Abstract
This review describes the multiple utilization of perennial grasses as resilient crops for a multifunctional agriculture. Beyond its role of producing food, feed and fiber, the concept of multifunctional agriculture includes many other functions, such as ecosystem services, renewable energy production and a contribution to the socio-economic viability of rural areas. Traditionally used for feed, some perennial grasses—known as perennial energy grasses (e.g., miscanthus—Miscanthus × giganteus Greef et Deuter, giant reed—Arundo donax L., switchgrass—Panicun virgatum L., reed canary grass—Phalaris arundinacea L.)—have been recommended as a biomass source for both energy and non-energy applications, and ecosystem services. Perennial grasses are lignocellulosic, low-cost feedstock, able to grow in variable environments including marginal lands. Due to their high yield, resilient traits, biomass composition, energy and environmental sustainability, perennial grasses are a candidate feedstock to foster the bio-based economy and adapt to a changing agriculture. However, perennial grasses for biomass production are largely undomesticated crops, or are at early stages of development. Hence, a great potential for improvements is expected, provided that research on breeding, agronomy, post-harvest logistic and bioconversion is undertaken in order to deliver resilient genotypes growing and performing well across a broad range of environmental conditions, climatic uncertainty, marginal land type and end-use destinations.
1. Introduction
Grasslands are among the largest habitat type in the world and, depending on its definitions, they occupy between 20 and 40% of the Earth’s landmass or 69% of the world’s agricultural area and 79 million ha in Europe or 38% of the agricultural land [1].
These herbaceous plants of monocotyledonous type belong to the Poaceae or Gramineae family and include many perennial forage and perennial tall grasses. The importance of grasslands conservation has been also recognized by the latest European directive on the promotion of renewable energies [2], suggesting, on the one hand, the feedstock to produce advanced biofuel, including perennial grasses and, on the other hand, the land categories excluded for such production such as highly biodiverse grassland, among others.
Perennial grasses are resistant and resilient to abiotic stresses [3], and together with their multiple uses, including feed and fiber, renewable energy production and a contribution to ecosystem services, they can be the leading herbaceous plants fitting a multifunctional agriculture [4].
The investigation of perennial grasses as biomass crops dates back 40 years in Europe [5]. These crops might be a key feedstock for modern biorefineries to produce a number of high-added value products (i.e., biopharmaceutical, nutrient supplements, biopolymers), biomaterials (i.e., building, phonic insulating, mulching and biodegradable products for gardening and animal bedding), energy carriers (advanced biofuels, heat and power), by-products (i.e., soil organic fertilizer and green chemistry products), along with ecosystem services (protection of soil erosion and degradation, C-sequestration, restoration of severely degraded and heavily contaminated lands).
Lewandowsky et al. [5] have indicated that miscanthus (Miscanthus × giganteus Greef et Deuter), reed canary grass (Phalaris arundinacea L.), switchgrass (Panicum virgatum L.) and giant reed (Arundo donax L.) are the most suitable perennial grasses for the growth in European environmental conditions. This review mainly focuses on these four, with particular emphasis on key traits as biomass crop, ideal feedstock for a circular economy and research challenges still to undertake to adopt perennial grasses in the context of a multifunctional agriculture.
Perennial energy grasses have the ability to grow in variable environments, including marginal lands, thus minimizing the competition with food crops on indirect land use change (iLUC) effects [6]. High environmental and energetic sustainability has also been recognized, as perennial grasses are established only once and harvested yearly, in a plantation life-time spanning from 10 to 25 years [7], resulting in highly positive energy and greenhouse gas (GHG) balances [8]. One of the most important sustainability characteristics of perennial grasses is the lignocellulosic structure of their cell walls that contributes to the natural resistance to pest and diseases [9]. Taken together, perennial grasses are a low-cost biomass feedstock in contrast with oil crops, sugars, cereals and other starch-rich crops used for biofuel production.
Perennial grasses are high resource-use efficient; most of them are C4 photosynthetic pathway with improved capture of solar radiation and water utilization, are low nutrient demanding and can store nutrients over winter in underground roots [10]. Furthermore, a great amount of crop residues are left to the ground at the onset of senescence, providing a mulch to control weeds and nutrients from decomposed residues that are re-utilized at regrowth [11]. Overall, growing high yielding perennial grasses expect an increased land-use-efficiency. In addition, there is a great potential to still improve their performances. Most of the perennial grasses used for biomass production are either undomesticated plants or are still at the early stages of development and improvement [12]. However, traditional breeding for these crop types is a long-term process, requiring a minimum of 15 years to allow the germplasm collection, parental selection, crossing and evaluation cycles to run [13].
Advanced research on breeding, agronomy, post-harvest logistic and bioconversion are a fundamental issue to exploit these species at farm-scale level and to deliver resilient genotypes growing and performing well across a broad range of environmental conditions, climatic uncertainty, marginal land type and end-use destinations.
2. Why Perennial Grasses as Bioenergy Crops?
Perennial energy grasses are warm-season C4 (miscanthus and swtchgrass) and C3 (giant reed) photosynthetic pathway plants, or cool-season C3 (reed canary grass), with quite different ecological requirements. In Europe, the low winter temperatures in the North and the summer drought in the South are the main climatic limitations to growing these crops [5].
Despite the environmental conditions, the ideal crop type (ideotype) for biomass production should include at least the traits listed in Table 1.

Table 1.
Key traits of miscanthus (Miscanthus spp.), giant reed (Arundo donax L.), switchgrass (Panicum virgatum L.), and reed canary grass (Phalaris arundinacea L.) as ideotype of biomass crop.
According to the climatic requirements, reed canary grass is most suited to north environmental zones of Europe as it shows frost tolerance traits and also good winter hardiness [5]. Due to its plasticity, it distributes and dominates a variety of wetland habitats, riparian zones and disturbed areas in cool temperate climate, can withstand flooding for long periods and exhibits excellent drought tolerance [14]. Switchgrass germinates when soil temperature is higher than 15.5 °C, while miscanthus sprouts from rhizomes at soil temperature higher than 10–12 °C. Both have a wider range of climatic adaptability than reed canary grass and are best fitted to central and southern Europe [12]. However, dry summer is a fundamental problem for these species, as optimal rainfall ranges between 700 and 800 mm in miscanthus and 450–750 in switchgrass [12]. Giant reed is a drought resistant crop, suited to warm, temperate and semi-arid environments with high temperatures and long summer dryness [15,16]. It grows even in areas with 250 mm of rainfall during the growing season; however, its biomass yield tends to increase almost linearly up to 450 mm, whereas yield increments turn less than proportional at higher rainfall amounts [15].
The reported biomass yield of perennial grasses are quite contrasting and vary with multiple factors, including environment, management, genotype used and time of harvest. In suitable environments and optimal growing conditions, biomass yields of more than 30 Mg DM ha−1 have been measured for warm-season grasses and around 12 Mg DM ha−1 for the cool-season one [5,17,18]. After the establishment year, biomass yield shows an upward trend in the first two to four years, then fluctuating yields around a quite high mean during the maturity stage and finally a gradual decrease associated with stand decline [7]. However, performances are affected at sub-optimal conditions and variable weather events to a different extent. A careful management of agricultural practices and monitoring of meteorological conditions throughout the growing season might help to stabilize yields in multiyear plantations.
2.1. Resource-Use-Efficiency
Perennial energy grasses are high resource-use-efficient crops in terms of light, water and nutrients and are low-input demanding. In accordance with the C4-type photosynthetic metabolism, a radiation-use-efficiency (RUE) of 1.6–5.0 g MJ−1 of intercepted photosynthetically active radiation (iPAR) has been measured in switchgrass [19], and 1.1–2.4 g MJ−1 in miscanthus grown in Texas under irrigation [20]. Cosentino et al. [18] reported a RUE of 2.33 g MJ−1 in fully irrigated miscanthus, and 1.24 g MJ−1 in rainfed conditions in a semi-arid Mediterranean environment. Although giant reed has a C3-pathway, its CO2 assimilation, light interception and biomass yield is similar to that of C4 plants [21]. RUE in giant reed was 1.26 g MJ−1 in rainfed and unfertilized and 1.94 g MJ−1 in well-watered and fertilized conditions in a semi-arid Mediterranean area [16]. In a more temperate and humid Mediterranean climate, a RUE of 5.74 g MJ−1 has been measured in giant reed [22]. In Central Italy, calculated RUE was 2.02 g MJ−1 for giant reed and 2.70 MJ−1 for miscanthus [23].
