta). Hydrobiologia 1996, 326, 393–400. [Google Scholar] [CrossRef]
- Yeong, H.-Y.; Phang, S.-M.; Reddy, C.R.K.; Khalid, N. Production of clonal planting materials from Gracilaria changii and Kappaphycus alvarezii through tissue culture and culture of G. changii explants in airlift photobioreactors. J. Appl. Phycol. 2014, 26, 729–746. [Google Scholar] [CrossRef]
- Munoz, J.; Cahue-López, A.C.; Patiño, R.; Robledo, D. Use of plant growth regulators in micropropagation of Kappaphycus alvarezii (Doty) in airlift bioreactors. J. Appl. Phycol. 2006, 18, 209–218. [Google Scholar] [CrossRef]
- Mussio, I.; Rusig, A.-M. Morphogenetic responses from protoplasts and tissue culture of Laminaria digitata (Linnaeus) J. V. Lamouroux (Laminariales, Phaeophyta): Callus and thalloid-like structures regeneration. J. Appl. Phycol. 2008, 21, 255–264. [Google Scholar] [CrossRef]
- Yoon, J.T.; Soh, W.Y. Developmental morphology on the regeneration of Pelvetia siliquosa Tseng et Chang (Phaeophyta) in Korea. Algae 1998, 13, 261–270. [Google Scholar]
- Muhamad, S.N.S.; Ling, A.P.-K.; Wong, C.-L. Effect of plant growth regulators on direct regeneration and callus induction from Sargassum polycystum C. Agardh. J. Appl. Phycol. 2018, 30, 3299–3310. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M.P. In vitro plant propagation: A review. J. For. Sci. 2011, 27, 61–72. [Google Scholar]
- Izumi, K.; Kamiya, Y.; Sakurai, A.; Oshio, H.; Takahashi, N. Studies of sites of action of a new plant growth retardant (E)–1–(4–chlorophenyl)–4,4–dimethyl–2–(1,2,4–triazol–1–yl)–1–penten–3–ol (s–3307) and comparative effects of its stereoisomers in a cell– free system from Cucurbita maxima. Plant Cell Physiol. 1985, 26, 821–827. [Google Scholar]
- Iwasaki, T.; Shibaoka, H. Brassinosteroids act as regulators of tracheary–element differentiation in isolated Zinnia mesophyll–cells. Plant Cell Physiol. 1991, 32, 1007–1014. [Google Scholar] [CrossRef]
- Saito, S.; Okamoto, M.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Hirai, N.; Todoroki, Y.; Sakata, K.; Nambara, E.; Mizutani, M. A plant growth retardant, uniconazole, is a potent inhibitor of ABA catabolism in Arabidopsis. Biosci. Biotechnol. Biochem. 2006, 70, 1731–1739. [Google Scholar] [CrossRef]
- Hofmannova, J.; Schwarzerova, K.; Havelkova, L.; Borikova, P.; Petrasek, J.; Opatrny, Z. A novel, cellulose synthesis inhibitory action of ancymidol impairs plant cell expansion. J. Exp. Bot. 2008, 59, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Ogura, T.; Takei, K.; Kojima, M.; Kitahata, N.; Sakakibara, H.; Asami, T.; Shimada, Y. Uniconazole, a cytochrome P450 inhibitor, inhibits trans–zeatin biosynthesis in Arabidopsis. Phytochemistry 2013, 87, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-J.; Seo, G.-M.; Cho, Y.C.; Hwang, E.; Sohn, C.H.; Hong, Y.-K. Gelling agents for tissue culture of the seaweed Hizikia fusiformis. J. Appl. Phycol. 1997, 9, 489–493. [Google Scholar]
- Robaina, R.R.; Garcia-Reina, G.; Luque, A. The effects of the physical characteristics of the culture medium on the development of red seaweeds in tissue culture. Hydrobiologia 1990, 204, 137–142. [Google Scholar] [CrossRef]
- Robledo, D.R.; Garcia-Reina, G. Apical callus formation in Solieria filiformis (Gigartinales, Rhodophyta) cultured in tanks. Hydrobiologia 1993, 260, 401–406. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.-H.; Jung, S.-P.; Choi, S.-J.; Chung, I.K.; Shin, J.-A. Cultivation of Laminaria japonica (Laminariales, Phaeophyta) in Udo Coast, Jeju, Korea. Algae 2005, 20, 167–176. [Google Scholar] [CrossRef]
