Improving the Ecological Performance of Miscanthus (Miscanthus × giganteus Greef et Deuter) through Intercropping with Woad (Isatis tinctoria L.) and Yellow Melilot (Melilotus officinalis L.)
Abstract
1. Introduction
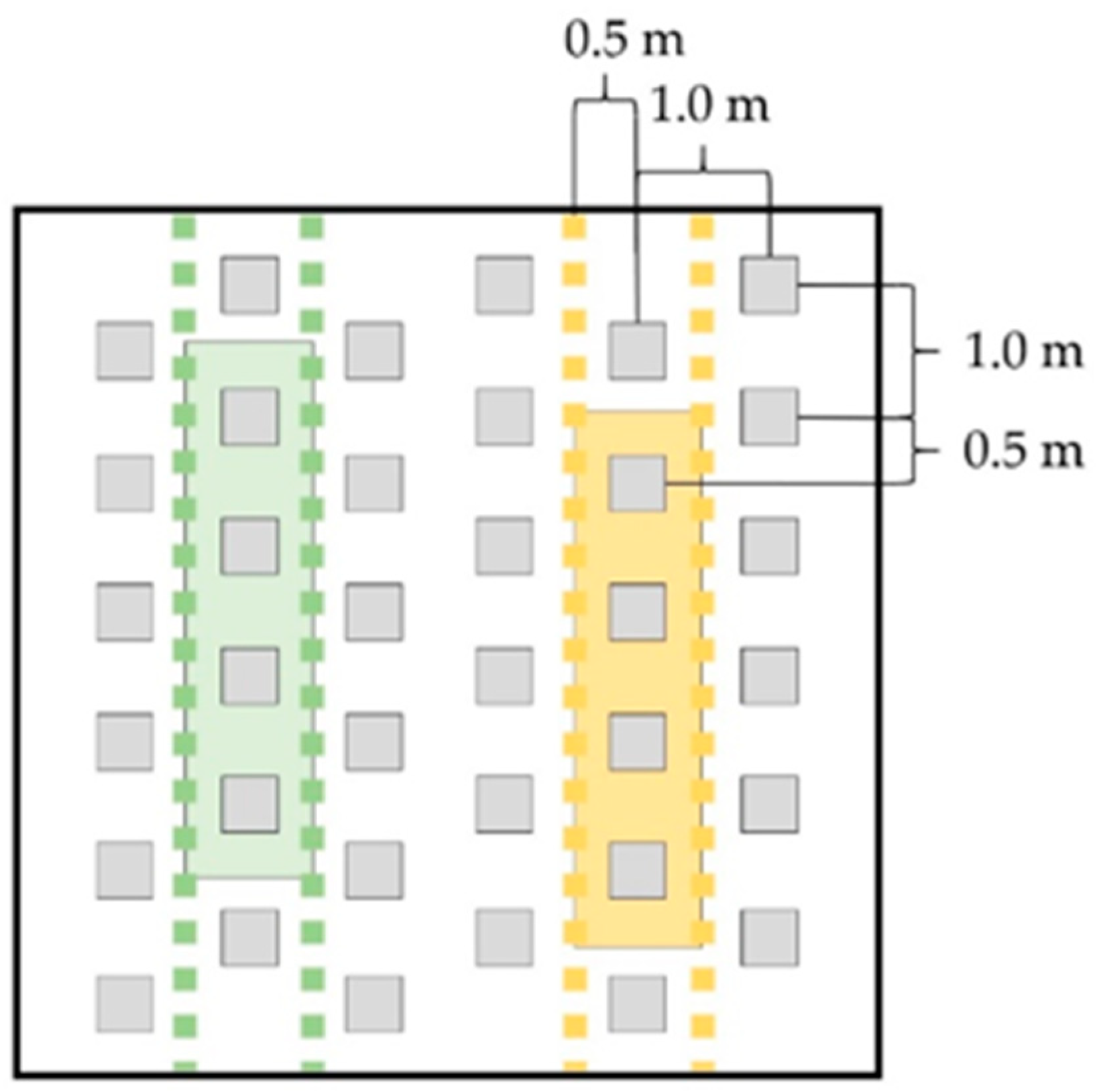
2. Materials and Methods
3. Results and Discussion
3.1. Woad and Miscanthus (WAM)
3.2. Yellow Melilot and Miscanthus (YAM)
3.3. Positive Ecological Effects of Miscanthus Intercropping with Biennial Wild Plant Species
3.4. Economic Implications
3.5. Recommendations for Future Investigations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Staffas, L.; Gustavsson, M.; McCormick, K. Strategies and policies for the bioeconomy and bio-based economy: An analysis of official national approaches. Sustainability 2013, 5, 2751–2769. [Google Scholar] [CrossRef]
- Isbell, F.; Gonzalez, A.; Loreau, M.; Cowles, J.; Díaz, S.; Hector, A.; Mace, G.M.; Wardle, D.A.; O’Connor, M.I.; Duffy, J.E.; et al. Linking the influence and dependence of people on biodiversity across scales. Nature 2017, 546, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N. Highlights of the Conference. In Proceedings of the 27th European Biomass Conference & Exhibition, Lisbon, Portugal, 27–30 May 2019; Available online: http://programme.eubce.com/search.php?close=all (accessed on 31 July 2019).
- Potts, S.G.; Imperatriz-Fonseca, V.L.; Ngo, H.T.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; Vanbergen, A.J. Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; IPBES: Bonn, Germany, 2016. [Google Scholar]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.C.; Segerer, A.; Ulrich, W.; Torchyk, O.; Weisser, W.W.; Schmitt, T. Butterfly community shifts over two centuries. Conserv. Biol. 2016, 30, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C. Beneficial biofuels—The food, energy, and environment trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef]