The high photosynthetic capacity of giant reed has been widely recognized, with rates higher than 30 µmol CO2 m−2 s−1 in optimal growing conditions [21,24,25], which is much higher than common values for C3 and very similar to C4 crops [26]. However, a substantial transpiration turns in less efficient water utilization (WUE) as compared with C4 [27,28]. Indeed, water use efficiency (biomass yield to the cumulative evapotranspiration) in giant reed ranged from 1.71 to 4.51 g L−1 [15], and that of miscanthus grown in the same area from 2.56 to 4.83 g L−1 [18]. In both crops, WUE was the highest at the lowest soil water content (i.e., rainfed) as compared with the half and full-irrigation treatments. Triana et al. [29] showed that WUE in miscanthus was higher than in giant reed; however, statistical differences between species were recorded only in the first growth year (4.3 and 2.9 g L−1, respectively), while both crops performed similarly in the subsequent years (about 3.5 g L−1). In switchgrass an average instantaneous WUE (net photosynthesis to the transpiration rate) of 6.5 for lowland and 4.4 µmol CO2 mmol H2O−1 for upland ecotypes has been reported. These ecotypic differences were mainly related to the different water requirements, morphology and productive traits [30]. The high WUE of switchgrass has been related not only to the C4 metabolism but also to its root length density and high water uptake capacity [31].
Although a high resource-use-efficiency has been associated to reed canary grass, studies on RUE and WUE are lacking, due probably to the growing conditions not limiting for light and water. On the other hand, many studies have investigated the effect of nitrogen (N) on reed canary grass, with contrasting results. No yield responses, or no significant increments were observed to N fertilization above 100 kg N ha−1 [32,33]. Smith and Slater [34] found no significant effect with N contents up to 87.5 kg ha−1, either with inorganic or organic fertilizers. Contrarily, yield increments were registered in Germany with N supply up to 163 kg ha−1 but with an associated decrease in N use efficiency (NUE) [35].
Although NUE is usually higher for perennial grasses than for annual crops, they are often less responsive, or even become inefficient at high N rates due partly to nutrients cycling.
In the C4 miscanthus, an average and significantly higher NUE was registered as compared with the C3 giant reed (442 vs. 382 g g−1, respectively) grown in Mediterranean area [36]. Monti and Zegada-Lizarazu [37] showed that NUE decreased as a giant reed plantation became older, and the N fertilization was raised from 0 to 160 kg N ha−1 year−1. Also, Cosentino et al. [15] achieved significant effects of N fertilization on NUE in giant reed only at the first and the second growing season, but not at the third one. NUE was maximized at a fertilization rate of 60 kg N ha−1 year −1 as compared with 120 kg N ha−1 year −1. In switchgrass NUE decreased as well when N was raised from 45 to 180 kg ha−1 [38]. Small increments (about 9 kg of biomass per kg of nutrient applied) were registered by Lemus et al. [39] at N fertilization from 0 to 270 kg N ha−1, showing also for switchgrass the general pattern of NUE decreases with increasing N supply. Similar trends were also confirmed by Lewandowski and Schmid [35] with miscanthus and reed canary grass suggesting, however, lower N fertilizer rates for the C4 miscanthus than for the C3 reed canary grass to maximize NUE.
2.2. Environmental Benefits
The multiple environmental benefits of perennial over annual species are widely recognized [3]. One of the most important sustainability characteristics of perennial grasses is the lignocellulosic structure of cell walls that contributes to the natural resistance to pest and diseases [9]. These crops have a few natural enemies and their cultivation is pesticide-free to date [12].
As with other perennials and deep-rooted crops, a deep soil tillage is essential to ensuring long-term performances. However, many studies reported outstanding performances under minimum soil tillage techniques [40]. Once established, agricultural management is quite simple, as perennial grasses compete quite well with weeds at regrowth and in some cases they require low or no-input, thus only biomass harvesting is conducted yearly. Mature stands improve the soil structure, its stability and health; in addition, they provide a canopy cover with a benefit for biodiversity [5]. The soil remains undisturbed for a long time (even up to 20 years), allowing the storage of carbon from both aboveground and underground plant residues, contributing to the carbon sequestration. Carbon dioxide emission savings may reach 7 Mg CO2 ha−1 year −1 from well-established plantations of miscanthus and giant reed [41], with calculated rates of soil organic carbon accumulation of 0.6–1.0 Mg C ha−1 year −1 in long-term giant reed stands [37]. Higher rates of CO2 savings have been reported with miscanthus grown on marginal sites limited by cold and by drought (19.2 and 24.0 Mg CO2 ha−1 year −1, respectively) [42].
Several studies have addressed the energetic and environmental sustainability of perennial grasses, even when they are cultivated on surplus agricultural land to avoid iLUC. The life-cycle energy use and GHG savings of reed canary grass, miscanthus, giant reed and switchgrass used in combined heat and power generation, has been ascribed as one of the most efficient option [43]. The environmental impact assessment (that included cultivation, post-harvest handling and logistic, conversion, use, and end of life), of perennial grasses on marginal Mediterranean lands showed a low erodibility potential with a few environmental side effects [8].
Energy balance studies always more frequently concluded on outstanding efficiencies of perennial grasses. Amaducci et al. [44] showed that the net energy gain (NEG) and the energy return on investment (EROI) at the farm gate were much higher for perennial grasses than for woody crops. Extremely high NEG was shown for giant reed and miscanthus (611.5 and 447.2 GJ ha−1, respectively) at their peak yield in south of Italy [45]. The EROI at the biorefinery gate for advanced bioethanol production of mature switchgrass, miscanthus and giant reed grown under North and South Italy showed values of 4.16–4.37 [46], well higher than the EROI for corn-based ethanol of 0.8–1.6 [47].
Ecosystem services contribute towards the overall sustainability of perennial grass systems. Soil erosion, for instance, is a crucial issue in Europe. The role of perennial grasses in contributing to soil erosion mitigation has been well documented [48,49]. In the Mediterranean area, soil losses were contained to 0.09 or 0.07 Mg ha−1 when miscanthus or giant reed were grown in a 26%–28% sloping area, as compared with 4.34, 4.81 and 10.1 Mg ha−1 of the fallow, Italian ryegrass and durum wheat cultivation systems, respectively [41]. Other sustainable provisions of environmental services include controlling nonpoint source pollutions, the restoration of degraded and contaminated lands, flood and nitrate leaching risk mitigation. Socio-economic benefits are expected from developments of new markets, economic structures and sources of employment in rural areas [12].
Owing to the little land disturbance compared to annual crops, perennial grasses have a high cover value for wildlife, scoring between forests and annual crops in terms of effects on biodiversity [8]. However, negative traits, such as invasiveness for instance, might affect biodiversity significantly. Invasiveness is a drawback of these fast-growing plants; if not well-managed, they can quickly invade new areas and inflict drastic ecological changes. Giant reed has been listed as noxious weeds in the US, demonstrating its invasiveness potential due to fragmentation and dispersal of rhizomes and other plant parts, especially in wet environments subjected to floods and proximity to riverside and stream channels [50]. In wetlands, moist meadows and riparian areas, reed canary grass can invade and displace desirable native plants, altering water circulation and ecosystem processes [51]. Precautionary measures are applied for fertile Miscanthus sinensis, as it has naturalized in several US states [52]. On the other hand, infertile miscanthus hybrids eliminate the risk of invasiveness from naturally dispersed, viable seed [13]. Like switchgrass in the US, multilocation trials in the North European Union (EU) observed that seeded miscanthus hybrids might not become invasive species due perhaps to low seed dormancy, poor overwintering and low seedling competitive strength [13]. Opportunities to manage the risk of invasiveness should be carefully investigated by breeding programs, as well as by selecting appropriate cultivation sites and tailored agricultural managements.
2.3. Feedstock for a Bio-Based Economy
The transition from a fossil-fuel to a bio-based industry mostly rely on the availability of high and stable biomass yield per unit land area, chemically consistent quality, biotechnology developments and economic, energetic, and environmental sustainability. Modern biorefineries to compete with fossil-fuel technologies, where several final products are being obtained by crude oil, should maximize the feedstock efficiency by adopting a cascading approach, thus extracting the highest added-value products and then downwards to those with lower value to final renewable energy production [53]. Lignocellulosic material consists of two structural polysaccharides, cellulose and hemicellulose, lignin, and small fractions of non-structural components, such as extractives, protein, lipids, pectin and minerals [54]. Cellulose is a linear chain of several hundred to many thousands of β-1,4-linked D-glucose units, while hemicelluloses are a class of branched polysaccharides, primarily C5 (xylans and arabinans) and a small fraction of C6 (galactans, mannans and glucans). Structural polysaccharides are biosynthesized in the primary wall and distribute in the secondary wall where are embedded by lignin, a complex three-dimensional polyphenolic polymer [55,56]. Generally, lignocellulosic biomass from woody species is more abundant in cellulose and lignin, whereas grass biomass has higher contents of hemicellulose (mainly xylans), extractives, and ashes [57].
