Evaluation of the Risks of Contaminating Low Erucic Acid Rapeseed with High Erucic Rapeseed and Identification of Mitigation Strategies
Abstract
1. Introduction
2. Methods
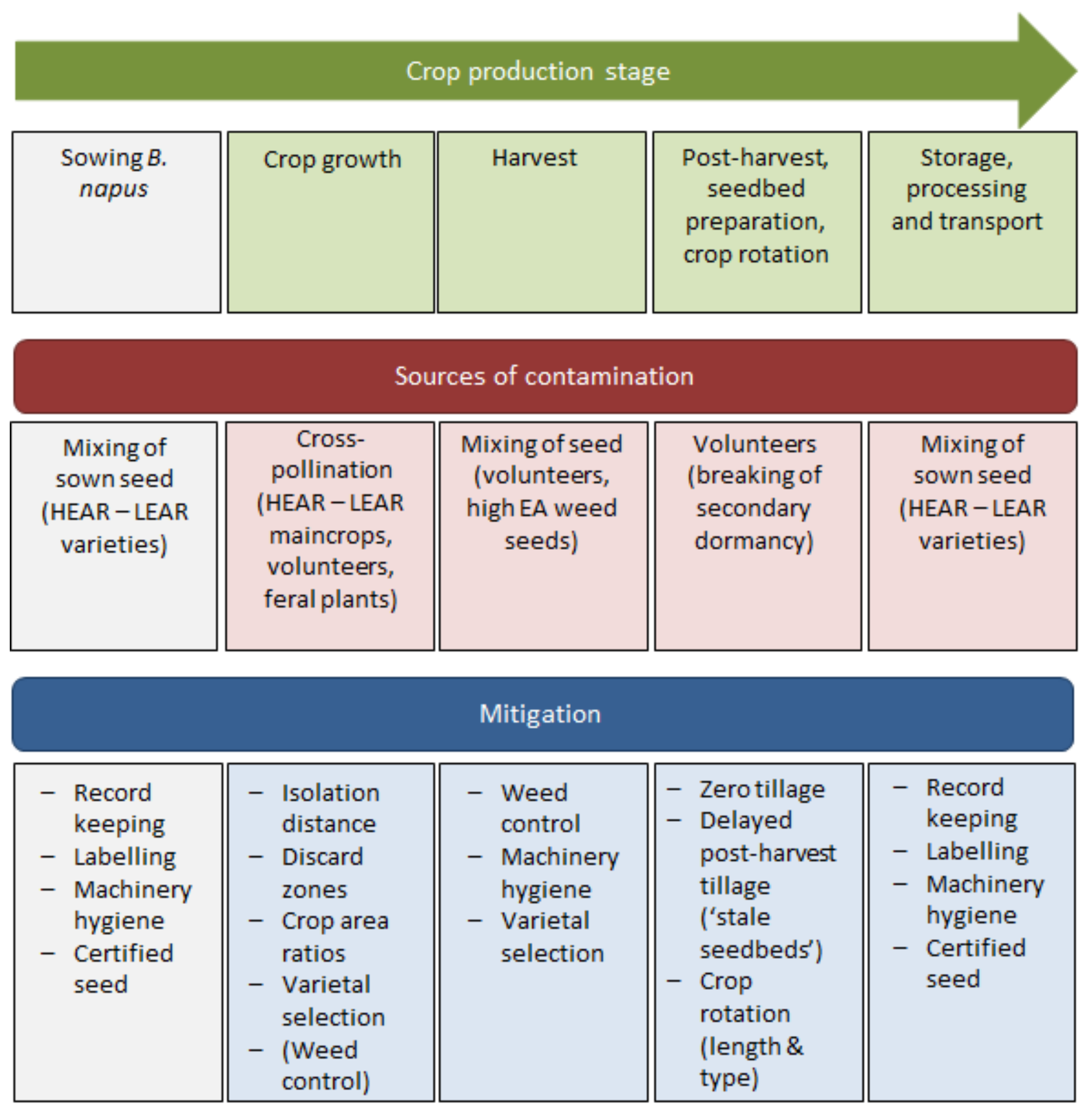
3. Review Findings
3.1. Sowing
3.2. Crop Growth
3.3. Harvest
3.4. Post-Harvest
3.5. Storage, Processing and Transport
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sovero, M. Rapeseed, a new oilseed crop for the United States. In New Crops; Janick, J.E., Ed.; Wiley: New York, NY, USA, 1993; pp. 302–307. [Google Scholar]
- Kramer, J.K. High and Low Erucic Acid in Rapeseed Oils; Academic Press Ltd.: London, UK, 2012. [Google Scholar]
- Carré, P.; Pouzet, A. Rapeseed market, worldwide and in Europe. Ocl 2004, 21, D102. [Google Scholar] [CrossRef]
- USDA—United States Department of Agriculture. World Agricultural Production; Circular Series WAP 08-17; United States Department of Agriculture: Washington, DC, USA, 2017. [Google Scholar]
- Cullen, D.W.; Squire, G.R.; McNicol, J.W.; Jacobs, J.H.; Osborne, J.L.; Ford, L.; Ramsay, G.; Scrimgeour, C.; Young, M.W. Development and validation of gas chromatography and real-time quantitative PCR for the quantification of landscape-scale gene flow from varieties of high erucic acid (HEAR) oilseed rape. J. Sci. Food Agric. 2008, 88, 2253–2264. [Google Scholar] [CrossRef]
- Cuthbert, R.D.; Crow, G.; McVetty, P.B.E. Assessment of seed quality performance and heterosis for seed quality traits in hybrid high erucic acid rapeseed (HEAR). Can. J. Plant Sci. 2011, 1, 837–846. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Erucic acid in feed and food. EFSA J. 2016, 14, 173. [Google Scholar]
- Brookes, G. Co-existence of GM and non-GM crops: Economic and market perspectives. Crop Biotech Update 2003, 27, 1–3. [Google Scholar]
- AGRII. We Are AGRII. 2017. Available online: http://www.agrii.co.uk/ (accessed on 8 June 2019).
- Moyes, C.L.; Dale, P.J. Organic Farming and Gene Transfer from Genetically Modified Crops; MAFF Research Project OF0157; John Innes Centre: Norwich, UK, 1999. [Google Scholar]
- Eastern, K.; Sweet, J. Genetically Modified Organisms (GMOs): The Significance of Gene Flow through Pollen Transfer; Environmental Issues Report No. 28; European Environment Agency: Copenhagen, Denmark, 2002; p. 75. [Google Scholar]
- EC Scientific Committee on Plants. Opinion of the Scientific Committee on Plants Concerning the Adventitious Presence of GM Seeds in Conventional Seeds; European Commission SCP/GMO-SEED-CONT/002-FINAL; European Commission: Brussels, Belgium, 2001. [Google Scholar]
- SCIMAC—Supply Chain Initiative on Modified Agricultural Crops. GM Crop Co-Existence in Perspective; SCIMAC: Cambridge, UK, 2006. [Google Scholar]
- Pearsall, D. GM crop co-existence. GM Crops Food 2013, 4, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Devos, Y.; Demont, M.; Dillen, K.; Reheul, D.; Kaiser, M.; Sanvido, O. Coexistence of genetically modified (GM) and non-GM crops in the European Union. A review. Agron. Sustain. Dev. 2009, e11–e30. [Google Scholar] [CrossRef]
- De La Pasture, L. Clear Solution for Weeds. Crop Protection Magazine. August 2016. Available online: http://www.cpm-magazine.co.uk/2016/08/08/clear-solution-for-weeds (accessed on 12 June 2019).
