Effect of GA3 and Gly Plant Growth Regulators on Productivity and Sugar Content of Sugarcane
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Design
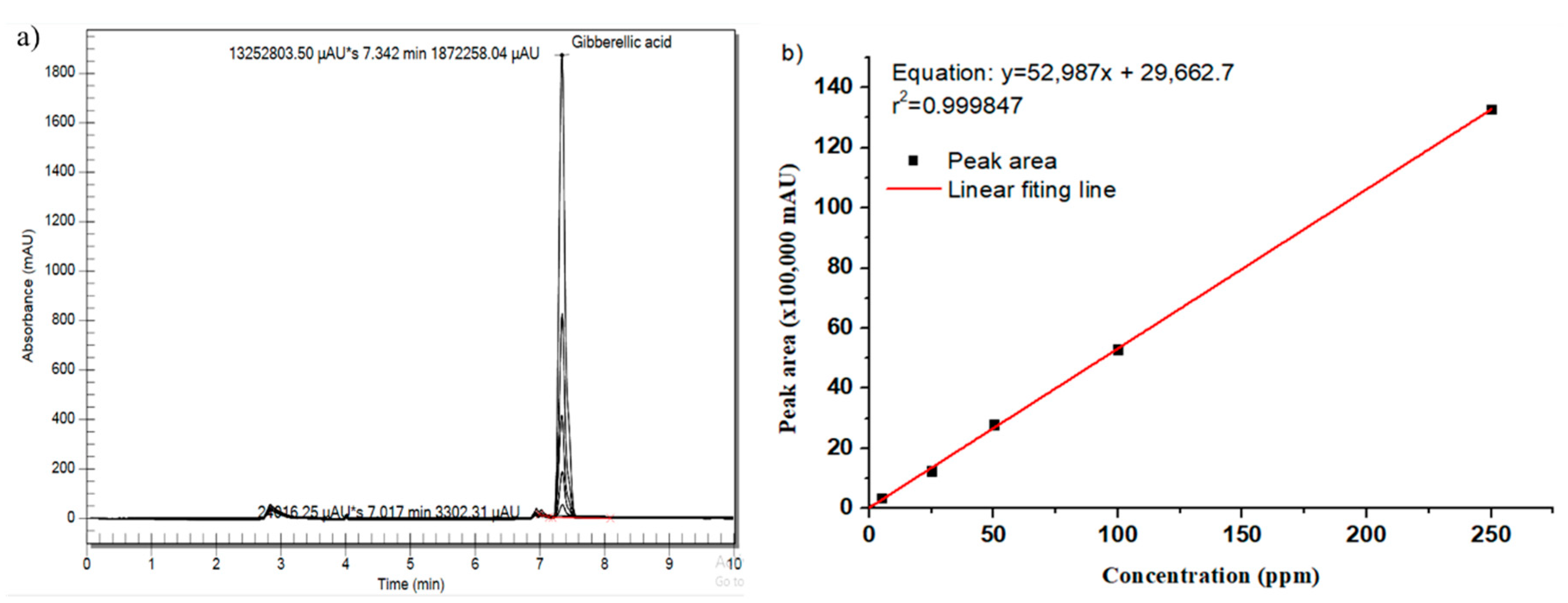
2.3. Method for Determining GA3 Residue in Sugarcane
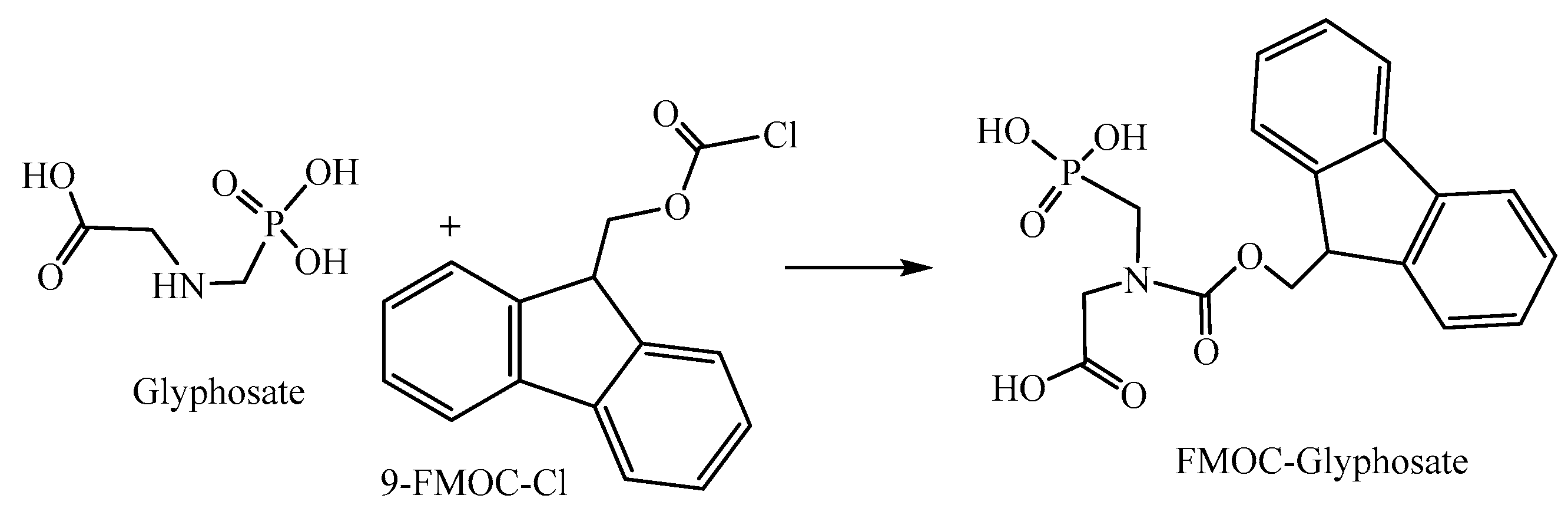
2.4. Method for Determining Gly Residues in Sugarcane
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Gibberellin Acid GA3 on the Production of Sugarcane
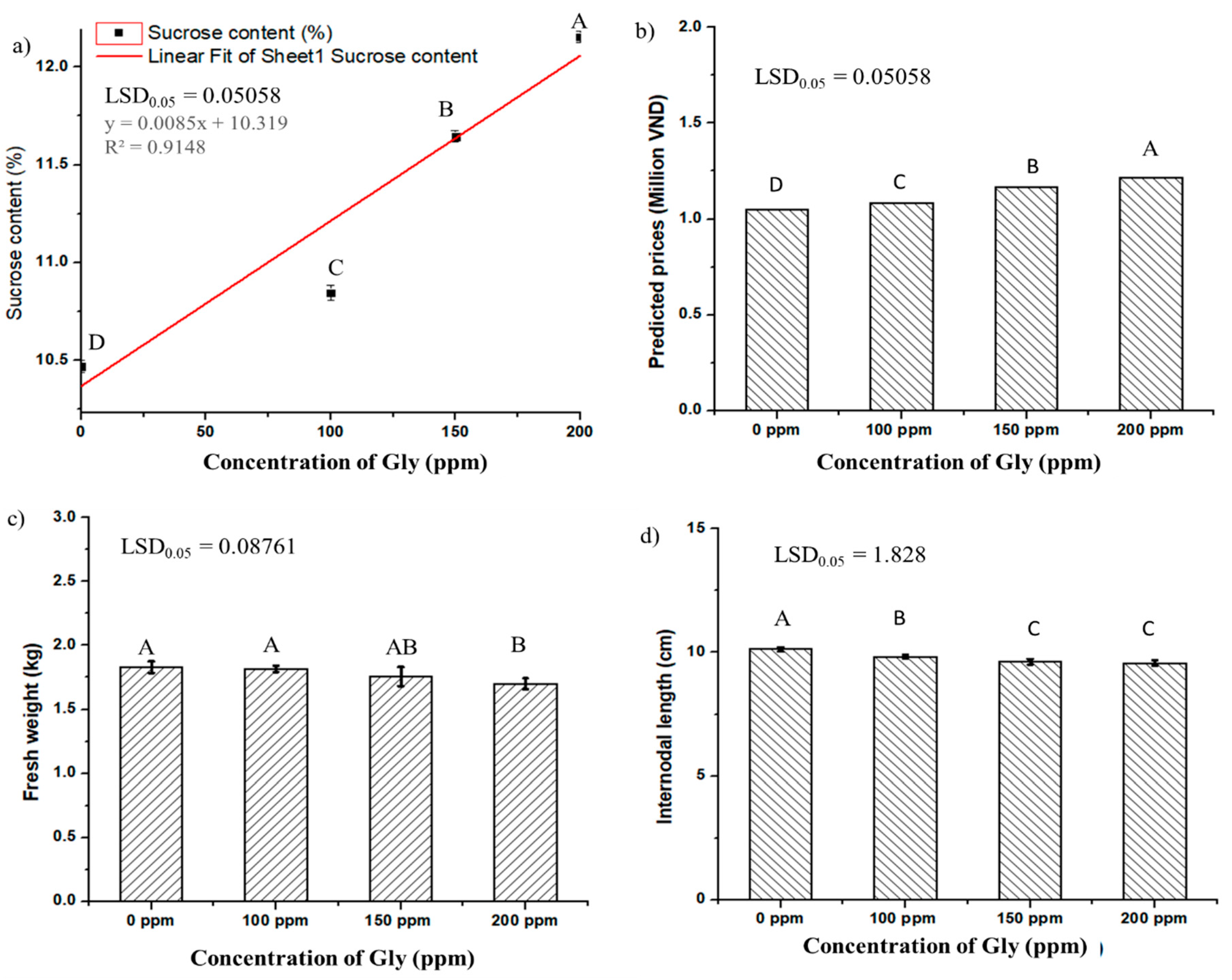
3.2. Effect of Gly on Sucrose Accumulation in Sugarcane
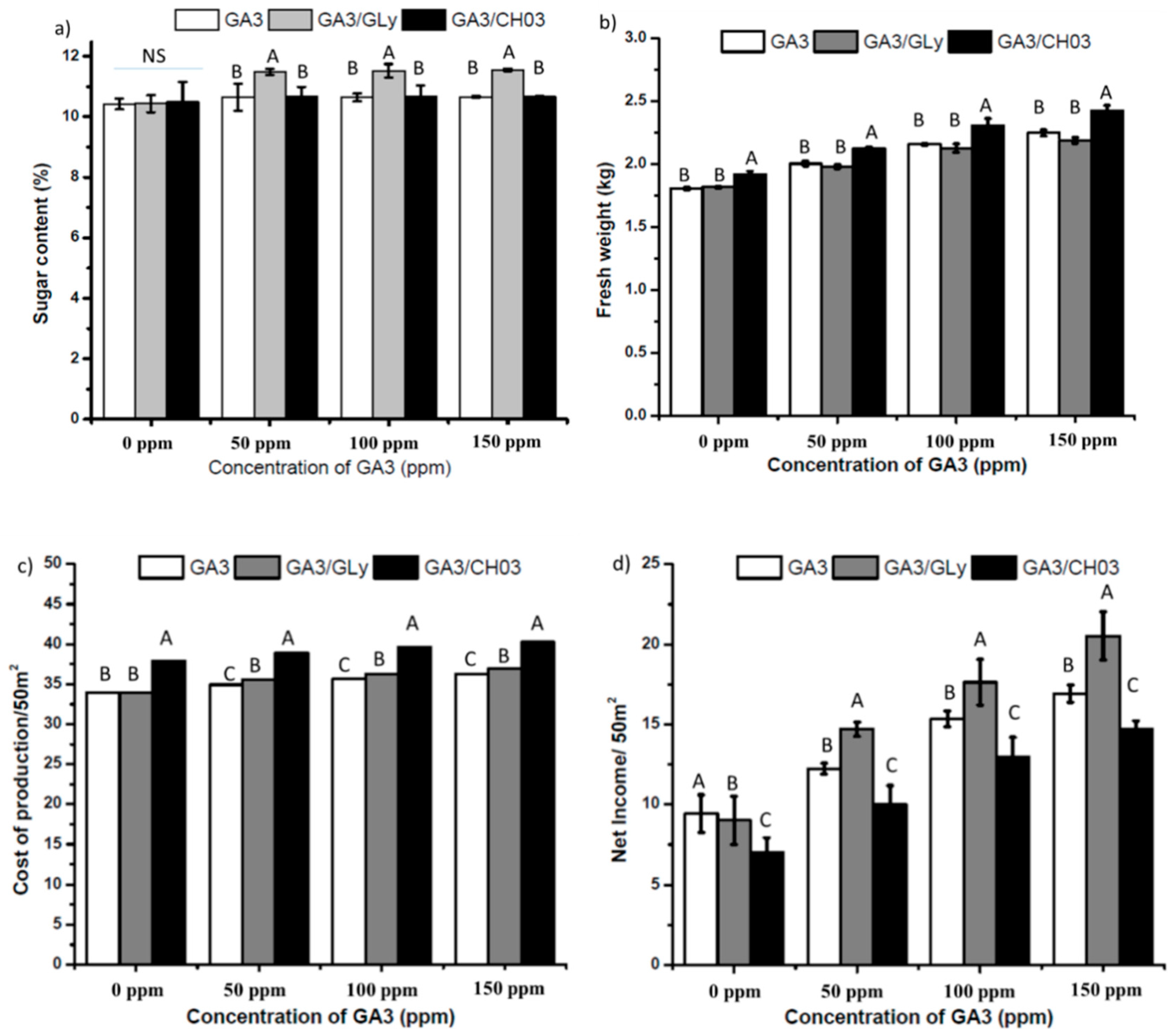
3.3. Dual Effect of GA3 and Gly on Sugarcane
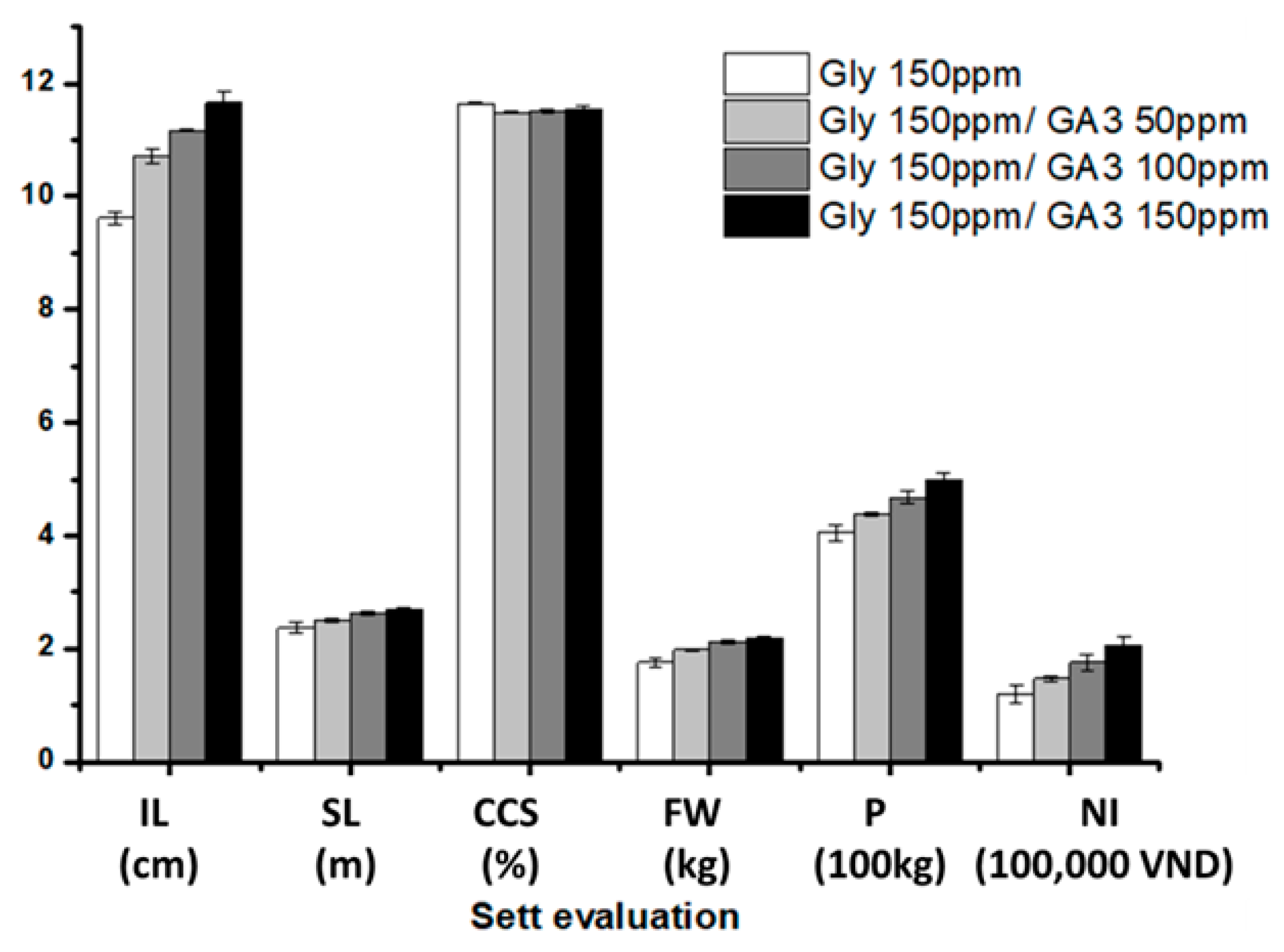
3.4. Evaluation of GA3 and Gly Residues in Sugarcane
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tew, T.L.; Cobill, R.M. Genetic improvement of sugarcane (Saccharum spp.) as an energy crop. In Genetic Improvement of Bioenergy Crops; Springer: New York, NY, USA, 2008; pp. 273–294. [Google Scholar]
- National Academy of Sciences (U.S.). Panel on the Plant Sciences, The Plant Sciences Now and in the Coming Decade; A Report on the Status, Trends, and Requirements of Plant Sciences in the United States; National Academy of Sciences (U.S.): Washington, DC, USA, 1966. [Google Scholar]
- Moore, P.H.; Osgood, R.V.; Carr, J.B.; Ginoza, H. Sugarcane studies with gibberellins. V. Plot harvests vs. stalk harvests to assess the effect of applied GA3 on sucrose yield. J. Plant Growth Regul. 1982, 1, 205–210. [Google Scholar]