Lignocellulose is a tremendous source of carbohydrates from which advanced biorefineries might enter numerous potential pathways to produce added-value biochemical and biofuels via the sugar platform. Broadly, lignocellulosic material from perennial grasses can be converted through biochemical or thermochemical conversion pathways for energy (e.g., heat, electricity, bioethanol, bio-oil, syngas, biomethane), non-energy (green chemistry products, non-wood fiber for papermaking, building material, phonic insulating material, mulching and biodegradable products for gardening and animal bedding, biochar, digestate) and other several applications. Figure 1 shows a number of products and by-products at different added-value that can be converted from thermochemical (torrefaction, pyrolysis, gasification, combustion), biochemical (fractionation into hemicellulose, cellulose and lignin) and non-energy (chemical, physical and biological) pathways.
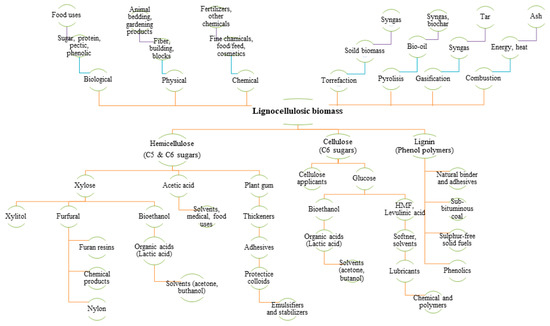
Figure 1.
Diagram flow of lignocellulosic biomass bioconversion processes for energy, biofuels, and added-value products. On the upper left side, the main conversions for non-energy applications; on the upper right side, the main thermochemical pathways. On the bottom, biomass fractionation (hemicellulose, cellulose and lignin) and biochemical pathways.
There is an extensive literature on the bioconversion of perennial grasses and the improved performances over woody material for biochemical conversions are due mainly to a lower biomass recalcitrance (i.e., characteristics of lignocellulose to protect its carbohydrates from degradation by micro-organisms or enzymes) [58]. Scordia et al. [59,60,61,62], using giant reed and miscanthus as raw material and micro-organisms capable of fermenting both C5 and C6 sugars into advanced bioethanol, found out quite high efficiencies (from 51 to 73% of the maximum theoretical). Switchgrass and reed canary grass have been used as well, with interesting results [63,64]. The theoretical bioethanol yield of perennial grasses, woody and crop residues have been compared [10]. By-products that can be recovered at different process stages include the un-hydrolyzed lignin, along with chemicals, organic (i.e., acetic acid, hydroxylmethylfurfural, furfural, levulinic and formic acid) and phenolic compounds [65]. These intermediate or by-products can be technologically processed into a spectrum of marketable products, from high-value niche to large volume applications to feed the circular economy [53].
Anaerobic digestion for advanced biomethane production by perennial grasses has shown its feasibility and comparable performances even with maize. Maize over-yielded both switchgrass and miscanthus in the most productive growing season; however, under sub-optimal conditions, miscanthus yielded more than maize, while switchgrass was the least productive [66]. In giant reed, very high biomethane productions per hectare were achieved, with a 20%–35% increase when it was harvested at a vegetative stage two-times per year, rather than under a single harvest at crop senescence [67]. Furthermore, Di Girolamo et al. [68], argued that bioconversion technologies, as biomass pre-treatment and fractionation without an acid catalyst, can enhance biomethane yield of giant reed by 4 to 23% as compared with an untreated material.
For thermochemical pathways, wood species are the leading feedstock, due mainly to a lower ash content, a higher ash melting temperature and heating value and a lower slagging index and alkali index as compared with perennial grasses [69,70]. For these reasons, perennial grasses are best suited to conversions that operate at low temperatures, such as torrefaction and pyrolysis, rather than gasification or combustion [71]. However, a strategy to improve the biomass of perennial grasses for higher temperature processes is the biomass densification or the torrefaction [72].
In non-energy applications, the use of perennial grasses is also abundant. The pulping and bleaching ability of giant reed for papermaking applications was investigated with satisfactory strength properties and bleachability [73,74]. In the building industry, miscanthus proved the feasibility for the production of panel boards and building blocks [75,76], and medium-density fibreboard (MDF) with quite comparable properties of wood chips [77]. Van Weyenberg et al. [78], showed no significant differences in cow comfort between miscanthus and straw in animal bedding applications, highlighting the better performance of the perennial grass over the straw due to much higher biomass yield.
Lignocellulosic biomass can thus be processed into a wide range of products; however, market development is essential for the successful implementation of lignocellulosic biorefinery. To date, large-scale, multi-process biorefineries are not mature enough and would require a huge amount of biomass and land. The selling price of lignocellulosic biomass is about 65 € dry Mg [79]. It becomes clear that biomass yield is the key driver for farmers from a financial point of view. It follows that comprehensive real yield data over a plant’s lifespan would be necessary for providing more reliable information to farmers and entrepreneurs with consistent and affordable economic plans, such as adequate plantation size and tailor-designed processing plants [7]. However, inter-seasonal variation in yields, and in some cases not suitable existing equipment for the new activity coupled with a lack of long-term contacts, makes the cultivation of perennial grasses less attractive to farmers [79]. In order to reduce investment costs, and in view of rapid biotechnology developments, Dahmen et al. [80] suggested designing small-scale, modular biorefineries tailored for specific locations, products and markets. An on-farm biorefinery, flexibly adapted for a range of feedstock and products would diversify farmers’ activity and will add value to their production. Nonetheless, the definition of the appropriate refinery scale remains an important question as it depends on biomass availability and logistic, bioconversion efficiency, products yield, demand and value.
2.4. Climate Change and Adaption to Marginal Lands
Global warming increases climate unpredictability all over the word. It is commonly agreed that a rise in CO2 and other greenhouse gases will affect current cropping systems. Crops respond differently to changing climatic variables. Generally, the global warming might extend the length of growing season at middle-high latitudes; however, late risk frost can have a severe impact on plant development and yield. On the contrary, a decrease in growing season length and yield is expected on more southern latitudes due mainly to the increase in evapotranspiration and water stress [81,82].
However, what is nowadays undeniable are unexpected extreme weather events, as prolonged heat waves and drought, hail, storms and flooding, making general projections on global warming effects still more unpredictable.
It has been shown that a direct increase in CO2 concentration enhances the yield of many crops, particularly C3 [83] and WUE by increasing net photosynthesis and reducing the transpiration rate [84,85]. For instance, giant reed significantly enhanced the instantaneous WUE raising the CO2 concentration from 400 to 800 μmol mol−1 in growth chambers (from 4.0 up to 12.0 μmol CO2 mol H2O−1), and the CO2 enrichment strongly reduced the transpiration rate delaying crop responses to drought [86].
However, predicting the interaction of the indirect increase in temperature and drought, and other side effects, rather than the direct increase in CO2 concentration alone, is quite complex and might affect crop response to a greater extent. It becomes even more complicated when other variables, as less productive, marginal soils unfavourable for food production are considered. Indeed, under optimal growth conditions any crop may achieve its potential production. However, in the field this is usually constrained by a number of abiotic factors leading to what can be called the attainable yield; this can be further reduced down to the actual yield due to biotic constraints [87]. In marginal conditions, a number of stresses, such as excess of temperature, water shortage, cold, flooding, poor chemical, physical and biological soil properties, slope, contaminants, among others, further reduce crop yield to various degrees, depending on the interaction and level of constraints (Figure 2). Therefore, when the implementation of yield-increasing measures (irrigation, fertilizer application, etc.) or protection measures (herbicide, pesticide, etc.) fails to realize either the attainable or actual yield, for a particular genotype × environment combination, the land can be considered “marginal” from an agronomic point of view and specific management interventions would be required to close yield gaps.
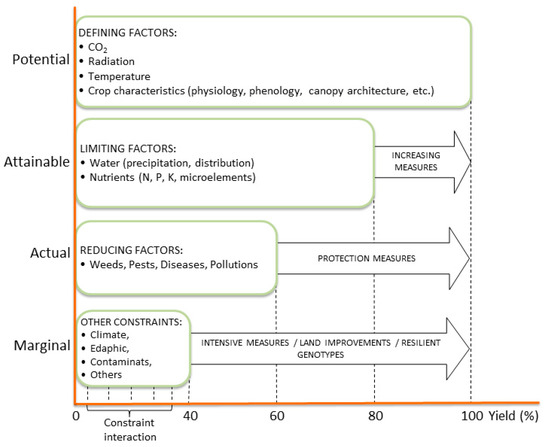
Figure 2.
Yield levels, and factors and constraints limiting biomass production. Modified from Rabbinge, 1993 [82].
A review of Jones et al. [88] proposed some morphological, phenological and physiological traits for perennial energy grasses and newly developed biotechnological methods worth being investigated to overcome these stresses under marginal conditions.
Although investigations of perennial energy grasses have proved traits of avoidance or tolerance to severe stresses, just to cite a few on salinity [24,89,90], on cold tolerance [91,92], on waterlogging or on water stress conditions [15,18,93,94], on sloping area [41], on shallow soil depth [95], on contaminated soils by heavy metals [96] and on brownfields [97]. As a matter of fact, the possibility of using very unproductive lands remains questionable [98]. This opens a massive research need on the resilience of perennial grasses to marginal lands, as knowledge of the production potential of species in these conditions is extremely limited.