- Gabrielle, B.; Denoeroy, P.; Gosse, G.; Justes, E.; Andersen, M.N. Development and evaluation of a CERES-type model for winter oilseed rape. Field Crops Res. 1998, 57, 95–111. [Google Scholar] [CrossRef]
- Messéan, A.; Squire, G.; Perry, J.; Angevin, F.; Gomez, M.; Townend, P.; Sausse, C.; Brekling, B.; Langrell, S.; Dzeroski, S.; et al. Sustainable introduction of GM crops into European agriculture: A summary report of the FP6 SIGMEA research project. Oléagineux, Corps Gras. Lipides 2009, 16, 37–51. [Google Scholar]
- Kightley, S.; Appleyard, H.; Evershed, D.; Maile, L.; Smith, W.; Solanki, P.; Wood, T. Investigation of High Levels of Erucic Acid in Consignments of Double-Zero Oilseed Rape Varieties; AHDB Cereals & Oilseeds Project Report No. 602; Agriculture and Horticulture Development Board: Warwickshire, UK, 2019. [Google Scholar]
- Sparrow, S.D.; Conn, J.S.; Knight, C.W. Canola seed survival over winter in the field in Alaska. Can. J. Plant Sci. 1990, 70, 799–807. [Google Scholar] [CrossRef]
- Pekrun, C.; Lutman, P.J.W.; Baeumer, K. Induction of secondary dormancy in rape seeds (Brassica napus L.) by prolonged imbibition under conditions of water stress or oxygen deficiency in darkness. Eur. J. Agron. 1997, 6, 245–255. [Google Scholar] [CrossRef]
- Momoh, E.J.J.; Zhou, W.J.; Kristiansson, B. Variation in the development of secondary dormancy in oilseed rape genotypes under conditions of stress. Weed Res. 2002, 42, 446–455. [Google Scholar] [CrossRef]
- Gulden, R.H.; Thomas, A.G.; Shirtliffe, S.J. Secondary dormancy, temperature, and burial depth regulate seedbank dynamics in canola. Weed Sci. 2004, 52, 382–388. [Google Scholar] [CrossRef]
- Gulden, R.H.; Shirtliffe, S.J.; Thomas, A.G. Harvest losses of canola (Brassica napus) cause large seedbank inputs. Weed Sci. 2003, 51, 83–86. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Li, Y.D.; Colbach, N.; Ma, K.P.; Wei, W.; Mi, X.C. Seed losses at harvest and seed persistence of oilseed rape (Brassica napus) in different cultural conditions in Chinese farming systems. Weed Res. 2012, 52, 317–326. [Google Scholar] [CrossRef]
- ADAS Summary Report. Audits of GM-HT Crops within the Farm Scale Evaluation Trial. Evaluation of Compliance with the SCIMAC Code of Practice on the Introduction of Genetically Modified Crops, and Guidelines for Growing Newly Developed Herbicide Tolerant Crops. Harvest Years 2000–2002; ADAS Consulting Ltd.: Helsby, UK, 2003. [Google Scholar]
- Sausse, C.; Colbach, N.; Young, M.W.; Squire, G.R. How to manage the impact of gene flow on oilseed rape grain quality? Simulation case studies of three contrasted landscapes. Eur. J. Agron. 2012, 38, 32–42. [Google Scholar] [CrossRef]
- Squire, G.R.; Begg, G.E. The Potential for Oilseed Rape Feral (Volunteer) Weeds to Cause Impurities in Later Oilseed Rape Crops; Final report of the DEFRA project: Consequences for Agriculture of the Introduction of Genetically Modified Crops, RG0114; DEFRA: London, UK, 2003. [Google Scholar]
- Cuthbert, R.D.; Crow, G.; McVetty, P.B.E. Assessment of agronomic performance and heterosis for agronomic traits in hybrid high erucic acid rapeseed (HEAR). Can. J. Plant Sci. 2009, 89, 227–237. [Google Scholar] [CrossRef][Green Version]