- Smeets, E.M.W.; Faaij, A.P.C.; Lewandowski, I.M.; Turkenburg, W.C. A bottom-up assessment and review of global bio-energy potentials to 2050. Prog. Energy Combust. Sci. 2007, 33, 56–106. [Google Scholar] [CrossRef]
- Philippidis, G.; Bartelings, H.; Helming, J.; M’barek, R.; Smeets, E.; Van Meijl, H. The Good, the Bad and the Uncertain: Bioenergy Use in the European Union. Energies 2018, 11, 2703. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal agricultural land low-input systems for biomass production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Norman, D.; Janke, R.; Freyenberg, S.; Schurle, B.; Kok, H. Defining and implementing sustainable agriculture. Kans. Sustain. Agric. Ser 1997, 1, 1–14. [Google Scholar]
- Fernando, A.L.; Costa, J.; Barbosa, B.; Monti, A.; Rettenmaier, N. Environmental impact assessment of perennial crops cultivation on marginal soils in the Mediterranean Region. Biomass Bioenergy 2018, 111, 174–186. [Google Scholar] [CrossRef]
- Araújo, K.; Mahajan, D.; Kerr, R.; Silva, M.D. Global biofuels at the crossroads: An overview of technical, policy, and investment complexities in the sustainability of biofuel development. Agriculture 2017, 7, 32. [Google Scholar] [CrossRef]
- Timsina, J. Can organic sources of nutrients increase crop yields to meet global food demand? Agronomy 2018, 8, 214. [Google Scholar] [CrossRef]
- Caslin, B.; Finnan, J.; Easson, L. Miscanthus best practice guidelines. Agric. Food Dev. Auth. Teagasc Agri-Food Biosci. Inst. 2010. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwjn0NrK37nkAhVGx4UKHTSRDn4QFjAAegQIABAC&url=https%3A%2F%2Fwww.agriculture.gov.ie%2Fmedia%2Fmigration%2Fruralenvironment%2Fenvironment%2Fbioenergyscheme%2FMiscanthusBestPracticeManual190913.pdf&usg=AOvVaw0resIY4jmSLVRNsXS8xsbg (accessed on 31 July 2019).