- Resende, P.A.P.; Soares, J.E.; Hudetz, M. Moddus, a plant growth regulator and management tool for sugarcane production in Brazil. Sugar Cane Int. 2000, 102, 5–9. [Google Scholar]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.H.; Ginoza, H. Gibberellin Studies with Sugarcane. III. Effects of Rate and Frequency of Gibberellic Acid Applications on Stalk Length and Fresh Weight 1. Crop Sci. 1980, 20, 78–82. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Khan, M.I.R.; Masood, A.; Khan, N.A. Role of gibberellins in regulation of source-sink relations under optimal and limiting environmental conditions. Curr. Sci. (Bangalore) 2011, 100, 998–1007. [Google Scholar]
- Verma, I.; Roopendra, K.; Sharma, A.; Chandra, A.; Kamal, A. Expression analysis of genes associated with sucrose accumulation and its effect on source–sink relationship in high sucrose accumulating early maturing sugarcane variety. Physiol. Mol. Biol. Plants 2019, 25, 207–220. [Google Scholar] [CrossRef]
- Chandra, A.; Verma, P.K.; Islam, M.N.; Grisham, M.P.; Jain, R.; Sharma, A.; Roopendra, K.; Singh, K.; Singh, P.; Verma, I.; et al. Expression analysis of genes associated with sucrose accumulation in sugarcane (Saccharum spp. hybrids) varieties differing in content and time of peak sucrose storage. Plant Biol. 2015, 17, 608–617. [Google Scholar] [CrossRef]
- Verma, I.; Roopendra, K.; Sharma, A.; Jain, R.; Singh, R.K.; Chandra, A. Expression analysis of genes associated with sucrose accumulation in sugarcane under normal and GA 3-induced source–sink perturbed conditions. Acta Physiol. Plant. 2017, 39, 133. [Google Scholar] [CrossRef]
