4.1. Late N Supply Increases Grain Yield and Grain Protein Concentration
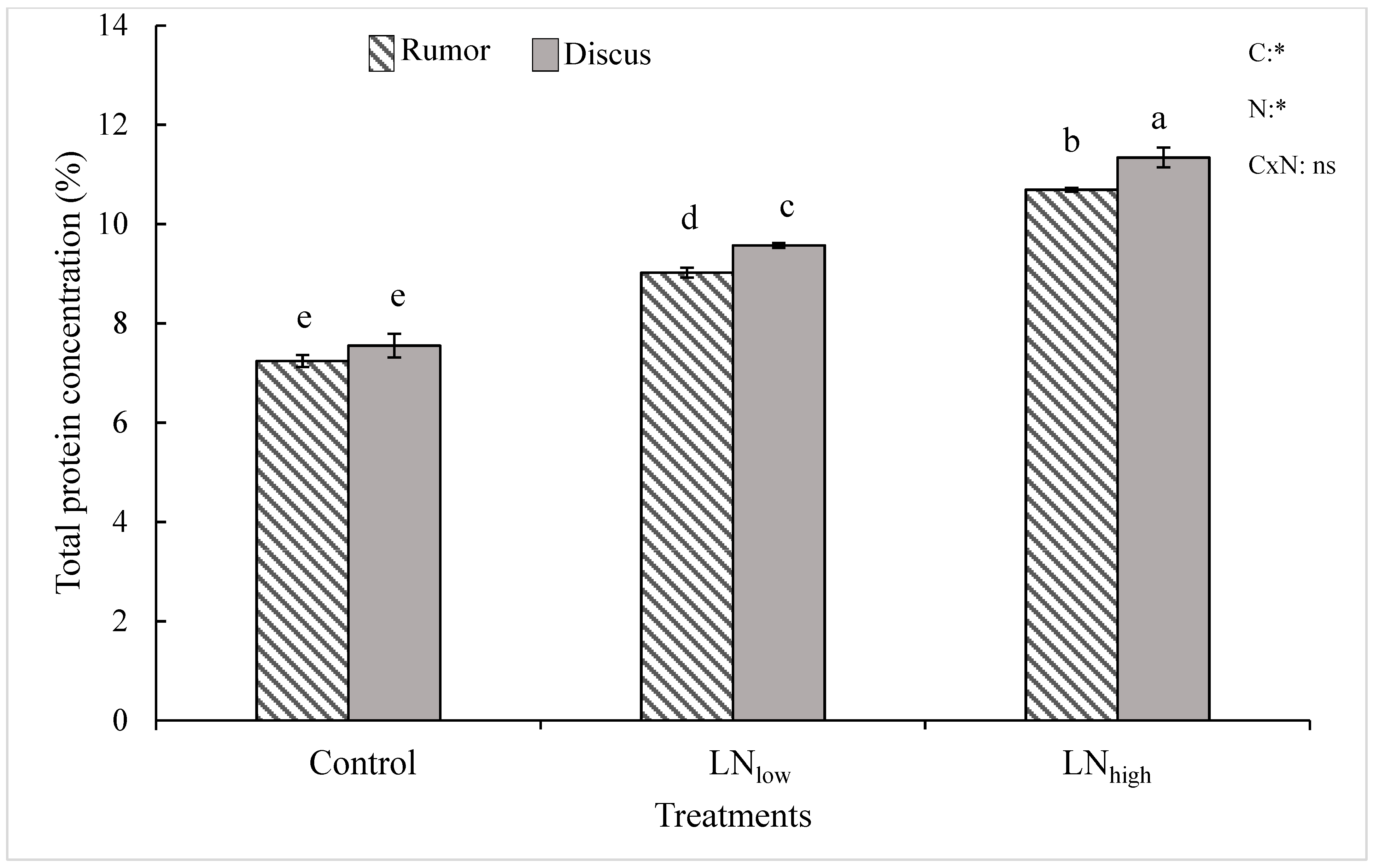
Results of the current study indicate that late N management is a viable option to improve the total protein content in winter wheat cultivars. This is in agreement with other studies where late N application increased the grain protein content of winter, spring and durum wheat cultivars within different growing seasons [
12,
13,
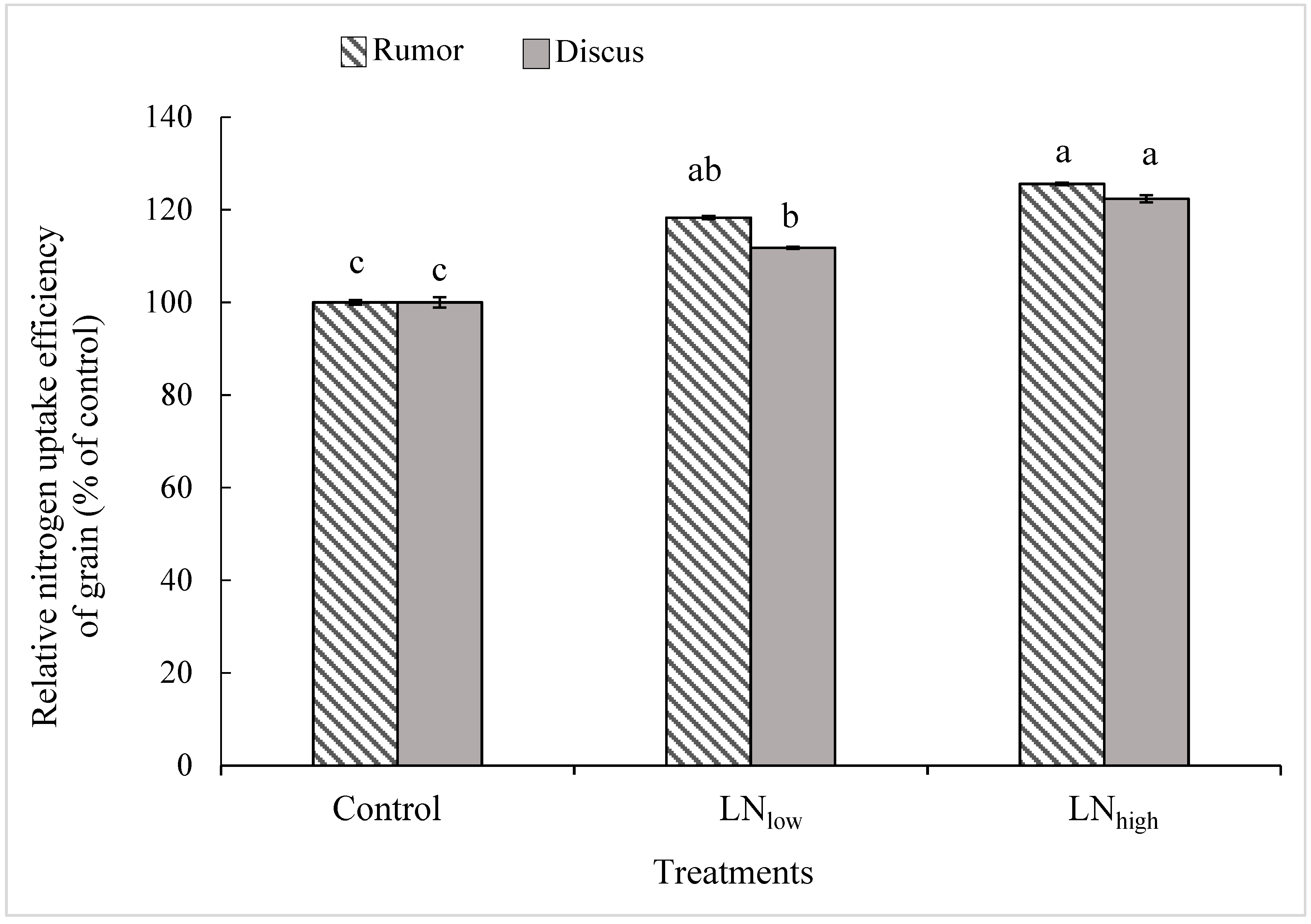
25]. Such an increase in grain protein content might be attributed to the fact that N applied after completion of vegetative growth would be directly available for grain protein synthesis, and thus increase N uptake efficiency [
1]. Accumulation of grain proteins can be fed by two different amino acid pools. The first pool represents amino acids that are assimilated during vegetative growth and are remobilized during grain filling, and the second pool represents amino acids that are assimilated during grain filling [
26]. It is plausible that amino acids synthesized from later added N are more readily available for export to the phloem to meet the demand of grain protein synthesis compared to those slowly released from the storage pool.