- Zegada-Lizarazu, W.; Monti, A. Energy crops in rotation. A review. Biomass Bioenergy 2011, 35, 12–25. [Google Scholar] [CrossRef]
- Bybee-Finley, K.A.; Ryan, M.R. Advancing Intercropping Research and Practices in Industrialized Agricultural Landscapes. Agriculture 2018, 8, 80. [Google Scholar] [CrossRef]
- Von Cossel, M. Agricultural Diversification of Biogas Crop Cultivation. Ph.D. Thesis, University of Hohenheim, Stuttgart, Germany, 2019. [Google Scholar]
- Heaton, E.A.; Schulte, L.A.; Berti, M.; Langeveld, H.; Zegada-Lizarazu, W.; Parrish, D.; Monti, A. Managing a second-generation crop portfolio through sustainable intensification: Examples from the USA and the EU. Biofuels Bioprod. Biorefining 2013, 7, 702–714. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Long, S.P. Meeting US biofuel goals with less land: The potential of Miscanthus. Glob. Chang. Biol. 2008, 14, 2000–2014. [Google Scholar] [CrossRef]
- Monti, A.; Zegada-Lizarazu, W.; Zanetti, F.; Casler, M. Chapter Two—Nitrogen Fertilization Management of Switchgrass, Miscanthus and Giant Reed: A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 153, pp. 87–119. [Google Scholar]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, N.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
- Kiesel, A.; Wagner, M.; Lewandowski, I. Environmental performance of miscanthus, switchgrass and maize: Can C4 perennials increase the sustainability of biogas production? Sustainability 2016, 9, 5. [Google Scholar] [CrossRef]
- Wagner, M.; Mangold, A.; Lask, J.; Petig, E.; Kiesel, A.; Lewandowski, I. Economic and environmental performance of miscanthus cultivated on marginal land for biogas production. GCB Bioenergy 2019, 11, 34–49. [Google Scholar] [CrossRef]
- Heaton, E.; Voigt, T.; Long, S.P. A quantitative review comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenergy 2004, 27, 21–30. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Zanetti, F.; Scordia, D.; Zegada-Lizarazu, W.; Christou, M.; Testa, G.; Cosentino, S.L.; Monti, A. Long-Term Yields of Switchgrass, Giant Reed, and Miscanthus in the Mediterranean Basin. Bioenergy Res. 2015, 8, 1492–1499. [Google Scholar] [CrossRef]
- Biala, K.; Terres, J.M.; Pointereau, P.; Paracchini, M.L. Low Input Farming Systems: An opportunity to develop sustainable agriculture. Proc. JRC Summer Univ. Ranco 2007. Available online: https://www.bezpecnostpotravin.cz/userfiles/file/ publikace/low-input.pdf (accessed on 31 July 2019). [CrossRef]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; van der Linden, G.C.; Schwarz, K.U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.L.; Dolstra, O.; Donnison, I.S.; et al. Progress on Optimizing Miscanthus Biomass Production for the European Bioeconomy: Results of the EU FP7 Project OPTIMISC. Front. Plant Sci. 2016, 7, 1620. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.; Mangold, A.; Von Cossel, M.; Clifton-Brown, J.; Pogrzeba, M.; Lewandowski, I.; Iqbal, Y.; Kiesel, A. Implementing miscanthus into sustainable farming systems: A review on agronomic practices, capital and labor demand. Renew. Sustain. Energy Rev. under review.
- Von Cossel, M.; Winkler, B.; Mangold, A.; Lewandowski, I.; Elbersen, B.; Wagner, M.; Magenau, E.; Lask, J.; Staritsky, I.; Van Eupen, M.; et al. Social-ecological implications of miscanthus (Miscanthus × giganteus Greef et Deuter) cultivation for isobutanol production. Manuscript in preparation.