- Shomeili, M.; Nabipour, M.; Meskarbashee, M.; Memari, H.R. Effects of gibberellic acid on sugarcane plants exposed to salinity under a hydroponic system. Afr. J. Plant Sci. 2011, 5, 609–616. [Google Scholar]
- Kumar, A.; Abbas, Z. Effect of pyridoxine and gibberellins spray on growth yield quality of Sugarcane Saccharum officinarum L. Int. J. Biol. Chem. Res. (JBCR) 2014, 31, 349–354. [Google Scholar]
- Crusciol, C.A.C.; Leite, G.H.P.; de Siqueira, G.F.; de Almeida Silva, M. Response of Application of Growth Inhibitors on Sugarcane Productivity and Sucrose Accumulation in the Middle of Cropping Season in Brazil. Sugar Tech 2017, 19, 155–164. [Google Scholar] [CrossRef]
- Cunha, C.P.; Roberto, G.G.; Vicentini, R.; Lembke, C.G.; Souza, G.M.; Ribeiro, R.V.; Machado, E.C.; Lagôa, A.M.; Menossi, M. Ethylene-induced transcriptional and hormonal responses at the onset of sugarcane ripening. Sci. Rep. 2017, 7, 43364. [Google Scholar] [CrossRef] [PubMed]
- Clowes, M.S.J. Ripening activity of the glyphosate salts Mon 8000 and Roundup. In Proceedings of the International Society of Sugar Cane Technologists; ISSCT: Quatre Bornes, Mauritius, 1980; Volume 17, pp. 676–693. [Google Scholar]
- Orgeron, A.J. Sugarcane Growth, Sucrose Content, and Yield Response to the Ripeners Glyphosate and Trinexapac-Ethyl. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2012. [Google Scholar]
- Zhang, J.; Du, P. Determination of forchlorfenuron and gibberellin acid in the grapes using high performance liquid chromatography-tandem mass spectrometry. Se Pu. 2011, 29, 1133–1136. [Google Scholar] [PubMed]