- Hwang, E.K.; Liu, F.; Lee, K.H.; Ha, D.S.; Park, C.S. Comparison of the cultivation performance between Korean (Sugwawon No. 301) and Chinese strains (Huangguan No. 1) of kelp Saccharina japonica in an aquaculture farm in Korea. Algae 2018, 33, 101–108. [Google Scholar] [CrossRef]
- Karsten, U. Research note: Salinity tolerance of Arctic kelps from Spitsbergen. Phycol. Res. 2007, 55, 257–262. [Google Scholar] [CrossRef]
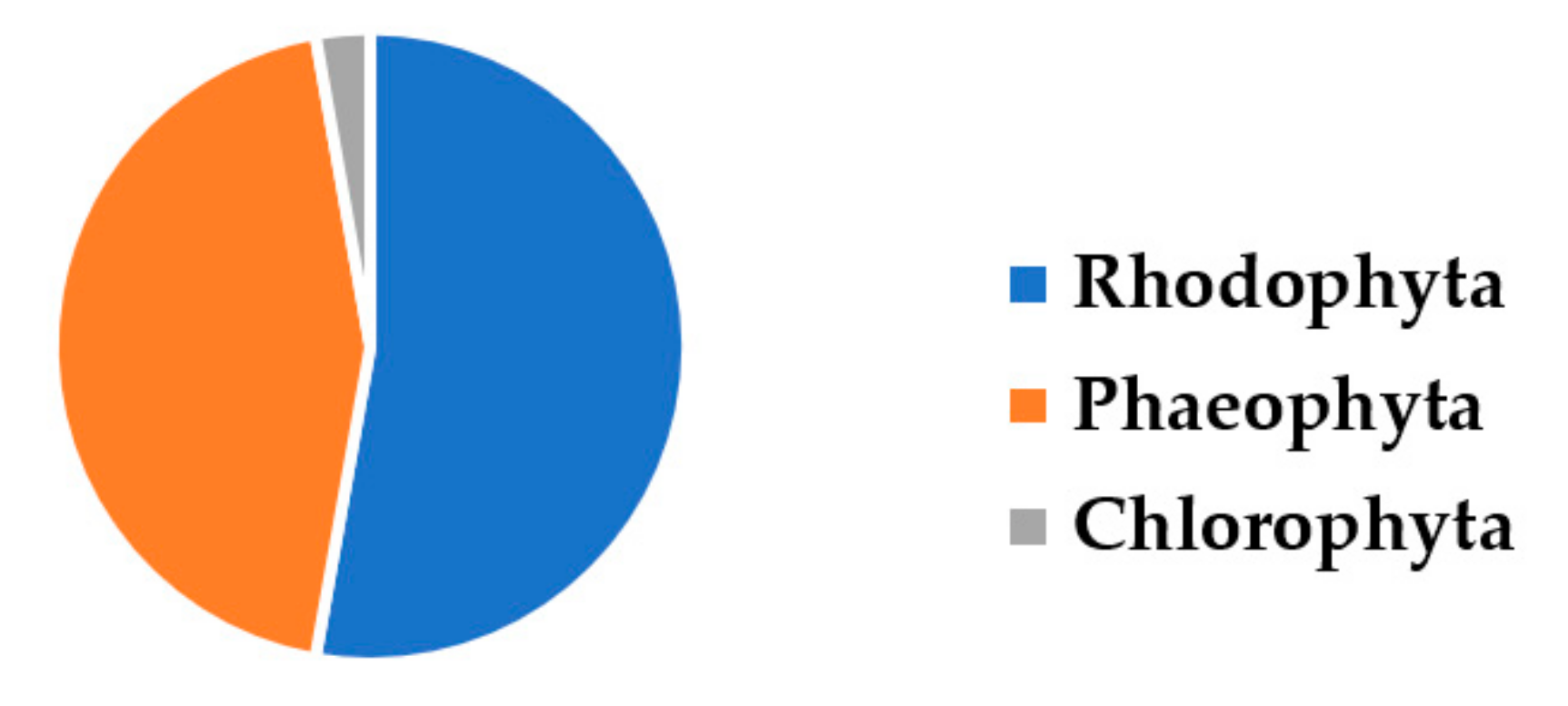
| Species | Light Condition | Result | Reference |
|---|---|---|---|
| Costaria costata | 13.5, 27, 54, and 108 µmol m−2 s−1 for 14 h. | Callus was induced optimally at 13.5–27 µmol m−2 s−1, and decreased at 108 µmol m−2 s−1. | [34] |
| Ecklonia cava | 160 µmol m−2 s−1 for 0 and 24 h. | Stipe explants induced higher callus in continuous dark (0 h) than continuous light (24 h). | [35] |
| Ecklonia radiata | 0, 3, 10, 20, 30, and 50 µmol m−2 s−1 for 24 h. | Unpigmented explants induced callus best at 0 µmol m−2 s−1; pigmented explants induced callus best at 30 µmol m−2 s−1. | [36] |
| Ecklonia radiata | Dark, red, blue, green, and white light. | Unpigmented explants induced callus best in the dark; pigmented explants induced best in red and white light. | [36] |
| Eisenia bicyclis | 13.5, 27, 54, and 108 µmol m−2 s−1 for 14 h. | Callus was induced optimally at 13.5 µmol m−2 s−1; thereafter, the induction decreased. | [36] |
| Fucus vesiculosus | 35, 45, and 60 µmol m−2 s−1 for 12, 14, and 16 h. | Only explants treated with 35 µmol m−2 s−1 for 14 h induced callus. | [37] |
| Gracilaria corticata | 5, 30, and 70 µmol m−2 s−1 for 12 h. | Explant induced callus (40%) only at 30 µmol m−2 s−1. | [26] |
| Gracilaria domingensis | 50 and 150 µmol m−2 s−1 for 14 h. | Apical segment induced callus (63%) optimally at 50 µmol m−2 s−1, but intercalary segment induced callus (81%) optimally at 150 µmol m−2 s−1. | [38] |
| Hizikia fusiformis | 7 and 27 µmol m−2 s−1 for 24 h. | Explant at 27 µmol m−2 s−1 induced callus better than at 6.75 µmol m−2 s−1. | [39] |
| Hypnea musciformis | 5, 30, and 70 µmol m−2 s−1 for 12 h. | Explant induced callus at 5 and 30 µmol m−2 s−1 by 10%. | [26] |
| Kappaphycus alvarezii | 5, 30, and 70 µmol m−2 s−1 for 12 h. | Both 5 and 30 µmol m−2 s−1 were suitable, but 70 µmol m−2 s−1 caused bleaching. | [40] |
| Kappaphycus striatus | 5 µmol m−2 s−1 for 12 h. | Explants induced callus 54–61%. | [41] |
| Kjellmaniella crassifolia | 0, 10, 20, 40, and 80 µmol m−2 s−1 for 14 h. | Highest growth rate of callus at 10 µmol m−2 s−1. | [42] |
| Saccharina japonica | White, red, and blue light. | Explants induced callus higher in red light (85.9%) than in white (48.0%) and blue (43.0%) lights. | [43] |
| Saccharina japonica | 13.5, 27, 54, and 108 µmol m−2 s−1 for 14 h. | Callus was induced optimally at 13.5 µmol m−2 s−1; an amount exceeding this caused the induction to decrease, and at 108 µmol m−2 s−1, the callus was not induced. | [34] |
| Saccharina japonica | 0, 15, 30, 60, and 120 µmol m−2 s−1 for 12 h. | 0–30 µmol m−2 s−1 induced callus (50–76%); optimal at 30 µmol m−2 s−1. Induction decreased at ≥60 µmol m−2 s−1. | [44] |
| Sargassum horneri | White, red, and blue light. | Explants induced callus better in blue light (45%) than in white (42.5%) and red (27.5%) lights. | [45] |
| Sargassum horneri | 20, 80, and 200 µmol m−2 s−1 for 14 h. | Highest callus induction rate of 50% at 20 µmol m−2 s−1. | [45] |