- Anderson, E.; Arundale, R.; Maughan, M.; Oladeinde, A.; Wycislo, A.; Voigt, T. Growth and agronomy of Miscanthus x giganteus for biomass production. Biofuels 2011, 2, 71–87. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lewandowski, I. Biomass composition and ash melting behaviour of selected miscanthus genotypes in Southern Germany. Fuel 2016, 180, 606–612. [Google Scholar] [CrossRef]
- O’Flynn, M.G.; Finnan, J.M.; Curley, E.M.; McDonnell, K.P. Effect of Harvest Timing and Soil Moisture Content on Compaction, Growth and Harvest Yield in a Miscanthus Cropping System. Agriculture 2018, 8, 148. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; Scalici, G.; Scordia, D.; Testa, G. Soil erosion mitigation by perennial species under Mediterranean environment. Bioenergy Res. 2015, 8, 1538–1547. [Google Scholar] [CrossRef]
- Felten, D.; Emmerling, C. Effects of bioenergy crop cultivation on earthworm communities—A comparative study of perennial (Miscanthus) and annual crops with consideration of graded land-use intensity. Appl. Soil Ecol. 2011, 49, 167–177. [Google Scholar] [CrossRef]
- Emmerling, C. Impact of land-use change towards perennial energy crops on earthworm population. Appl. Soil Ecol. 2014, 84, 12–15. [Google Scholar] [CrossRef]
- Ruf, T.; Emmerling, C. Impact of premature harvest of Miscanthus x giganteus for biogas production on organic residues, microbial parameters and earthworm community in soil. Appl. Soil Ecol. 2017, 114, 74–81. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Alexopoulou, E. Future yields assessment of bioenergy crops in relation to climate change and technological development in Europe. Ital. J. Agron. 2012, 7, 22. [Google Scholar] [CrossRef]
- Krasuska, E.; Cadórniga, C.; Tenorio, J.L.; Testa, G.; Scordia, D. Potential land availability for energy crops production in Europe. Biofuels Bioprod. Biorefining 2010, 4, 658–673. [Google Scholar] [CrossRef]
- Tuck, G.; Glendining, M.J.; Smith, P.; House, J.I.; Wattenbach, M. The potential distribution of bioenergy crops in Europe under present and future climate. Biomass Bioenergy 2006, 30, 183–197. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Von Cossel, V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; et al. Prospects of Bioenergy Cropping Systems for a more Social-Ecologically Sound Bioeconomy. Agronomy. under review.
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Folke, C. Resilience: The emergence of a perspective for social–ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Xue, S.; Lewandowski, I.; Wang, X.; Yi, Z. Assessment of the production potentials of Miscanthus on marginal land in China. Renew. Sustain. Energy Rev. 2016, 54, 932–943. [Google Scholar] [CrossRef]
- Greef, J.M.; Deuter, M. Syntaxonomy of Miscanthus × giganteus GREEF et DEU. Angew. Bot. 1993, 67, 87–90. [Google Scholar]
- Von Cossel, M.; Mangold, A.; Iqbal, Y.; Hartung, J.; Lewandowski, I.; Kiesel, A. How to Generate Yield in the First Year—A Three-Year Experiment on Miscanthus (Miscanthus × giganteus (Greef et Deuter)) Establishment under Maize (Zea mays L.). Agronomy 2019, 9, 237. [Google Scholar] [CrossRef]
- Riffell, S.; Verschuyl, J.; Miller, D.; Wigley, T.B. Potential Biodiversity Response to Intercropping Herbaceous Biomass Crops on Forest Lands. J. For. 2012, 110, 42–47. [Google Scholar] [CrossRef]
- Dondini, M.; Hastings, A.; Saiz, G.; Jones, M.B.; Smith, P. The potential of Miscanthus to sequester carbon in soils: Comparing field measurements in Carlow, Ireland to model predictions. GCB Bioenergy 2009, 1, 413–425. [Google Scholar] [CrossRef]
