Evaluation of the Optimum Harvesting Maturity of Makhwaen Fruit for the Perfumery Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Essential Oils Extraction
2.3. Chemical Composition Analysis
2.4. NIR Analysis
2.5. Sensory Analysis
2.6. Statistical Analyses
3. Results and Discussion
3.1. Fruit Size, Weight and Colour
3.2. Chemical Compounds
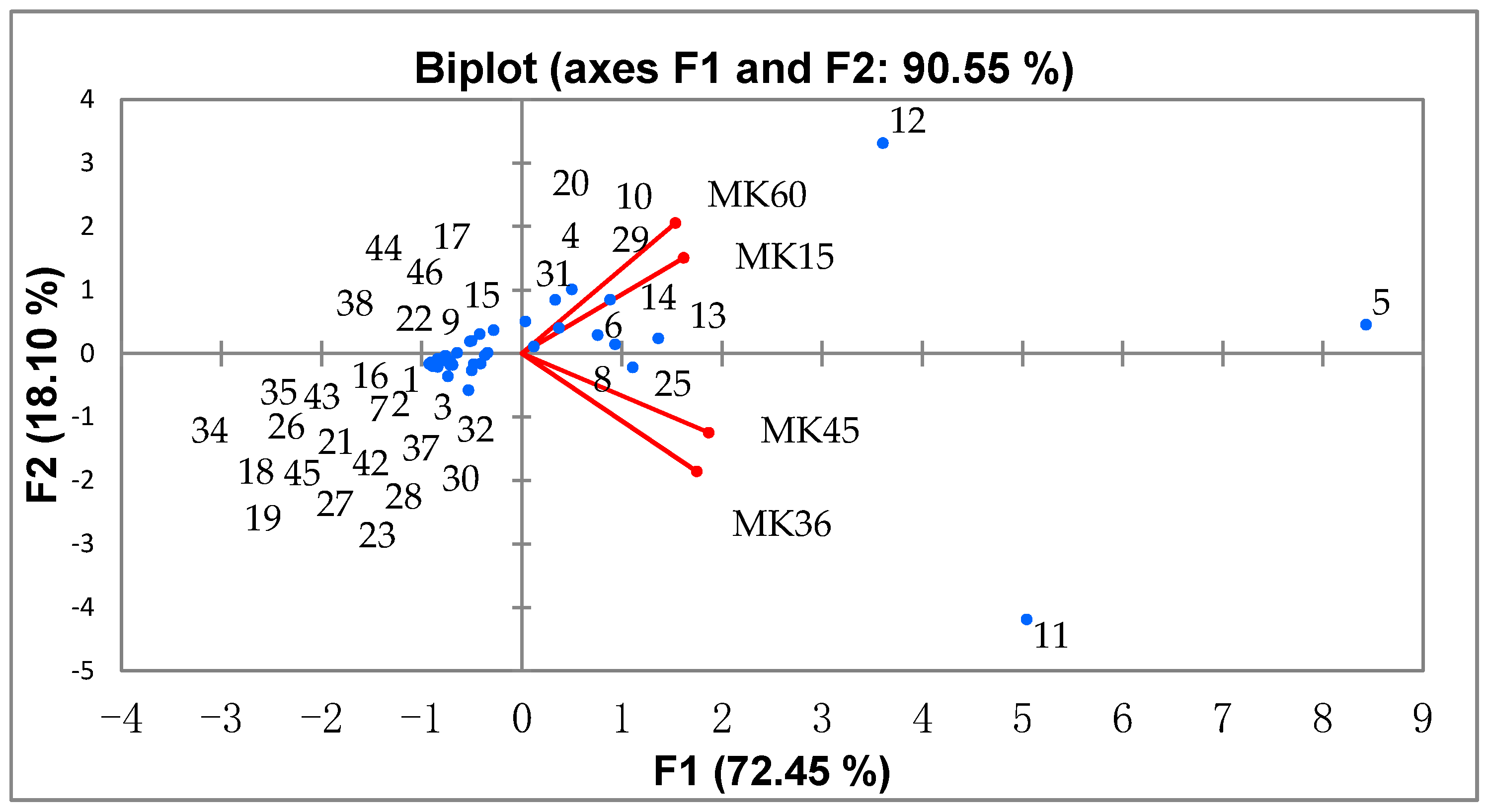
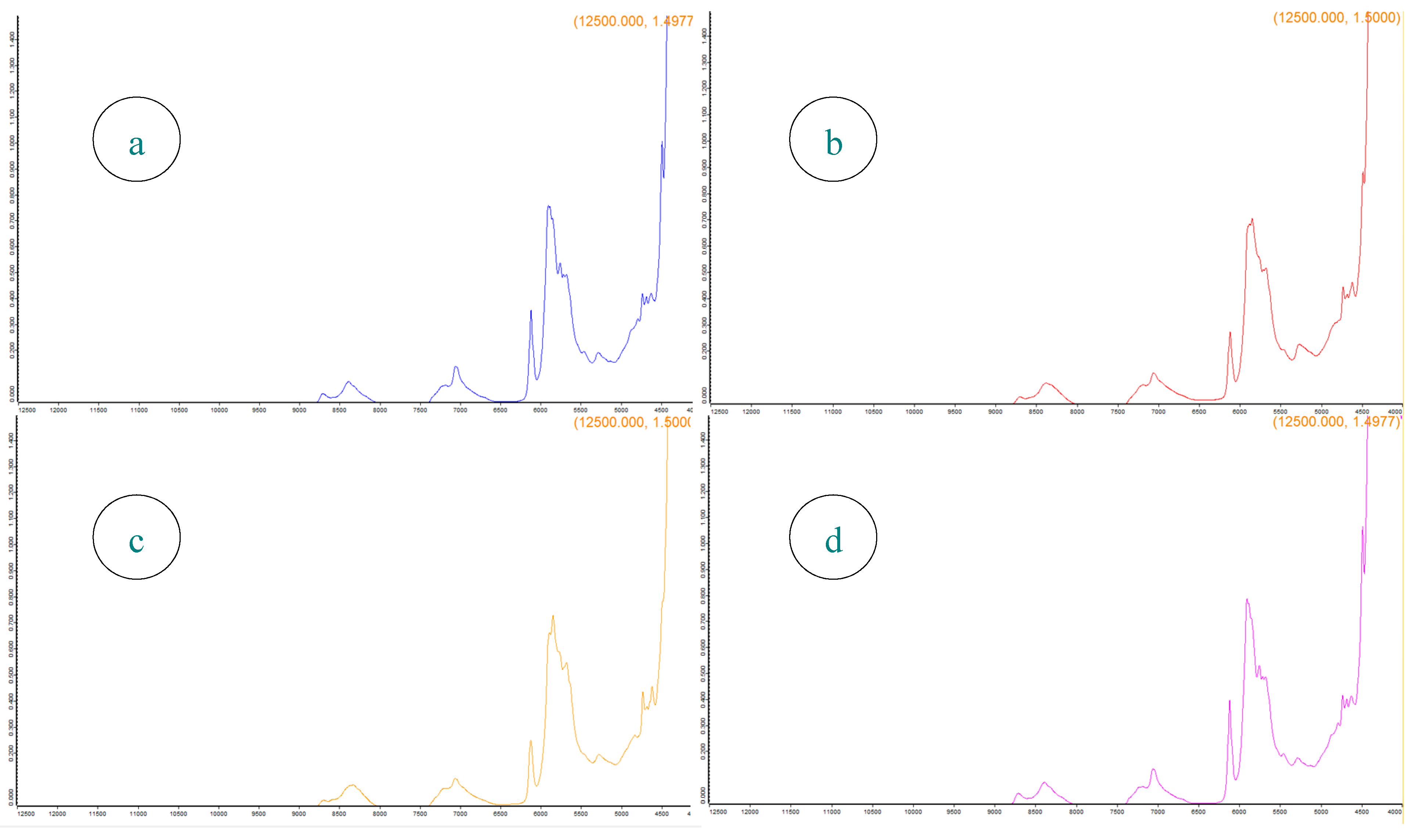
3.3. NIR Analysis
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum maxim. (Rutaceae): A systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef] [PubMed]
- Toyin, Y.M.; Olakunle, A.T.; Adewunmi, A.M. Toxicity and Beneficial Effects of Some African Plants on the Reproductive System. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 445–492. [Google Scholar]
- Brijwal, L.; Pandey, A.; Tamta, D.S. An overview on phytomedicinal approaches of Zanthoxylum armatum DC.: An important magical medicinal plant. J. Med. Plants Res. 2013, 7, 366–370. [Google Scholar]
- Patiño, L.O.J.; Prieto, R.J.A.; Cuca, S.L.E. Zanthoxylum Genus as Potential Source of Bioactive Compounds. In Bioactive Compounds in Phytomedicine; Rasooli, I., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 185–220. [Google Scholar]
- Suksathan, R.; Trisonthi, C.; Trisonthi, P.; Wangpakapattanawong, P. Notes on Spice Plants in the Genus Zanthoxylum (Rutaceae) in Northern Thailand. Available online: https://www.tci-thaijo.org/index.php/ThaiForestBulletin/article/view/24374 (accessed on 16 April 2019).