| Sargassum tenerrimum | 5, 30, and 70 µmol m−2 s−1 for 12 h. | Explant induced callus (10%) only at 30 µmol m−2 s−1. | [26] |
| Turbinaria conoides | 5, 30, and 70 µmol m−2 s−1 for 12 h. | Explant induced callus (40%) only at 30 µmol m−2 s−1. | [26] |
| Undaria pinnatifida | 13.5, 27, 54, and 108 µmol m−2 s−1 for 14 h. | Callus was induced optimally at 13.5–27 µmol m−2 s−1, after which the induction decreased, and at 108 µmol m−2 s−1, the callus was not induced. | [34] |
| Species | Temperature Condition | Result | Reference |
|---|---|---|---|
| Ceramium kondoi | 12–15, 18–20, and 26 °C | Highest callus induction rate 2% at 12–15 °C. | [50] |
| Costaria costata | 10, 15, 20, and 25 °C | Callus was induced optimally at 15 °C. At 25 °C, callus gradually died. | [34] |
| Ecklonia cava | 8, 13, 18, and 23 °C | Meristem explants induced callus at 13 °C (86%) better than at 18 °C (29%). Stipe explants induced callus better at temperatures ranging from 8–13 °C. | [35] |
| Eisenia bicyclis | 10, 15, 20, and 25 °C | The induced callus increased with increasing temperature, with an optimum at 20 °C. At 25 °C, the induction decreased. | [34] |
| Furcellaria fastigiata | 12–15, 18–20, and 26 °C | Highest callus induction rate 16% at 18–20 °C. | [50] |
| Gelidium vagum | 12–15, 18–20, and 26 °C | Highest callus induction rate 15% at 18–20 °C. | [50] |
| Gracillaria verrucosa | 12–15, 18–20, and 26 °C | Highest callus induction rate 4% at 18–20 °C. | [50] |
| Kappaphycus striatus | 24 ± 2 °C | Explants induced callus 54–61%. | [41] |
| Kjellmaniella crassifolia | 5, 10, 15, 20, and 25 °C | Explant induced callus and grew at 5–15 °C, highest growth at 10 °C. At 20–25 °C, explants died. | [42] |
| Phyllophora nervosa | 12–15, 18–20, and 26 °C | Highest callus induction rate 18% at 18–20 °C. | [50] |
| Saccharina japonica | 10, 15, 20, and 25 °C | Callus was induced optimally at 15 °C. Explants died at 25 °C. | [34] |
| Saccharina japonica | 5, 10, 15, and 20 °C | Callus was induced optimally at 10 °C. Increasing temperature higher than 10 °C decreased callus induction. | [44] |
| Sargassum horneri | 5, 20, and 30 °C | Highest callus induction rate 37.5% at 20 °C. | [45] |
| Undaria pinnatifida | 8, 13, and 18 °C | Stipe induced callus better at 13–18 °C (20%), compared with 8 °C (10%). Meristem induced best at 13 °C (33%), compared with 8 and 18 °C (20 and 22%, respectively). | [51] |
| Undaria pinnatifida | 10, 15, 20, and 25 °C | Callus was induced optimally at 15 °C. Explants died at 25 °C. | [34] |
| Species | Media Type | Result | Reference |
|---|---|---|---|
| Cytoseira osmundacea | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in MS medium, but induced in seawater (10%), PES (9%), TC-1 (11%), ASP-C-1 (4%), ASP-12-NTA (2%), and ASP-6-F2 (4%). | [23] |
| Ecklonia cava | SWA, SWII, SWIICH, PESI, ASP 12, and ASP-C-1. | Stipe and meristem did not induce callus in ASP-C-1. In other media, the callus was induced without any particular media preference. | [35] |
| Ecklonia radiata | 0.7% agar PES medium supplemented with different carbon sources (1 and 10 mM): arabinose, aspartic acid, glyceric acid, glycerol, mannitol, mannose, and sodium pyruvate. | Growth of callus of unpigmented cells was inhibited in the dark by aspartic acid (1 and 10 mM), mannitol, or glycerol (10 mM); other carbon sources had no effect. In light, all the carbon sources stimulated the callus growth. In pigmented cells, only glycerol showed a small stimulatory effect. | [36] |