In some circumstances, the exploitation of genetic resources from the wild germplasm adapted to specific-marginal conditions might result a winning strategy. Sulas et al. [99] showed outstanding performances of several native Mediterranean populations of Piptatherum miliaceum (L.) Coss, evidencing a combination of interesting traits for bioenergy production. Drought tolerance to severe water stress and agronomic desirable traits have been observed in Saccharum spontaneum L. spp. aegyptiacum (Willd.) Hackel, recommending growing this species in rainfed, marginal lands in hot drought prone climates [100,101,102].
3. Research Challenges
Overall, the reviewed studies hereto agree that perennial energy grasses are resistant and resilient plants to abiotic and biotic stresses, and are suited to a wide range of environmental conditions, including marginal lands. These species are able to use the natural resource efficiently and their cultivation is environmentally friendly. In addition, their biomass composition is suited to many bioconversions to foster a circular economy, hence they are able to rapidly adapt to a changing agriculture. However, advanced research in several areas is still required to implement effective supply chains from the field to the biorefinery.
3.1. Breeding
Unlike traditional crops, perennial energy grasses are still at the first phase of improvement. Breeding is relatively recent and for some of them, such as the unfertile giant reed and the naturally occurring interspecies triploid hybrid Miscanthus × giganteus Greef et Deuter, it is not possible by traditional methods. The research on perennial energy grasses started on the mid-1980s in Europe and USA [5], while breeding programs about one decade later. The initial objective was to improve biomass yield and quality for bioenergy purpose [103]; nowadays, plants suited to a range of growing conditions and tolerant to abiotic stresses that characterize marginal lands with a high net energy yield and low agriculture management costs are prioritized [13]. The extensive review of Clifton-Brown et al. [13] reports the state of the art in breeding for some perennial grasses, particularly miscanthus (M. sinensis, M. sacchariflorus, M. floridulus) and switchgrass (upland and lowland ecotypes), highlighting the timeframe required by conventional breeding cycle for commercial scale development of hybrids in 11–15 years. Nowadays, there are no commercial switchgrass hybrids on the market, although 36 pre-commercial cultivars are registered in USA, while cloning material of M. × giganteus and M. sinensis are available in Europe, China and USA. It is expected that pre-commercial interspecific and intraspecific seed-based hybrids of miscanthus, displaying exceptional yield under drought (~30% greater than control M. × giganteus), variation in biomass quality for specific end-use, and minimal risk of invasiveness, will be available on the European market in 3 years [13]. In reed canary grass key traits for its use in agriculture, as seedling vigour and establishment capacity, seed retention and alkaloid profiles were initially selected for breeding programs [104]. Recently, the highest yielding population was derived from local accessions in the United Kingdom and Sweden to be used to create a synthetic variety [105]. Although phenotypic variability exists in giant reed [17], the restricted genetic diversity, inability to produce viable seeds and highly polyploidy of this species basically precludes breeding efforts. Thus, biotechnological methods, both GM and non-GM (i.e., genome editing) technologies hold considerable promise for creating targeted changes in phenotype [13,88]. Physical mutagenesis was successfully employed to induce genetic and phenotypic variations in giant reed. This methodology proved that genetic improvement may be achieved to generate remarkable genetic and phenotypic variation for relevant traits [106].
3.2. Agronomy
The cultivation system in perennial grasses is quite simple once established. Low input requirements and absence of crop protection measures leads in most cases to only harvest the biomass at a selected time for specific end uses. However, a drawback of agricultural management is the establishment, both for seeded (i.e., switchgrass and reed canary grass) and cloned species (i.e., miscanthus and giant reed). Establishment failure due to the small seed size and morphology, seed dormancy and low early seedling vigour has been reported for the former [5,30,107], while high establishment costs due to considerable work to prepare clonal material for transplant are main limiting factors at the first growth year for the latter [40]. Furthermore, the low growth rate for switchgrass and reed canary grass, and the low plant density for miscanthus and giant reed (10,000–20,000 plants ha−1), along with trade-off between sink- and source-limited growth, imposes a careful management of weeds in most type of soils. A lot of work has been done to increase the propagule ratio of miscanthus and giant reed as compared with the typical rhizome cutting for propagation. Micropropagation, stem-cuttings and single node-cuttings propagation methods have been successfully used to increase the propagule multiplication rates and to reduce the considerable work involved in digging up, breaking apart and replanting rhizomes by still lacking effective mechanization systems [40,108,109,110,111].
Alternative sowing techniques have been investigated for seed sown crops; hydro-seeding can be considered a valuable alternative for particular conditions (i.e., sloping areas), although further optimization still need to be done [40]. In cool climates, mulch films originally developed for maize have improved the establishment rate and reliability of seed-based hybrid miscanthus transplanted with plugs [112]. The response of different ecotypes to seedbed preparation methods (minimum and conservation tillage), sowing time, seedling density and weed control remain a field of investigation. Agronomic research and techniques to improve yields and reduce input specifically in the first growth year should be addressed. Intercropping with perennial grasses and legumes would be an option, although dynamics regarding potential nitrogen limitations with legume nitrogen-fixation, symbiotic relationships with soil microbiota (e.g., rhizobacteria, arbuscular mycorrhizal fungi), and relationships with macrofauna should be thoroughly evaluated [113].
Determination of energy inputs versus energy outputs is essential for the maximization of sustainable yields. Zanetti et al. [46] showed that cultivation under low-or-no-input regimes (unfertilized, rainfed, no-weed and pest management) of long-term plantations (>10 years) of switchgrass, miscanthus and giant reed applied only harvesting practice at an energy cost of 3.40 GJ ha−1. The resulting net energy gain (output-input) was between 107 and 298 GJ ha−1, depending on the biomass yield and the biomass lower heating value.
A careful management of agronomic input can achieve yield maximization. Improved WUE and NUE has been reported for giant reed grown in a semi-arid environment by using meteorological information and soil moisture measurements, allowing to reduce irrigation demands (in the range of 40 to 60% of field capacity) and fertilization rate at 60 kg N ha−1 [16]. NUE could be strategically improved through symbiotic associations with mycorrhiza. Studies have identified some strains of Flavobacterium nitrogenifigens in the rhizosphere of switchgrass [114] and Ker et al. [115] proposed the possibility of inoculating switchgrass with endophytes capable of fixating N with encouraging results.
Increasing the knowledge of input application on output gains and the offset between production systems and the efficient use of resources in specific sites, agricultural management and plant species is advisable, which, however, can greatly diverge depending on the cultivation goal, namely biomass yield maximization or ecosystem services for instance.
3.3. Quality
A stable biomass composition delivered at the bioconversion site avoids continual process modifications, allowing optimization of post-harvest logistics, process reactions, energy flows, time and costs [46]. Many factors influence biomass compositions of perennial grasses, such as genotype, environmental conditions, plant phenological phase, stand maturity, field practices and storage.
Biomass moisture content dictate biomass handling and logistics and bioconversion processes. The relationship between biomass moisture content and bioconversion technology is straight forward; relatively dry biomass (<30% d.w.) is more suited to thermochemical conversions, while biochemical can utilize higher moisture content feedstock [116].
Harvest time and location significantly affect cell wall composition, ash and moisture content. Generally, delayed harvest improves cell-wall content and reduces the ash [46]. Thus, a higher biomass quality is expected with winter harvests, as the lower heating value (LHV) is negatively correlated with the ash and positively with structural polysaccharides and lignin content [71,117]. Moisture content of switchgrass was reduced by a winter as compared with an autumn harvest in North Italy (35 and 56%, respectively), while giant reed kept quite stable values in the South (52% and 49% in autumn and winter harvest, respectively). Miscanthus harvested in winter, was much drier in the South than in the North Italy (16 and 47%, respectively) [46].
Increases in neutral detergent fibre, cellulose and lignin content (up to 3.5, 7.5 and 10.0%, respectively) have been reported for reed canary grass and switchgrass by increasing N fertilization [118]. Miscanthus, on the other hand, responds differently to N fertilizer than switchgrass and reed canarygrass, as it reduced cell wall content following increasing N applications [119]. Furthermore, N fertilization not only improves biomass productivity, it increases also the ash melting behaviour raising the slagging index, but at LHV expenses [71].
Phenological phase and stand maturity also affect biomass composition. Rhizomatous perennial grasses naturally mobilize and store nutrients to rhizomes during the cold season, to re-use them for next season regrowth with a direct effect on stand longevity and biomass quality [120,121,122,123]. Jensen et al. [124] showed that flowering and senescence of 16 miscanthus genotypes tended towards lower P, K, Cl and moisture contents as compared with miscanthus genotypes that did not flower. Senescence changes also the leaf to stem ratio, in turn reducing the ash, mineral and alkali, improving the quality for thermochemical conversions [125].