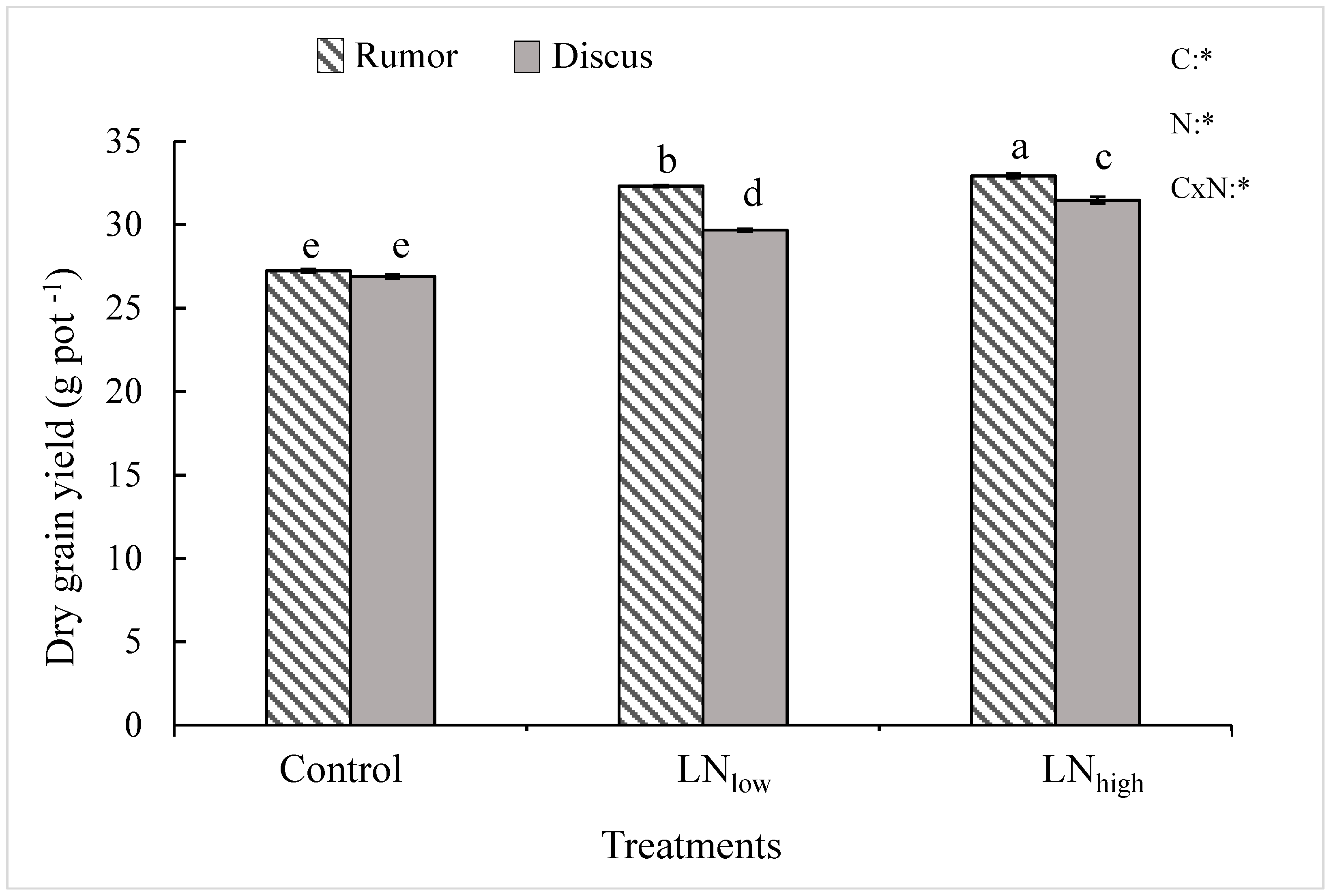
In the present study, late N application not only increased grain protein content but also grain yield in both cultivars. It is possible that the N applied before heading was mostly used up by the plant during vegetative growth but possibly the N supply during prior growth stages was below optimum to reach maximum yield. Therefore, plants used the late N at least in part for kernel filling, and partly for synthesis of proteins.