| Ecklonia radiata | 0.7% agar PES medium supplemented with different vitamin sources (0, 0.1, 1, 10, 100, and 1000 µg L−1): biotin, thiamine HCl, nicotinic acid, cyanocobalamin, pyridoxine HCl, and tocopherol. | Nicotinic acid or thiamine (1 µg L−1) or biotin (10–100 µg L−1) supported callus growth of unpigmented cells. Other vitamins had no effect. | [36] |
| Egregia menziesii | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in TC-1 and MS, but was induced in seawater (5%), PES (9%), ASP-C-1 (1%), ASP-12-NTA (7%), and ASP-6-F2 (1%). | [23] |
| Enteromorpha intestinalis | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus induced in all media 83–91%. Highest rate in seawater. | [23] |
| Eucheuma uncinatum | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in seawater and ASP-C-1, but was induced in PES (3%), TC-1 (1%), MS (1%), ASP-12-NTA (3%), and ASP-6-F2 (2%). | [23] |
| Gelidium robustum | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in seawater and TC-1, and ASP-12-NTA, but was induced in PES (2%), MS (1%), ASP-C-1 (0.3%), and ASP-6-F2 (0.5%). | [23] |
| Gigartina exasperata | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in MS, ASP-C-1 and ASP-6-F2, but was induced in seawater (1%), PES (3%), TC-1 (1%), and ASP-12-NTA (1%). | [23] |
| Gracilaria papenfussii | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in seawater and TC-1, and ASP-C-1 medium, but was induced in PES (0.5%), MS (0.6%), ASP-12-NTA (2%), and ASP-6-F2 (0.3%). | [23] |
| Grateloupia filicina | f/2, f/2 + glycerol (0.5, 1.0, and 1.5%), and f/2 + NAA or BAP or kinetin (10−5, 10−6, and 10−7 M) in 1.5% agar. | Explants only induced callus in f/2 (30%), f/2 + glycerol 0.5% (70%), f/2 + glycerol 1.0% (10%), f/2 + NAA 10−5 M (40%), f/2 + NAA 10−6 M (25%), and f/2 + BAP 10−6 M (30%). | [59] |
| Grateloupia filicina | PES, PES + glycerol (0.5, 1.0, and 1.5%), and PES + NAA or BAP or kinetin (10−5, 10−6 and 10−7 M) in 1.5% agar. | Explants only induced callus in PES (20%), PES + glycerol 0.5% (25%), PES + glycerol 1.0% (10%), PES + glycerol 1.5% (10%), PES + NAA 10−5 M (25%), PES + NAA 10−6 M (10%), and PES + kinetin 10−5 M (5%). | [59] |
| Grateloupia filicina | ESW, ESW + glycerol (0.5, 1.0, and 1.5%), and ESW + NAA or BAP or kinetin (10−5, 10−6 and 10−7 M) in 1.5% agar. | Explants only induced callus in ESW (10%) | [59] |
| Kappaphycus alvarezii | CW medium in 0.8% agar. | Explants induced callus 40%. | [60] |
| Kappaphycus alvarezii | PES medium in 1.5% agar. | Explants induced callus 82%. | [40] |
| Kappaphycus alvarezii | 50% VS, f/2, and ASP-12-NTA in 0.5% agar. | Explants induced callus highest in 50% VS (>95%) and lowest in ASP-12-NTA (~35%). In f/2 medium, induced callus was up to 82%. | [61] |
| Kappaphycus alvarezii | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus induced in all media 0.8–10%. Highest and lowest rates in PES and ASP-6-F2, respectively. | [23] |
| Kappaphycus striatus | PES medium in 1.5% agar. | Explants induced callus 54–61%. | [41] |
| Laminaria sinclairii | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in ASP-C-1 medium, but was induced in seawater (28%), PES (23%), TC-1 (9%), MS (4%), ASP-12-NTA (3%), and ASP-6-F2 (25%). | [23] |
| Macrocystis pyrifera | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in ASP-C-1 medium, but was induced in seawater (30%), PES (35%), TC-1 (11%), MS (2%), ASP-12-NTA (14%), and ASP-6-F2 (20%). | [23] |