- Prasad, K.; Kumar Das, A.; Oza, M.D.; Brahmbhatt, H.; Siddhanta, A.K.; Meena, R.; Eswaran, K.; Rajyaguru, M.R.; Ghosh, P.K. Detection and quantification of some plant growth regulators in a seaweed-based foliar spray employing a mass spectrometric technique sans chromatographic separation. J. Agric. Food Chem. 2010, 58, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Felton, D.E.; Ederer, M.; Steffens, T.; Hartzell, P.L.; Waynant, K.V. UV–Vis Spectrophotometric Analysis and Quantification of Glyphosate for an Interdisciplinary Undergraduate Laboratory. J. Chem. Educ. 2017, 95, 136–140. [Google Scholar] [CrossRef]
- Sprankle, P.; Meggitt, W.F.; Penner, D. Absorption, action, and translocation of glyphosate. Weed Sci. 1975, 23, 235–240. [Google Scholar] [CrossRef]
- Sujatha, P.; Kumar, B.R.; Naidum, N.V.; Charumathi, M.; Beby, P.; Jayachandra, K. Plant growth promoters effect on cane, quality and yield parameters in sugarcane (Saccharum officinarum L.). Int. J. Chem. Stud. 2018, 6, 737–743. [Google Scholar]
- Schaffer; Arthur, A.; Zamski, E. (Eds.) Photoassimilate Distribution in Plants and Crops: Source—Sink Relationships; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- MOORE, P.H. Additive and nonadditive effects of serial applications of gibberellic acid on sugarcane internode growth. Physiol. Plant. 1980, 49, 271–276. [Google Scholar] [CrossRef]