Independently of field practices and bioconversions, logistic options such as transport, conditioning and storage are influenced by the biomass bulk density and the moisture content [115]. The bulk density of stacked giant reed and miscanthus biomass was 169–297 kg m−3 and 113–125 kg m−3, respectively [71]. However, when randomly measured, it was 49%–70% lower in giant reed and 65%–70% in miscanthus. Modulating harvesting time, field drying and bailing the biomass are strategies to improve bulk density and moisture directly on field, as reported for miscanthus and giant reed [126,127]. Round bale density of switchgrass and reed canary grass averaged 163 kg m−3 with no significant differences between crops [128]. The same authors pointed out that storage can greatly change the biomass composition, the moisture content and the dry matter loss. Storage outdoor for 9–11 months lost from 3.8 to 14.9% dry matter depending on bales cover (i.e., wrapped with plastic film or sisal twine); moisture content and the loss of hemicellulose and cellulose varied spatially within the bales (i.e., on the base, outer layer or core). On the other hand, dry bales stored under cover averaged 3.0% dry matter loss with uniform feedstock properties.
3.4. Marginal Lands
As pointed out by Monti and Alexopoulou [98], effective biomass production chains rely on having the right crops on the right location and drawing up effective logistics concepts, from the field to the biorefinery.
Current EU policies directed towards the support of bioenergy crop farming on “Areas of Natural Constraint or affected by Biophysical Constraints” are based primarily on soil, landscape and climatic criteria, which are easily quantified and quite stable through time and therefore define agriculturally unproductive lands in a comparatively objective way. However, they do not capture other important aspects, including social, environmental, institutional and economic factors that, in some cases, may be more important than bio-physical characteristics [129].
There is an increasing interest in the definition, crop suitability, cultivation techniques, logistic systems, environmental advantages, and other issues concerning the use of marginal lands (Figure 3). An analysis of terms “marginal land” and “marginal land, perennial grass” filtering by keyword and title on Scopus database (Copyright © 2019 Elsevier B.V.), highlights a steady increase of published studies on both search terms, with 5-fold and 19-fold increase for the first and second term, respectively, in a 10-year timeframe (Figure 3A). Interesting, the sharp increase of documents on “marginal land, perennial grass” was on 2015, when the 1513/2015 EU directive [130] was issued to include provisions to address the impact of iLUC given from biofuels produced from crops grown on existing agricultural land. Furthermore, in that directive, amended by (EU) 2018/2001 [2], perennial energy grasses, such as switchgrass, miscanthus, giant reed and ryegrass were included as non-food cellulosic material to produce advanced biofuels and to minimize the overall direct and indirect LUC impacts. Indeed, Figure 3B shows that most of the published studies on “marginal land” and “marginal land, perennial grass” terms, come from European scientists (41.2 and 40.3%, respectively), followed by United States (29.5 and 32.8%, respectively) and China (16.2 and 10.4%, respectively). On both terms, the majority of documents have been published on Agricultural and Biological Science (26.2 and 39.2%, respectively), Environmental Science (25.1 and 21.9%, respectively) and Energy (14.9 and 32.7%, respectively) journals. Social, Earth, Engineering and Economic Science journals published a significant number of documents only on the “marginal land” term (Figure 3C).
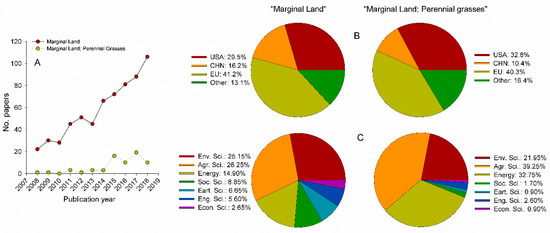
Figure 3.
Scientific literature on “marginal land,” and “marginal land, perennial grasses” search terms. Analysis has been restricted on “keyword” and “title” from 2008 to 2018. (A) Number of published documents. (B) Documents by country/territory. (C) Documents by subject area.
Although a great effort has been placed on studying plant traits of tolerance to abiotic stresses that characterize marginal lands, most of the reviewed studies have been conducted at experimental scale, while scalable case studies and coordinated research actions specifically addressing replicable logistic concepts on marginal land are still required [93]. The development of on-farm technology, which allows decentralized biomass use and valorisation, might have a synergistic effect, since it can help to involve farmers in the local bio-based value chains and gain knowledge on real case studies to provide more reliable information and consistent economic/technical plans to farmers, entrepreneurs and industries. Attractive market programs should be developed to support biomass production and derived products, as well as remuneration for beneficial services to ecosystems such as carbon sequestration, mitigation of soil erosion, nitrate leaching and flooding risks and the restoration of severely degraded and contaminated lands.
Author Contributions
Draft preparation, writing, review and editing, D.S. Supervision, S.L.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations. Grasslands of the World; Suttie, J.M., Reynolds, S.G., Batello, C., Eds.; FAO: Rome, Italy, 2005. [Google Scholar]
- DIRECTIVE (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources (Recast). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L2001&from=EN (accessed on 2 May 2019).
- Volaire, F.; Barkaoui, K.; Norton, M. Designing resilient and sustainable grasslands for a drier future: Adaptive strategies, functional traits and biotic interactions. Eur. J. Agron. 2014, 52, 81–89. [Google Scholar] [CrossRef]
- Renting, H.; Rossing, W.; Groot, J.; van der Ploeg, J.; Laurent, C.; Perraud, D.; Stobbelaar, D.; van Ittersum, M.; van Ittersum, M. Exploring multifunctional agriculture. A review of conceptual approaches and prospects for an integrative transitional framework. J. Environ. Manag. 2009, 90, S112–S123. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Schmidt, T.; Fernando, A.L.; Monti, A.; Rettenmaier, N. Life Cycle Assessment of Bioenergy and Bio-Based Products from Perennial Grasses Cultivated on Marginal Land in the Mediterranean Region. Bioenergy Res. 2015, 8, 1548–1561. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Zanetti, F.; Scordia, D.; Zegada-Lizarazu, W.; Christou, M.; Testa, G.; Cosentino, S.L.; Monti, A. Long-Term Yields of Switchgrass, Giant Reed, and Miscanthus in the Mediterranean Basin. Bioenergy Res. 2015, 8, 1492–1499. [Google Scholar] [CrossRef]
- Fernando, A.L.; Boléo, S.; Barbosa, B.; Costa, J.; Duarte, M.P.; Monti, A. Perennial Grass Production Opportunities on Marginal Mediterranean Land. Bioenergy Res. 2015, 8, 1523–1537. [Google Scholar] [CrossRef]
- Himmel, M.E.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D.; Ding, S.-Y.; Johnson, D.K. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Testa, G.; Cosentino, S.L. Perennial grasses as lignocellulosic feedstock for second-generation bioethanol production in Mediterranean environment. Ital. J. Agron. 2014, 9, 84. [Google Scholar] [CrossRef]
- Strullu, L.; Cadoux, S.; Preudhomme, M.; Jeuffroy, M.H.; Beaudoin, N. Biomass production and nitrogen accumulation and remobilization by Miscanthus × giganteus as influenced by nitrogen stocks in belowground organs. Field Crop Res. 2011, 121, 381–391. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Elbersen, H.W.; Cosentino, S.L.; Zatta, A.; Alexopoulou, E.; Monti, A. Agronomic aspects of future energy crops in Europe. Biofuels Bioprod. Biorefining 2010, 4, 674–691. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D.; Jones, H.D.; Macalpine, W.J.; Murphy-Bokern, D.; Smart, L.B.; Adler, A.; Ashman, C.; Awty-Carroll, D.; et al. Breeding progress and preparedness for mass-scale deployment of perennial lignocellulosic biomass crops switchgrass, miscanthus, willow and poplar. GCB Bioenergy 2019, 11, 118–151. [Google Scholar] [CrossRef] [PubMed]