- Sriwichai, T.; Suksathan, R.; Charoenlertthanakit, N.; Sommano, S. Zanthoxylum spp.: A new potential sources of essential oil for the perfumery and pharmaceutical industries in Thailand. Med. Plants 2019, 11, 26–45. [Google Scholar] [CrossRef]
- Charoensup, R.; Duangyod, T.; Phuneerub, P.; Singharachai, C. Pharmacognostic specifcation of Zanthoxylum limonella (Dennst.) alston: Fruits and seeds in Thailand. J. Adv. Pharm. Technol. Res. 2016, 7, 134–138. [Google Scholar] [CrossRef]
- Wongsrisom, N.; Jinata, J.; Manosan, B.; Kuntakhoo, J.; Wankuan, S.; Sriyam, S. Anti-bacterial activities of essential oils from mah-khwuaen (Zanthoxylum limonella alston). KMUTT Res. Dev. J. 2014, 37, 3–15. [Google Scholar]
- Li, R.; Yang, J.; Shi, Y.; Zhao, M.; Ji, K.; Zhang, P.; Xu, Y.; Hu, H. Chemical compositions antimicrobial and anti-inflammatory activities of the essential oil from maqian (Zanthoxylum myriacanthum var. Pubescens) in Xishuangbanna, southwest China. J. Ethnopharmacol. 2014, 158, 43–48. [Google Scholar] [CrossRef]
- Tangpao, T.; Chung, H.H.; Sommano, S.R. Aromatic profiles of essential oils from five commonly used Thai basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Kader, A. Postharvest Technology of Horticultural Crops, 3rd ed.; The University of California Division of Agriculture and Natural Resources: Davis, CA, USA, 2002. [Google Scholar]
- Carrubba, A.; Catalano, C. Essential Oil Crops for Sustainable Agriculture—A Review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Springer: Dordrecht, The Netherlands, 2009; pp. 247–287. [Google Scholar]
- Hazarika, P. Impact of harvesting cycle, maturity stage, drying and storage on essential oil content of patchouli leaves grown in northeast region of India. J. Essent. Oil Bear. Plants 2014, 17, 1389–1396. [Google Scholar]
- Inan, M.; Kirpik, M.; Kaya, D.; Kirici, S. Effect of harvest time on essential oil composition of Thymbra spicata L. growing in flora of adıyaman. J. Environ. Biol. 2011, 5, 356–358. [Google Scholar]
- Andrianoelisoa, H.; Danthu, P.; Tomazello, M.; Menut, C.; Chaix, G. Near infrared (NIR) spectroscopy for determination of essential oil chemotypes from Ravensara aromatic. In NIR2013 Proceedings; Maurel, V.B., Williams, P., Downey, G., Eds.; RSTEA—France Institut National de Recherche en Sciences et Technologies Pour L’environnement et L’agriculture: La Grande-Motte, France, 2013. [Google Scholar]