- Rai, R.K.; Tripathi, N.; Gautam, D.; Singh, P. Exogenous application of ethrel and gibberellic acid stimulates physiological growth of late planted sugarcane with short growth period in sub-tropical India. J. Plant Growth Regul. 2017, 36, 472–486. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Geijskes, R.J.; Aitken, K.S.; Grof, C.L.; Bonnett, G.D.; Smith, G.R. Sugarcane biotechnology: The challenges and opportunities. Vitr. Cell. Dev. Biol. Plant 2005, 41, 345–363. [Google Scholar] [CrossRef]
- Didier, K.E.K.; Crépin, P.E.E.B.; Mélanie, B.B.; Yah, O.; Michel, Z. Effect of glyphosate used as a sugarcane chemical ripener in Cte dIvoire. Afr. J. Plant Sci. 2017, 11, 341–350. [Google Scholar]
- Su, L.Y.; Cruz, A.D.; Moore, P.H.; Maretzki, A. The relationship of glyphosate treatment to sugar metabolism in sugarcane: New physiological insights. J. Plant Physiol. 1992, 140, 168–173. [Google Scholar] [CrossRef]
- Solomon, S.; Jain, N.; Chaab, A.; Hamdi, H.; Berghe, D.V. Improvement of the sucrose content of early harvested sugarcane using stabilised orthosilicic acid. In Proceedings of the International Society of Sugar Cane Technologists; ISSCT: Quatre Bornes, Mauritius, 2016; Volume 29. [Google Scholar]
- Carbonari, C.A.; Gomes, G.L.G.C.; Velini, E.D.; Machado, R.F.; Simões, P.S.; de Castro Macedo, G. Glyphosate effects on sugarcane metabolism and growth. Am. J. Plant Sci. 2014, 5, 3585. [Google Scholar] [CrossRef]
- Marques, T.A.; Marques, P.A.; Suriani, M.W.; Santos, A.T.D.; Mendonça, F.C. Water absorbent polymer in sugarcane crop. Eng. Agrícola 2013, 33, 99–108. [Google Scholar] [CrossRef]