- Fargue, A.; Meynard, J.M.; Colbach, N.; Vallee, P.; Grandeau, G.; Renard, M. Contamination of rapeseed harvest by volunteers of other varieties: A study of intergenotypic competition. Eur. J. Agron. 2004, 21, 193–207. [Google Scholar] [CrossRef]
- Thöle, H.; Dietz-Pfeilstetter, A. Molecular marker-based identification of oilseed rape volunteers with different secondary dormancy levels in oilseed rape fields. Eur. J. Agron. 2012, 43, 194–200. [Google Scholar] [CrossRef]
- Gruber, S.; Pekrun, C.; Claupein, W. Seed persistence of oilseed rape (Brassica napus): Variation in transgenic and conventionally bred cultivars. J. Agric. Sci. 2004, 142, 29–40. [Google Scholar] [CrossRef]
- Bilsborrow, P.E.; Evans, E.J.; Bland, B.F. Pollen transfer between high and low erucic acid oilseed rape crops. Asp. Appl. Biol. 1994, 35, 163–166. [Google Scholar]
- Becker, H.C.; Karle, R.; Han, S.S. Environmental variations for outcrossing rates in rapeseed (Brassica napus). Theor. Appl. Genet. 1992, 84, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Dietz-Pfeilstetter, A.; Langhof, M.; Rühl, G. Outcrossing frequencies from multiple high erucic acid oilseed rape fields to a central receptor field. Euphytica 2013, 191, 1–7. [Google Scholar] [CrossRef]
- Knispel, A.L.; Mclachan, S.M. Landscape-scale distribution and persistence of genetically modified oilseed rape (Brassica napus) in Manitoba, Canada. Environ. Sci. Poll Res. 2010, 17, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, J.L.; McVetty, P.B.E. Plot-to-plot, row-to-row and plant-to-plant outcrossing studies in oilseed rape. Can. J. Plant Sci. 2001, 81, 657–664. [Google Scholar] [CrossRef]
- Gruber, S.; Claupein, W. Fecundity of volunteer oilseed rape and estimation of potential gene dispersal by a practice-related model. Agric. Ecosyst. Environ. 2007, 119, 401–408. [Google Scholar] [CrossRef]
- Ingram, J. Report on the Separation Distances Required to Ensure Cross Pollination Is below Specified Limits in Non-Seed Crops of Sugar Beet, Maize and Oilseed Rape; MAFF: London, UK, 2000. [Google Scholar]
- Hüsken, A.; Dietz-Pfeilstetter, A. Pollen mediated intraspecific gene flow from herbicide resistant oilseed rape (Brassica napus L.). Transgenic Res. 2007, 16, 557–569. [Google Scholar] [CrossRef]
- Downey, R. Gene flow and rape—The Canadian experience. In Gene Flow and Agriculture—Relevance for Transgenic Crops; Lutman, P.J.W., Ed.; British Crop Protection Council: Hampshire, UK, 1999; Volume 72, pp. 109–116. [Google Scholar]
- Joint Research Centre. Scenarios for Co-Existence of GM, Conventional and Organic Crops in European Agriculture; IPTS: Seville, Spain, 2002. [Google Scholar]
- Colbach, N.; Monod, H.; Lavigne, C. A simulation study of the medium-term effects of field patterns on cross-pollination rates in oilseed rape. Ecol. Model. 2009, 220, 662–672. [Google Scholar] [CrossRef]
- Norris, C.; Sweet, J. Monitoring Large Scale Releases of Genetically Modified Crops; DEFRA: London, UK, 2000. [Google Scholar]