- Casler, M.; Cherney, J.; Brummer, E. Biomass yield of naturalized populations and cultivars of reed canarygrass. Bioenergy Res. 2009, 2, 165–173. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Scordia, D.; Sanzone, E.; Testa, G.; Copani, V. Response of giant reed (Arundo donax L.) to nitrogen fertilization and soil water availability in semi-arid Mediterranean environment. Eur. J. Agron. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Patanè, C.; Sanzone, E.; Testa, G.; Scordia, D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; D’Agosta, G.M.; Sanzone, E.; Mantineo, M. First results on evaluation of Arundo donax L. clones collected in Southern Italy. Ind. Crop. Prod. 2006, 23, 212–222. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Patane, C.; Sanzone, E.; Copani, V.; Foti, S. Effect of soil water content and nitrogen supply on the productivity of Miscanthus × giganteus Greef and Deu. in Mediterranean environment. Ind. Crop. Prod. 2007, 25, 75–88. [Google Scholar] [CrossRef]
- Kiniry, J.; Tischler, C.; van Esbroeck, G. Radiation use efficiency and leaf CO2 exchange for diverse C4 grasses. Biomass Bioenergy 1999, 17, 95–112. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Schmer, M.R.; Vogel, K.P.; Mitchell, R.B. Switchgrass Biomass Simulation at Diverse Sites in the Northern Great Plains of the U.S. Bioenergy Res. 2008, 1, 259–264. [Google Scholar] [CrossRef]
- Rossa, B.; Tuffers, A.V.; Naidoo, G.; von Willert, D.J. Arundo donax L. (Poaceae)—A C3 species with unusually high photosynthetic capacity. Biol. Plant 1998, 111, 216–221. [Google Scholar]
- Ceotto, E.; Di Candilo, M.; Castelli, F.; Badeck, F.W.; Rizza, F.; Soave, C.; Volta, A.; Villanig, G.; Marletto, V. Comparing solar radiation interception and use efficiency for the energy crops giant reed (Arundo donax L.) and sweet sorghum (Sorghum bicolor L. Moench). Field Crop Res. 2013, 149, 159–166. [Google Scholar] [CrossRef]
- Di Nasso, N.N.; Roncucci, N.; Triana, F.; Tozzini, C.; Bonari, E. Productivity of giant reed (Arundo donax L.) and miscanthus (Miscanthus × giganteus Greef et Deuter) as energy crops: Growth analysis. Ital. J. Agron. 2011, 6, 22. [Google Scholar] [CrossRef]
- Sanchez, E.; Scordia, D.; Lino, G.; Arias, C.; Cosentino, S.L.; Nogues, S.; Cosentino, S. Salinity and Water Stress Effects on Biomass Production in Different Arundo donax L. Clones. Bioenergy Res. 2015, 8, 1461–1479. [Google Scholar] [CrossRef]
- Haworth, M.; Cosentino, S.L.; Marino, G.; Brunetti, C.; Scordia, D.; Testa, G.; Riggi, E.; Avola, G.; Loreto, F.; Centritto, M. Physiological responses of Arundo donax ecotypes to drought: A common garden study. GCB Bioenergy 2017, 9, 132–143. [Google Scholar] [CrossRef]
- Webster, R.J.; Driever, S.M.; Kromdijk, J.; McGrath, J.; Leakey, A.D.B.; Siebke, K.; Demetriades-Shah, T.; Bonnage, S.; Peloe, T.; Lawson, T.; et al. High C3 photosynthetic capacity and high intrinsic water use efficiency underlies the high productivity of the bioenergy grass Arundo donax. Sci. Rep. 2016, 6, 20694. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.E.; Soikaew, A.; Sollenberger, L.E.; Bennett, J.M. Water Use and Water-Use Efficiency of Three Perennial Bioenergy Grass Crops in Florida. Agriculture 2012, 2, 325–338. [Google Scholar] [CrossRef]
- Mann, J.J.; Barney, J.N.; Kyser, G.B.; Di Tomaso, J.M. Miscanthus × giganteus and Arundo donax shoot and rhizome tolerance of extreme moisture stress. GCB Bioenergy 2013, 5, 693–700. [Google Scholar] [CrossRef]
- Triana, F.; Nassi o Di Nasso, N.; Ragaglini, G.; Roncucci, N.; Bonari, E. Evapotranspiration, crop coefficient and water use efficiency of giant reed (Arundo donax L.) and miscanthus (Miscanthus × giganteus Greef et Deu.) in a Mediterranean environment. GCB Bioenergy 2015, 7, 811–819. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Wullschleger, S.D.; Nair, S.S.; Monti, A. Crop physiology. In Switchgrass; Springer: London, UK, 2012; pp. 55–86. [Google Scholar]
- Monti, A.; Zatta, A. Root distribution and soil moisture retrieval in perennial and annual energy crops in Northern Italy. Agric. Ecosyst. Environ. 2009, 132, 252–259. [Google Scholar] [CrossRef]
- Landstrom, S. Sustainability of reed canary grass in cold climate. In Proceedings of the Alternative Crops for Sustainable Agriculture Workshop Proceedings, BioCity, Turku, Finland, 13–15 June 1999; pp. 194–197. [Google Scholar]
- Lindvall, E. Nutrient Supply to Reed Canary Grass as a Bioenergy Crop. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2014. Available online: http://pub.epsilon.slu.se/11239/ (accessed on 10 May 2019).
- Smith, R.; Slater, F. The effects of organic and inorganic fertilizer applications to Miscanthus × giganteus, Arundo donax and Phalaris arundinacea, when grown as energy crops in Wales, UK. GCB Bioenergy 2010, 2, 169–179. [Google Scholar] [CrossRef]
- Lewandowski, I.; Schmidt, U. Nitrogen, energy and land use efficiencies of miscanthus, reed canary grass and triticale as determined by the boundary line approach. Agric. Ecosyst. Environ. 2006, 112, 335–346. [Google Scholar] [CrossRef]
- Di Nasso, N.N.; Roncucci, N.; Triana, F.; Tozzini, C.; Bonari, E. Seasonal nutrient dynamics and biomass quality of giant reed (Arundo donax L.) and miscanthus (Miscanthus × giganteus Greef et Deuter) as energy crops. Ital. J. Agron. 2011, 6, 24. [Google Scholar] [CrossRef]
- Monti, A.; Zegada-Lizarazu, W. Sixteen-year biomass yield and soil carbon storage of giant reed (Arundo donax L.) grown under variable nitrogen fertilization rates. Bioenergy Res. 2016, 9, 248–256. [Google Scholar] [CrossRef]
- Obour, A.K.; Harmoney, K.; Holman, J.D. Nitrogen Fertilizer Application Effects on Switchgrass Herbage Mass, Nutritive Value and Nutrient Removal. Crop. Sci. 2017, 57, 1754. [Google Scholar] [CrossRef]
- Lemus, R.; Parrish, D.J.; Abaye, O. Nitrogen-Use Dynamics in Switchgrass Grown for Biomass. Bioenergy Res. 2008, 1, 153–162. [Google Scholar] [CrossRef]
- Scordia, D.; Zanetti, F.; Varga, S.S.; Alexopoulou, E.; Cavallaro, V.; Monti, A.; Copani, V.; Cosentino, S.L. New Insights into the Propagation Methods of Switchgrass, Miscanthus and Giant Reed. Bioenergy Res. 2015, 8, 1480–1491. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; Scalici, G.; Scordia, D.; Testa, G. Soil Erosion Mitigation by Perennial Species Under Mediterranean Environment. Bioenergy Res. 2015, 8, 1538–1547. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; van der Linden, G.C.; Schwarz, K.U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.L.; Dolstra, O.; Donnison, I.; et al. Progress on optimizing miscanthus biomass production for the European bioeconomy: Results of the EU FP7 project OPTIMISC. Front. Plant Sci. 2016, 7, 1620. [Google Scholar] [CrossRef]
- Rettenmaier, N.; Köppen, S.; Gärtner, S.O.; Reinhardt, G.A. Life cycle assessment of selected future energy crops for Europe. Biofuels Bioprod. Biorefining 2010, 4, 620–636. [Google Scholar] [CrossRef]
- Amaducci, S.; Facciotto, G.; Bergante, S.; Perego, A.; Serra, P.; Ferrarini, A.; Chimento, C. Biomass production and energy balance of herbaceous and woody crops on marginal soils in the Po valley. GCB Bioenergy 2017, 9, 31–45. [Google Scholar] [CrossRef]
- Mantineo, M.; D’Agosta, G.; Copani, V.; Patane, C.; Cosentino, S.; Cosentino, S. Biomass yield and energy balance of three perennial crops for energy use in the semi-arid Mediterranean environment. Field Crop. Res. 2009, 114, 204–213. [Google Scholar] [CrossRef]
- Zanetti, F.; Scordia, D.; Calcagno, S.; Acciai, M.; Grasso, A.; Cosentino, S.L.; Monti, A. Trade-off between harvest date and lignocellulosic crop choice for advanced biofuel production in the Mediterranean area. Ind. Crop. Prod. 2019, 138, 111439. [Google Scholar] [CrossRef]
- Hall, C.A.; Lambert, J.G.; Balogh, S.B. EROI of different fuels and the implications for society. Energy Policy 2014, 64, 141–152. [Google Scholar] [CrossRef]
- Wuest, S.B.; Williams, J.D.; Gollany, H.T. Tillage and perennial grass effects on ponded infiltration for seven semi-arid loess soils. J. Soil Water Conserv. 2006, 61, 218–223. [Google Scholar]
- Cosentino, S.L.; Mantineo, M.; Copani, V. Sod seeding and soil erosion in a semi-arid Mediterranean environment of south of Italy. Ital. J. Agron. 2008, 3, 47–48. [Google Scholar]
- Global Invasive Species Database. Species Profile: Arundo donax. 2019. Available online: http://www.iucngisd.org/gisd/speciesname/Arundo+donax (accessed on 14 July 2019).
- Global Invasive Species Database. Species Profile: Phalaris arundinacea. 2019. Available online: http://www.iucngisd.org/gisd/speciesname/Phalaris+arundinacea (accessed on 14 July 2019).