- Hosseinchi, M.; Soltanalinejad, F.; Najafi, G.; Roshangar, L. Effect of gibberellic acid on the quality of sperm and in vitro fertilization outcome in adult male rats. In Veterinary Research Forum: An International Quarterly Journal; Faculty of Veterinary Medicine, Urmia University: Urmia, Iran, 2013; Volume 4, p. 259. [Google Scholar]
- Csaba, G.; Darvas, S.; Laszlo, V. Effects of treatment with the plant hormone gibberellin on neonatal rats. Acta Biol. Med. Ger. 1977, 36, 1487–1488. [Google Scholar] [PubMed]
- Surulirajan, M.; Sarbhoy, A.K. Gibberellic acid production by Fusarium moniliforme. J. Mycopathol. Res. 2000, 38, 101–104. [Google Scholar]
- Dalley, C.D.; Richard, E.P. Herbicides as ripeners for sugarcane. Weed Sci. 2010, 58, 329–333. [Google Scholar] [CrossRef]
- Nascentes, R.F.; Carbonari, C.A.; Simões, P.S.; Brunelli, M.C.; Velini, E.D.; Duke, S.O. Low doses of glyphosate enhance growth, CO2 assimilation, stomatal conductance and transpiration in sugarcane and eucalyptus. Pest Manag. Sci. 2018, 74, 1197–1205. [Google Scholar] [CrossRef]
- Dusky, J.A.; Kang, M.S.; Glaz, B.; Miller, J.D. Response of eight sugarcane cultivars to glyphosine and glyphosate ripeners. J. Plant Growth Regul. 1985, 4, 225–235. [Google Scholar] [CrossRef]
- Millhollon, R.W.; Legendre, B.L. Sugarcane yield as affected by annual glyphosate ripener treatments. JASSCT 1996, 16, 7–16. [Google Scholar]
- Millhollon, R.W.; Legendre, B.L. Growth and yield response of Louisiana sugarcane cultivars to annual preharvest treatments with the ripener glyphosate. Sugar Cane Int. 2000, 18, 5–9. [Google Scholar]