- Maisnam, D.; Rasane, P.; Dey, A.; Kaur, S.; Sarma, C. Recent advances in conventional drying of foods. J. Food Technol. Preserv. 2017, 1, 25–34. [Google Scholar]
- Chopra, R.; Folstad, N.; Lyons, J.; Ulmasov, T.; Gallaher, C.; Sullivan, L.; McGovern, A.; Mitacek, R.; Frels, K.; Altendorf, K.; et al. The adaptable use of brassica NIRS calibration equations to identify pennycress variants to facilitate the rapid domestication of a new winter oil seed crop. Ind. Crops Prod. 2019, 128, 55–61. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Belz, H.-H.; Rösch, P.; Strehle, M.A.; Popp, J. Chemotaxonomic characterisation of essential oil plants by vibrational spectroscopy measurements. Vib. Spectrosc. 2004, 35, 81–86. [Google Scholar] [CrossRef]
- Steuer, B.; Schulz, H.; Läger, E. Classification and analysis of citrus oils by NIR spectroscopy. Food Chem. 2001, 72, 113–117. [Google Scholar] [CrossRef]
- Schulz, H.; Quilitzsch, R.; Krüger, H. Rapid evaluation and quantitative analysis of thyme, origano and chamomile essential oils by ATR-IR and NIR spectroscopy. J. Mol. Struct. 2003, 661, 229–306. [Google Scholar] [CrossRef]
- Suksathan, R. Botanical Characteristics of Some Spice Plants in Genus Zanthoxylum in Upper North of Thailand; Chiang Mai University: Chiang Mai, Thailand, 2002; Chapter 3; pp. 13–26. [Google Scholar]
- Patil, J.B.; Shivanna, H.; Hegde, K. Seed source variation on fruit and seed traits of Zanthoxylum rhetsa a medicinal tree under high exploitation in central western Ghats, India. Int. J. Pharm. Life Sci. 2014, 5, 4081–4085. [Google Scholar]
- Mashkani, D.; Reza, M.; Larijani, K.; Mehrafarin, A.; Naghdi Badi, H. Changes in the essential oil content and composition of Thymus daenensis celak. under different drying methods. Ind. Crops Prod. 2018, 112, 389–395. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Pub. Corporation: Carol Stream, IL, USA, 2001. [Google Scholar]
- Wedding, B.; White, R.; Grauf, S.; Coomans, D.; Nottingham, S.; Gadek, P. The Determination of Essential Oils in Sandalwood via NIR Spectroscopy. In Proceedings of the 12th Australian Near Infrared Spectroscopy Group (ANISG), Rockhampton, Australia, 7–10 May 2006. [Google Scholar]
- Jiang, L.; Kubota, K. Differences in the volatile components and their odor characteristics of green and ripe fruits and dried pericarp of Japanese pepper (Xanthoxylum piperitum DC.). J. Agric. Food Chem. 2006, 52, 4197–4203. [Google Scholar] [CrossRef]