| Pelvetia fastigiata | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in MS, ASP-C-1, and ASP-6-F2 medium, but was induced in seawater (20%), PES (21%), TC-1 (18%), and ASP-12-NTA (6%). | [23] |
| Porphyra lanceolata | MS, ASP-C-1, ASP 12 NTA, or ASP-6-F2 in 1.5% agar medium. | Callus induced in all media 75–84%. Highest rate in ASP-6-F2 medium. | [23] |
| Porphyra nereocystis | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus induced in all media 70–90%. Highest and lowest rates in ASP-12-NTA and ASP-6-F2, respectively. | [23] |
| Porphyra perforata | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus induced in all media 76–89%. Highest rate in ASP-12-NTA. | [23] |
| Prionitis lanceolata | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in seawater, TC-1, MS, and ASP-6-F2 medium, but was induced in PES (1%), ASP-C-1 (0.8%), and ASP-12-NTA (0.3%). | [23] |
| Saccharina japonica | MS-S-V, PESI, and ASP-C-1 in 1.5% agar medium and MS liquid medium. | Callus induced only in MS-S-V (67.3%) and PESI (75.5%). | [62] |
| Saccharina japonica | ASP-12-NTA in 0.5% agar with various Ca2+ concentrations (2.5–15 mM). | 5 mM of Ca2+ supplementation was the highest callus induction rate (46.3%); a concentration higher than this decreased its induction rate. | [63] |
| Sargassum fluitans | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in ASP-6-F2, but was induced in seawater (15%), PES (12%), TC-1 (12%), MS (2%), ASP-C-1 (1%), and ASP-12-NTA (4%). | [23] |
| Sargassum muticum | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus was not induced in ASP-C-1, but was induced in seawater (25%), PES (27%), TC-1 (23%), MS (0.7%), ASP-12-NTA (5%), and ASP-6-F2 (7%). | [23] |
| Smithora naiadum | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus induced in all media 5–85%. Highest and lowest rates in seawater and ASP-6-F2, respectively. | [23] |
| Ulva angusta | Seawater, PES, TC-1, MS, ASP-C-1, ASP-12-NTA, or ASP-6-F2 in 1.5% agar medium. | Callus induced in all media 80–92%. Highest rate was in MS. | [23] |
| Undaria pinnatifida | SWA, SWII, PESI, and ASP 12. | Stipe and meristem induced callus best in PESI (20 and 30%, respectively). | [51] |
| Species | PGR Treatment | Result | Reference |
|---|---|---|---|
| Chondracanthus chamissoi | 0.5–50 µM of IAA, 2,4-D and BAP. | Highest callus induction (6–8%) with BAP 50 µM in the apical segment, but IAA 50 µM showed the highest callus induction rate (18–20%) in the intercalary segment. | [71] |
| Eucheuma denticulatum | Combination of NAA or PAA (0–10 mg L−1) and 2iP (0–1 mg L−1) or BAP (1 mg L−1). | 0.1–1.0 mg L−1 of NAA and 1.0 mg L−1 of BAP induced callus, but explants were killed by 10 mg L−1 of NAA. | [72] |
| Gracilaria perplexa | 0.1–100.0 µM of IAA, 2,4-D and KIN. | 1 µM of IAA, 2,4-D or kinetin was optimal in increasing the callus induction of intercalary segments. | [73] |
| Gracilaria tenuistipitata | 0.1–100.0 µM of IAA, 2,4-D and KIN. | 100.0 µM of IAA or 2,4-D optimally induced callus on the apical segments (>80%). Kinetin decreased the induction rate at higher concentrations. | [73] |
| Gracilaria vermiculophylla | 0.1 and 1.0 mg L−1 of IAA, 0.1, 1.0 and 10.0 mg L−1 of 2,4-D, 0.1 and 1.0 mg L−1 of BAP, and the combinations 0.1 + 0.1, 0.1 + 1.0, 1.0 + 0.1 and 1.0 + 1.0 mg L−1 of IAA + BAP. | 0.1 mg L−1 of IAA; 0.1 mg L−1 of 2,4-D; and 1 mg L−1 of BAP were the best for callus growth in apical segments. In the intercalary segments, 0.1 mg L−1 of IAA, 10.0 mg L−1 of 2,4-D, and the combinations 0.1 + 0.1, 0.1 + 1.0 and 1.0 + 0.1 (IAA + BAP) were optimum for callus growth. | [74] |