- Stewart, J.; Toma, Y.; Fernandez, F.G.; Nishiwaki, A.; Yamada, T.; Bollero, G. The ecology and agronomy of Miscanthus sinensis, a species important to bioenergy crop development, in its native range in Japan: A review. GCB Bioenergy 2009, 1, 126–153. [Google Scholar] [CrossRef]
- Semere, T.; Slater, F.M. Ground flora, small mammal and bird species diversity in miscanthus (Miscanthus×giganteus) and reed canary-grass (Phalaris arundinacea) fields. Biomass Bioenergy 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crops Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Nabel, M.; Barbosa, D.B.P.; Horsch, D.; Jablonowski, N.D. Energy Crop (Sida Hermaphrodita) Fertilization Using Digestate under Marginal Soil Conditions: A Dose-response Experiment. Energy Procedia 2014, 59, 127–133. [Google Scholar] [CrossRef]
- Nabel, M.; Temperton, V.M.; Poorter, H.; Lücke, A.; Jablonowski, N.D. Energizing marginal soils—The establishment of the energy crop Sida hermaphrodita as dependent on digestate fertilization, NPK, and legume intercropping. Biomass Bioenergy 2016, 87, 9–16. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Kollmann, T.; Nabel, M.; Damm, T.; Klose, H.; Müller, M.; Bläsing, M.; Seebold, S.; Krafft, S.; Kuperjans, I.; et al. Valorization of Sida (Sida hermaphrodita) biomass for multiple energy purposes. GCB Bioenergy 2017, 9, 202–214. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I. Perennial wild plant mixtures for biomass production: Impact of species composition dynamics on yield performance over a five-year cultivation period in southwest Germany. Eur. J. Agron. 2016, 79, 74–89. [Google Scholar] [CrossRef]
- Von Cossel, M.; Steberl, K.; Hartung, J.; Agra Pereira, L.; Kiesel, A.; Lewandowski, I. Methane yield and species diversity dynamics of perennial wild plant mixtures established alone, under cover crop maize (Zea mays L.) and after spring barley (Hordeum vulgare L.). GCB Bioenergy 2019, in press. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/gcbb.12640 (accessed on 5 September 2019). [CrossRef]
- Kohli, L.; Daniel, O.; Schönholzer, F.; Hahn, D.; Zeyer, J. Miscanthus sinensis and wild flowers as food resources of Lumbricus terrestris L. Appl. Soil Ecol. 1999, 11, 189–197. [Google Scholar] [CrossRef]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dainese, M.; González-Varo, J.P.; Mudri-Stojnić, S.; Riedinger, V.; Rundlöf, M.; Scheper, J.; Wickens, J.B.; Wickens, V.J.; Bommarco, R.; et al. Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 2016, 19, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Woodcock, B.A.; Roberts, S.P.M.; Tscheulin, T.; Pilgrim, E.S.; Brown, V.K.; Tallowin, J.R. Enhancing pollinator biodiversity in intensive grasslands. J. Appl. Ecol. 2009, 46, 369–379. [Google Scholar] [CrossRef]
- Emmerling, C.; Pude, R. Introducing Miscanthus to the greening measures of the EU Common Agricultural Policy. GCB Bioenergy 2017, 9, 274–279. [Google Scholar] [CrossRef]
- European Commission. Commission Delegated Regulation; Report from the Commission to the European Parliament and the Council; European Commission: Brussels, Belgium, 2018; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1490786763554&uri=COM:2017:152:FIN (accessed on 31 July 2019).
- GRACE. GRowing Advanced Industrial Crops on Marginal Lands for BiorEfineries. 2019. Available online: https://www.grace-bbi.eu/project/ (accessed on 14 June 2019).
- Frick, M.; Pfender, G. AG Wildpflanzen-Biogas Kißlegg. In Biogas aus Wildpflanzen-Chancen und Herausforderungen Mehrjähriger Wildpflanzenmischungen zur Biogasnutzung aus Sicht der Forschung und der Praxis; University of Hohenheim: Stuttgart, Germany, 2019; Available online: https://baden-wuerttemberg.nabu.de/natur-und-landschaft/landwirtschaft/biogas/index.html (accessed on 31 July 2019).