- Zouaoui, K.; Dulaurent, S.; Gaulier, J.M.; Moesch, C.; Lachâtre, G. Determination of glyphosate and AMPA in blood and urine from humans: About 13 cases of acute intoxication. Forensic Sci. Int. 2013, 226, e20–e25. [Google Scholar] [CrossRef] [PubMed]
- Morishita, D.W. Impact of glyphosate-resistant sugar beet. Pest Manag. Sci. 2018, 74, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Velini, E.D.; Alves, E.; Godoy, M.C.; Meschede, D.K.; Souza, R.T.; Duke, S.O. Glyphosate applied at low doses can stimulate plant growth. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Johal, G.S.; Huber, D.M. Glyphosate effects on diseases of plants. Eur. J. Agron. 2009, 31, 144–152. [Google Scholar] [CrossRef]
- Reddy, K.N.; Cizdziel, J.V.; Williams, M.M.; Maul, J.E.; Rimando, A.M.; Duke, S.O. Glyphosate resistance technology has minimal or no effect on maize mineral content and yield. J. Agric. Food Chem. 2018, 66, 10139–10146. [Google Scholar] [CrossRef] [PubMed]
| Time (min) | %A | %B |
|---|---|---|
| 1 | 80 | 20 |
| 3 | 80 | 20 |
| 7 | 65 | 35 |
| 15 | 80 | 20 |
| 0 ppm | 50 ppm | 100 ppm | 150 ppm | CV (%) | |
|---|---|---|---|---|---|
| Internode length (mm) | 101.2 C ± 0.95 | 111.9 B ± 0.49 | 116.0 B ± 1.84 | 122.8 A ± 3.82 | 2.48 |
| Stem length (m) | 2.38 C ± 0.07 | 2.60 B ± 0.04 | 2.67 B ± 0.08 | 2.79 A ± 0.04 | 2.38 |
| Diameter (mm) | 25.75 C ± 0.21 | 26.31 BC ± 0.51 | 27.03 B ± 0.52 | 27.86 A ± 0.21 | 1.74 |
| Fresh weight (1 stem/kg) | 1.81 D ± 0.01 | 2.010 C ± 0.02 | 2.160 B ± 0.01 | 2.250 A ± 0.03 | 0.88 |
| CCS (%) | 10.43 B ± 0.02 | 10.66 A ± 0.05 | 10.65 A ± 0.01 | 10.66 A ± 0.03 | 0.36 |