- Sweet, J.B. Monitoring the impact of releases of genetically modified herbicide tolerant oilseed rape in the UK. In Methods for Risk Assessment of Transgenic Plants; Ammann, K., Jacot, Y., Kjellsson, G., Simonsen, V., Eds.; Birkhäuser: Basel, Switzerland, 1999; pp. 159–169. [Google Scholar]
- Damgaard, C.; Kjellson, G. Gene flow of oilseed rape (Brassica napus) according to isolation distance and buffer zone. Agric. Ecosyst. Environ. 2005, 108, 291–301. [Google Scholar] [CrossRef]
- Beckie, H.J.; Warwick, S.I.; Nair, H.; Séguin-Swartz, G. Gene flow in commercial fields of herbicide-resistant canola (Brassica napus). Ecol. Appl. 2003, 13, 1276–1294. [Google Scholar] [CrossRef]
- Chifflet, R.; Klein, E.K.; Lavigne, C.; Le Feon, V.; Ricroch, A.E.; Lecomte, J.; Vaissiere, B.E. Spatial scale of insect-mediated pollen dispersal in oilseed rape in an open agricultural landscape. J. Appl. Ecol. 2011, 48, 689–696. [Google Scholar] [CrossRef]
- Rieger, M.A.; Lamond, M.; Preston, C.; Powles, S.B.; Roush, R.T. Pollen-mediated movement of herbicide resistance between commercial canola fields. Science 2002, 296, 2386–2388. [Google Scholar] [CrossRef] [PubMed]
- Colbach, N.; Molinari, N.; Meynard, J.M.; Messéan, A. Spatial aspects of geneflow between rapeseed varieties and volunteers: An application of the GENESYS model based on a spatio-temporal sensitivity analysis. Agron. Sustain. Dev. 2005, 25, 355–368. [Google Scholar] [CrossRef]
- Squire, G.R.; Allnutt, T.; Boffey, C.W.H.; Cullen, D.; Daniels, R.E.; Ford, L.; Henry, C.; Jacobs, J.H.; Kightley, S.; Kilpatrick, J.B.; et al. Factors Affecting Cross Pollination of Oilseed Rape Growing under UK Conditions; Final report of project RG0125 funded by Defra and RERAD; DEFRA: London, UK, 2008. [Google Scholar]
- Adler, L.S.; Wilker, K.; Wyndham, F.S.; Linder, C.R.; Schmitt, J. Potential for persistence of genes escaped form canola: Germination cues in crop, wild, and crop-wild hybrid Brassica rapa. Funct Ecol. 1993, 7, 736–745. [Google Scholar] [CrossRef]
- Rees, M.; Long, M.J. Germination biology and the ecology of annual plants. Am. Nat. 1992, 139, 484–508. [Google Scholar] [CrossRef]
- Banks, G. Feral Oilseed Rape Populations within a Scottish Landscape: Implications for GM Coexistence and Environmental Risk Assessment. Ph.D. Thesis, University of Dundee, Dundee, UK, 2014. [Google Scholar]
- Bailleul, D.; Ollier, S.; Lecomte, J. Genetic diversity of oilseed rape fields and feral populations in the context of coexistence with GM crops. PLoS ONE 2016, 11, e0158403. [Google Scholar] [CrossRef]
- Bond, J.M.; Mogg, R.J.; Squire, G.R.; Johnstone, C. Microsatellite amplification in Brassica napus cultivars: Cultivar variability and relationship to a long-term feral population. Euphytica 2004, 139, 173–178. [Google Scholar] [CrossRef]
- Pivard, S.; Adamczyk, K.; Lecomte, J.; Lavigne, C.; Bouvier, A.; Deville, A.; Gouyon, P.H.; Huet, S. Where do the feral oilseed rape populations come from? A large-scale study of their possible origin in a farmland area. J. Appl. Ecol. 2008, 45, 476–485. [Google Scholar] [CrossRef]
- Scott, S.E.; Wilkinson, M.J. Trangene risk is low. Nature 1998, 393, 320. [Google Scholar] [CrossRef]