- Quinn, L.D.; Allen, D.J.; Stewart, J.R. Invasiveness potential of Miscanthus sinensis: Implications for bioenergy production in the United States. GCB Bioenergy 2010, 2, 310–320. [Google Scholar] [CrossRef]
- Keegan, D.; Kretschmer, B.; Elbersen, B.; Panoutsou, C. Cascading use: A systematic approach to biomass beyond the energy sector. Biofuels Bioprod. Biorefining 2013, 7, 193–206. [Google Scholar] [CrossRef]
- Wyman, C.E. Ethanol from lignocellulosic biomass: Technology, economics, and opportunities. Bioresour. Technol. 1994, 50, 3–15. [Google Scholar] [CrossRef]
- Somerville, C. Cellulose Synthesis in Higher Plants. Annu. Rev. Cell Dev. Boil. 2006, 22, 53–78. [Google Scholar] [CrossRef]
- Chundawat, S.P.; Beckham, G.T.; Himmel, M.E.; Dale, B.E. Deconstruction of Lignocellulosic Biomass to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121–145. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Zhu, J.; Pan, X. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresour. Technol. 2010, 101, 4992–5002. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Cosentino, S.L.; Lee, J.-W.; Jeffries, T.W. Dilute oxalic acid pretreatment for biorefining giant reed (Arundo donax L.). Biomass Bioenergy 2011, 35, 3018–3024. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Lee, J.-W.; Jeffries, T.W. Bioconversion of giant reed (Arundo donax L.) hemicellulose hydrolysate to ethanol by Scheffersomyces stipitis CBS6054. Biomass Bioenergy 2012, 39, 296–305. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Enzymatic hydrolysis, simultaneous saccharification and ethanol fermentation of oxalic acid pretreated giant reed (Arundo donax L.). Ind. Crop. Prod. 2013, 49, 392–399. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Effectiveness of dilute oxalic acid pretreatment of Miscanthus × giganteus biomass for ethanol production. Biomass Bioenergy 2013, 59, 540–548. [Google Scholar] [CrossRef]
- Mitchell, R.; Vogel, K.P.; Uden, D.R. The feasibility of switchgrass for biofuel production. Biofuels 2012, 3, 47–59. [Google Scholar] [CrossRef]
- Kallioinen, A.; Uusitalo, J.; Pahkala, K.; Kontturi, M.; Viikari, L.; Von Weymarn, N.; Siika-Aho, M. Reed canary grass as a feedstock for 2nd generation bioethanol production. Bioresour. Technol. 2012, 123, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Kiesel, A.; Wagner, M.; Lewandowski, I. Environmental Performance of Miscanthus, Switchgrass and Maize: Can C4 Perennials Increase the Sustainability of Biogas Production? Sustainability 2017, 9, 5. [Google Scholar] [CrossRef]
- Ragaglini, G.; Dragoni, F.; Simone, M.; Bonari, E. Suitability of giant reed (Arundo donax L.) for anaerobic digestion: Effect of harvest time and frequency on the biomethane yield potential. Bioresour. Technol. 2014, 152, 107–115. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Grigatti, M.; Barbanti, L.; Angelidaki, I. Effects of hydrothermal pre-treatments on Giant reed (Arundo donax) methane yield. Bioresour. Technol. 2013, 147, 152–159. [Google Scholar] [CrossRef]
- 96/04085 Boiler deposits from firing biomass fuels. Fuel Energy Abstr. 1996, 37, 285. [CrossRef]
- Tanger, P.; Field, J.L.; Jahn, C.E.; DeFoort, M.W.; Leach, J.E. Biomass for thermochemical conversion: Targets and chellenges. Front Plant Sci. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Berg, D.V.D.; Van Sleen, P.; Alexopoulou, E.; Cosentino, S.L. Are herbaceous perennial grasses suitable feedstock for thermochemical conversion pathways? Ind. Crop. Prod. 2016, 91, 350–357. [Google Scholar] [CrossRef]
- Xue, G.; Kwapinska, M.; Kwapinski, W.; Czajka, K.M.; Kennedy, J.; Leahy, J.J. Impact of torrefaction on properties of Miscanthus×giganteus relevant to gasification. Fuel 2014, 121, 189–197. [Google Scholar] [CrossRef]
- Bhat, R.V.; Virmani, K.C. Indigenous cellulosic raw material for the production of pulp, paper and board. Part 1. Pulps for writing and printing papers from Arundo donax L. Indian For. Leaflet 1951, 123, 1–9. [Google Scholar]
- Di Felippo, J. Twenty-five years of Argentine industrial experience in the pulping of straw and canes. In Pulp and Paper Prospect in Latin America; United Nations, FAO: New York, NY, USA, 1955. [Google Scholar]
- Mangan, C.L. Non-food crops and non-food uses in EC research programmes. In Proceedings of the 7th E.C. Conference on Biomass for Energy, Environment, Agriculture and Industry, Florence, Italy, 5–9 October 1992; pp. 341–347. [Google Scholar]
- Visser, P.; Pignatelli, V. Utilization of Miscanthus. In Miscanthus for Energy and Fibre; Michael, B.J., Walsh, M., Eds.; Earthscan: London, UK, 2001; ISBN 978-1-84971-097-8. [Google Scholar]
- Harvey, J.; Hutchens, M. Progress in commercial development of Miscanthus in England. In Proceedings of the 8th E.C. Conference on Biomass for Energy, Environment, Agriculture and Industry, Vienna, Austria, 3–5 October 1994; pp. 587–593. [Google Scholar]
- Van Weyenberg, S.; Ulens, T.; de Reu, K.; Zwertvaegher, I.; Demeyer, P.; Pluym, L. Feasibility of Miscanthus as alternative bedding for dairy cows. Vet. Med. Czech 2015, 60, 121–132. [Google Scholar] [CrossRef]
- Soldatos, P.; Lychnaras, V.; Panoutsou, C.; Cosentino, S.L. Economic viability of energy crops in the EU: The farmer’s point of view. Biofuels Bioprod. Bioref. 2010, 4, 637–657. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Olesen, J.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.; Seguin, B.; Peltonen-Sainio, P.; Rossi, F.; Kozyra, J.; Micale, F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011, 34, 96–112. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Alexopoulou, E. Future yields assessment of bioenergy crops in relation to climate change and technological development in Europe. Ital. J. Agron. 2012, 7, 22. [Google Scholar] [CrossRef]
- Poorter, H. Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 1993, 104, 77–97. [Google Scholar] [CrossRef]
- Drake, B.G.; Gonzalez-Meler, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2? Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Ort, D.R. How Do We Improve Crop Production in a Warming World? Plant Physiol. 2010, 154, 526–530. [Google Scholar] [CrossRef]
- Nackley, L.L.; Vogt, K.A.; Kim, S.-H. Arundo donax water use and photosynthetic responses to drought and elevated CO2. Agric. Water Manag. 2014, 136, 13–22. [Google Scholar] [CrossRef]
- Rabbinge, R. The ecological background of food production. Ciba Found. Symp. 1993, 177, 2–22. [Google Scholar]
- Jones, M.B.; Finnan, J.; Hodkinson, T.R. Morphological and physiological traits for higher biomass production in perennial rhizomatous grasses grown on marginal land. GCB Bioenergy 2015, 7, 375–385. [Google Scholar] [CrossRef]
- Nackley, L.D.L.; Kim, S.H. A salt on the bioenergy and biological invasions debate: Salinity tolerance of the invasive biomass feedstock Arundo donax. GCB Bioenergy 2015, 7, 752–762. [Google Scholar] [CrossRef]
- Stavridou, E.; Hastings, A.; Webster, R.J.; Robson, P.R.H. The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus × giganteus. GCB Bioenergy 2016, 9, 92–104. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Lewandowski, I. Overwintering problems with newly established Miscanthus plantations can be overcome by identifying genotypes with improved rhizome cold tolerance. New Phytol. 2000, 148, 287–294. [Google Scholar] [CrossRef]
- Naidu, S.L.; Long, S.P. Potential mechanisms of low temperature tolerance of C4 photosynthesis in Miscanthus × giganteus: An in vivo analysis. Planta 2004, 220, 145–155. [Google Scholar] [CrossRef]
- McDonald, M.P.; Galwey, N.W.; Colmer, T.D. Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant Cell Environ. 2002, 25, 441–451. [Google Scholar] [CrossRef]