| Productivity (kg)/50 m2 | 416.3 D ± 11.2 | 442.8 C ± 3.11 | 479.5 B ± 4.77 | 499.3 A ± 5.07 | 1.57 |
| Net income (VND)/50 m2 | 94,315.75 D ± 11710.74 | 122,438.25 C ± 3316.08 | 153,518 B ± 5077.74 | 169,192.5 A ± 5041.95 | 5.62 |
| Sample | NT1 | NT2 | NT3 | NT4 | Residue | ||||
|---|---|---|---|---|---|---|---|---|---|
| GA3 (µg/mL) | ND | ND | ND | ND | ND | ||||
| Gly (µg/mL) | 1.05 | 1.06 | 1.05 | 1.05 | 1.06 | 1.06 | 1.07 | 1.06 | 1.06 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, C.T.; Dang, L.H.; Nguyen, D.T.; Tran, K.P.; Giang, B.L.; Tran, N.Q. Effect of GA3 and Gly Plant Growth Regulators on Productivity and Sugar Content of Sugarcane. Agriculture 2019, 9, 136. https://doi.org/10.3390/agriculture9070136
Nguyen CT, Dang LH, Nguyen DT, Tran KP, Giang BL, Tran NQ. Effect of GA3 and Gly Plant Growth Regulators on Productivity and Sugar Content of Sugarcane. Agriculture. 2019; 9(7):136. https://doi.org/10.3390/agriculture9070136
Chicago/Turabian StyleNguyen, Cong Truc, Le Hang Dang, Dinh Trung Nguyen, Kim Phu Tran, Bach Long Giang, and Ngoc Quyen Tran. 2019. "Effect of GA3 and Gly Plant Growth Regulators on Productivity and Sugar Content of Sugarcane" Agriculture 9, no. 7: 136. https://doi.org/10.3390/agriculture9070136
APA StyleNguyen, C. T., Dang, L. H., Nguyen, D. T., Tran, K. P., Giang, B. L., & Tran, N. Q. (2019). Effect of GA3 and Gly Plant Growth Regulators on Productivity and Sugar Content of Sugarcane. Agriculture, 9(7), 136. https://doi.org/10.3390/agriculture9070136