- Allainguillaume, J.; Alexander, M.; Bullock, J.M.; Saunders, M.; Allender, C.J.; King, G.; Ford, C.S.; Wilkinson, M.J. Fitness of hybrids between rapeseed (Brassica napus) and wild Brassica rapa in natural habitats. Mol. Ecol. 2006, 15, 1175–1184. [Google Scholar] [CrossRef]
- Halfhill, M.D.; Zhu, B.; Warwick, S.I.; Raymer, P.L.; Millwood, J.R.; Weissinger, A.K.; Stewart, C.N., Jr. Hybridization and backcrossing between transgenic oilseed rape and two related species under field conditions. Environ. Biosaf. Res. 2004, 3, 73–81. [Google Scholar] [CrossRef][Green Version]
- Elliott, L.J.; Mason, D.C.; Wilkinson, M.J.; Allainguillaume, J.; Norris, C.; Alexander, M.; Welters, R. Methodological Insights: The role of satellite image-processing for national-scale estimates of gene flow from genetically modified crops: Rapeseed in the UK as a model. J. Appl. Ecol. 2004, 41, 1174–1184. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Davenport, I.J.; Charters, Y.M.; Jones, A.E.; Allainguillaume, J.; Butler, H.T.; Mason, D.C.; Raybould, A.F. A direct regional scale estimate of transgene movement from genetically modified oilseed rape to its wild progenitors. Mol. Ecol. 2000, 9, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Norris, C. Components of Gene Flow in Oilseed Rape (Brassica napus). Ph.D. Thesis, The University of Cambridge, Cambridge, UK, 2002. [Google Scholar]
- Leaper, D.J.; Melloul, S. The impact of Clearfield® Production System on the quality of oilseed rape. In Proceedings of the 13th International Rapeseed Congress, Prague, Czech Republic, 5–9 June 2011. [Google Scholar]
- Blackshaw, R.E.; Lemerle, D.; Mailer, R.; Young, K.R. Influence of wild radish on yield and quality of canola. Weed Sci. 2002, 50, 344–349. [Google Scholar] [CrossRef]
- Jones, D. Weedy oilseed rape may be culprit in rejected loads. Farmers Weekly, 24 January 2017. [Google Scholar]
- Oxley, S. Oilseed Rape Market Seeks Growers: Any ‘Volunteers’? AHDB Blog. Available online: http://cereals-blog.ahdb.org.uk/oilseed-rape-market-seeks-growers-any-volunteers/2016 (accessed on 9 June 2019).
- Friedt, W.; Tu, J.; Fu, T. Academic and economic importance of Brassica napus rapeseed. In The Brassica napus Genome. Compendium of Plant Genomes; Liu, S., Snowdon, R., Chalhoub, B., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Stephenson, P.; Stacey, N.; Brüser, M.; Pullen, N.; Ilyas, M.; O’Neill, C.; Østergaard, L. The power of model-to-crop translation illustrated by reducing seed loss from pod shatter in oilseed rape. BioRxiv 2019, 604769. [Google Scholar] [CrossRef] [PubMed]
- Colbach, V.; Durr, C.; Roger-Estrade, J.; Chauvel, B.; Caneill, J. AlomySys: Modelling black-grass (Alopecurus myosuroides Huds.) germination and emergence, in interaction with seed characteristics, tillage and soil climate I. Construction. Eur. J. Ecol. 2006, 24, 95–112. [Google Scholar] [CrossRef]
- Soltani, E.; Soltani, A.; Galeshi, S.; Ghaderi-far, F.; Zeinali, E. Seedbank modelling of volunteer oil seed rape: From seeds fate in the soil to seedling emergence. Planta Daninha. 2013, 31, 267–279. [Google Scholar] [CrossRef]
- Gruber, S.; Bühler, A.; Möhring, J.; Claupein, W. Sleepers in the soil—Vertical distribution by tillage and long-term survival of oilseed rape seeds compared with plastic pellets. Eur. J. Agron. 2010, 33, 81–88. [Google Scholar] [CrossRef]