- Sunanta, P.; Sriwichai, T.; Tangpao, T.; Sommano, S.R. Chok-anan aroma analysis: Descriptive analysis of volatiles from selected tropical plants. J. Postharvest Technol. 2018, 6, 35–43. [Google Scholar]
- Choi, S.M.; Lee, D.-J.; Kim, J.-Y.; Lim, S.-T. Volatile composition and sensory characteristics of onion powders prepared by convective drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef]
- Sriwichai, T.; Sookwong, P.; Siddiqui, M.W.; Sommano, S.R. Aromatic profiling of Zanthoxylum myriacanthum (makwhaen) essential oils from dried fruits using different initial drying techniques. Ind. Crops Prod. 2019, 133, 284–291. [Google Scholar] [CrossRef]
- Gonçalves, D.; Costa, P.; Rodrigues, C.E.C.; Rodrigues, A.E. Effect of Citrus sinensis essential oil deterpenation on the aroma profile of the phases obtained by solvent extraction. J. Chem. Thermodyn. 2018, 116, 166–275. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peris, J.E.; Redondo, A.; Shimada, T.; Peña, L. Principal component analysis (PCA) of volatile terpene compounds dataset emitted by genetically modified sweet orange fruits and juices in which a D-limonene synthase was either up- or down-regulated vs. empty vector controls. Data Brief 2016, 9, 355–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Gamal, S.M.A.; Ahmed, H.M.I. Influence of different maturity stages on fruit yield and essential oil content of some Apiaceae family plants. Plant Prod. Mansoura Univ. 2017, 8, 127–133. [Google Scholar]
- Alam, F.; Us Saqib, Q.N. Pharmacognostic study and development of quality control parameters for fruit, bark and leaf of Zanthoxylum armatum (Rutaceae). Anc. Sci. Life 2015, 34, 147–155. [Google Scholar] [CrossRef]
- Costa, E.C.C.; Christofoli, M.; Costa, G.C.S.; Peixoto, M.F.; Fernandes, J.B.; Forim, M.R.; Pereira, K.C.; Silva, F.G.; Cazal, C.M. Essential oil repellent action of plants of the genus Zanthoxylum against Bemisia tabaci biotype B (Homoptera: Aleyrodidae). Sci. Hortic. 2017, 226, 327–332. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Zhang, Z.; Chen, Z.-Y.; Liang, J.-Y.; Geng, Z.-F.; Guo, S.-S.; Du, S.-S.; Deng, Z.-W. Chemical composition of essential oils from six Zanthoxylum species and their repellent activities against two stored-product insects. J. Chem. 2017, 2017, 1287362. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Kim, G.-H. Safety evaluation of Zanthoxylum piperitum-derived essential oil by assessing micronucleus abnormalities, mutagenicity, and chromosomal aberration. Food Res. Int. 2012, 47, 267–271. [Google Scholar] [CrossRef]