- Kuhn, W.; Zeller, J.; Bretschneider-Herrmann, N.; Drenckhahn, K. Energy from Wild Plants—Practical Tips for the Cultivation of Wild Plants to Create Biomass for Biogas Generation Plants; Jagdverband e.V.: Berlin, Germany, 2014; Volume 1, ISBN 978-3-936802-16-0. Available online: lebensraum-brache.de/wp-content/uploads/2014/09/NLF_Praxisratgeber_Englisch_140905.pdf (accessed on 31 July 2019).
- Xiong, G.R.; Zheng, J.F.; Wu, Y.X.; He, Y.Q. A new disease, clubroot of Isatis tinctoria L. J. Agric. Catastro 2012, 2, 3–4. [Google Scholar]
- Chai, A.L.; Xie, X.W.; Shi, Y.X.; Li, B.J. Research status of clubroot (Plasmodiophora brassicae) on cruciferous crops in China. Can. J. Plant Pathol. 2014, 36, 142–153. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Temperton, V.M.; Harrison, L.; Jablonowski, N.D. Legume Intercropping with the Bioenergy Crop Sida hermaphrodita on Marginal Soil. Front. Plant Sci. 2018, 9, 905. [Google Scholar] [CrossRef]
- Xue, S.; Kalinina, O.; Lewandowski, I. Present and future options for Miscanthus propagation and establishment. Renew. Sustain. Energy Rev. 2015, 49, 1233–1246. [Google Scholar] [CrossRef]
- Iqbal, Y.; Gauder, M.; Claupein, W.; Graeff-Hönninger, S.; Lewandowski, I. Yield and quality development comparison between miscanthus and switchgrass over a period of 10 years. Energy 2015, 89, 268–276. [Google Scholar] [CrossRef]






| Treatment | Dry Matter Yield (Mg ha−1) | Dry Matter Content (% of Fresh Matter) | Number of Shoots per Plant | Plant Height (cm) |
|---|---|---|---|---|
| WAM | 2.2 | 85.0 | 25.3 ± 7.0 | 0.91 |
| YAM | 1.7 | 87.9 | 20.8 ± 2.9 | 0.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Cossel, M.; Iqbal, Y.; Lewandowski, I. Improving the Ecological Performance of Miscanthus (Miscanthus × giganteus Greef et Deuter) through Intercropping with Woad (Isatis tinctoria L.) and Yellow Melilot (Melilotus officinalis L.). Agriculture 2019, 9, 194. https://doi.org/10.3390/agriculture9090194
von Cossel M, Iqbal Y, Lewandowski I. Improving the Ecological Performance of Miscanthus (Miscanthus × giganteus Greef et Deuter) through Intercropping with Woad (Isatis tinctoria L.) and Yellow Melilot (Melilotus officinalis L.). Agriculture. 2019; 9(9):194. https://doi.org/10.3390/agriculture9090194
Chicago/Turabian Stylevon Cossel, Moritz, Yasir Iqbal, and Iris Lewandowski. 2019. "Improving the Ecological Performance of Miscanthus (Miscanthus × giganteus Greef et Deuter) through Intercropping with Woad (Isatis tinctoria L.) and Yellow Melilot (Melilotus officinalis L.)" Agriculture 9, no. 9: 194. https://doi.org/10.3390/agriculture9090194
APA Stylevon Cossel, M., Iqbal, Y., & Lewandowski, I. (2019). Improving the Ecological Performance of Miscanthus (Miscanthus × giganteus Greef et Deuter) through Intercropping with Woad (Isatis tinctoria L.) and Yellow Melilot (Melilotus officinalis L.). Agriculture, 9(9), 194. https://doi.org/10.3390/agriculture9090194