4.2. Late N Supply Alters Protein Composition and Improves Baking Quality in High Protein Cultivars
Late N fertilization caused an increase in all protein fractions, though mainly in the glutenins and gliadins. The stronger increase in glutenins and gliadins with application of late N fertilizer indicates that gluten proteins were preferentially biosynthesized compared to albumins and globulins. Even though it has been suggested that N distribution within grain protein fractions is mostly dependent upon the genetic background [
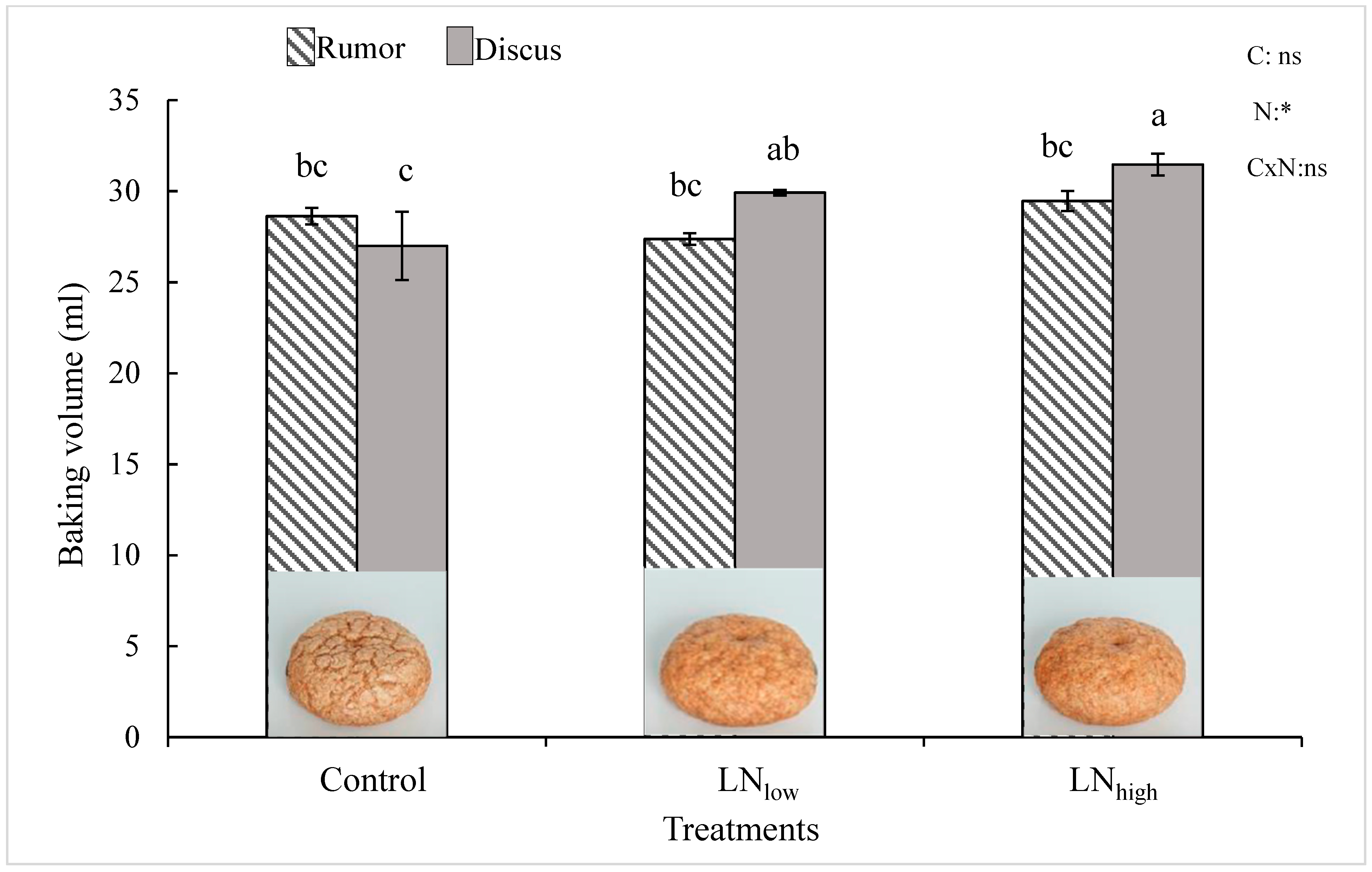
27], our results clearly indicate that protein composition can also be modified by external factors such as the concentration of N available after heading. However, the overall increase in gluten proteins did not seem to be directly correlated with an improvement of the baking volume. While gluten increased to a similar extent in both cultivars with late N application, baking quality was enhanced only in Discus, i.e., not in Rumor (
Figure 4).
We therefore conclude that the composition changes of glutenin and to some extent gliadin with late N application might be decisive for the baking quality, rather than the overall total gluten concentration. This is further supported by the fact that changes in the composition of gluten fractions, especially HMW glutenins and ω-gliadins, in response to late N fertilization were significant only in Discus, i.e., not in Rumor (
Table 4,
Table 5 and
Table 6). The HMW glutenin subunit is known to be extremely important for dough properties, and it was suggested that variation in quantity and quality of HMW-GS in European wheat cultivars is responsible for 45–70% of the variation in baking quality [
28]. HMW-GS and ω-gliadins have relatively low sulfur contents [
9] and previous reports have shown that N fertilization enhanced the percentages of storage proteins containing low to medium sulfur, while those of sulfur rich proteins (e.g., LMW-GS) remained constant or decreased [
28,
29,
30]. This is fully in line with our results where HMW-GS and ω-gliadins tended to increase with late N fertilizer, while LMW-GS rather decreased, at least in Discus. Both ω-gliadins and HMW-GS mainly consist of metabolically inexpensive amino acids (glutamine and proline), which may represent sinks for readily available N, which is not needed for vegetative growth [
31]. As protein concentration was improved in both cultivars by application of LN
low and LN
high, the same improvement was not recorded for different protein subunits; therefore, it can be assumed that the composition of gluten subunits is independent from total protein concentration.