- Zanettti, F.; Mosca, G.; Rampin, E.; Vameralti, T. Adaptability and sustainable management of high-erucic Brassicaceae in Mediterranean environment. In Oilseeds; Akpan, U.G., Ed.; IntechOpen: London, UK, 2012; Chapter 3; p. 99. [Google Scholar]
- Söchting, H.P.; Gummert, A.; Zwerger, P. Temporal emergence and growth processes of oilseed rape volunteers in oilseed rape crops (Auflaufdynamik und Wachstumsverlauf von Ausfallraps in Winterrapsbeständen). J. Plant Dis. Protect. 2008, XXI, 303–308. [Google Scholar]
- Pessel, D.; Lecomte, J.; Emeriau, V.; Krouti, M.; Messéan, A.; Gouyon, P.H. Persistence of oilseed rape (Brassica napus L.) outside of cultivated fields. Theor. Appl. Genet. 2001, 102, 841–846. [Google Scholar] [CrossRef]
- Jørgensen, T.; Hauser, T.P.; Jørgensen, R.B. Adventitious presence of other varieties in oilseed rape (Brassica napus) from seedbanks and certified seed. Seed Sci. Res. 2007, 17, 115–125. [Google Scholar] [CrossRef]
- D’Hertefeldt, T.; Jørgensen, R.B.; Pettersson, L.B. Long-term persistence of GM oilseed rape in the seedbank. Biol. Let. 2008, 4, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.S.; Rasmussen, J.; Jørgensen, R.B. You reap what you sow—Or do you? Volunteers in organic row-sown and broadcast-sown oilseed rape fields. Eur. J. Agron. 2010, 32, 121–126. [Google Scholar] [CrossRef]
- Simard, M.J.; Légère, A.; Pageau, D.; Lajeunesse, J.; Warwick, S. The frequency and persistence of volunteer canola (Brassica napus) in Quebec cropping systems. Weed Tech. 2002, 16, 433–439. [Google Scholar] [CrossRef]
- Beckie, H.J.; Warwick, S.I. Persistence of an oilseed rape transgene in the environment. Crop Protect. 2010, 29, 509–512. [Google Scholar] [CrossRef]
- Lutman, P.J.W.; Berry, K.; Payne, R.W.; Simpson, E.; Sweet, J.B.; Champion, G.T.; May, M.J.; Wightman, P.; Walker, K.; Lainsbury, M. Persistence of seeds from crops of conventional and herbicide tolerant oilseed rape (Brassica napus). Proc. R. Soc. B 2005, 272, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Thöle, H.; Dietz-Pfeilstetter, A.; Hüsken, A. Statistical approach to predict abundances of oilseed rape volunteers. In Proceedings of the 13th International Rapeseed Congress, Prague, Czech Republic, 5–9 June 2011; pp. 5–9. [Google Scholar]
- Lutman, P.J.W. Dormancy and Persistence of Volunteer Oilseed Rape; HGCA Topic Sheet No.24; Home-Grown Cereals Authority: London, UK, 1999. [Google Scholar]
- Begg, G.S.; Hockaday, S.; McNicol, J.W.; Askew, M.; Squire, G.R. Modelling the persistence of volunteer oilseed rape (Brassica napus). Ecol. Model. 2006, 198, 195–207. [Google Scholar] [CrossRef]
- Debeljak, M.; Squire, G.R.; Demsar, D.; Young, M.W.; Dzeroski, S. Relations between the oilseed rape volunteer seedbank, and soil factors, weed functional groups and geographical location in the UK. Ecol. Model. 2008, 212, 138–146. [Google Scholar] [CrossRef]
- Bennett, A.; Gosling, P.; Mullan, B.; Nicholls, C.; Watts, J. Oilseed Rape Guide, 2nd ed.; AHDB Cereals and Oilseeds: Kenilworth, UK, 2018; Available online: https://ahdb.org.uk/knowledge-library/oilseed-rape-guide (accessed on 12 June 2019).