- Li, K.; Zhou, R.; Wang Jia, W.; Li, Z.; Li, J.; Zhang, P.; Xiao, T. Zanthoxylum bungeanum essential oil induces apoptosis of HaCaT human keratinocytes. J. Ethnopharmacol. 2016, 186, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Śmigielski, K.; Prusinowska, R.; Raj, A.; Sikora, M.; Woliñska, K.; Gruska, R. Effect of drying on the composition of essential oil from lavandula angustifolia. J. Essent. Oil Bear. Plants 2013, 14, 532–542. [Google Scholar] [CrossRef]
- Ogbonna, C.N.; Nozaki, K.; Yajima, H. Antimicrobial activity of Xylopia aethiopica, Aframomum melegueta and Piper guineense ethanolic extracts and the potential of using Xylopia aethiopica to preserve fresh orange juice. Afr. J. Biotechnol. 2013, 12, 1993–1998. [Google Scholar] [CrossRef]

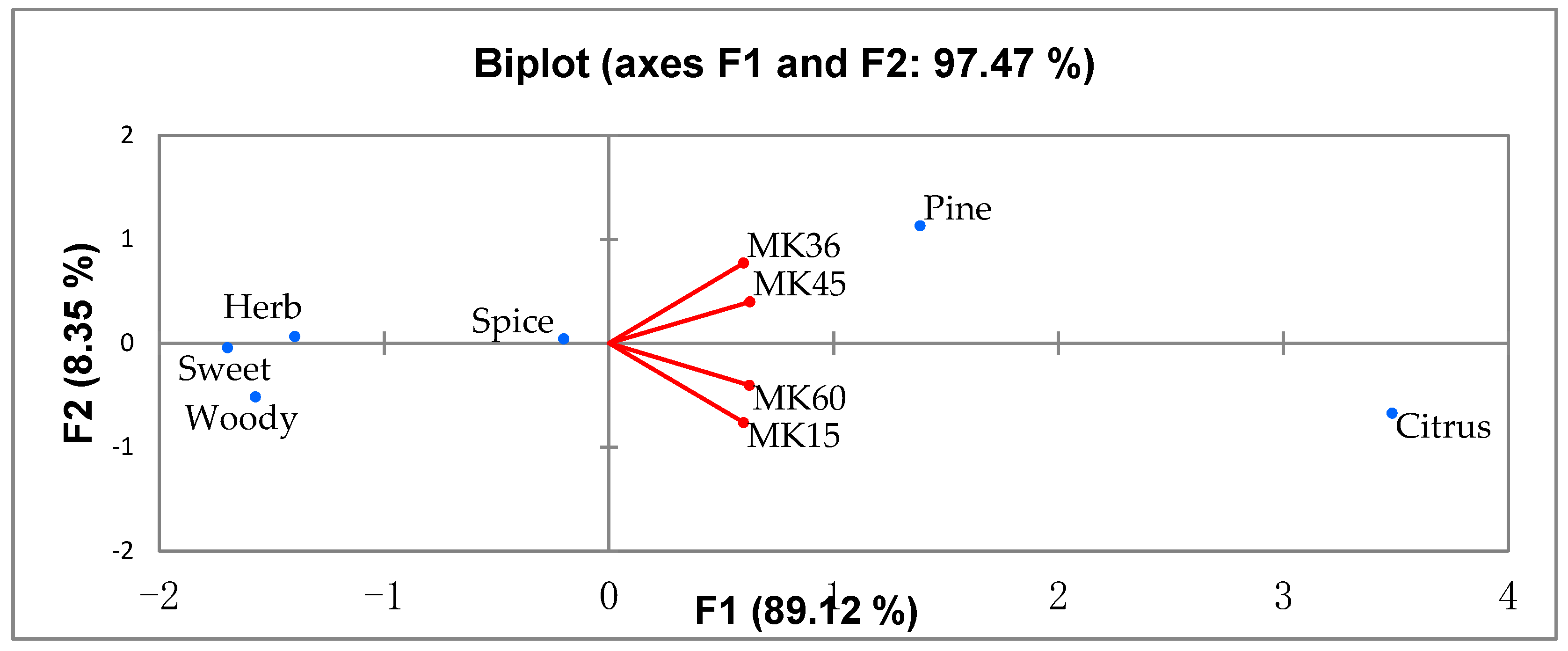
| Odour Attributes | Reference Standards | n/15 * |
|---|---|---|
| Citrus | Lemon extract (McCormick), 200 µL | 8/15 |
| Herb | Thyme (McCormick), 0.5 g | 10/15 |
| Pine | Pine/cypress essential oil | 10/15 |
| Spice | Ground allspice (McCormick), 0.5 g | 8/15 |
| Sweet | Vanilla flavour (McCormick), 200 µL | 10/15 |
| Woody | Peanut peel 2 g with 100 µL DI water | 7/15 |
| Treatment | Size (mm) | Weight (g) |
|---|---|---|
| MK15 | 3.73 ± 0.08 b | 0.026 ± 0.006 ab |
| MK36 | 3.72 ± 0.08 b | 0.034 ± 0.024 b |
| MK45 | 3.55 ± 0.07 ab | 0.023 ± 0.005 ab |
| MK60 | 3.45 ± 0.06 a | 0.016 ± 0.010 a |
| No. | Chemical Compounds | Amount of Chemical (µg·mL−1 Essential Oil a) | |||||
|---|---|---|---|---|---|---|---|
| RIcal | RIref | MK15 | MK36 | MK45 | MK60 | ||
| 1 | α-thujene | 800.5 | 926 | 2.21 ± 0.01 | ND | 4.30 ± 0.02 | ND |
| 2 | α-phellandrene | 800.5 | 1004 | 4.26 ± 0.01 | ND | ND | 2.23 ± 0.01 |
| 3 | α-pinene | 800.6 | 937 | 2.01 ± 0.01 | 5.16 ± 0.01 | 11.66 ± 0.01 | 6.10 ± 0.01 |
| 4 | cis-ocimene | 886.2 | 3.91 ± 0.37 | 1.92 ± 0.01 | 3.45 ± 0.01 | 1.96 ± 0.01 | |
| 5 | sabinene | 900.0 | 1132 | 85.81 ± 0.02 | 118.89 ± 0.05 | 115.48 ± 0.11 | 146.27 ± 0.07 |
| 6 | β-mycrene | 900.1 | 992 | 22.45 ± 0.18 | 14.69 ± 0.01 | 23.47 ± 0.02 | 20.30 ± 0.01 |