| Gracilariopsis tenuifrons | 0.5 and 5.0 mg L−1 of IAA, 0.5 and 5.0 mg L−1 of BAP, and 1:1, 1:5 and 5:1 mg L−1 of IAA:BAP. | 0.5 mg L−1 of IAA and 5:1 mg L−1 of IAA:BAP showed the highest callus formation in apical segments. A total of 5.0 mg L−1 of IAA stimulated the highest callus formation in intercalary segments. | [75] |
| Grateloupia dichotoma | 0.5 and 5.0 mg L−1 of IAA, 0.5 and 5.0 mg L−1 of 2,4-D, 0.5 and 5.0 mg L−1 of BAP, 1:5 and 5:1 mg L−1 of IAA:BAP. | All PGRs induced callus, but the maximal induction was using 2,4-D or IAA:BAP (1:5 mg L−1). | [76] |
| Kappaphycus alvarezii | 0–25 mg L−1 of 2,4-D in solid PES (1%). | 5 mg L−1 of 2,4-D induced the highest rate of callus formation (50%). | [77] |
| Kappaphycus alvarezii | Combination of 2,4-D (0.1 and 1.0) and KIN (0.1 and 1.0 mg L−1). | 0.1 mg L−1 of 2,4-D and 1 mg L−1 of kinetin induced the highest rate of callus formation (40.7%). | [77] |
| Kappaphycus alvarezii | 0.5–1 mg L−1 of BAP and 0.5–1 mg L−1 of NAA in solid CW (0.8%). | Explants induced callus 10–60%. | [60] |
| Kappaphycus alvarezii | 2.5–5 mg L−1 of IAA in solid CW (0.8%). | Explants induced callus 0–20%. | [60] |
| Kappaphycus alvarezii | 2.5–5 mg L−1 of IAA in solid PES (0.8%). | Explants induced callus 40–50%. | [60] |
| Kappaphycus alvarezii | 0.5–1 mg L−1 of BAP in solid PES (0.8%). | Explants induced callus 30–70%. | [60] |
| Kappaphycus alvarezii | 1 mg mL−1 of BAP and 2.5 mg L−1 of IAA in solid CW and PES (0.8%). | Explants induced callus 30–50%. | [60] |
| Kappaphycus alvarezii | 0.5 mg mL−1 of BAP and 0–1 mg L−1 of NAA in solid PES (0.8%). | Explants induced callus 40–50%. | [60] |
| Kappaphycus alvarezii | 0.5–1 mg L−1 of NAA in solid CW and PES (0.8%). | Explants induced callus 20–50%. | [60] |
| Kappaphycus alvarezii | 0.1–1 mg L−1 of NAA or BAP in solid PES media. | Explants induced callus 84%. | [40] |
| Kappaphycus alvarezii | Combination of NAA or PAA (0–10 mg L−1) and 2iP (0–1 mg L−1) or BAP (1 mg L−1). | The presence of 2iP induced callus. Increasing NAA to 1 mg mL−1 favored callus induction. The combination of NAA or PAA (1 mg L−1) and BAP (1 mg L−1) was as effective as NAA and 2iP in inducing callus. NAA and PAA at higher concentration (10 mg L−1) decreased the callus induction rate. | [72] |
| Kappaphycus alvarezii | Sixteen combinations of NAA (0 or 1 mg L−1), KIN (0 or 1 mg L−1), BAP (0 or 1 mg L−1), and spermine (0 or 0.0018 mg L−1). | NAA or BAP at 1 mg L−1 induced calluses at a 129% higher rate than the control. The combination of NAA, kinetin, and spermine (1, 1, and 0.018 mg L−1, respectively) increased callus induction 85% higher than the control. | [78] |
| Laminaria digitata | 2,4-D (0.45–45 μM), NAA (0.53–53 μM), PIC (0.04–4 μM)/BAP (0.44 μM), NAA (0.53 μM)/CPPU (0.04–4 μM), PIC (4 μM)/KIN (0.46–2.3 μM), PIC (4 μM)/CPPU (0.4–2 μM), PIC (4 μM)/ZEA (0.45–0.9 μM), PIC (4 μM)/CPPU (0.45–0.9 μM). | High callus induction (38–50%) in media containing 2,4-D alone (0.45–45 μM). The combination of Pi (4 μM)–CPPU (2 μM) and CPPU (4 μM)–NAA (0.54 μM) also induced callus at a high rate (50 and 29.5%, respectively). | [79] |
| Pelvetia siliquosa | 1, 2, 3, 4, and 5 mg L−1 of IAA | Callus induced (90%) optimally at an IAA concentration of 1–3 mg L−1. More than 3 mg L−1 of IAA drastically decreased callus induction (~5%). | [80] |