- Robson, M.C.; Fowler, S.M.; Lampkin, N.H.; Leifert, C.; Leitch, M.; Robinson, D.; Litterick, A.M. The agronomic and economic potential of break crops for ley/arable rotations in temperate organic agriculture. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2002; Volume 77, pp. 369–427. [Google Scholar]
- Saji, H.; Nakajima, N.; Aono, M.; Tamaoki, M.; Kubo, A.; Wakiyama, S.; Hatase, Y.; Nagatsu, M. Monitoring the escape of transgenic oilseed rape around Japanese ports and roadsides. Environ. Biosaf. Res. 2005, 4, 217–222. [Google Scholar] [CrossRef]
- Von der Lippe, M.; Kowarik, I. Long-distance dispersal of plants by vehicles as a driver of plant invasions. Conserv. Biol. 2007, 21, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, D.; Ollier, S.; Huet, S.; Gardarin, A.; Lecomte, J. Seed spillage from grain trailers on road verges during oilseed rape harvest: An experimental survey. PLoS ONE 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leflon, M.; Hüsken, A.; Kightley, S.; Pinochet, X. Cleistogamy of oilseed rape, a way to prevent cross-fertilization between adjacent fields. Plant Breed. 2011, 130, 338–344. [Google Scholar] [CrossRef]
- Fargue, A.; Colbach, N.; Pierre, J.; Picault, H.; Renard, M.; Meynard, J.M. Predictive study of the advantages of cleistogamy in oilseed rape in limiting unwanted gene flow. Euphytica 2006, 151, 1–13. [Google Scholar] [CrossRef]
- Sausse, C.; Le Bail, M.; Lecroart, B.; Remy, B.; Messéan, A. How to manage the coexistence between genetically modified and conventional crops in grain and oilseed collection areas? Elaboration of scenarios using role playing games. Land Use Pol. 2013, 30, 719–729. [Google Scholar] [CrossRef]
- SCIMAC—Supply Chain Initiative on Modified Agricultural Crops. Code of Practice on the Introduction of Genetically Modified Crops; SCIMAC: Cambridge, UK, 1999. [Google Scholar]
- Friesen, L.F.; Nelson, A.G.; Van Acker, R.C. Evidence of contamination of pedigreed canola (Brassica napus) seedlots in western Canada with genetically engineered herbicide resistance traits. Agron. J. 2003, 95, 1342–1347. [Google Scholar] [CrossRef]
- Carter, M.R. Conservation tillage. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 306–311. [Google Scholar]
- Firbank, L.G.; Dewar, A.M.; Hill, M.O.; May, M.J.; Perry, J.M.; Rothery, P.; Squire, G.R.; Woiwood, I.P. Farm-scale evaluation of GM crops explained. Nature 1999, 399, 727. [Google Scholar] [CrossRef][Green Version]
- Henry, C.; Morgan, D.; Weeks, R.; Daniels, R.; Boffey, C. Farm Scale Evaluations of GM Crops: Monitoring Gene Flow from GM Crops to Non-GM Equivalent Crops in the Vicinity. Part I: Forage Maize; Final report to DEFRA, contract reference EPG 1/5/138; DEFRA: London, UK, 2003. [Google Scholar]
| Trait | Description | Advantage/Disadvantage | Reference |
|---|---|---|---|
| Flowering time | HEAR F1 hybrids flower at time similar to that of the earliest parent. Growth vigour is not associated with flowering time. | Strong competition for nutrients, water & light. Where flowering with the crop is simultaneous outcrossing is likely. | [29] |
| Plant maturity | HEAR F1 hybrids mature at time similar to that of the earliest parent. Growth vigour is not associated with maturing time. | Strong competition for nutrients, water & light. | [29] |
| Growth rate & biomass production | HEAR—LEAR F1 plants often demonstrate superior growth rates and biomass accumulation compared to their parents. | The period between sowing & volunteer emergence may be critical regarding the intensity of competition. | [30] |
| Plant height & stem elongation | Hybrid plant height tends to be intermediate falling between that of each parent. | If the crop is shorter than the hybrid especially if hybrid has increased vigour, the volunteer has a selective advantage. However, this additional height allows volunteers to be more easily identified and so removed. | [29,30,31] |
| Lodging resistance | No evidence of high parent heterosis for lodging resistance in any HEAR hybrids. | No advantage conferred. | [31] |
| Seed yield | HEAR F1 hybrid may produce seed yields of up to 140% more than the highest yielding parent. | High potential for seed and so volunteer occurrence and persistence. | [29] |
| EA content | F1 hybrids may display greater EA content, although screening has identified this potential in only 6% of cultivars. | Enhanced potential for HEAR EA contamination in the crop. | [29] |
| Activity | The Issue | Mitigation Options | References |
|---|---|---|---|