| 7 | octanal | 900.2 | ND | ND | 1.20 ± 0.01 | 1.10 ± 0.01 | |
| 8 | L-phellandrene | 900.2 | 14.18 ± 0.07 | 14.81 ± 0.01 | 52.41 ± 0.02 | 22.72 ± 0.02 | |
| 9 | α-terpinene | 900.3 | 1196 | 6.03 ± 0.03 | 6.37 ± 0.03 | 10.78 ± 0.01 | 9.21 ± 0.01 |
| 10 | benzene, methyl (1-methylethyl) | 900.4 | 49.34 ± 0.30 | 4.82 ± 0.02 | 2.77 ± 0.01 | 2.78 ± 0.01 | |
| 11 | L-limonene | 900.4 | 1035 | 25.31 ± 0.18 | 139.04 ± 0.09 | 140.65 ± 0.08 | 135.64 ± 0.01 |
| 12 | β-phellandrene | 900.5 | 1227 | 45.22 ± 0.29 | 28.48 ± 0.14 | 39.13 ± 0.02 | 125.30 ± 0.06 |
| 13 | 1,3,6 octatriene, 3,7-dimethyl | 900.6 | 38.45 ± 0.35 | 23.71 ± 0.02 | 30.49 ± 0.02 | 12.83 ± 0.01 | |
| 14 | γ-terpinene | 900.7 | 1017 | 22.04 ± 0.18 | 11.76 ± 0.06 | 17.81 ± 0.01 | 14.80 ± 0.01 |
| 15 | p-menth-2-en-1-ol | 967.1 | 34.49 ± 0.22 | ND | ND | ND | |
| 16 | α -terpinolene | 920.7 | 2.14 ± 0.01 | 2.93 ± 0.01 | 4.89 ± 0.01 | 3.94 ± 0.01 | |
| 17 | cis-sabnenehydrate | 900.6 | 1578 | 37.31 ± 0.18 | ND | ND | ND |
| 18 | trans-sabinene hydrate | 900.7 | ND | 2.33 ± 0.01 | ND | 4.27 ± 0.01 | |
| 19 | α-terpineol | 900.9 | 1594 | ND | 2.16 ± 0.01 | ND | ND |
| 20 | cyclohexane, 1-methyl-4-(1-methylethylidene) | 920.7 | 30.84 ± 0.15 | 5.39 ± 0.03 | 4.03 ± 0.01 | ND | |
| 21 | L-linalool | 925.7 | 1566 | 3.93 ± 0.01 | 5.10 ± 0.01 | 14.12 ± 0.01 | 8.77 ± 0.01 |
| 22 | 1-terpineol | 1000.1 | ND | ND | 3.44 ± 0.03 | ND | |
| 23 | Δ-3-carene | 900.6 | 1011 | ND | 9.86 ± 0.10 | ND | ND |
| 24 | 3-cyclohexane-1-ol, 4-methyl-1-(1-methylethylidene) | 1000.5 | 9.01 ± 0.04 | 27.06 ± 0.13 | 39.49 ± 0.03 | 35.65 ± 0.02 | |
| 25 | 2-cyclohexane-1-ol, 1-methyl-4-(1-methylethylidene) | 901.1 | ND | 2.18 ± 0.11 | ND | ND | |
| 26 | 2-β -pinene | 901.3 | ND | 1.35 ± 0.03 | ND | ND | |
| 27 | cryptone | 1000.5 | 2.20 ± 0.01 | 6.39 ± 0.01 | 1.34 ± 0.01 | 2.69 ± 0.01 | |
| 28 | β-fenchyl alcohol | 1013.0 | 42.98 ± 0.38 | 11.03 ± 0.01 | 11.19 ± 0.04 | 12.69 ± 0.01 | |
| 29 | decanal | 1000.7 | 7.59 ± 0.02 | 5.85 ± 0.03 | 10.72 ± 0.03 | 5.97 ± 0.01 | |
| 30 | acetic acid, octyl ester | 1025.6 | 16.76 ± 0.14 | 10.65 ± 0.01 | 11.86 ± 0.01 | 9.36 ± 0.01 | |
| 31 | 2-undrcanone | 1128.8 | 2.78 ± 0.01 | 4.30 ± 0.02 | 12.82 ± 0.01 | 5.28 ± 0.01 | |
| 32 | benzaldehyde, 4-(1-methylethylidene) | 1066.9 | 1.45 ± 0.01 | 1.30 ± 0.01 | ND | 1.02 ± 0.01 | |
| 33 | 2-decanone | 1050.8 | ND | 9.49 ± 0.01 | ND | ND | |