| Saccharina japonica | 0, 1, 5, 10, 50, 50, 100, and 500 mg L−1 of ALA. | 100 mg L−1 of ALA was optimal to induce callus in apical (58.2%), middle (94.1%), and basal (100%) segments. | [44] |
| Sargassum horneri | IAA, 2,4-D, BAP, GA, and 5–10 µM of UNI. | IAA, 2,4-D, BAP, and GA treatments did not induce calluses, but 5 µM of uniconazole induced the highest number of calluses. | [45] |
| Sargassum polycystum | 4.52–22.62 µM of 2,4-D, 5.71–28.54 µM of IAA, 4.92–24.60 µM of IBA, 5.37–26.85 of NAA, 1.00–10.00 µM of PIC, 4.44–22.20 µM of BAP, 4.65–23.23 µM of KIN, 1.00–10.00 CPPU, 1.00–10.00 µM of UNI. | Calluses were only induced in media containing kinetin at 4.65–13.94 µM (11.11–14.81%) or UNI 3–10 µM (14.81–22.22%). UNI at 7 µM caused the highest induction rate (22.22%) of all treatments. | [81] |
| Species | Gelling Condition | Result | Reference |
|---|---|---|---|
| Cytoseira osmundacea | 0 or 1.5% of agar. | No calli were induced from explants in liquid media, but in 1.5% agar media, explants showed callus induction up to 11%. | [23] |
| Egregia menziesii | 0 or 1.5% of agar. | No calli were induced from explants in liquid media, but in 1.5% agar media, explants showed callus induction up to 9%. | [23] |
| Enteromorpha intestinalis | 0 or 1.5% of agar. | Callus induction rate of 0.07–5% from explants in liquid media, but in 1.5% agar media, explants showed callus induction up to 91%. | [23] |
| Eucheuma uncinatum | 0 or 1.5% of agar. | No calli were induced from explants in liquid media, but in 1.5% agar media, explants showed callus induction up to 3%. | [23] |
| Gelidium robustum | 0 or 1.5% of agar. | No calli were induced from explants in liquid media, but in 1.5% agar media, explants showed callus induction up to 2%. | [23] |
| Gigartina exasperata | 0 or 1.5% of agar. | No calli were induced from explants in liquid media, but in 1.5% agar media, explants showed callus induction up to 3%. | [23] |
| Gracilaria papenfussii | 0 or 1.5% of agar. | No calli were induced from explants in liquid media, but in 1.5% agar media, explants showed callus induction up to 2%. | [23] |
| Grateloupia doryphora | 0.3, 0.8 or 1.5% of agar. | Increasing the agar concentration increased the callus formation, optimal range 0.8–1.5% of agar. | [89] |
| Hizikia fusiformis | 0.1, 0.2, 0.5, 0.7, 1.0, or 1.5% of agar or agarose or alginic acid or high gel strength agar or phytagel or purified agar or transfer agar. | The highest induction rate (47%) occurred with 1% agar and 2% high gel strength agar. All gelling agents showed induction rates ≤ 20% at concentrations less than 1%. | [88] |
| Kappaphycus alvarezii | 0.8–3% of agar | Explants at media with 0.8% agar showed a low callus induction rate. The induction rate was high at 1.5% agar (82%), and decreased at 3% agar (64%). | [40] |
| Kappaphycus alvarezii | 0, 0.8, or 1.0% of agar PES medium | Explants induced calluses only on agar 0.8 and 1.0% (20 and 38%, respectively). | [60,77] |
| Kappaphycus alvarezii | 0 or 1.5% of agar. | Explants in liquid media showed a callus induction up to 2%, but in 1.5% agar media, they showed callus induction up to 10%. | [23] |
| Laminaria sinclairii | 0 or 1.5% of agar. | Explants in liquid media induced no calli, but in 1.5% agar media, showed callus induction up to 28%. | [23] |
| Laurencia sp. | 0.3, 0.8, or 1.5% of agar. | The increasing of agar concentration increased the callus formation; optimal at 0.8–1.5% of agar. | [89] |