| Varietal choice | Varietal choice will affect the strength of the traits most likely to affect the occurrence of volunteer and feral plants. |
| [29,30,31,69,71,91,92] |
| Machinery hygiene | B. napus seeds are small & difficult to remove from machinery. |
| [18,26,93] |
| Seed storage | Seed contamination is unlikely to be the source if low levels of EA contamination are detected in LEAR seed but will be a potential source if levels are very high |
| [16,26,94] |
| Seed quality | Seed batch contamination and potentially farm-saved seed can introduce EA contamination. |
| [35] |
| Transport | The cultivar diversity of feral populations is greatest along roadside verges suggesting dispersal via farm vehicles. |
| [26,55] |
| Crop Production Stage and Sources of Contamination | Risk | Score | Mitigation | Score | Priority Score | Priority |
|---|---|---|---|---|---|---|
| Crop growth | ||||||
| Cross-pollination: from volunteers in OSR crop | H | 3 | Varietal selection (cleistogamous varieties) | 2 | 6 | H |
| H | 3 | Weed control using the ‘Clearfield’ system | 3 | 9 | VH | |
| H | 3 | Increased crop plant density | 1 | 3 | M | |
| Cross-pollination: between HEAR–LEAR maincrops | M | 2 | Isolation distance of 100 m | 3 | 6 | H |
| M | 2 | Isolation distance of 10 m | 2 | 4 | M | |
| M | 2 | Isolation distance of 1 m | 1 | 2 | L | |
| M | 2 | Discard zones in the outer 10 m | 2 | 4 | M | |
| M | 2 | Low crop edge to crop area ratio | 2 | 4 | M | |
| M | 2 | Increased landscape heterogeneity | 2 | 4 | M | |
| M | 2 | Varietal selection (cleistogamous varieties) | 2 | 4 | M | |
| Cross-pollination: feral plants outside crop | L | 1 | Varietal selection (cleistogamous varieties) | 2 | 2 | L |
| L | 1 | Weed control withconventional herbicide | 2 | 2 | L | |
| Cross-pollination: compatible wild relatives (inside crop) | L | 1 | Varietal selection (cleistogamous varieties) | 2 | 2 | L |
| L | 1 | Weed control using the ‘Clearfield’ system | 3 | 3 | M | |
| Cross-pollination: compatible wild relatives (outside crop) | L | 1 | Varietal selection (cleistogamous varieties) | 2 | 2 | L |
| L | 1 | Weed control with conventional herbicide | 2 | 2 | L | |
| Harvest | ||||||
| Mixing of seed: weeds with high EA content | M | 2 | Weed control using the ‘Clearfield’ system | 3 | 6 | H |
| Mixing of seed: volunteer HEAR/hybrid HEAR | H | 3 | Weed control using the ‘Clearfield’ system | 3 | 9 | VH |
| H | 3 | Varietal selection (varieties with enhanced shatter resistance) | 2 | 6 | H | |
| H | 3 | Farm machinery hygiene | 2 | 6 | H | |
| Post-harvest | ||||||
| Volunteers (preventing secondary dormancy) | H | 3 | Zero tillage | 3 | 9 | VH |
| H | 3 | Delayed post-harvest tillage (‘stale seedbeds’) with following winter crops | 2 | 6 | H | |
| H | 3 | Delayed post-harvest tillage (‘stale seedbeds’) with following spring crops | 3 | 9 | VH | |
| H | 3 | Varietal selection (varieties less susceptible to secondary dormancy) | 2 | 6 | H | |
| Volunteers in subsequent crops | H | 3 | Crop rotation: increase the time between OSR crops (>5 years) | 2 | 6 | H |
| H | 3 | Crop rotation: increase the time between OSR crops (>10 years) | 3 | 9 | VH | |
| Storage, processing & transport | ||||||
| Impurities in seed batches | H | 3 | Seed testing | 3 | 9 | VH |
| H | 3 | Use certified seed | 3 | 9 | VH | |
| H | 3 | Concise record keeping and labelling | 3 | 9 | VH | |
| Accidental commingling of crops via machinery and processing | H | 3 | Farm machinery hygiene | 3 | 9 | VH |
| Seed spillage (feral plants) | L | 1 | Cover trailer, do not overfill | 3 | 3 | M |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warner, D.J.; Lewis, K.A. Evaluation of the Risks of Contaminating Low Erucic Acid Rapeseed with High Erucic Rapeseed and Identification of Mitigation Strategies. Agriculture 2019, 9, 190. https://doi.org/10.3390/agriculture9090190
Warner DJ, Lewis KA. Evaluation of the Risks of Contaminating Low Erucic Acid Rapeseed with High Erucic Rapeseed and Identification of Mitigation Strategies. Agriculture. 2019; 9(9):190. https://doi.org/10.3390/agriculture9090190
Chicago/Turabian StyleWarner, Douglas J., and Kathleen A. Lewis. 2019. "Evaluation of the Risks of Contaminating Low Erucic Acid Rapeseed with High Erucic Rapeseed and Identification of Mitigation Strategies" Agriculture 9, no. 9: 190. https://doi.org/10.3390/agriculture9090190
APA StyleWarner, D. J., & Lewis, K. A. (2019). Evaluation of the Risks of Contaminating Low Erucic Acid Rapeseed with High Erucic Rapeseed and Identification of Mitigation Strategies. Agriculture, 9(9), 190. https://doi.org/10.3390/agriculture9090190