| 34 | 1-decanol | 1100.2 | ND | ND | ND | 1.10 ± 0.01 | |
| 35 | 2,6-octadiene-1-ol, 3,7-dimethyl-, acetate | 1001.8 | ND | 3.23 ± 0.02 | ND | ND | |
| 36 | tetradecanal | 1150.7 | ND | 1.87 ± 0.01 | 1.95 ± 0.01 | ND | |
| 37 | D-germacrene | 1226.3 | 1490 | ND | 1.45 ± 0.01 | 2.50 ± 0.01 | ND |
| 38 | neryl acetate | 1200.0 | ND | 2.34 ± 0.01 | 6.56 ± 0.01 | 3.65 ± 0.01 | |
| 39 | dodecanal | 1200.2 | ND | ND | 2.32 ± 0.01 | 2.02 ± 0.01 | |
| 40 | 1-tetradecanol | 1299.8 | 1257 | ND | 1.80 ± 0.01 | ND | ND |
| 41 | linalyl acetate | 1051.2 | 5.51 ± 0.04 | ND | ND | ND | |
| 42 | geranly acetate | 1233.3 | 1385 | 14.55 ± 0.12 | ND | ND | ND |
| 43 | 2-tridecanone | 1288.7 | 1820 | 5.97 ± 0.02 | 2.18 ± 0.01 | 1.89 ± 0.01 | 1.35 ± 0.01 |
| 44 | hexadecanoic acid | 1501.1 | ND | 2.69 ± 0.01 | 3.57 ± 0.01 | 10.18 ± 0.04 | |
| Day/Odour Attributes | Citrus | Herb | Pine | Spice | Sweet | Woody |
|---|---|---|---|---|---|---|
| MK15 | 10.0 ± 1.10 a | 3.1 ± 1.03 a | 5.2 ± 1.38 a | 5.2 ± 1.24 a | 3.9 ± 0.90 b | 4.2 ± 0.49 b |
| MK36 | 8.1 ± 1.17 a | 3.5 ± 0.77 a | 8.4 ± 0.51 b | 5.6 ± 1.03 a | 2.8 ± 0.73 ab | 2.6 ± 0.53 a |
| MK45 | 10.1 ± 0.95 a | 2.7 ± 0.73 a | 8.5 ± 0.45 b | 3.7 ± 0.89 a | 3.1 ± 0.40 ab | 1.8 ± 0.58 a |
| MK60 | 9.5 ± 0.58 a | 3.6 ± 0.93 a | 5.4 ± 1.34 a | 4.3 ± 1.34 a | 1.7 ± 0.44 a | 3.2 ± 0.37 ab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriwichai, T.; Junmahasathien, T.; Sookwong, P.; Potapohn, N.; Sommano, S.R. Evaluation of the Optimum Harvesting Maturity of Makhwaen Fruit for the Perfumery Industry. Agriculture 2019, 9, 78. https://doi.org/10.3390/agriculture9040078
Sriwichai T, Junmahasathien T, Sookwong P, Potapohn N, Sommano SR. Evaluation of the Optimum Harvesting Maturity of Makhwaen Fruit for the Perfumery Industry. Agriculture. 2019; 9(4):78. https://doi.org/10.3390/agriculture9040078
Chicago/Turabian StyleSriwichai, Trid, Taepin Junmahasathien, Phumon Sookwong, Nuttha Potapohn, and Sarana Rose Sommano. 2019. "Evaluation of the Optimum Harvesting Maturity of Makhwaen Fruit for the Perfumery Industry" Agriculture 9, no. 4: 78. https://doi.org/10.3390/agriculture9040078
APA StyleSriwichai, T., Junmahasathien, T., Sookwong, P., Potapohn, N., & Sommano, S. R. (2019). Evaluation of the Optimum Harvesting Maturity of Makhwaen Fruit for the Perfumery Industry. Agriculture, 9(4), 78. https://doi.org/10.3390/agriculture9040078