Moreover, it was suggested that bread wheat cultivars contain between three and five HMW-GS. On the other hand it was assumed that the relative proportion of individual HMW-GS with application of N fertilizer stayed constant [
17]. Our results point out that relative quantity of specific HMW-GS was improved with late N application at least in one cultivar. In addition, it was proposed that amongst HMW-GS, x-type subunits had higher effects on bread making quality than y-type subunits [
32]. In our study, x-type HMW-GS present in both wheat cultivars was the largest HMW-GS (
Table 5). The change of x-type HMW-GS in Discus was significant (
Table 5 band #1 and #2). As a result, the relative change of this subunit may have excessive impacts on baking quality, as was observed in our study.
Additionally, the ratio of protein subunits (HMW/LMW or gliadin/glutenin) strongly affects baking performance of flour [
33]. Therefore, it is essential to study the effect of late N application not only on protein fractions and subunits but also on their ratios.
It was suggested that the increase of the HMW/LMW ratio may improve baking volume [
33]. This effect was observed in our study. While the ratio of HMW/LMW and further baking volume stayed unchanged in Rumor, application of LN
low increased HMW/LMW and baking volume significantly in Discus.
It was suggested [
34] with an increase in nitrogen supply over unchanged sulfur supply, more N is available for biosynthesis, which causes a higher increase in glutenin with respect to gliadin. Simultaneously, the bread volume and gliadin to glutenin ratio has a negative correlation [
35]. This effect was observed in our study where the gliadin/glutenin ratio was decreased with the application of LN
low in Discus and baking volume improved. In contrast, neither the gliadin/glutenin ratio nor baking volume significantly changed in Rumor. This can be due to the higher gliadin concentration in Rumor compared to Discus.
While grain yield and total grain protein concentration overall increased with increasing amount of supplied N, significant changes in glutenin and gliadin subunits were already observed in the LNlow treatment, while a higher late N supply did not bring about additional changes in protein composition, nor did further improve baking volumes. These results seem to further indicate not only that baking quality is more strongly determined by the gluten composition rather than the overall protein concentration, but also that a small increase in late N availability might be sufficient for improving baking quality at least in cultivars with a higher grain protein yield capacity. For these cultivars, higher rates of late N supply would not seem to be economically advantageous and might potentially lead to N leaching and losses into the environment.
In summary, it can be suggested that a LN
low application of late N fertilizer might be a feasible way to improve the baking quality of wheat flour. However, one of the main findings of the present study is that the compositional changes of gluten protein fractions were much less pronounced in Rumor compared to Discus. Rumor is a cultivar characterized by optimum baking quality at lower grain protein content compared with Discus. In other words, it reaches its optimal baking volume already at lower protein levels, and consequently, additional N supplied at late stages of development can be used for kernel filling and thus increase grain yield, but they do not induce major compositional protein changes and improved baking quality. A similar result was also obtained in a previous study using a different pair of wheat cultivars [
13], which further supports that different N management strategies might be adequate for different wheat cultivars. This opens up an opportunity for some reduction of N fertilization rates in wheat production. Overall in this study gliadin, glutenin and protein concentrations were not well correlated with different protein subunits and loaf volume in both cultivars. Therefore, our results confirm that total grain protein concentration alone may not be a reliable parameter for the evaluation of flour quality in high protein wheat cultivars, while an increased amount of gluten fractions is not a promising breeding target for lower protein wheat cultivars. It can be suggested that combined analysis of protein concentration and composition should take a place in order to evaluate the wheat baking quality.
Based on this study, the effect of late N application was observed in protein concentration, composition and baking quality of wheat flour. Many other factors such as the rate and type of late N, cultivar differences and environmental conditions (availability of rainfall, especially during spring) need to be considered. This study provides knowledge on effects of late N fertilizer under controlled condition with two cultivars. Therefore, it is essential to bring this result to the field level with different cultivars and locations to evaluate the further G*E effects.