- Yue, Y.; Hou, X.; Fan, X.; Zhu, Y.; Zhao, C.; Wu, J. Biomass yield components of 12 switchgrass cultivars grown in Northern China. Biomass Bioenergy 2017, 102, 44–51. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Zanetti, F.; Papazoglou, E.G.; Christou, M.; Papatheohari, Y.; Tsiotas, K.; Papamichael, I. Long-term studies on switchgrass grown on a marginal area in Greece under different varieties and nitrogen fertilization rates. Ind. Crop. Prod. 2017, 107, 446–452. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus spp. and Arundo donax L. Bioenergy Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Lord, R.; Lord, R. Reed canarygrass (Phalaris arundinacea) outperforms Miscanthus or willow on marginal soils, brownfield and non-agricultural sites for local, sustainable energy crop production. Biomass Bioenergy 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Monti, A.; Alexopoulou, E. Non-food crops in marginal land: An illusion or a reality? Biofuels Bioprod. Bioref. 2017, 11, 937–938. [Google Scholar] [CrossRef]
- Sulas, L.; Franca, A.; Sanna, F.; Re, G.A.; Melis, R.; Porqueddu, C. Biomass characteristics in Mediterranean populations of Piptatherum miliaceum—A native perennial grass species for bioenergy. Ind. Crop. Prod. 2015, 75, 76–84. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; Testa, G.; Scordia, D. Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hack. a potential perennial grass for biomass production in marginal land in semi-arid Mediterranean environment. Ind. Crops Prod. 2015, 75, 93–102. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Cosentino, S.L.; Copani, V.; Patanè, C. Soil water effect on crop growth, leaf gas exchange, water and radiation use efficiency of Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hackel in semi-arid Mediterranean environment. Ital. J. Agron. 2015, 10, 185. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Copani, V.; Patanè, C.; Cosentino, S.L. Lignocellulosic biomass production of Mediterranean wild accessions (Oryzopsis miliacea, Cymbopogon hirtus, Sorghum halepense and Saccharum spontaneum) in a semi-arid environment. Field Crop. Res. 2017, 214, 56–65. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Robson, P.; Allison, G.G.; Lister, S.J.; Sanderson, R.; Morris, C.; Hodgson, E.; Farrar, K.; Hawkins, S.; Jensen, E.; et al. Miscanthus: Breeding our way to a better future. Asp. App. Biol. 2008, 90, 109–206. [Google Scholar]
- Casler, M. Genetics, breeding, and ecology of reed canarygrass. Int. J. Plant Breed. 2010, 4, 30–36. [Google Scholar]
- Jensen, E.F.; Casler, M.D.; Farrar, K.; Finnan, J.M.; Lord, R.; Palmborg, C.; Valentine, J.; Donnison, I.S. Reed Canary Grass: From Production to End Use. In Perennial Grasses for Bioenergy and Bioproducts; Elsevier: London, UK, 2018; pp. 153–173. [Google Scholar]
- Valli, F.; Trebbi, D.; Zegada-Lizarazu, W.; Monti, A.; Tuberosa, R.; Salvi, S.; Zegada-Lizarazu, W. In vitro physical mutagenesis of giant reed (Arundo donax L.). GCB Bioenergy 2017, 9, 1380–1389. [Google Scholar] [CrossRef]
- Parrish, D.; Berti, M.; Monti, A.; Zegada-Lizarazu, W.; Zegada-Lizarazu, W. Dedicated crops for advanced biofuels: Consistent and diverging agronomic points of view between the USA and the EU-27. Biofuels Bioprod. Biorefining 2013, 7, 715–731. [Google Scholar]
- Boersma, N.N.; Heaton, E.A. Effects of Temperature, Illumination and Node Position on Stem Propagation of Miscanthus × giganteus. GCB Bioenergy 2012, 4, 680–687. [Google Scholar] [CrossRef]
- Copani, V.; Cosentino, S.L.; Testa, G.; Scordia, D. Agamic propagation of giant reed (Arundo donax L.) in semi-arid Mediterranean environment. Ital. J. Agron. 2013, 8, 18–24. [Google Scholar]
- Cavallaro, V.; Patanè, C.; Cosentino, S.L.; Di Silvestro, I.; Copani, V. Optimizing in vitro large scale production of giant reed (Arundo donax L.) by liquid medium culture. Biomass Bioenergy 2014, 69, 21–27. [Google Scholar] [CrossRef]
- Cavallaro, V.; Scordia, D.; Cosentino, S.L.; Copani, V. Up-scaling agamic propagation of giant reed (Arundo donax L.) by means of single-node stem cuttings. Ind. Crop. Prod. 2019, 128, 534–544. [Google Scholar] [CrossRef]
- Hastings, A.; Mos, M.; Yesufu, J.A.; McCalmont, J.; Schwarz, K.; Shafei, R.; Ashman, C.; Nunn, C.; Schuele, H.; Cosentino, S.; et al. Economic and Environmental Assessment of Seed and Rhizome Propagated Miscanthus in the UK. Front. Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Bybee-Finley, K.A.; Ryan, M.R. Advancing Intercropping Research and Practices in Industrialized Agricultural Landscapes. Agriculture 2018, 8, 80. [Google Scholar] [CrossRef]
- Kämpfer, P.; Busse, H.-J.; McInroy, J.A.; Xu, J.; Glaeser, S.P. Flavobacterium nitrogenifigens sp. nov. isolated from switchgrass (Panicum virgatum). Int. J. Syst. Evol. Microbiol. 2015, 65, 2803–2809. [Google Scholar] [CrossRef]
- Ker, K.; Seguin, P.; Driscoll, B.T.; Fyles, J.W.; Smith, D.L. Evidence for enhanced N availability during switchgrass establishment and seeding year production following inoculation with rhizosphere endophytes. Arch. Agron. Soil Sci. 2014, 60, 1553–1563. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.; Ghosal, G. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Allison, G.G.; Morris, C.; Lister, S.J.; Barraclough, T.; Yates, N.; Shield, I.; Donnison, I.S. Effect of nitrogen fertiliser application on cell wall composition in switchgrass and reed canary grass. Biomass Bioenergy 2012, 40, 19–26. [Google Scholar] [CrossRef]
- Hodgson, E.M.; Fahmi, R.; Yates, N.; Barraclough, T.; Shield, I.; Allison, G.; Bridgwater, A.V.; Donnison, I.S. Miscanthus as a feedstock for fast-pyrolysis: Does agronomic treatment affect quality? Bioresour. Technol. 2010, 101, 6185–6691. [Google Scholar] [CrossRef]
- Beale, C.V.; Long, S.P. Seasonal dynamics of nutrient accumulation and partitioning in the perennial C4-grasses Miscanthus × giganteus and Spartina cynosroides. Biomass Bioenergy 1997, 12, 419–428. [Google Scholar] [CrossRef]
- Himken, M.; Lammel, J.; Neukirchen, D.; Czypionka-Krause, U.; Olfs, H.-W. Cultivation of Miscanthus under West European conditions: Seasonal changes in dry matter production, nutrient uptake and remobilization. Plant Soil 1997, 189, 117–126. [Google Scholar] [CrossRef]
- Christian, D.; Poulton, P.; Riche, A.; Yates, N.; Todd, A. The recovery over several seasons of 15N-labelled fertilizer applied to Miscanthus×giganteus ranging from 1 to 3 years old. Biomass Bioenergy 2006, 30, 125–133. [Google Scholar] [CrossRef]
- Monti, A.; Zanetti, F.; Scordia, D.; Testa, G.; Cosentino, S.L. What to harvest when? Autumn, winter, annual and biennial harvesting of giant reed, miscanthus and switchgrass in northern and southern Mediterranean area. Ind. Crop. Prod. 2015, 75, 129–134. [Google Scholar] [CrossRef]
- Jensen, E.; Robson, P.; Farrar, K.; Jones, S.T.; Clifton-Brown, J.; Payne, R.; Donnison, I. Towards Miscanthus combustion quality improvement: The role of flowering and senescence. GCB Bioenergy 2016, 9, 891–908. [Google Scholar] [CrossRef]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef]
- Nolan, A.; Mc Donnell, K.; Mc Siurtain, M.; Carroll, J.; Finnan, J.; Rice, B. Conservation of miscanthus in bale form. Biosyst. Eng. 2009, 104, 345–352. [Google Scholar] [CrossRef]
- Pari, L.; Curt, M.D.; Sánchez, J.; Santangelo, E. Economic and energy analysis of different systems for giant reed (Arundo donax L.) harvesting in Italy and Spain. Ind. Crop. Prod. 2016, 84, 176–188. [Google Scholar] [CrossRef]
- Shinners, K.J.; Boettcher, G.C.; Muck, R.E.; Weimer, P.J.; Casler, M.D. Harvest and Storage of Two Perennial Grasses as Biomass Feedstocks. Trans. ASABE 2010, 53, 359–370. [Google Scholar] [CrossRef]
- Kang, S.; Post, W.; Wang, D.; Nichols, J.; Bandaru, V.; West, T. Hierarchical marginal land assessment for land use planning. Land Use Policy 2013, 30, 106–113. [Google Scholar] [CrossRef]
- Directive (EU) 2015/1513 of the European Parliament and of the Council of 9 September 2015 Amending Directive 98/70/EC Relating to the Quality of Petrol and Diesel Fuels and Amending Directive 2009/28/EC on the Promotion of the Use of Energy from Renewable Source. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015L1513&from=EN (accessed on 18 May 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).