| Macrocystis pyrifera | 0 or 1.5% of agar. | Explants in liquid media showed callus induction by up to 2%, but in 1.5% agar media, they showed callus induction up to 35%. | [23] |
| Pelvetia fastigiata | 0 or 1.5% of agar. | Explants in liquid media induced no calli, but in 1.5% agar media, they showed callus induction up to 20%. | [23] |
| Porphyra lanceolata | 0 or 1.5% of agar. | Explants in liquid media induced calluses up to 2%, but in 1.5% agar media, they showed callus induction up to 84%. | [23] |
| Porphyra nereocystis | 0 or 1.5% of agar. | Explants in liquid media induced calluses up to 4%, but in 1.5% agar media, they showed callus induction up to 90%. | [23] |
| Porphyra perforata | 0 or 1.5% of agar. | Explants in liquid media induced calluses up to 4%, but in 1.5% agar media, they showed callus induction up to 89%. | [23] |
| Prionitis lanceolata | 0 or 1.5% of agar. | Explants in liquid media induced no calli, but in 1.5% agar media, they showed callus induction up to 1%. | [23] |
| Sargassum fluitans | 0 or 1.5% of agar. | Explants in liquid media induced no calli, but in 1.5% agar media, they showed callus induction up to 15%. | [23] |
| Sargassum muticum | 0 or 1.5% of agar. | Explants in liquid media induced callus up to 0.1%, but in 1.5% agar media, they showed callus induction up to 27%. | [23] |
| Smithora naiadum | 0 or 1.5% of agar. | Explants in liquid media induced callus up to 4%, but in 1.5% agar media, they showed callus induction up to 85%. | [23] |
| Ulva angusta | 0 or 1.5% of agar. | Explants in liquid media induced callus up to 3%, but in 1.5% agar media, they showed callus induction up to 92%. | [23] |
| Species | Culture Condition | Result | Reference |
|---|---|---|---|
| Laurencia sp. | Different osmolality: 0.5, 0.7, 1.0, or 1.5 Os kg−1 | Callus induced at 0.7–1.0 Os kg−1. At 0.5 or 1.5 Os kg−1, callus induction was inhibited or reduced. | [89] |
| Saccharina japonica | Different salinity: 15, 17.5, 20, 22.5, 25, 27.5, 30, or 35 psu | Callus induced at salinity 25–35 psu (30–60%); optimal at 27.5–30 psu. | [44] |
| Solieria filiformis | Different forms of turbulence: stationary, air, or water turbulence | Explants in media with air turbulence showed a higher callus induction rate (90.3%), than with water turbulence (4.0%) and stationary (0.0%). | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirtawijaya, G.; Negara, B.F.S.P.; Lee, J.-H.; Cho, M.-G.; Kim, H.K.; Choi, Y.-S.; Lee, S.-H.; Choi, J.-S. The Influence of Abiotic Factors on the Induction of Seaweed Callus. J. Mar. Sci. Eng. 2022, 10, 513. https://doi.org/10.3390/jmse10040513
Tirtawijaya G, Negara BFSP, Lee J-H, Cho M-G, Kim HK, Choi Y-S, Lee S-H, Choi J-S. The Influence of Abiotic Factors on the Induction of Seaweed Callus. Journal of Marine Science and Engineering. 2022; 10(4):513. https://doi.org/10.3390/jmse10040513
Chicago/Turabian StyleTirtawijaya, Gabriel, Bertoka Fajar Surya Perwira Negara, Jin-Hwa Lee, Man-Gi Cho, Hye Kyung Kim, Yun-Sik Choi, Sang-Hoon Lee, and Jae-Suk Choi. 2022. "The Influence of Abiotic Factors on the Induction of Seaweed Callus" Journal of Marine Science and Engineering 10, no. 4: 513. https://doi.org/10.3390/jmse10040513
APA StyleTirtawijaya, G., Negara, B. F. S. P., Lee, J.-H., Cho, M.-G., Kim, H. K., Choi, Y.-S., Lee, S.-H., & Choi, J.-S. (2022). The Influence of Abiotic Factors on the Induction of Seaweed Callus. Journal of Marine Science and Engineering, 10(4), 513. https://doi.org/10.3390/jmse10040513








